Effect of Al (III) Ions on the Separation of Cassiterite and Clinochlore Through Reverse Flotation
Abstract
:1. Introduction
2. Materials and Methods
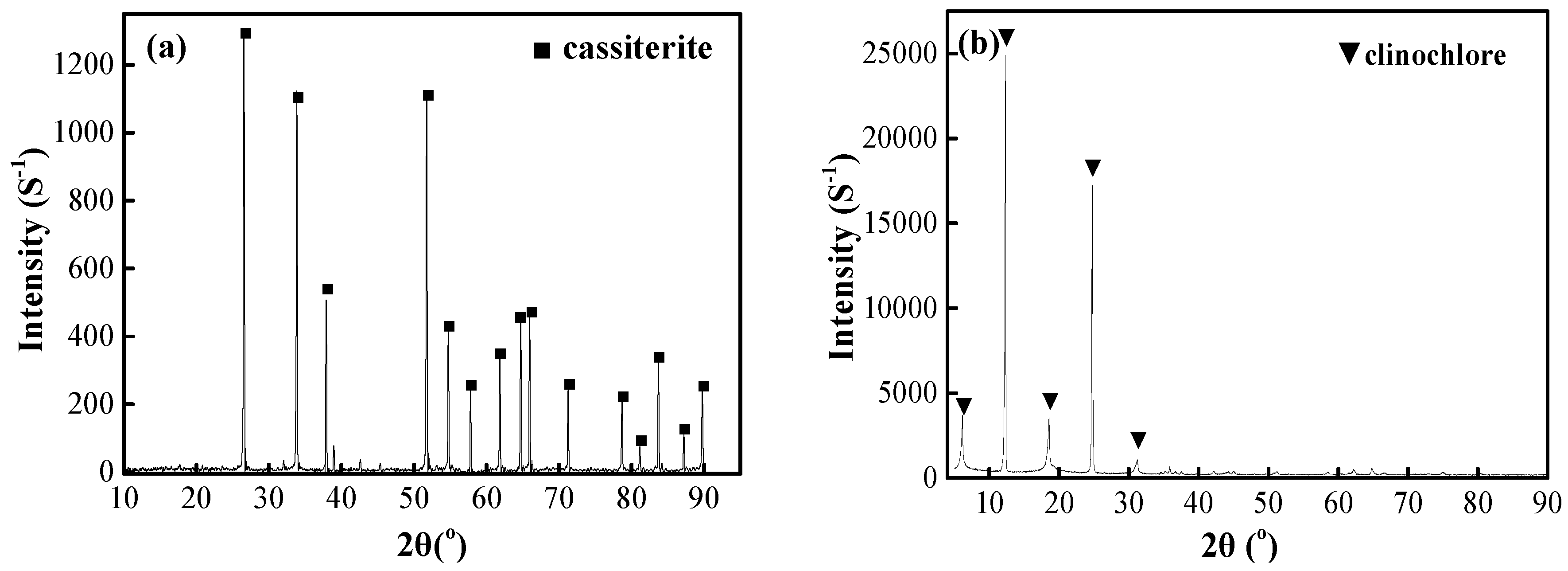
2.1. Materials
2.2. Methods
2.2.1. Flotation Tests
2.2.2. Adsorption Tests
2.2.3. Contact Angle Measurements
2.2.4. XPS Analysis
3. Results and Discussion
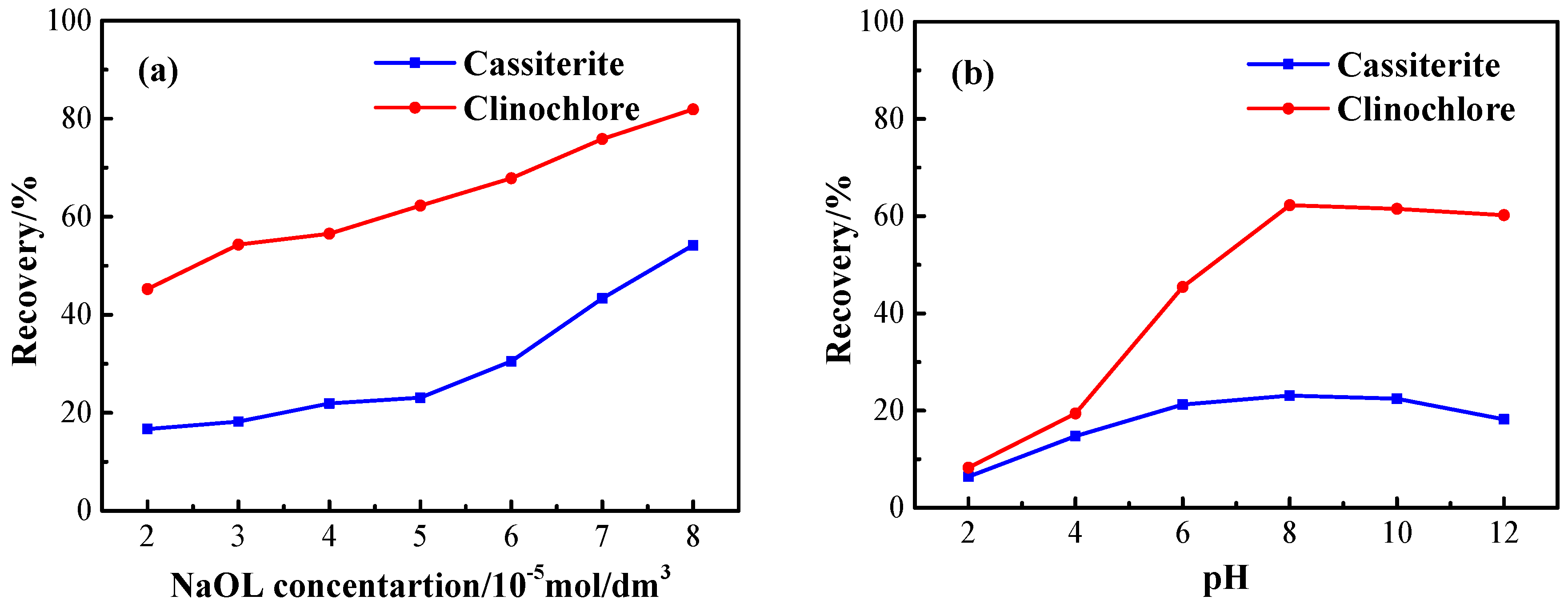
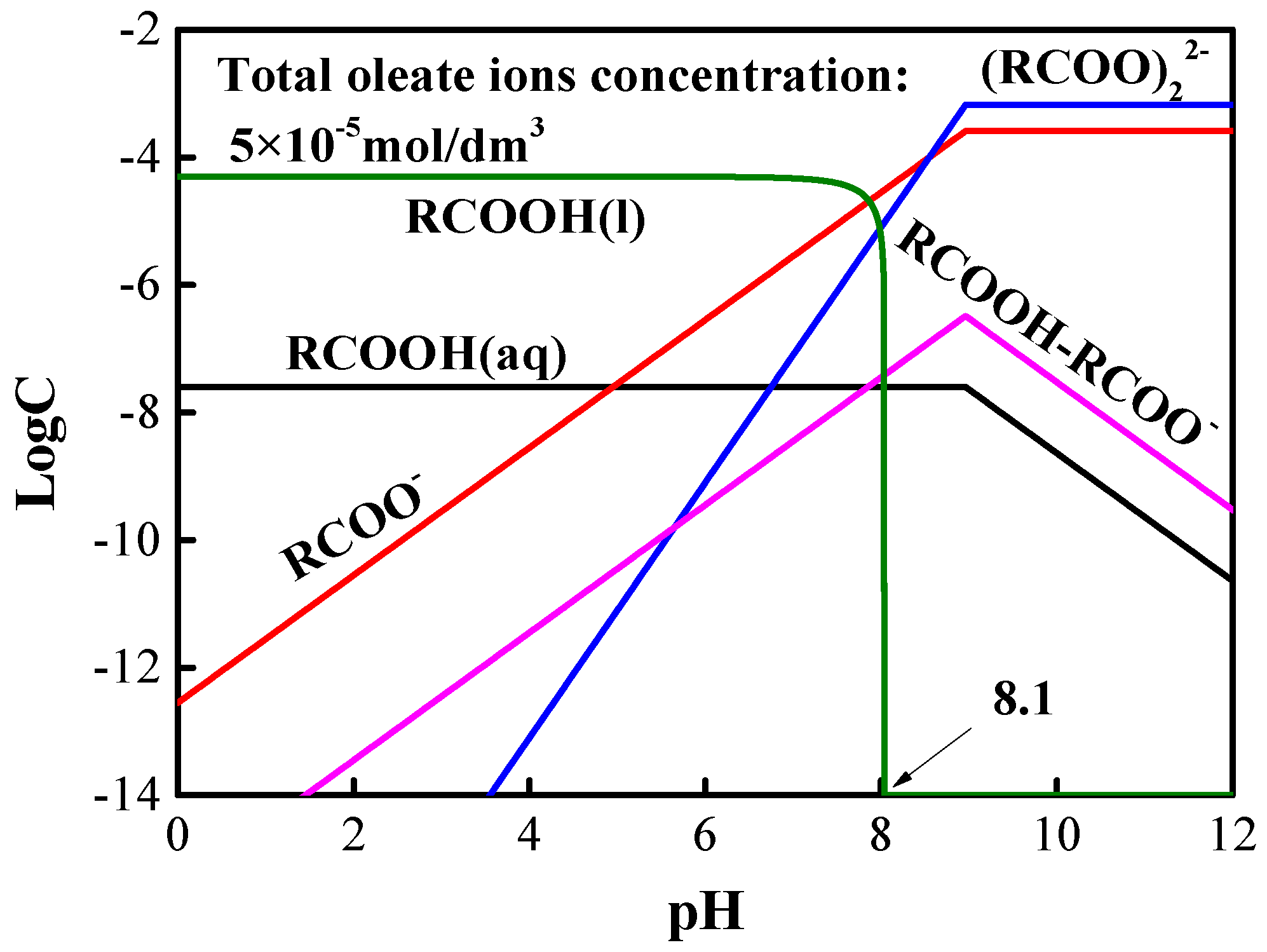
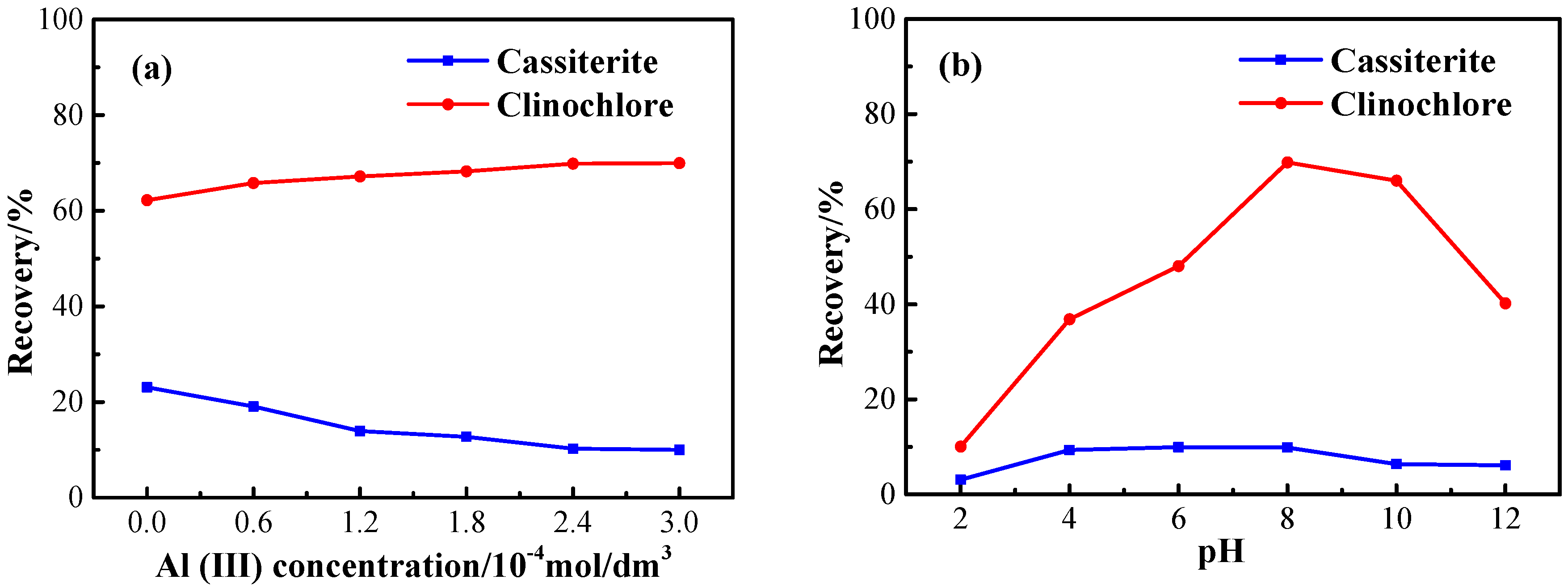
3.1. Flotation Tests
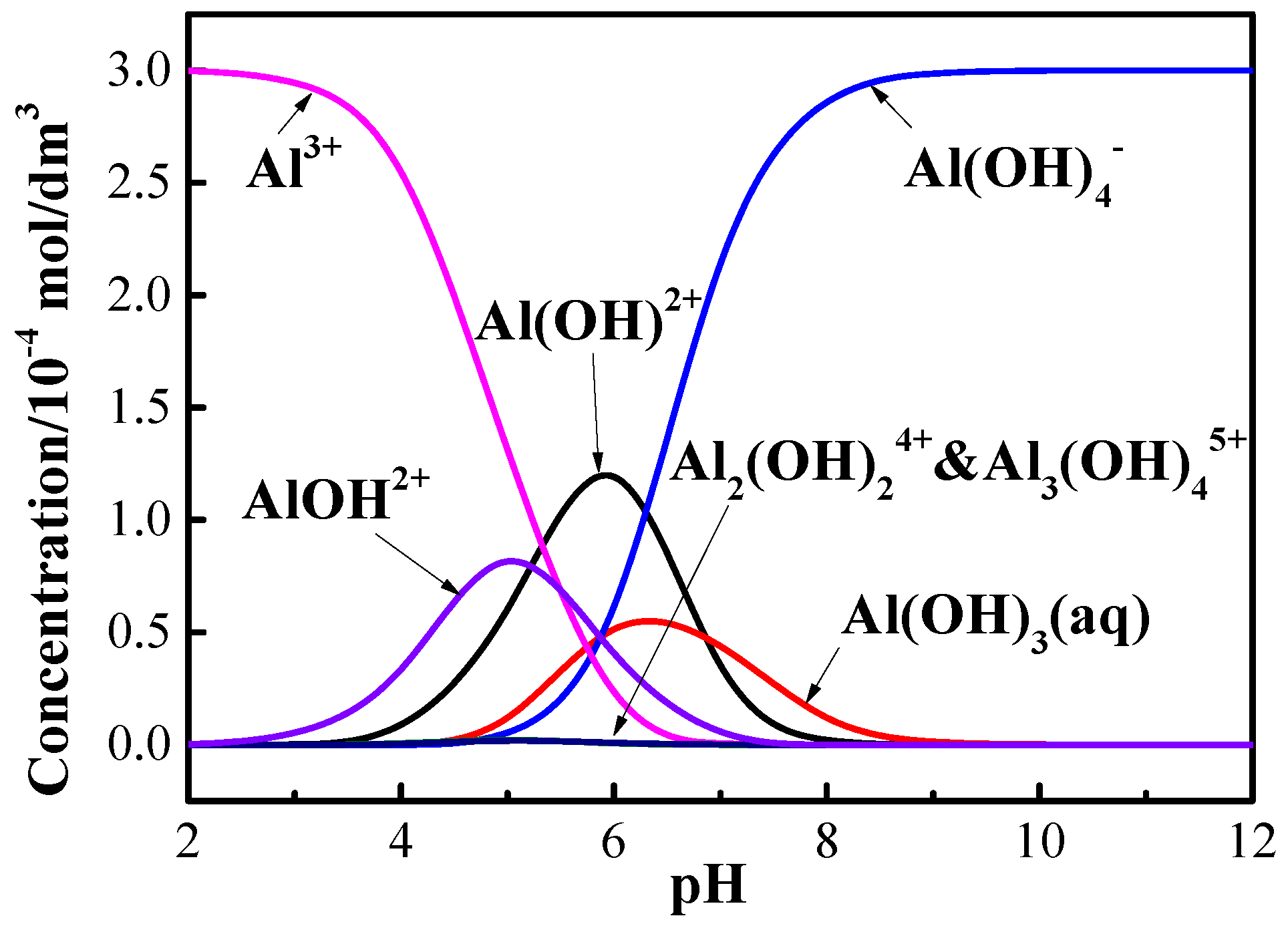
3.2. Adsorption Tests
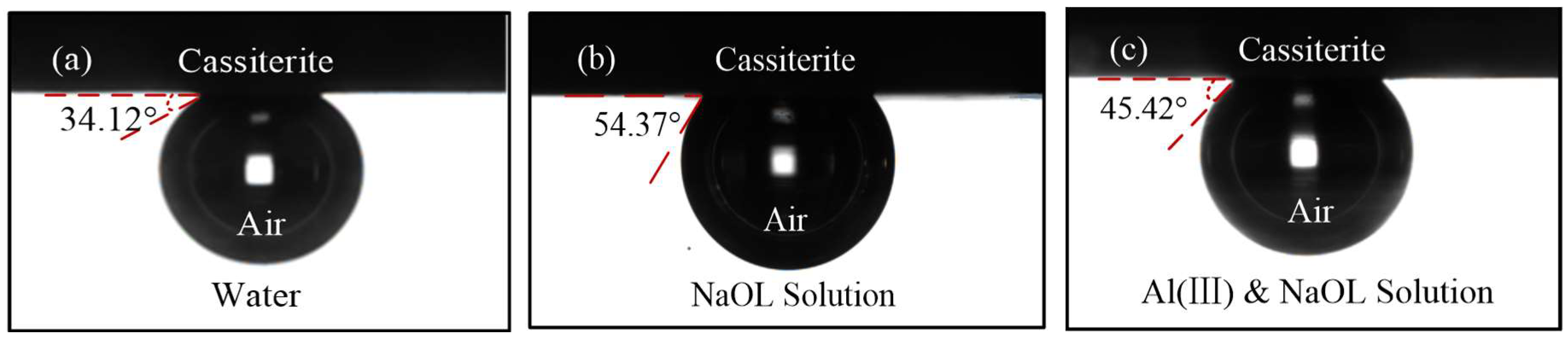
3.3. Contact Angle Measurements
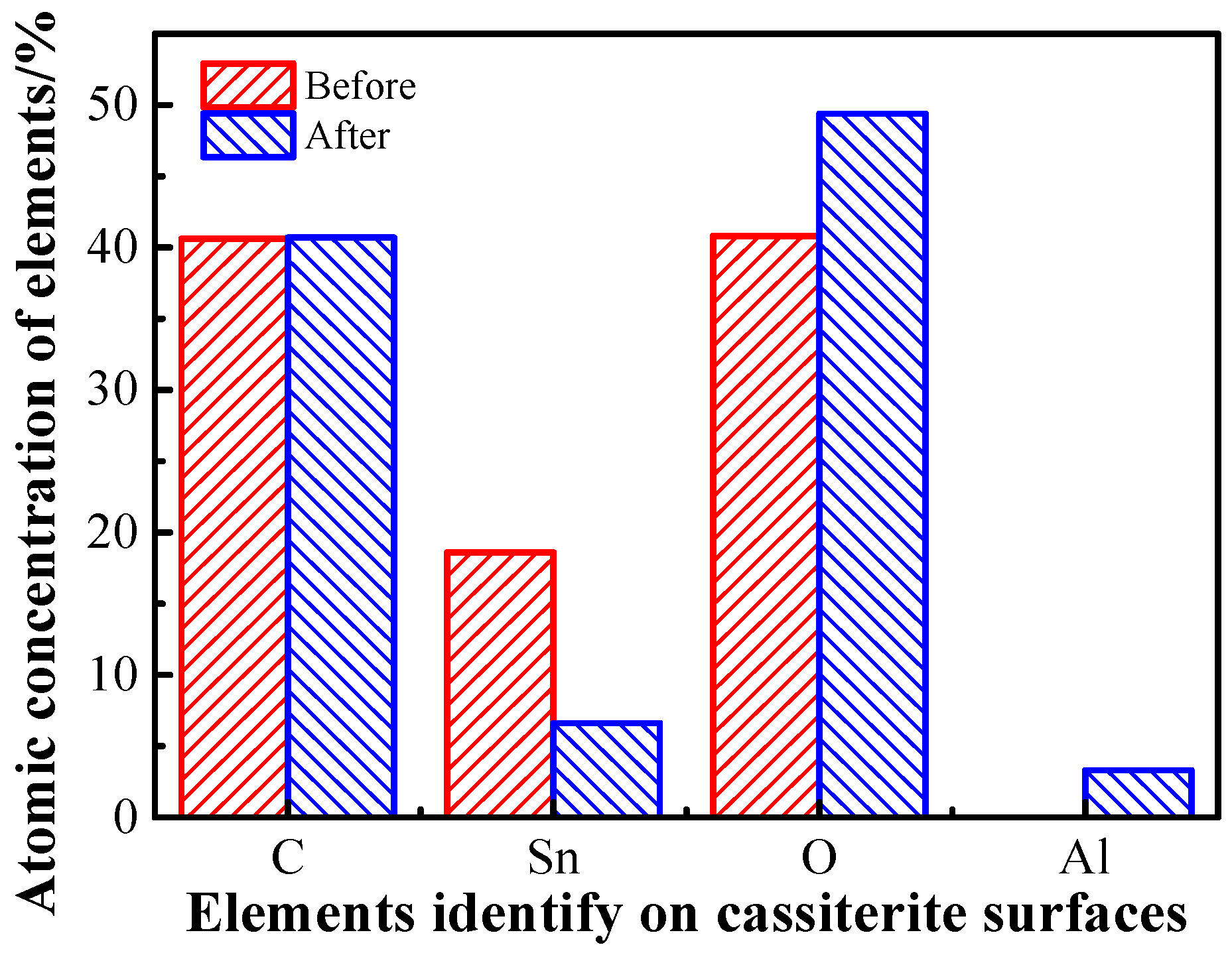
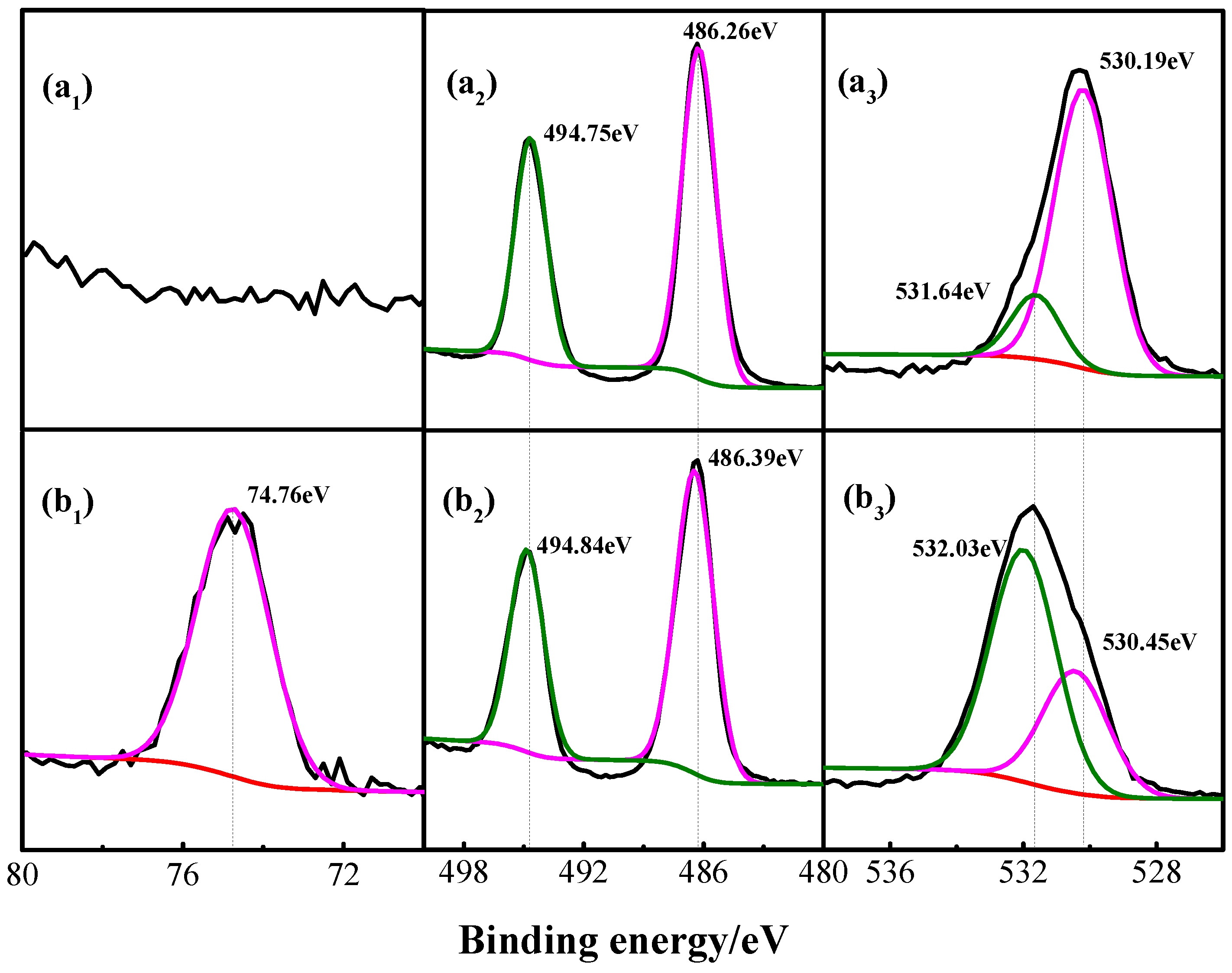
3.4. XPS Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Angadi, S.I.; Sreenivas, T.; Jeon, H.S.; Baek, S.H.; Mishra, B.K. A review of cassiterite beneficiation fundamentals and plant practices. Miner. Eng. 2015, 70, 178–200. [Google Scholar] [CrossRef]
- Tian, M.; Liu, R.; Gao, Z.; Chen, P.; Han, H.; Wang, L.; Zhang, C.; Sun, W.; Hu, Y. Activation mechanism of Fe (III) ions in cassiterite flotation with benzohydroxamic acid collector. Miner. Eng. 2018, 119, 31–37. [Google Scholar] [CrossRef]
- Liao, Z.; Liu, Y.P.; Li, C.Y. Characteristics of chlorites from dulong Sn-Zn deposit and their metallogenic implications. Miner. Deposit. 2010, 29, 169–176. [Google Scholar]
- Edwards, C.R.; Kipkie, W.B.; Agar, G.E. The effect of slime coatings of the serpentine minerals, chrysotile and lizardite, on pentlandite flotation. Int. J. Miner. Process. 1980, 7, 33–42. [Google Scholar] [CrossRef]
- Peng, Y.; Zhao, S. The effect of surface oxidation of copper sulfide minerals on clay slime coating in flotation. Miner. Eng. 2011, 24, 1687–1693. [Google Scholar] [CrossRef]
- Wu, X.Q.; Zhu, J.G. Selective flotation of cassiterite with benzohydroxamic acid. Miner. Eng. 2006, 19, 1410–1417. [Google Scholar] [CrossRef]
- Cheng, T.W.; Holtham, P.N.; Tran, T. Froth flotation of monazite and xenotime. Miner. Eng. 1993, 6, 341–351. [Google Scholar] [CrossRef]
- Leistner, T.; Embrechts, M.; Leißner, T.; Chelgani, S.C.; Osbahr, I.; Möckel, R.; Peuker, U.A.; Rudolph, M. A study of the reprocessing of fine and ultrafine cassiterite from gravity tailing residues by using various flotation techniques. Miner. Eng. 2016, 96–97, 94–98. [Google Scholar] [CrossRef]
- Xing, Y.; Gui, X.; Pan, L.; Pinchasik, B.E.; Cao, Y.; Liu, J.; Kappl, M.; Butt, H.J. Recent experimental advances for understanding bubble-particle attachment in flotation. Adv. Colloid Interface Sci. 2017, 246, 105–132. [Google Scholar] [CrossRef] [PubMed]
- Chau, T.T. A review of techniques for measurement of contact angles and their applicability on mineral surfaces. Miner. Eng. 2009, 22, 213–219. [Google Scholar] [CrossRef]
- Ren, L.; Qiu, H.; Zhang, M.; Feng, K.; Liu, P.; Guo, J.; Feng, J. The behavior of lead ions in cassiterite flotation using octanohydroxamic acid. Ind. Eng. Chem. Res. 2017, 56, 8723–8728. [Google Scholar] [CrossRef]
- Yin, W.; Fu, Y.; Yao, J.; Yang, B.; Cao, S.; Sun, Q. Study on the dispersion mechanism of citric acid on chlorite in hematite reverse flotation system. Minerals 2017, 7, 221. [Google Scholar]
- Zheng, G.S.; Liu, L.J.; Liu, J.T.; Wang, Y.T.; Cao, Y.J. Study of chlorite flotation and its influencing factors. Procedia Earth. Planetary Sci. 2009, 1, 830–837. [Google Scholar]
- Neethling, S.J.; Cilliers, J.J. The entrainment of gangue into a flotation froth. Int. J. Miner. Process. 2002, 64, 123–134. [Google Scholar] [CrossRef]
- Yu, Y.; Ma, L.; Cao, M.; Liu, Q. Slime coatings in froth flotation: A review. Miner. Eng. 2017, 114, 26–36. [Google Scholar] [CrossRef]
- Feng, Q.; Zhao, W.; Wen, S.; Cao, Q. Activation mechanism of lead ions in cassiterite flotation with salicylhydroxamic acid as collector. Sep. Purif. Technol. 2017, 178, 193–199. [Google Scholar] [CrossRef]
- Shrimali, K.; Atluri, V.; Wang, Y.; Bacchuwar, S.; Wang, X.; Miller, J.D. The nature of hematite depression with corn starch in the reverse flotation of iron ore. J. Colloid Interface Sci. 2018, 524, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, Y.; He, P.; Gu, G. Reverse flotation for removal of silicates from diasporic-bauxite. Miner. Eng. 2004, 17, 63–68. [Google Scholar] [CrossRef]
- Yao, J.; Yin, W.; Gong, E. Depressing effect of fine hydrophilic particles on magnesite reverse flotation. Int. J. Miner. Process. 2016, 149, 84–93. [Google Scholar] [CrossRef] [Green Version]
- Xing, Y.; Xu, M.; Gui, X.; Cao, Y.; Babel, B.; Rudolph, M.; Weber, S.; Kappl, M.; Butt, H.J. The application of atomic force microscopy in mineral flotation. Adv. Colloid Interface Sci. 2018, 256, 373–392. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, M.; Liu, Q. The effect of non-polar oil on fine hematite flocculation and flotation using sodium oleate or hydroxamic acids as a collector. Miner. Eng. 2018, 119, 105–115. [Google Scholar] [CrossRef]
- Roonasi, P.; Yang, X.; Holmgren, A. Competition between sodium oleate and sodium silicate for a silicate/oleate modified magnetite surface studied by in situ atr-ftir spectroscopy. J. Colloid Interface Sci. 2010, 343, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Qin, W. Surface analysis of cassiterite with sodium oleate in aqueous solution. Sep. Sci. Technol. 2012, 47, 502–506. [Google Scholar] [CrossRef]
- Liu, J.; Gong, G.; Han, Y.; Zhu, Y. New insights into the adsorption of oleate on cassiterite: A DFT study. Minerals 2017, 7, 236. [Google Scholar] [CrossRef]
- Schubert, H.; Schoenherr, J.; Schubert, H. Alkane dicarboxylic acids and aminonaphthol-sulfonic acids—A new reagent regime for cassiterite flotation. Int. J. Miner. Process. 1985, 15, 117–133. [Google Scholar]
- Tian, M.; Gao, Z.; Han, H.; Sun, W.; Hu, Y. Improved flotation separation of cassiterite from calcite using a mixture of lead (ii) ion/benzohydroxamic acid as collector and carboxymethyl cellulose as depressant. Miner. Eng. 2017, 113, 68–70. [Google Scholar] [CrossRef]
- Ren, Z.; Yu, F.; Gao, H.; Chen, Z.; Peng, Y.; Liu, L. Selective separation of fluorite, barite and calcite with valonea extract and sodium fluosilicate as depressants. Minerals 2017, 7, 24. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, Z.; Sun, W.; Hu, Y. Selective flotation of scheelite from calcite: A novel reagent scheme. Int. J. Miner. Process. 2016, 154, 10–15. [Google Scholar] [CrossRef]
- Zeng, Q.; Hong, Z.; Wang, D. Influence of metal cations on cassiterite flotation. Trans. Nonferr. Metal. Soc. 2000, 10, 98–101. [Google Scholar]
- Choi, W.; Zeng, Q.; Jiang, E.; SeokJeon, H. Cassiterite flotation with sulphosuccinamate collector. Geosyst. Eng. 1998, 1, 30–34. [Google Scholar] [CrossRef]
- Quast, K. Literature review on the interaction of oleate with non-sulphide minerals using zeta potential. Miner. Eng. 2016, 94, 10–20. [Google Scholar] [CrossRef]
- Feng, Q.; Wen, S.; Zhao, W.; Chen, Y. Effect of calcium ions on adsorption of sodium oleate onto cassiterite and quartz surfaces and implications for their flotation separation. Sep. Purif. Technol. 2018, 200, 300–306. [Google Scholar] [CrossRef]
- Liu, C.; Chen, Y.; Song, S.; Li, H. The effect of aluminum ions on the flotation separation of pentlandite from lizardite. Colloid Surf. A-Physicochem. Eng. Asp. 2018, in press. [Google Scholar] [CrossRef]
- Medeiros, A.R.S.D.; Baltar, C.A.M. Importance of collector chain length in flotation of fine particles. Miner. Eng. 2018, 122, 179–184. [Google Scholar] [CrossRef]
- Hauert, R.; Patscheider, J.; Tobler, M.; Zehringer, R. XPS investigation of the a-C: H/Al interface. Surf. Sci. 1993, 292, 121–129. [Google Scholar] [CrossRef]
- Li, F.; Zhong, H.; Zhao, G.; Wang, S.; Liu, G. Flotation performances and adsorption mechanism of α-hydroxyoctyl phosphinic acid to cassiterite. Appl. Surf. Sci. 2015, 353, 856–864. [Google Scholar] [CrossRef]
- Nowak, P.; Laajalehto, K.; Kartio, I. A flotation related X-ray photoelectron spectroscopy study of the oxidation of galena surface. Colloid Surf. A-Physicochem. Eng. Asp. 2000, 161, 447–460. [Google Scholar] [CrossRef]
- Tian, M.; Zhang, C.; Han, H.; Liu, R.; Gao, Z.; Chen, P.; He, J.; Hu, Y.; Sun, W.; Yuan, D. Novel insights into adsorption mechanism of benzohydroxamic acid on lead (II)-activated cassiterite surface: An integrated experimental and computational study. Miner. Eng. 2018, 122, 327–338. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Tong, X.; Feng, D.; Xie, X. Effect of Al (III) Ions on the Separation of Cassiterite and Clinochlore Through Reverse Flotation. Minerals 2018, 8, 347. https://doi.org/10.3390/min8080347
Chen Y, Tong X, Feng D, Xie X. Effect of Al (III) Ions on the Separation of Cassiterite and Clinochlore Through Reverse Flotation. Minerals. 2018; 8(8):347. https://doi.org/10.3390/min8080347
Chicago/Turabian StyleChen, Yumeng, Xiong Tong, Dongxia Feng, and Xian Xie. 2018. "Effect of Al (III) Ions on the Separation of Cassiterite and Clinochlore Through Reverse Flotation" Minerals 8, no. 8: 347. https://doi.org/10.3390/min8080347
APA StyleChen, Y., Tong, X., Feng, D., & Xie, X. (2018). Effect of Al (III) Ions on the Separation of Cassiterite and Clinochlore Through Reverse Flotation. Minerals, 8(8), 347. https://doi.org/10.3390/min8080347



