Evaluation of Sulfonate-Based Collectors with Different Hydrophobic Tails for Flotation of Fluorite
Abstract
:1. Introduction
2. Materials and Methods
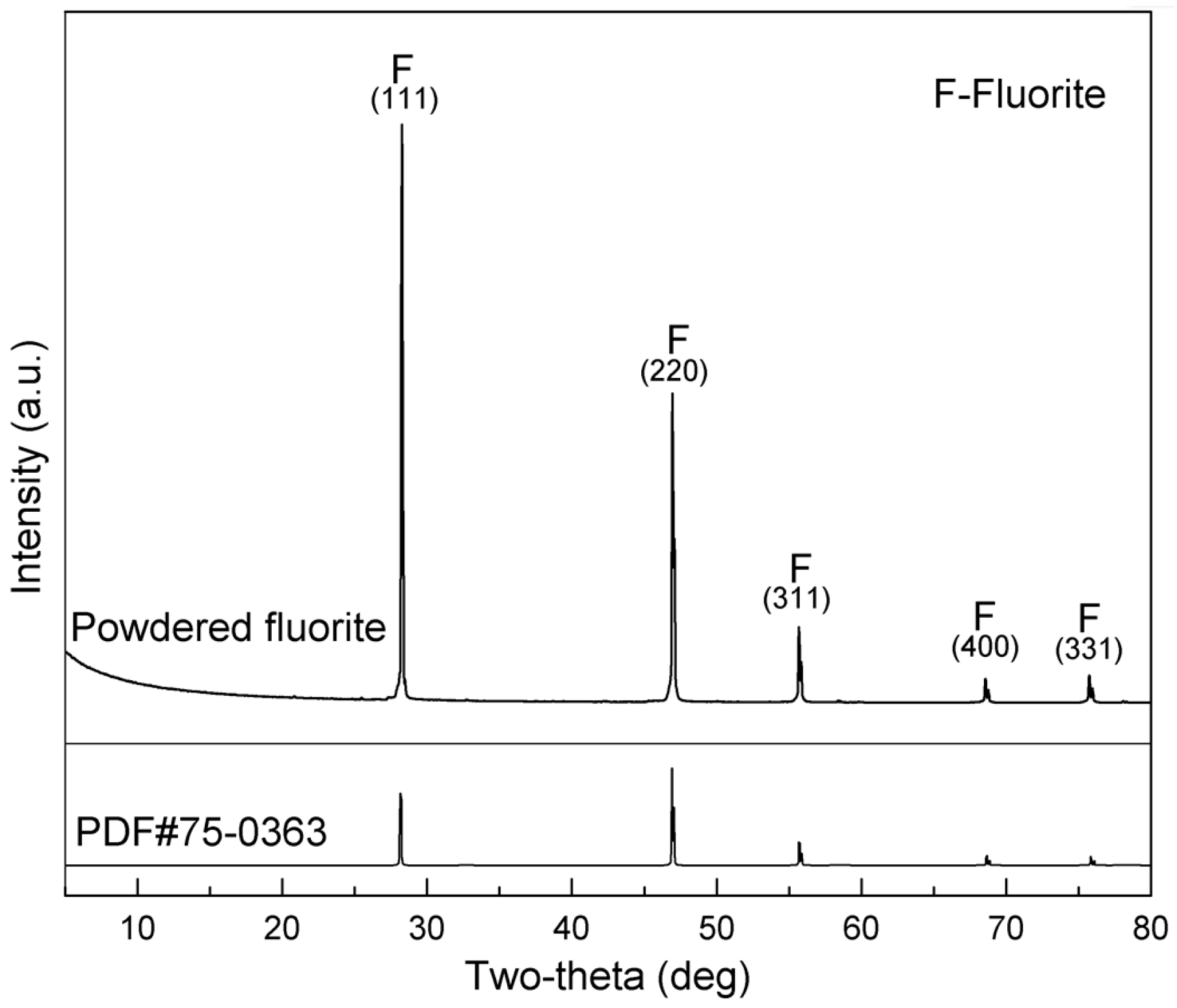
2.1. Materials and Reagents
2.2. Methods
2.2.1. Micro-Flotation Tests
2.2.2. Zeta Potential Measurements
2.2.3. Surface Tension Measurements
2.2.4. FTIR Measurements
2.2.5. XPS Analyses
2.2.6. Calculation of Frontier Orbital Energy
3. Results and Discussion
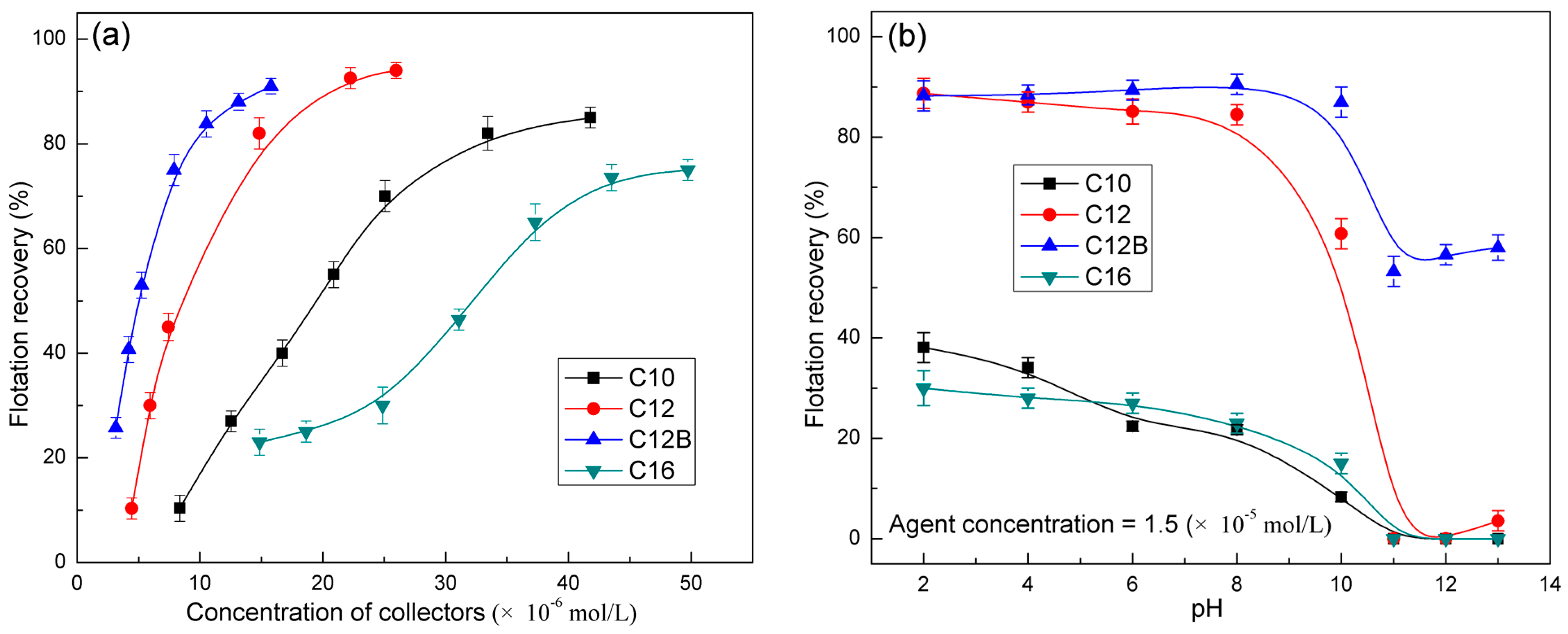
3.1. Micro-Flotation Test Results
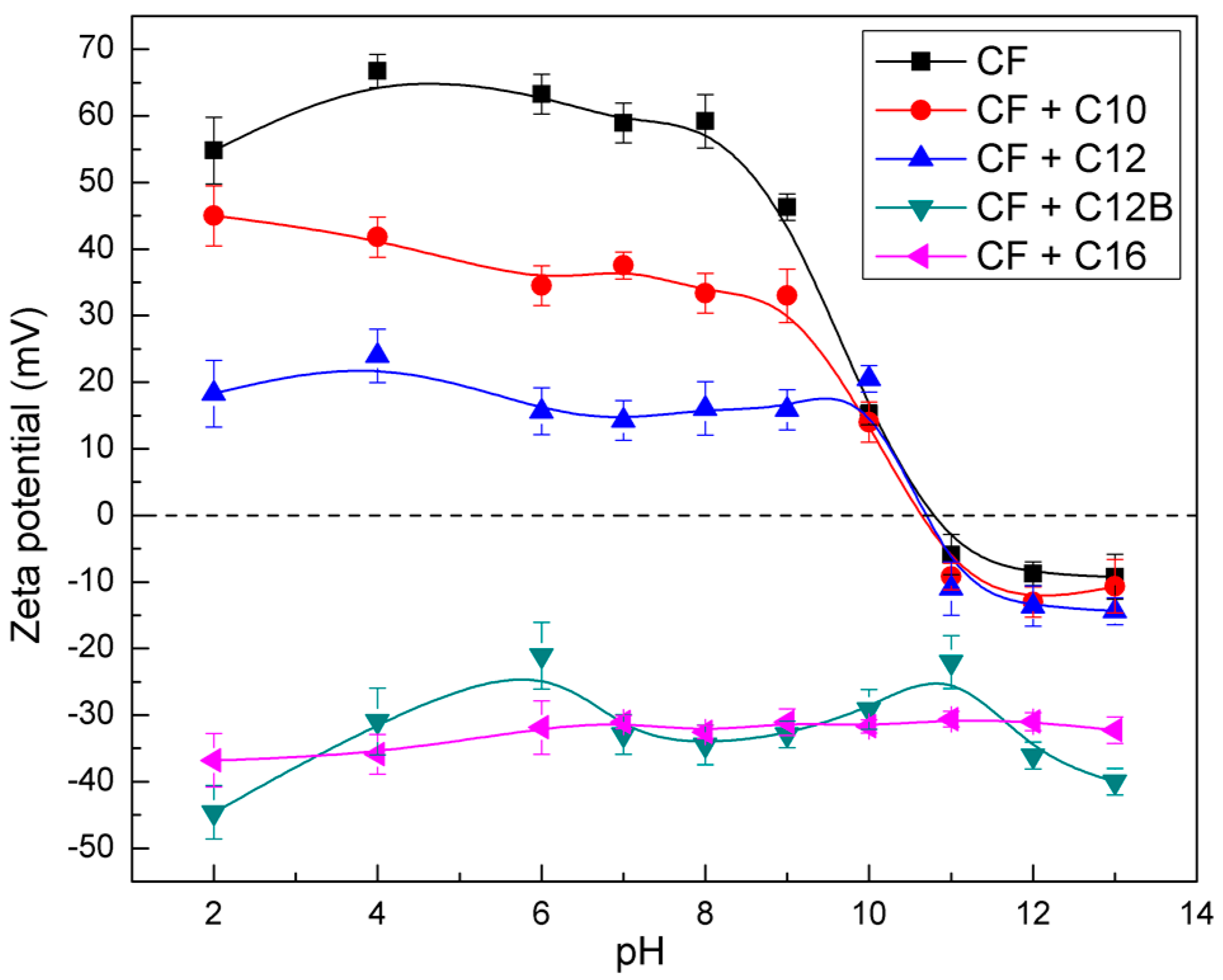
3.2. Zeta Potential Measurement Results
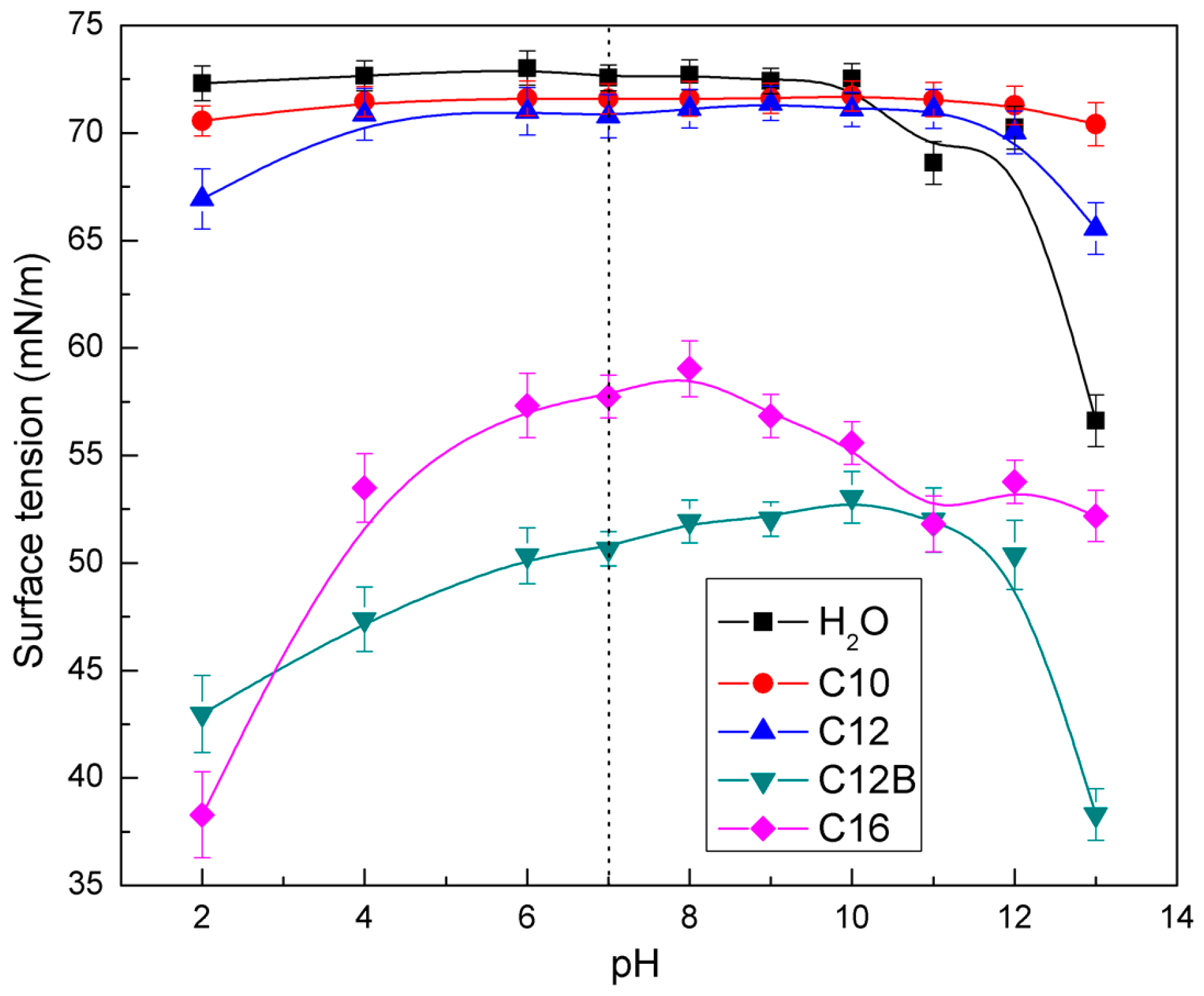
3.3. Surface Tension Measurement Results
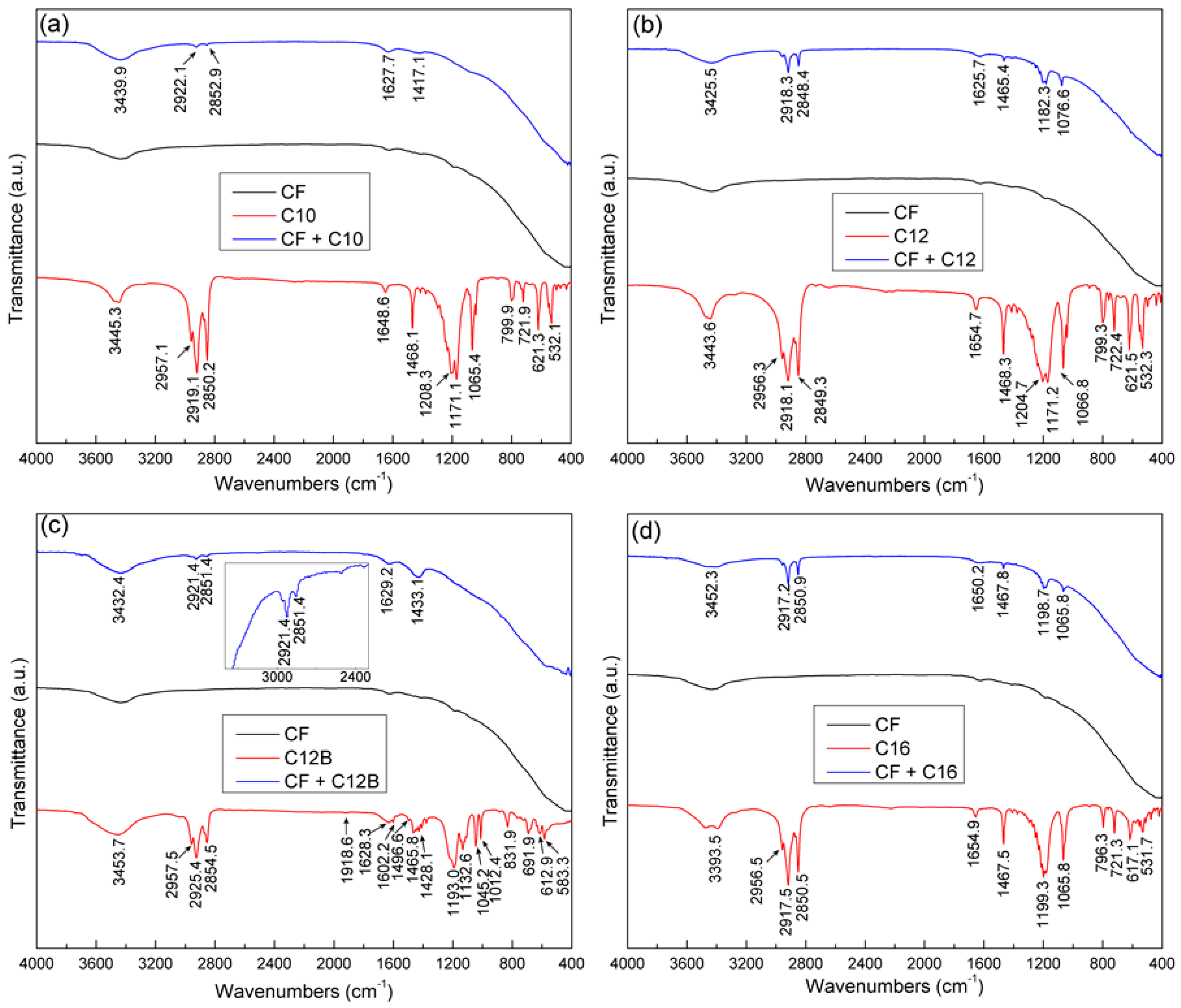
3.4. IR Spectroscopic Analysis Results
3.5. XPS Analyses
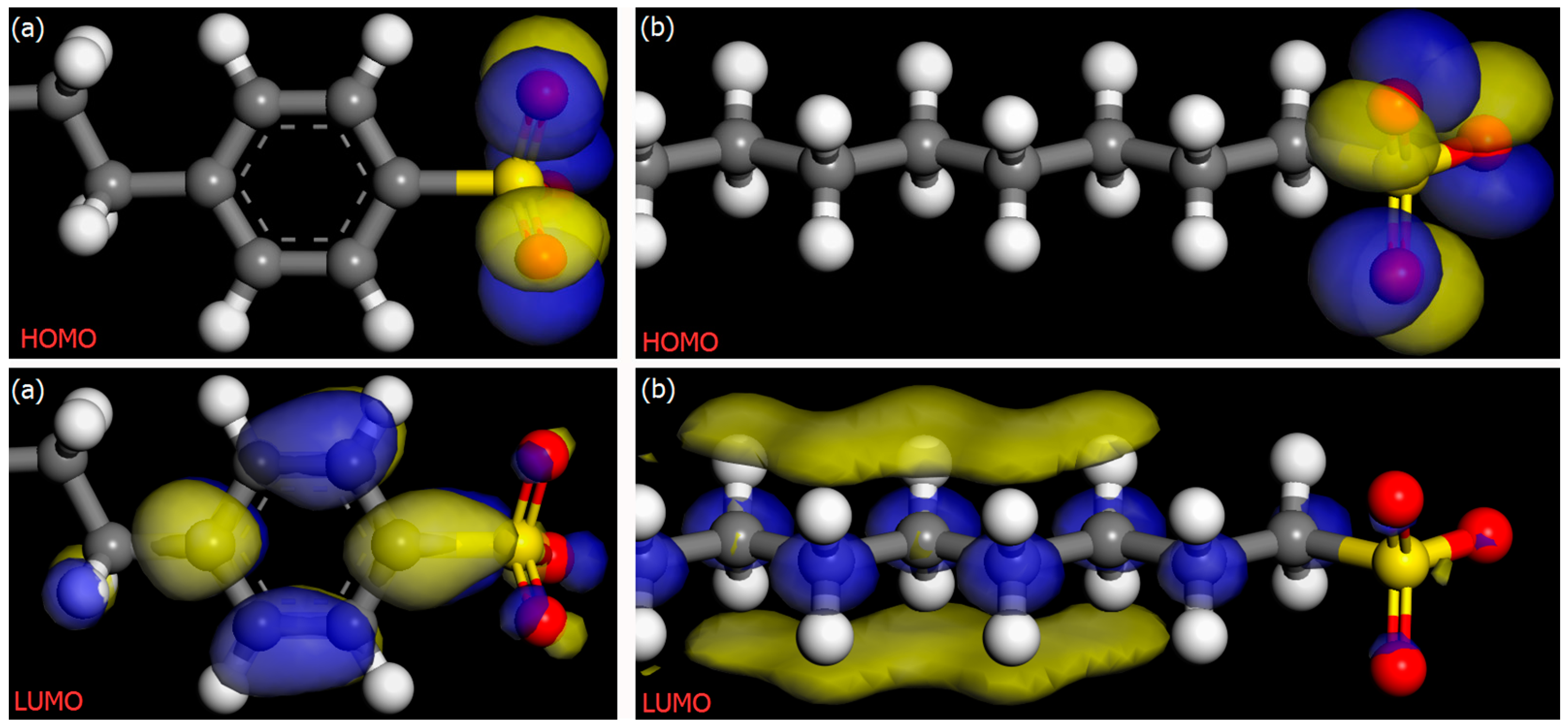
3.6. Molecular Frontier Orbital Energy and HLB Calculation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jiang, W.; Gao, Z.; Khoso, S.A.; Gao, J.; Sun, W.; Pu, W.; Hu, Y. Selective adsorption of benzhydroxamic acid on fluorite rendering selective separation of fluorite/calcite. Appl. Surf. Sci. 2017, 435, 752–758. [Google Scholar] [CrossRef]
- Zhang, C.; Wei, S.; Hu, Y.; Tang, H.; Gao, J.; Yin, Z.; Guan, Q. Selective adsorption of tannic acid on calcite and implications for separation of fluorite minerals. J. Colloid Interface Sci. 2018, 512, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Ren, Z.; Gao, H.; Qian, Y. Flotation behavior of different colored fluorites using sodium oleate as a collector. Minerals 2017, 7, 159. [Google Scholar] [CrossRef]
- Industrie, D.E.E.; Isi, F. Critical Raw Materials for the EU Report of the Ad-hocWorking Group on Defining Critical Raw Materials; European Commission: Brussels, Belgium, 2010. [Google Scholar]
- Gao, Y.; Gao, Z.; Sun, W.; Hu, Y. Selective flotation of scheelite from calcite: A novel reagent scheme. Int. J. Miner. Process. 2016, 154, 10–15. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, Z. Interactions of amphoteric amino phosphoric acids with calcium-containing minerals and selective flotation. Int. J. Miner. Process. 2003, 72, 87–94. [Google Scholar] [CrossRef]
- Fa, K.; Nguyen, A.V.; Miller, J.D. Interaction of calcium dioleate collector colloids with calcite and fluorite surfaces as revealed by AFM force measurements and molecular dynamics simulation. Int. J. Miner. Process. 2006, 81, 166–177. [Google Scholar] [CrossRef]
- Pradip; Rai, B.; Rao, T.K.; Krishnamurthy, S.; Vetrivel, R.; Mielczarski, J.; Cases, J.M. Molecular modeling of interactions of alkyl hydroxamates with calcium minerals. J. Colloid Interface Sci. 2002, 256, 106–113. [Google Scholar] [CrossRef]
- González-Martín, M.L.; Bruque, J.M.; González-Caballero, F.; Perea-Carpio, R.; Janczuk, B. The mechanism of adsorption of sodium dodecylsulfonate on fluorite and its surface free energy. Appl. Surf. Sci. 1996, 103, 395–402. [Google Scholar] [CrossRef]
- Zhu, H.; Deng, H.; Chen, C. Flotation separation of andalusite from quartz using sodium petroleum sulfonate as collector. Trans. Nonferr. Met. Soc. 2015, 25, 1279–1285. [Google Scholar] [CrossRef]
- Chen, Z.; Gao, H.; Li, Y.; Jin, J.; Ren, Z.; Wang, W. Evaluation of sodium petroleum sulfonates with different molecular weights for flotation of kyanite ore. Physicochem. Probl. Miner. 2017, 53, 956–968. [Google Scholar]
- Martı́nez-L, A.; Uribe, S.A.; Carrillo, P.F.R.; Coreño, A.J.; Ortiz, J.C. Study of celestite flotation efficiency using sodium dodecyl sulfonate collector: Factorial experiment and statistical analysis of data. Int. J. Miner. Process. 2003, 70, 83–97. [Google Scholar] [CrossRef]
- Cao, Q.; Cheng, J.; Wen, S.; Li, C.; Liu, J. Synergistic effect of dodecyl sulfonate on apatite flotation with fatty acid collector. Sep. Sci. Technol. 2016, 51, 1389–1396. [Google Scholar] [CrossRef]
- Taguta, J.; O’Connor, C.T.; Mcfadzean, B. The effect of the alkyl chain length and ligand type of thiol collectors on the heat of adsorption and floatability of sulphide minerals. Miner. Eng. 2017, 110, 145–152. [Google Scholar] [CrossRef]
- Ackerman, P.K.; Harris, G.H.; Klimpel, R.R.; Aplan, F.F. Evaluation of flotation collectors for copper sulfides and pyrite, III. Effect of xanthate chain length and branching. Int. J. Miner. Process. 1987, 21, 141–156. [Google Scholar] [CrossRef]
- Fuerstenau, M.C.; Miller, J.D.; Kuhn, M.C. Chemistry of Flotation; Metallurgical: New York, NY, USA, 1985; pp. 17–34. [Google Scholar]
- Chakraborty, T.; Hens, A.; Kulashrestha, S.; Murmu, N.C.; Banerjee, P. Calculation of diffusion coefficient of long chain molecules using molecular dynamics. Physica E 2015, 69, 371–377. [Google Scholar] [CrossRef]
- Gao, Z.; Sun, W.; Gao, J.; Hu, Y. A density functional theory study on the effect of lattice impurities on the electronic structures and reactivity of fluorite. Minerals 2017, 7, 160. [Google Scholar] [CrossRef]
- Liu, Z.; Fan, C.; Zhang, T.; Ji, X.; Chen, S.; Sun, S.; Ho, S. Density functional theory study of the interaction between sodium dodecylbenzenesulfonate and mineral cations. Acta Phys. Chim. Sin. 2016, 32, 445–452. [Google Scholar]
- Yu, F.; Wang, Y.; Zhang, L.; Zhu, G. Role of oleic acid ionic−molecular complexes in the flotation of spodumene. Miner. Eng. 2015, 71, 7–12. [Google Scholar] [CrossRef]
- Weissenborn, P.K.; Pugh, R.J. Surface tension of aqueous solutions of electrolytes: Relationship with ion hydration, oxygen solubility, and bubble coalescence. J. Colloid Interface Sci. 1996, 184, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sun, W.; Hu, Y.; Xu, L. Adsorption mechanism of mixed anionic/cationic collectors in Muscovite-Quartz flotation system. Miner. Eng. 2014, 64, 44–50. [Google Scholar] [CrossRef]
- Rao, K.H.; Forssberg, K.S.E. Mechanism of oleate interaction on salt-type minerals part III. Adsorption, zeta potential and diffuse reflectance FT-IR studies of scheelite in the presence of sodium oleate. Colloids Surf. 1991, 54, 161–187. [Google Scholar] [CrossRef]
- Roonasi, P.; Yang, X.; Holmgren, A. Competition between sodium oleate and sodium silicate for a silicate/oleate modified magnetite surface studied by in situ ATR-FTIR spectroscopy. J. Colloid Interface Sci. 2010, 343, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Yamabe, S.; Minato, T. Frontier orbital theory. Chem. Educ. 1992, 40, 450–454. [Google Scholar]
- Zhou, Z.; Parr, R.G. Activation hardness: New index for describing the orientation of electrophilic aromatic substitution. J. Am. Chem. Soc. 1990, 112, 5720–5724. [Google Scholar] [CrossRef]
- Gece, G. The use of quantum chemical methods in corrosion inhibitor studies. Corros. Sci. 2008, 50, 2981–2992. [Google Scholar] [CrossRef]
- Davies, J.T. Interfacial phenomena. Phys. Today 1962, 15, 78. [Google Scholar] [CrossRef]
- Ho, O.B. Electrokinetic studies on emulsions stabilized by ionic surfactants: The electroacoustophoretic behavior and estimation of Davies’ HLB increments. J. Colloid Interface Sci. 1998, 198, 249–260. [Google Scholar] [CrossRef]
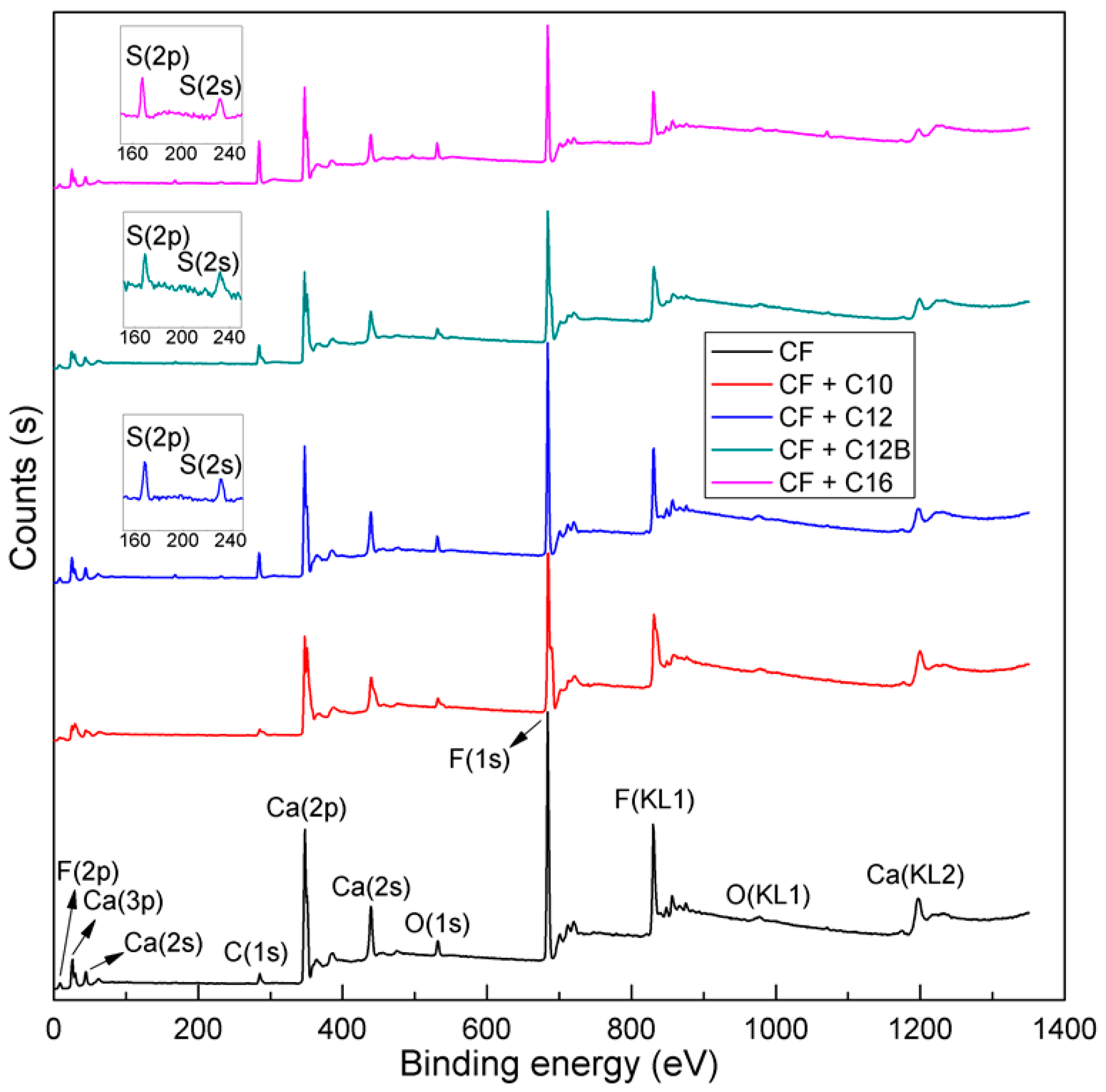
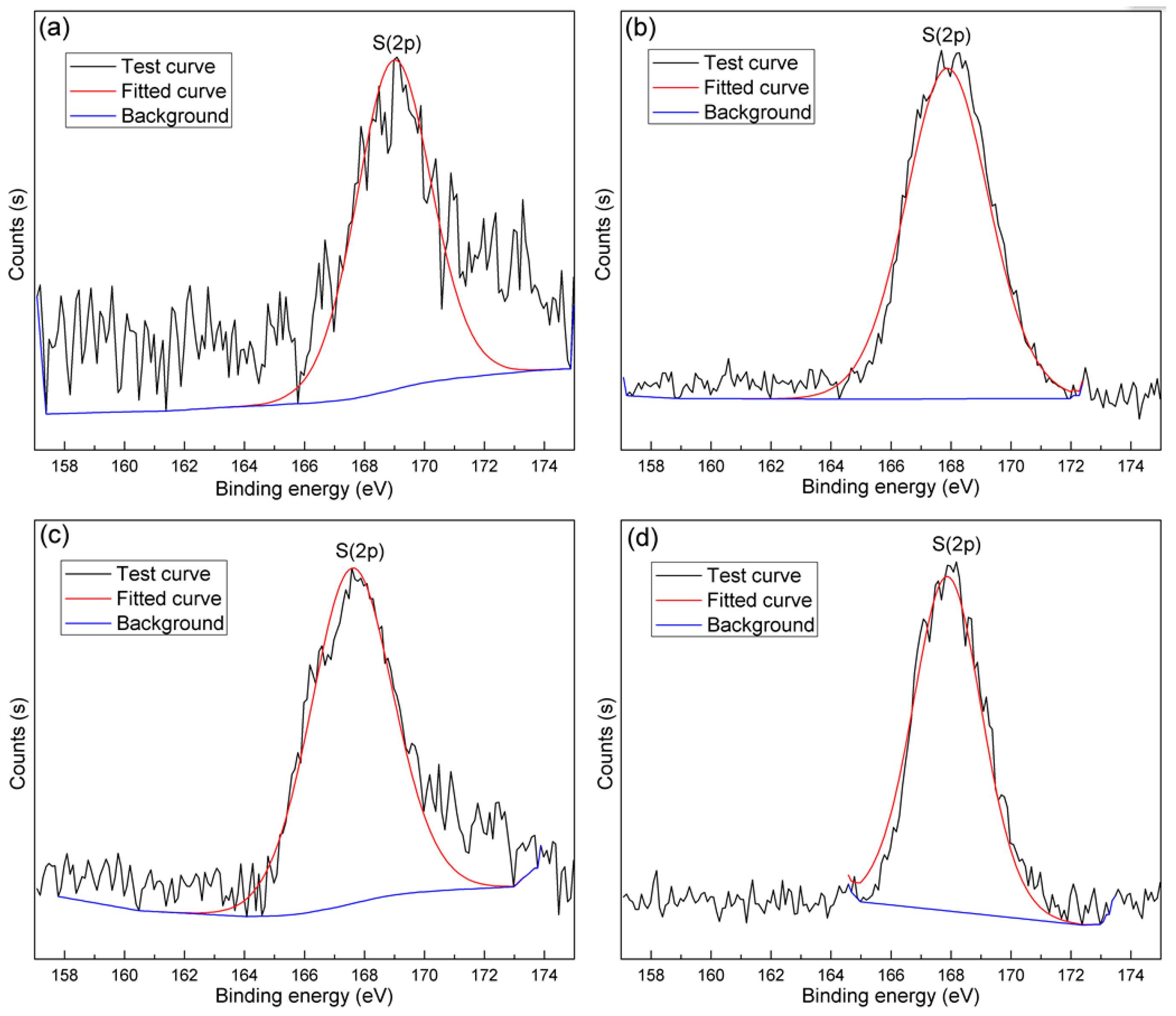
| Sample | Relative Atomic Concentration (%) | ||||
|---|---|---|---|---|---|
| Ca(2p) | F(1s) | C(1s) | O(1s) | S(2p) | |
| CF | 29.72 | 59.24 | 6.71 | 4.33 | - |
| CF + C10 | 28.71 | 58.91 | 7.48 | 4.67 | 0.23 |
| CF + C12 | 26.47 | 51.16 | 15.96 | 5.45 | 0.96 |
| CF + C12B | 26.09 | 49.26 | 17.95 | 5.57 | 1.13 |
| CF + C16 | 23.42 | 46.12 | 22.58 | 6.49 | 1.39 |
| Sulfonate Type | EHOMO (eV) | ELUMO (eV) | △E (eV) |
|---|---|---|---|
| C10 | −5.559 | 1.362 | 6.921 |
| C12 | −5.561 | 1.352 | 6.913 |
| C16 | −5.561 | 1.340 | 6.901 |
| C12B | −5.709 | −1.448 | 4.261 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, R.; Ren, Z.; Gao, H.; Qian, Y. Evaluation of Sulfonate-Based Collectors with Different Hydrophobic Tails for Flotation of Fluorite. Minerals 2018, 8, 57. https://doi.org/10.3390/min8020057
Zheng R, Ren Z, Gao H, Qian Y. Evaluation of Sulfonate-Based Collectors with Different Hydrophobic Tails for Flotation of Fluorite. Minerals. 2018; 8(2):57. https://doi.org/10.3390/min8020057
Chicago/Turabian StyleZheng, Renji, Zijie Ren, Huimin Gao, and Yupeng Qian. 2018. "Evaluation of Sulfonate-Based Collectors with Different Hydrophobic Tails for Flotation of Fluorite" Minerals 8, no. 2: 57. https://doi.org/10.3390/min8020057





