Intercalation and Exfoliation of Kaolinite with Sodium Dodecyl Sulfate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation
2.3. Characterization
3. Results and Discussion
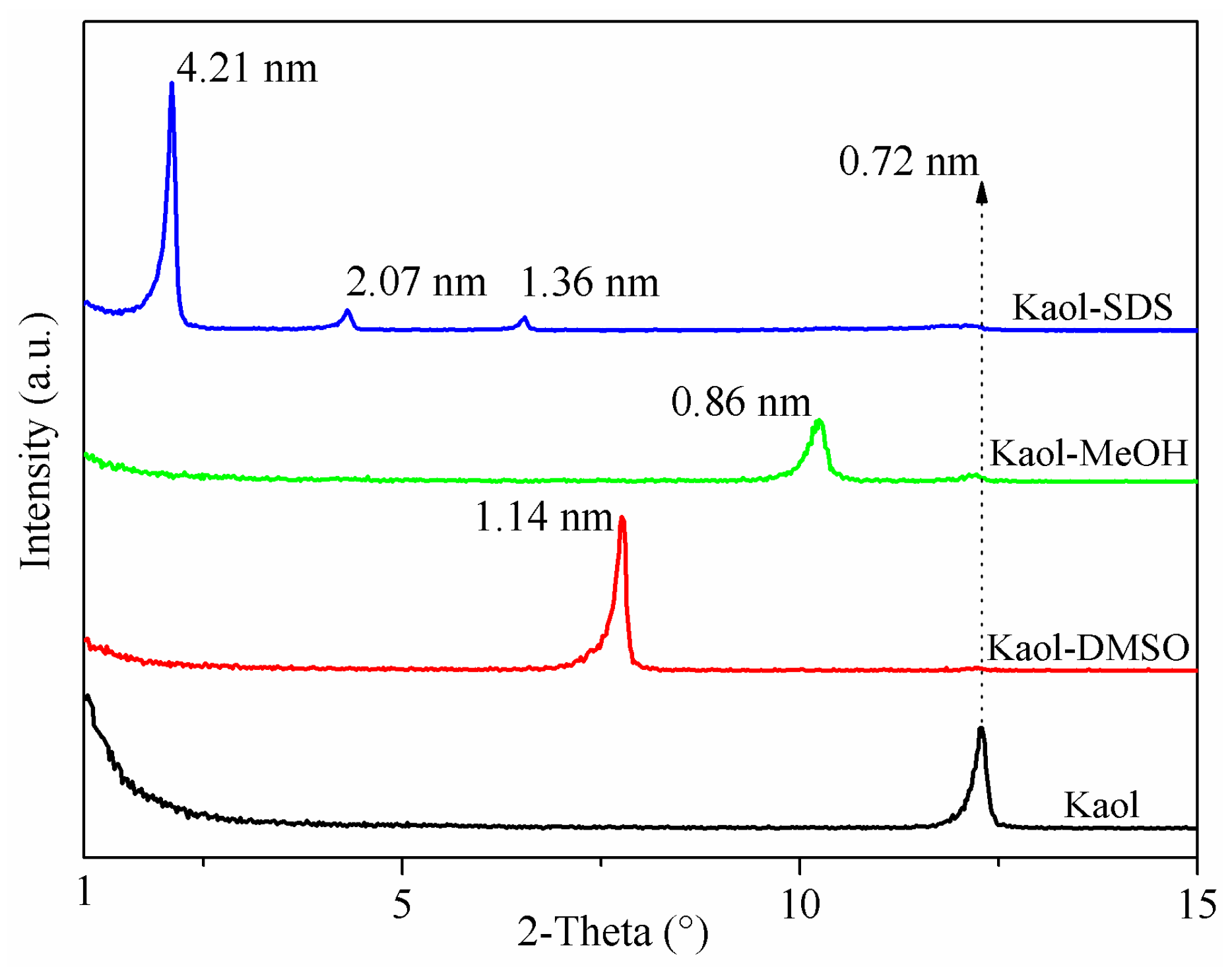
3.1. XRD Analysis
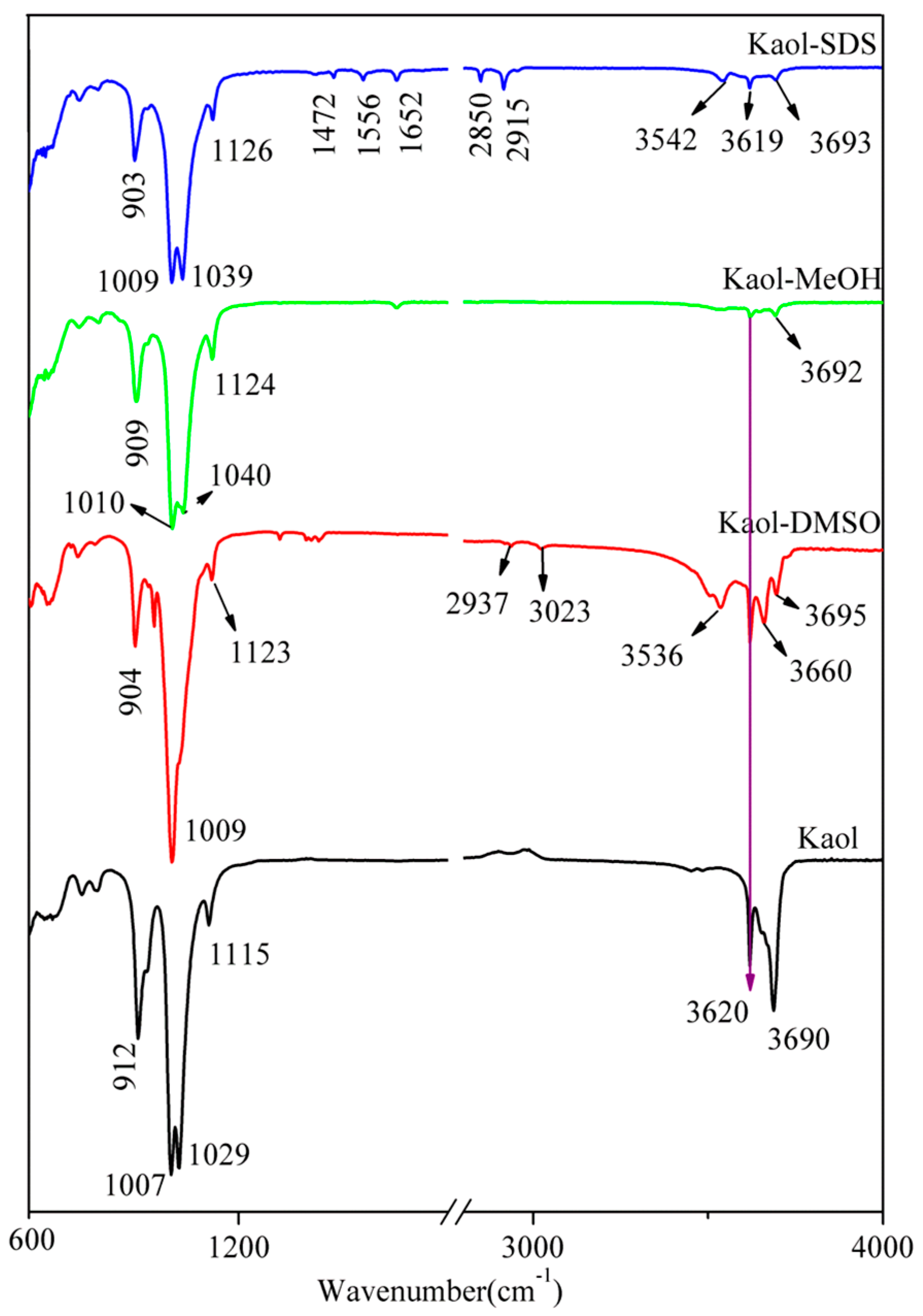
3.2. FTIR Analysis
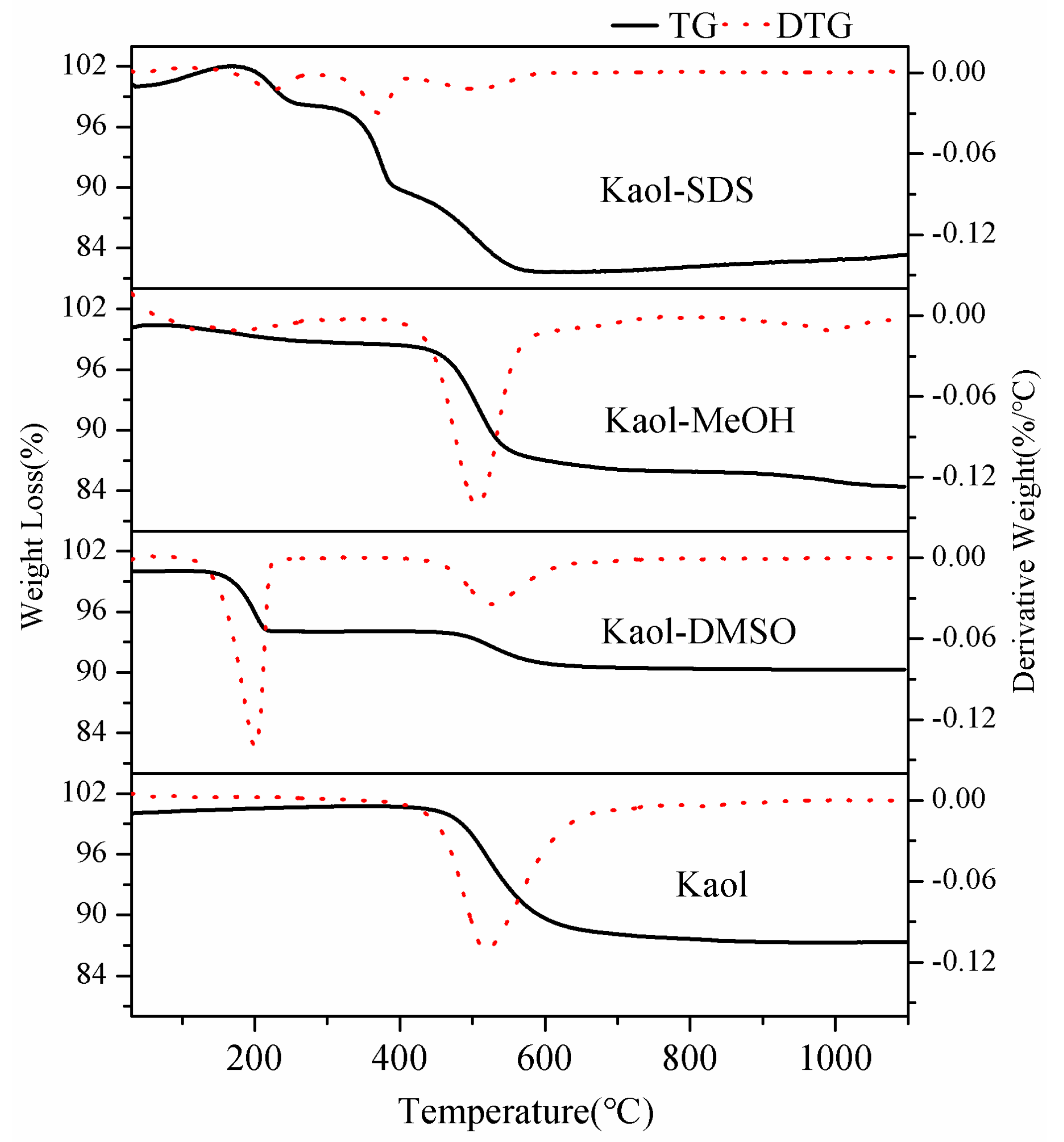
3.3. Thermal Analysis
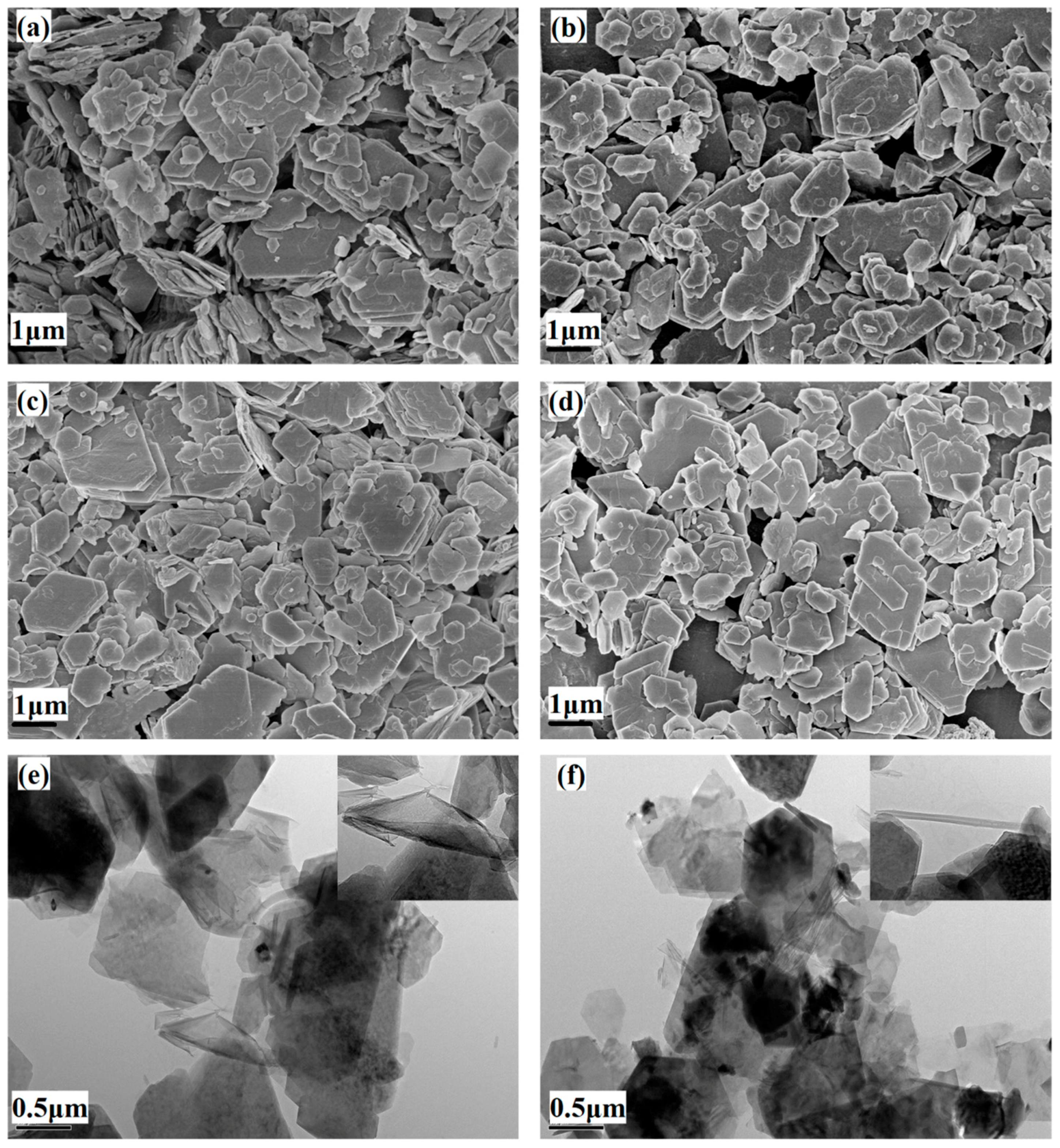
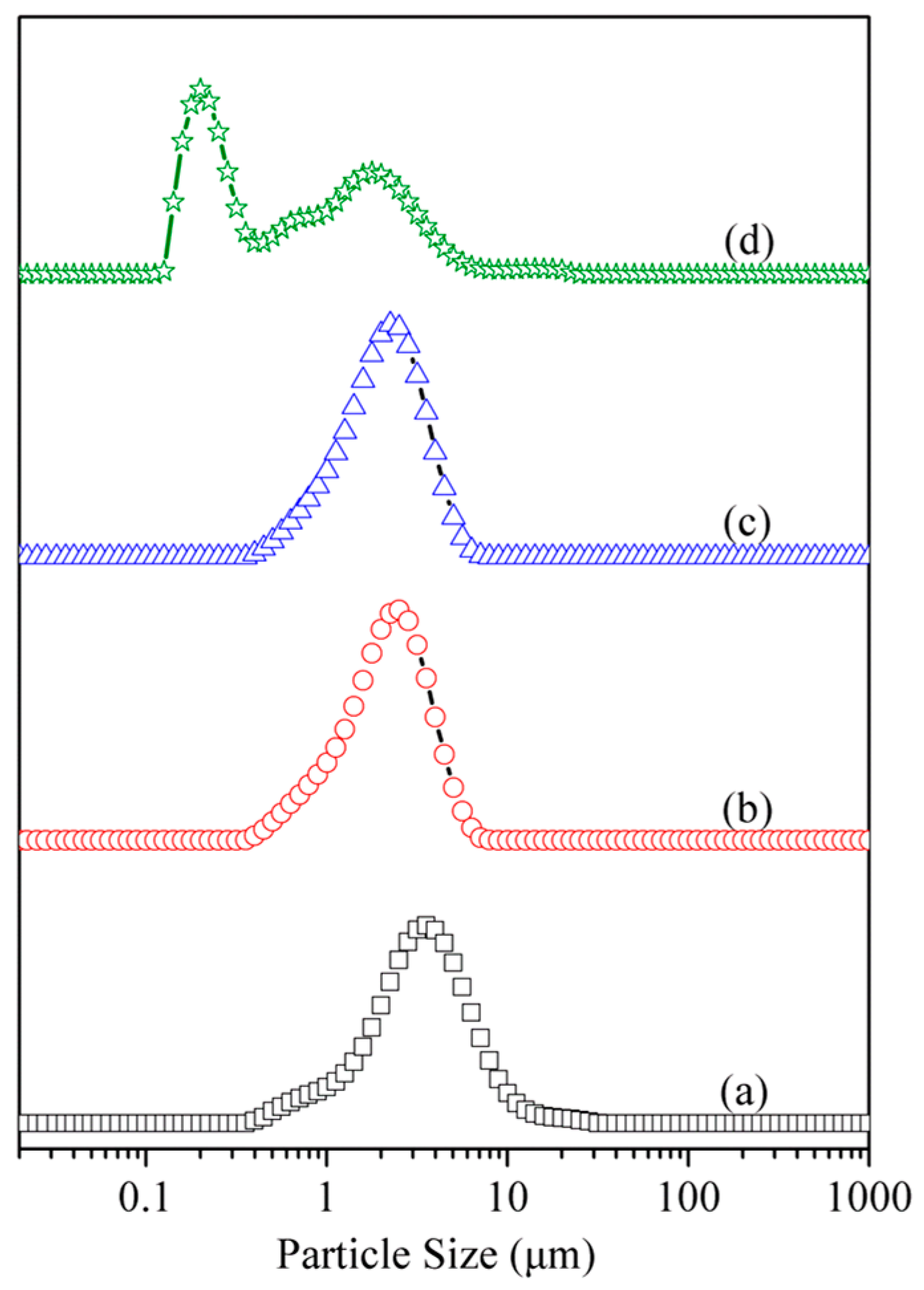
3.4. Particle Size and Morphology Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kotal, M.; Bhowmick, A.K. Polymer nanocomposites from modified clays: Recent advances and challenges. Prog. Polym. Sci. 2015, 51, 127–187. [Google Scholar] [CrossRef]
- Cui, Y.B.; Kumar, S.; Kona, B.R.; van Houcke, D. Gas barrier properties of polymer/clay nanocomposites. RSC Adv. 2015, 5, 63669–63690. [Google Scholar] [CrossRef]
- Zhang, Y.D.; Liu, Q.F.; Xiang, J.J.; Zhang, S.L.; Frost, R.L. Influence of the structural characteristic of pyrolysis products on thermal stability of styrene-butadiene rubber composites reinforced by different particle sized kaolinites. J. Therm. Anal. Calorim. 2014, 117, 1201–1210. [Google Scholar] [CrossRef]
- Bromberg, L.; Straut, C.M.; Centrone, A.; Wilusz, E.; Hatton, T.A. Montmorillonite functionalized with pralidoxime as a material for chemical protection against organophosphorous compounds. ACS Appl. Mater. Interface 2011, 3, 1479–1484. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.D.; Liu, Q.F.; Zhang, Q.; Lu, Y.P. Gas barrier properties of natural rubber/kaolin composites prepared by melt blending. Appl. Clay Sci. 2010, 50, 255–259. [Google Scholar] [CrossRef]
- Yahaya, L.E.; Adebowale, K.O.; Menon, A.R.R. Mechanical properties of organomodified kaolin/natural rubber vulcanizates. Appl. Clay Sci. 2009, 46, 283–288. [Google Scholar] [CrossRef]
- Mirzadeh, A.; Kokabi, M. The effect of composition and draw-down ratio on morphology and oxygen permeability of polypropylene nanocomposite blown films. Eur. Polym. J. 2007, 43, 3757–3765. [Google Scholar] [CrossRef]
- Cabeda, L.; Gimenez, E.; Lagaron, J.M.; Gavara, R.; Saura, J.J. Development of EVOH-kaolinite nanocomposites. Polymer 2004, 45, 5233–5238. [Google Scholar] [CrossRef]
- Bundy, W.; Ishley, J. Kaolin in paper filling and coating. Appl. Clay Sci. 1991, 5, 397–420. [Google Scholar] [CrossRef]
- Xia, X.N.; Zeng, X.L.; Liu, J.; Xu, W.J. Preparation and characterization of epoxy/kaolinite nanocomposites. J. Appl. Polym. Sci. 2010, 118, 2461–2466. [Google Scholar] [CrossRef]
- Cheng, H.F.; Zhang, Z.L.; Liu, Q.F.; Leung, J. A new method for determining platy particle aspect ratio: A kaolinite case study. Appl. Clay Sci. 2014, 97–98, 125–131. [Google Scholar] [CrossRef]
- Lu, C.S.; Mai, Y.W. Influence of aspect ratio on barrier properties of polymer-clay nanocomposites. Phys. Rev. Lett. 2005, 95, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.L.; Liu, Q.F.; Yang, Y.J.; Wang, D.; He, J.K.; Sun, L.Y. Preparation; morphology; and structure of kaolinites with various aspect ratios. Appl. Clay Sci. 2017, 147, 117–122. [Google Scholar] [CrossRef]
- Zoromba, M.S.; Belal, A.A.M.; Ali, A.E.M.; Helaly, F.M.; Abd El-Hakim, A.A.; Badran, A.S. Preparation and characterization of some NR and SBR formulations containing different modified kaolinite. Polym. Plast. Technol. Eng. 2007, 46, 529–535. [Google Scholar] [CrossRef]
- Zhang, Y.D.; Zhang, Q.; Liu, Q.F.; Cheng, H.F.; Frost, R.L. Thermal stability of styrene butadiene rubber (SBR) composites filled with kaolinite/silica hybrid filler. J. Therm. Anal. Calorim. 2014, 115, 1013–1020. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Liu, Q.F.; Zhang, S.L.; Zhang, Y.D.; Zhang, Y.F.; Liang, P. Characterization of kaolinite/styrene butadiene rubber composite: Mechanical properties and thermal stability. Appl. Clay Sci. 2016, 124–125, 167–174. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Q.F.; Zhang, Y.D.; Cheng, H.F.; Lu, Y.P. Silane-grafted silica-covered kaolinite as filler of styrene butadiene rubber. Appl. Clay Sci. 2012, 65–66, 134–138. [Google Scholar] [CrossRef]
- Cheng, H.F.; Liu, Q.F.; Zhang, J.S.; Yang, J.; Frost, R.L. Delamination of kaolinite-potassium acetate intercalates by ball-milling. J. Colloid Interface Sci. 2010, 348, 355–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuroda, Y.; Ito, K.; Itabshi, K.; Kuroda, K. One-Step Exfoliation of kaolinites and their transformation into nanoscrolls. Langmuir 2011, 27, 2028–2035. [Google Scholar] [CrossRef] [PubMed]
- Nicolosi, V.; Chhowalla, M.; Kanatzidis, M.G.; Strano, M.S.; Coleman, J.N. Liquid exfoliation of layered materials. Science 2013, 340, 1226419. [Google Scholar] [CrossRef]
- Pi, Z.B.; Liu, Z.Q.; Yang, C.; Tian, X.K.; Fei, J.B.; Zheng, J.H. Exfoliation of kaolinite by urea-intercalation precursor and microwave irradiation assistance process. Front. Earth Sci. China 2007, 1, 26–29. [Google Scholar] [CrossRef]
- Li, X.G.; Liu, Q.F.; Cheng, H.F.; Zhang, S.; Frost, R.L. Mechanism of kaolinite sheets curling via the intercalation and delamination process. J. Colloid Interface Sci. 2015, 444, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.C.; Wang, D.; Zhang, S.L.; Liu, Q.F.; Yang, H.M. Effect of intercalation agents on morphology of exfoliated kaolinite. Minerals 2017, 7, 249. [Google Scholar] [CrossRef]
- Makó, É.; Kristóf, J.; Horváth, E.; Vágvölgyi, V. Kaolinite-urea complexes obtained by mechanochemical and aqueous suspension techniques—A comparative study. J. Colloid Interface Sci. 2009, 330, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.F.; Liu, Q.F.; Liu, J.; Sun, B.; Kang, Y.X.; Frost, R.L. TG-MS-FTIR (evolved gas analysis) of kaolinite-urea intercalation complex. J. Therm. Anal. Calorim. 2014, 116, 195–203. [Google Scholar] [CrossRef]
- Long, M.; Zhang, Y.; Shu, Z.; Tang, A.D.; Ouyang, J.; Yang, H.M. Fe2O3 nanoparticles anchored on 2D kaolinite with enhanced antibacterial activity. Chem. Commun. 2017, 5346, 6255–6258. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Zhang, Y.; Huang, P.; Chang, S.; Hu, Y.H.; Yang, Q.; Mao, L.F.; Yang, H.M. Emerging nanoclay composite for effective hemostasis. Adv. Funct. Mater. 2017. [Google Scholar] [CrossRef]
- Zhang, Y.; Long, M.; Huang, P.; Chang, S.; Hu, Y.H.; Yang, H.M.; Tang, A.D.; Mao, L.F. Intercalated 2D nanoclay for emerging drug delivery in cancer therapy. Nano Res. 2017, 10, 2633–2643. [Google Scholar] [CrossRef]
- Frost, R.L.; Kristof, J.; Paroz, G.N.; Kloprogge, J.T. Modification of the kaolinite hydroxyl surfaces through intercalation with potassium acetate under pressure. J. Colloid Interface Sci. 1998, 208, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Y.; Yang, H.M. Composite of coal-series kaolinite and capric-lauric acid as form-stable phase-change material. Energy Technol. 2015, 3, 77–83. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Q.F.; Cheng, H.F.; Zeng, F.G. Combined experimental and theoretical investigation of interactions between kaolinite inner surface and intercalated dimethyl sulfoxide. Appl. Surf. Sci. 2015, 331, 234–240. [Google Scholar] [CrossRef]
- Fu, L.J.; Yang, H.M. Structure and electronic properties of transition metal doped kaolinite nanoclay. Nanoscale Res. Lett. 2017, 12, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.L.; Fu, L.J.; Hu, Y.H.; Yang, H.M. Functionalized 2D Clay derivative: Hybrid nanosheets with unique lead sorption behaviors and interface structure. Adv. Mater. Interface 2017. [Google Scholar] [CrossRef]
- Yan, Z.L.; Fu, L.J.; Zuo, X.C.; Yang, H.M. Green assembly of stable and uniform silver nanoparticles on 2D silica nanosheets for catalytic reduction of 4-nitrophenol. Appl. Catal. B Environ. 2018, 226, 23–30. [Google Scholar] [CrossRef]
- Yan, Z.L.; Fu, L.J.; Zuo, X.C.; Yang, H.M.; Ouyang, J. Amino-functionalized Hierarchical Porous SiO2-AlOOH Composite Nanosheets with Enhanced Adsorption Performance. J. Hazard. Mater. 2018, 344, 1090–1100. [Google Scholar] [CrossRef]
- Fu, L.J.; Yang, H.M.; Hu, Y.H.; Wu, D.; Navrotsky, A. Tailoring mesoporous γ-Al2O3 properties by transition metal doping: A combined experimental and computational study. Chem. Mater. 2017, 29, 1338–1349. [Google Scholar] [CrossRef]
- Yan, Z.L.; Yang, H.M.; Ouyang, J.; Tang, A.D. In situ loading of highly-dispersed CuO nanoparticles on hydroxyl-group-rich SiO2-AlOOH composite nanosheets for CO catalytic oxidation. Chem. Eng. J. 2017, 316, 1035–1046. [Google Scholar] [CrossRef]
- Peng, K.; Fu, L.J.; Li, X.Y.; Ouyang, J.; Yang, H.M. Stearic acid modified montmorillonite as emerging microcapsules for thermal energy storage. Appl. Clay Sci. 2017, 138, 100–106. [Google Scholar] [CrossRef]
- Liu, S.Y.; Yan, Z.L.; Fu, L.J.; Yang, H.M. Hierarchical nano-activated silica nanosheets for thermal energy storage. Sol. Energy Mater. Sol. Cells 2017, 167, 140–149. [Google Scholar] [CrossRef]
- Shu, Z.; Zhang, Y.; Yang, Q.; Yang, H.M. Halloysite nanotubes supported Ag and ZnO nanoparticles with synergistically enhanced antibacterial activity. Nanoscale Res. Lett. 2017, 12, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Wen, X.; Yan, P.; Tang, A.D.; Yang, H.M. Tin oxide-carbon coated sepiolite nanofibers with enhanced lithium-ion storage property. Nanoscale Res. Lett. 2017, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Ouyang, J.; Yang, H.M. Pd nanoparticles and MOFs synergistically hybridized halloysite nanotubes for hydrogen storage. Nanoscale Res. Lett. 2017, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Ouyang, J.; Zheng, C.H.; Zhang, J.H.; Yang, H.M. Chemically modified sepiolite fibers for reinforcing resin brake composites. Mater. Express 2017, 7, 104–112. [Google Scholar] [CrossRef]
- Shen, Q.; Ouyang, J.; Zhang, Y.; Yang, H.M. Lauric acid/modified sepiolite composite as a form-stable phase change material for thermal energy storage. Appl. Clay Sci. 2017, 146, 14–22. [Google Scholar] [CrossRef]
- Peng, K.; Yang, H.M. Carbon hybridized montmorillonite nanosheets: Preparation, structural evolution and enhanced adsorption performance. Chem. Commun. 2017, 53, 6085–6088. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.J.; Yang, H.M.; Tang, A.D.; Hu, Y.H. Engineering a tubular mesoporous silica nanocontainer with well-preserved clay shell from natural halloysite. Nano Res. 2017, 10, 2782–2799. [Google Scholar] [CrossRef]
- Komori, Y.; Sugahara, Y. A kaolinite-NMF-methanol intercalation compound as a versatile intermediate for further intercalation reaction of kaolinite. J. Mater. Res. 1998, 13, 930–934. [Google Scholar] [CrossRef]
- Komori, Y.; Sugahara, Y.; Kuroda, K. Intercalation of alkylamines and water into kaolinite with methanol kaolinite as an intermediate. Appl. Clay Sci. 1999, 15, 241–252. [Google Scholar] [CrossRef]
- Matusik, J.; Kłapyta, Z. Characterization of kaolinite intercalation compounds withbenzylalkylammonium chlorides using XRD; TGADTA and CHNS elemental analysis. Appl. Clay Sci. 2013, 83–84, 433–440. [Google Scholar] [CrossRef]
- Matusik, J.; Kłapyta, Z.; Olejniczak, Z. NMR and IR study of kaolinite intercalation compounds with benzylalkylammonium chlorides. Appl. Clay Sci. 2013, 83–84, 426–432. [Google Scholar] [CrossRef]
- Gardolinski, J.E.F.C.; Lagaly, G. Grafted organic derivatives of kaolinite: I. Synthesis; chemical and rheological characterization. Clay Miner. 2005, 40, 537–546. [Google Scholar] [CrossRef]
- Gardolinski, J.E.F.C.; Lagaly, G. Grafted organic derivatives of kaolinite: II. Intercalation of primary n-alkylamines and delamination. Clay Miner. 2005, 40, 547–556. [Google Scholar] [CrossRef]
- Liu, Q.F.; Li, X.; Cheng, H.F. Insight into the self-adaptive deformation of kaolinite layers into nanoscrolls. Appl. Clay Sci. 2016, 124–125, 175–182. [Google Scholar] [CrossRef]
- Sidheswaran, P.; Bhat, A.N.; Ganguli, P. Intercalation of salts of fatty acids into kaolinite. Clays Clay Miner. 1990, 38, 29–32. [Google Scholar] [CrossRef]
- Wang, S.; Zuo, X.; Cheng, H.F.; Yang, Y.; Liu, Q.F. Structural model and de-intercalation kinetics of kaolinite-methanol-sodium stearate intercalation compound. J. Braz. Chem. Soc. 2016, 27, 1311–1318. [Google Scholar] [CrossRef]
- Li, X.G.; Cui, X.J.; Wang, S.; Wang, D.; Li, K.; Liu, Q.F.; Komarneni, S. Methoxy-grafted kaolinite preparation by intercalation of methanol: Mechanism of its structural variability. Appl. Clay Sci. 2017, 137, 241–248. [Google Scholar] [CrossRef]
- Horváth, E.; Kristóf, J.; Frost, R.L. Vibrational spectroscopy of intercalated kaolinites. Part I. Appl. Spectrosc. Rev. 2010, 45, 130–147. [Google Scholar] [CrossRef] [Green Version]
- Dong, W.J.; Li, W.J.; Yu, K.F.; Krishna, K.; Song, L.Z.; Wang, X.F.; Wang, Z.C.; Marc-Oliver, C.; Feng, S.H. Synthesis of silica nanotubes from kaolin clay. Chem. Commun. 2003, 11, 1302–1303. [Google Scholar] [CrossRef]
- Liu, Q.F.; Wang, D.; Guo, P.; Zhang, S.L.; Cheng, H.F.; Li, X.G.; Zhang, S. Preparation and structural characterization of kaolinite intercalation compound with Series of quaternary anmmonium salt. J. Chin. Ceram. Soc. 2015, 2, 222–230. [Google Scholar]
- Yuan, P.; Tan, D.Y.; Annabi-Bergaya, F.; Yan, W.C.; Liu, D.; Liu, Z.W. From platy kaolinite to aluminosilicate nanoroll via one-step delamination of kaolinite: Effect of the temperature of intercalation. Appl. Clay Sci. 2013, 83–84, 68–76. [Google Scholar] [CrossRef]
| Composition | SiO2 | Al2O3 | Fe2O3 | TiO2 | MgO | CaO | Na2O | K2O | P2O5 | LOI |
|---|---|---|---|---|---|---|---|---|---|---|
| Content (mass %) | 44.64 | 38.05 | 0.22 | 1.13 | 0.06 | 0.11 | 0.27 | <0.10 | 0.13 | 15.06 |
| Samples | DTG Peak (°C) | Ti (°C) | Te (°C) | W (°C) |
|---|---|---|---|---|
| Kaol | 520 | 450 | 632 | 182 |
| Kaol-DMSO | 524 | 456 | 627 | 171 |
| Kaol-MeOH | 507 | 440 | 564 | 124 |
| Kaol-SDS | 502 | 406 | 587 | 181 |
| Samples | D10 (µm) a | D50 (µm) a | D90 (µm) a | Daverage (µm) |
|---|---|---|---|---|
| Kaol | 1.225 | 3.142 | 6.614 | 3.729 |
| Kaol-MeOH | 0.885 | 2.054 | 3.702 | 2.205 |
| Kaol-SDS | 0.885 | 1.955 | 3.507 | 2.104 |
| Kaol-SDS-U | 0.165 | 0.635 | 2.811 | 1.274 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, X.; Wang, D.; Zhang, S.; Liu, Q.; Yang, H. Intercalation and Exfoliation of Kaolinite with Sodium Dodecyl Sulfate. Minerals 2018, 8, 112. https://doi.org/10.3390/min8030112
Zuo X, Wang D, Zhang S, Liu Q, Yang H. Intercalation and Exfoliation of Kaolinite with Sodium Dodecyl Sulfate. Minerals. 2018; 8(3):112. https://doi.org/10.3390/min8030112
Chicago/Turabian StyleZuo, Xiaochao, Ding Wang, Shilong Zhang, Qinfu Liu, and Huaming Yang. 2018. "Intercalation and Exfoliation of Kaolinite with Sodium Dodecyl Sulfate" Minerals 8, no. 3: 112. https://doi.org/10.3390/min8030112
APA StyleZuo, X., Wang, D., Zhang, S., Liu, Q., & Yang, H. (2018). Intercalation and Exfoliation of Kaolinite with Sodium Dodecyl Sulfate. Minerals, 8(3), 112. https://doi.org/10.3390/min8030112






