Forecasting Geoenvironmental Risks: Integrated Applications of Mineralogical and Chemical Data
Abstract
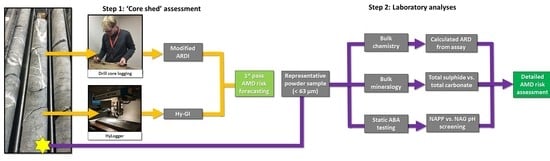
:1. Introduction
2. Materials and Methods
2.1. Drill Hole and Pulp Sampling
2.2. Geoenvironmental Logging
2.3. Mineralogical Evaluations
2.3.1. Hyperspectral Mineralogy
2.3.2. Bulk Mineralogy
2.4. Bulk Chemistry
2.5. Static Testing
3. Results
3.1. Geoenvironmental Logging Assessments
3.1.1. W-1
3.1.2. W-2
3.1.3. W-3
3.1.4. W-4
3.1.5. W-5
3.2. Carbonate Mineralogy
3.3. Calculated ARD from Assay
3.4. ABA Classification
4. Discussion
4.1. Waste Mangement Planning
- Type I: total S: <0.1%; ARDI: <0/50; Hy-GI: >10,000 Lowest risk/ANC offered
- Type II: total S: 0.1 to 0.3%; ARDI: 1 to 20/50; Hy-GI: 1000 to 10,000 Low risk/NAF
- Type III: total S: 0.3 to 1%; ARDI score: 20 to 30/50; Hy-GI: <1000 High risk/AMD probable
- Type IV: total S: >1%; ARDI score: >30/50; Hy-GI: <500 Highest risk/rapid AMD
4.2. Mineralogical Approaches to Waste Classification
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AF | Acid forming |
| ANC | Acid neutralising capacity |
| ARDI | Acid rock drainage index |
| EAF | Extremely acid forming |
| GMTG | Geochemistry-mineralogy-texture-geometallurgy approach |
| Hy-Gi | HyLogger geoenvironmental index |
| LOM | Life-of-mine |
| NAF | Non-acid forming |
| NAG | Net acid generation |
| NAPP | Net acid producing potential |
| PAF | Potentially acid forming |
| PNC | Potential neutralising capacity |
| SWIR | Shortwave infrared |
| TIR | Thermal infrared |
| XRF | X-ray fluorescence |
| XRD | X-ray diffractometry |
References
- Sillitoe, R. Porphyry copper systems. Econ. Geol. 2010, 105, 3–41. [Google Scholar] [CrossRef]
- Sillitoe, R. A plate tectonic model for the origin of porphyry copper deposits. Econ. Geol. 1972, 67, 184–197. [Google Scholar] [CrossRef]
- Richards, J.P. Tectono-magmatic precursors for geophysical data over a copper gold porphyry Cu-(Mo-Au) deposit formation. Econ. Geol. 2003, 96, 1419–1431. [Google Scholar]
- Singer, D.A.; Cox, D.P.; Mosier, D.L. Grade and tonnage model of porphyry Cu–Mo. In Mineral Deposit Models; Cox, D.P., Singer, D.A., Eds.; U.S. Geological Survey Bulletin: Washington, DC, USA, 1986; Volume 1693, pp. 116–119. [Google Scholar]
- Cooke, D.R.; Hollings, P.; Wilkinson, J.J.; Tosdal, R.M. Geochemistry of Porphyry Deposits. In Treatise on Geochemistry; Holland, H., Turekian, K., Eds.; Elsevier: Milan, Italy, 2014; pp. 357–381. ISBN 978-0-08-098300-4. [Google Scholar]
- Cooke, D.R.; Hollings, P.; Walshe, J. Giant porphyry deposits: Characteristics, distribution and tectonic controls. Econ. Geol. 2005, 100, 801–818. [Google Scholar] [CrossRef]
- Mudd, G.M.; Jowitt, S.M. Growing global copper resources, reserves and production: Discovery is not the only control on supply. Econ. Geol. 2018, 113, 1235–1267. [Google Scholar] [CrossRef]
- Plumlee, G. The environmental geology of mineral deposits. Rev. Econ. Geol. 1999, 6, 71–116. [Google Scholar]
- Mudd, G.M. The environmental sustainability of mining in Australia: Key mega trends and looming contraints. Res. Pol. 2010, 35, 98–115. [Google Scholar] [CrossRef]
- Cox, L.J.; Chaffee, M.A.; Cox, D.P.; Klein, D.P. Porphyry Cu deposits: U.S. Geological Survey Open-File Report 95–831; U.S. Geological Survey: Reston, VA, USA, 1995; pp. 75–89.
- Parbhakar-Fox, A.; Lottermoser, B.G. A critical review of acid rock drainage prediction methods and practices. Miner. Eng. 2015, 82, 107–124. [Google Scholar] [CrossRef]
- Dold, B. Acid rock drainage prediction: A critical review. J. Geochem. Explor. 2017, 172, 120–132. [Google Scholar] [CrossRef]
- Leon, E.A.; Westhuizen, C.; Du, L. Acid mine and metalliferous drainage (AMD); sample selection an intricate task. In Proceedings of the 10th International Conference on Acid Rick Drainage and IMWA Annual Conference, Santiago, Chile, 21–24 April 2015; pp. 1–10. [Google Scholar]
- Wang, R.; Cudahy, T.; Laukamp, C.; Walshe, J.L.; Bath, A.; Mei, Y.; Young, C.; Roache, T.J.; Jenkins, A.; Roberts, M.; et al. White Mica as a Hyperspectral Tool in Exploration for the Sunrise Dam and Kanowna Belle Gold Deposits, Western Australia. Econ. Geol. 2017, 112, 1153–1176. [Google Scholar] [CrossRef]
- Montero, I.C.; Brimhall, G.H.; Alpers, C.H.; Swayze, G.A. Characterization of waste rock associated with acid drainage at the Penn Mine, California, by ground-based visible to short-wave infrared reflectance spectroscopy assisted by digital mapping. Chem. Geol. 2005, 215, 453–472. [Google Scholar] [CrossRef] [Green Version]
- Francisco Velasco, F.; Alvaro, A.; Suarez, S.; Herrero, J.-M.; Yusta, I. Mapping Fe-bearing hydrated sulphate minerals with short wave infrared (SWIR) spectral analysis at San Miguel mine environment, Iberian Pyrite Belt (SW Spain). J. Geochem. Explor. 2005, 87, 45–72. [Google Scholar] [CrossRef]
- Laakso, K.; Middleton, M.; Heinig, T.; Bärs, R.; Lintinen, P. Assessing the ability to combine hyperspectral imaging (HSI) data with Mineral Liberation Analyzer (MLA) data to characterize phosphate rocks. Int. J. Appl. Earth Obs. Geoinf. 2018, 69, 1–12. [Google Scholar] [CrossRef]
- Fox, N.; Parbhakar-Fox, A.; Moltzen, J.; Feig, S.; Goemann, K.; Huntington, J. Applications of hyperspectral mineralogy for geoenvironmental characterisation. Miner. Eng. 2017, 107, 63–77. [Google Scholar] [CrossRef]
- Schodlok, M.C.; Whitbourn, L.; Huntington, J.; Mason, P.; Green, A.; Berman, M.; Coward, D.; Connor, P.; Wright, W.; Jolivet, M.; et al. HyLogger-3, a visible to shortwave and thermal infrared reflectance spectrometer system for drill core logging: Functional description. Aust. J. Earth Sci. 2016, 63, 929–940. [Google Scholar]
- Berry, R.; Hunt, J.; Parbhakar-Fox, A.; Lottermoser, B. Prediction of Acid Rock Drainage (ARD) from calculated mineralogy. In Proceedings of the 10th International Conference on Acid Rock Drainage and IMWA Annual Conference, Santiago, Chile, 21–24 April 2015; pp. 1–10, ISBN 978-956-9393-28-0. [Google Scholar]
- Taylor, J.; Winchester, S.; Tyler, M.; Ehrig, K.; Waters, J.; Beavis, F. Converting chemistry to mineralogy for acid and metalliferous drainage risk management. In Proceedings of the Ninth Australian Workshop on Acid and Metalliferous Drainage, Burnie, Tasmania, 20–23 November 2017; Bell, L.C., Edraki, M., Gerbo, C., Eds.; The University of Queensland: Brisbane, Australia, 2017; pp. 14–21. [Google Scholar]
- Brough, C.; Strongman, J.; Bowell, R.; Warrender, R.; Prestia, A.; Barnes, A.; Fletcher, J. Automated environmental mineralogy; the use of liberation analysis in humidity cell testwork. Miner. Eng. 2017, 107, 112–122. [Google Scholar] [CrossRef]
- Becker, M.; Dyantyi, N.; Broadhurst, J.L.; Harrison, S.T.L.; Franzidis, J.-P. A mineralogical approach to evaluating laboratory scale acid rock drainage characterisation tests. Miner. Eng. 2015, 80, 33–36. [Google Scholar] [CrossRef]
- Pooler, R.; Dold, B. Optimisation and quality control of automated quality control of automated quantitative mineralogy analysis for acid rock drainage prediction. Minerals 2017, 7, 12. [Google Scholar] [CrossRef]
- Parbhakar-Fox, A.; Lottermoser, B.; Hartner, R.; Berry, R.F.; Noble, T.L. Prediction of Acid Rock Drainage from Automated Mineralogy. In Environmental Indicators in Metal Mining; Lottermoser, B., Ed.; Springer International Publishing: Basel, Switzerland, 2017; pp. 139–156. ISBN 978-3-319-42729-4. [Google Scholar]
- Parbhakar-Fox, A. Predicting Waste Properties Using the Geochemistry-Mineralogy-Texture-Geometallurgy Approach. In Environmental Indicators in Metal Mining; Lottermoser, B., Ed.; Springer International Publishing: Basel, Switzerland, 2017; pp. 73–96. ISBN 978-3-319-42729-4. [Google Scholar]
- Parbhakar-Fox, A.; Edraki, M.; Walters, S.; Bradshaw, D. Development of a textural index for the prediction of acid rock drainage. Miner. Eng. 2011, 24, 1277–1287. [Google Scholar] [CrossRef]
- Cornelius, R.; Parbhakar-Fox, A.; Cooke, D.R. A geoenvironmental characterisation tool for the coreshed during early life-of-mine assessments. In Proceedings of the 9th Australian Workshop on Acid and Metalliferous Drainage, Burnie, Tasmania, 20–23 November 2017; pp. 102–113. [Google Scholar]
- Jackson, L.; Parbhakar-Fox, A.; Fox, N.; Cooke, D.R.; Harris, A.C.; Savinova, E. Intrinsic neutralisation potential from automated drill core logging for improved geoenvironmental domaining. In Proceedings of the 9th Australian Workshop on Acid and Metalliferous Drainage, Burnie, Tasmania, 20–23 November 2017; pp. 378–392. [Google Scholar]
- Jambor, J.L.; Dutrizac, J.E.; Raudsepp, M. Measured and computed neutralization potentials from static tests of diverse rock types. Environ. Geol. 2007, 52, 1019–1031. [Google Scholar] [CrossRef]
- Sverdrup, H.U. The Kinetics of Base Cation Release Due to Chemical Weathering; Lund University Press: Lund, Switzerland, 1990; 246p. [Google Scholar]
- ASTM D4972-18, Standard Test Methods for pH of Soils; ASTM International: West Conshohocken, PA, USA, 2018. [CrossRef]
- Parbhakar, A.; Fox, N.; Ferguson, T.; Hill, R.; Maynard, B. Dissection of the NAG pH test: Tracking efficacy through examining reaction products. In Proceedings of the 11th International Conference for Acid Rock Drainage, Pretoria, South Africa, 10–14 September 2018. in press. [Google Scholar]
- Paktunc, A.D. Mineralogical constraints on the determination of neutralising potential and prediction of acid mine drainage. Environ. Geol. 1999, 39, 103–112. [Google Scholar] [CrossRef]
- Vriens, B.; Peterson, H.; Laurenzi, L.; Smith, L.; Aranda, C.; Mayer, K.U.; Beckie, R.D. Long-term monitoring of waste rock weathering at the Antamina mine, Peru. Chemosphere 2019, 215, 858–869. [Google Scholar] [CrossRef] [PubMed]
- Pashkevich, M.A.; Petrova, T.A. Technogenic impact of sulphide-containing wastes produced by ore mining and processing at the Ozernoe deposit: Investigation and forecast. J. Ecol. Eng. 2017, 18, 127–133. [Google Scholar] [CrossRef]
- Michaud, M.L.; Plante, B.; Bussière, B.; Benzaazoua, M.; Lerous, J. Development of a modified kinetic test using EDTA and citric acid for the prediction of contaminated neutral drainage. J. Geochem. Explor. 2017, 181, 58–68. [Google Scholar] [CrossRef]
- Garvie, A.; Kentwell, D. The influence of sample numbers and distribution on the assessment of AMD potential. In Proceedings of the 9th Australian Workshop on Acid and Metalliferous Drainage, Burnie, Tasmania, 20–23 November 2017; pp. 92–101. [Google Scholar]
- Zhou, X.; Jara, C.; Bardoux, M.; Pasencia, C. Multi-scale integrated application of spectral geology and remote sensing for mineral exploration. In Proceedings of the 6th Decennial International Conference on Mineral Exploration, Toronto, ON, Canada, 21–25 October 2017; Tschirhart, V., Thomas, M.D., Eds.; Spectral Geology and Remote Sensing Paper 82. Decennial Mineral Exploration Conferences: Toronto, ON, Canada, 2017; pp. 899–910. [Google Scholar]
- Jackson, L.M.; Parbhakar-Fox, A.; Fox, N.; Cooke, D.R.; Harris, A.C.; Meffre, S.; Danyushevsky, L.; Goemann, K.; Rodemann, T.; Gloy, G.; et al. Assessing geo-environmental risk using intact materials for early life-of-mine planning—A review of established techniques and emerging tools. In From Start to Finish: A Life-of-Mine Perspective; The Australasian Institute of Mining and Metallurgy: Carlton, Australia, 2018; pp. 1–18. ISBN 9781925100723. [Google Scholar]
- Langman, J.B.; Moore, M.L.; Ptacek, C.J.; Smith, L.; Sego, D.; Blowes, D. Diavik waste rock project: Evolution of mineral weathering, element release, and acid generation and neutralization during a five-year humidity cell experiement. Minerals 2014, 4, 257–278. [Google Scholar] [CrossRef]
- Pearce, S.; Lehane, S.; Pearce, J. Waste material placement options during construction and closure risk reduction—Quantifying the how, the why and the how much. In Proceedings of the 11th International Conference on Mine Closure, Perth, Australia, 15–17 March 2016; Fourie, A.B., Tibbett, M., Eds.; Australian Centre for Geomechanics: Perth, Australia, 2016; pp. 691–706. [Google Scholar]
- Barritt, R.; Scott, P.; Taylor, I. Managing the waste rock storage design-can we build a waste rock dump that works? In Proceedings of the 11th International Conference on Mine Closure, Perth, Australia, 15–17 March 2016; Fourie, A.B., Tibbett, M., Eds.; Australian Centre for Geomechanics: Perth, Australia, 2016; pp. 125–134. [Google Scholar]
- Amos, R.; Blowes, D.W.; Bailey, B.L.; Sego, D.C.; Smith, L.; Ritchie, I.M. Waste rock hydrogeology and geochemistry. Appl. Geol. 2015, 57, 140–156. [Google Scholar] [CrossRef]
- Sinclair, S.A.; Pham, N.; Amos, R.T.; Sego, D.C.; Smith, L.; Blowes, D. Influence of freeze-thaw dynamics on internal evolution of low sulfide waste rock. Appl. Geochem. 2015, 61, 160–174. [Google Scholar] [CrossRef]
- Merrill, J.; Martínez, P.; Urrutia, N.; Voisin, L.; Merrill, J.; Martínez, P.; Urrutia, N.; Voisin, L. Sulphides detection by hyperspectral analysis in the thermal infrared range. In Proceedings of the Geomet Conference, Lima, Peru, 11–13 December 2016. [Google Scholar]
- Smart, R.; Skinner, W.M.; Levay, G.; Gerson., A.R.; Thomas, J.E.; Sobieraj, H.; Schumann, R.; Weisener, C.G.; Weber, P.A.; Miller, S.D.; et al. ARD Test Handbook: Project P387, A Prediction and Kinetic Control of Acid Mine Drainage; AMIRA, International Ltd.: Melbourne, Australia, 2002. [Google Scholar]
- Hirsch, M.; Kettler, J.; Havenith, A. Online prompt gamma neutron activation analysis for the characterization of raw materials. In Proceedings of the Seventh Sensor-Based Sorting and Control, Aachen, Germany, 23–24 February 2016; Pretz, T., Wortruba, H., Eds.; Shaker Verlag: Aachen, Germany, 2016; pp. 239–247. [Google Scholar]
- Mutina, A.; Bruyndonckx, P. Combined micro-X-ray tomography and micro-X-ray fluorescence study of reservoir rocks: Applicability to core analysis. Microsc. Anal.–Compos. Anal. Suppl. 2013, 27, 4–6. [Google Scholar]
- Kuhn, K.; Meima, J.A.; Rammlmair, D.; Ohlendorf, C. Chemical mapping of mine waste drill cores with laser-induced breakdown spectroscopy (LIBS) and energy dispersive X-ray fluorescence (EDXRF) for mineral resource exploration. J. Geochem. Explor. 2016, 161, 72–84. [Google Scholar] [CrossRef]











| Drill Hole ID | Depth (m) | Number of Half Drill Core Samples | Number of Pulp Sub-Samples |
|---|---|---|---|
| W1 | 7.1–226.25 | 17 | 19 |
| W2 | 1–282 | 39 | 70 |
| W3 | 2–342 | 86 | not available |
| W4 | 5.6–217 | 28 | 23 |
| W5 | 23.4–304 | 69 | 75 |
| OZ1 | 24.4–170.6 | 21 | 19 |
| Total | 260 | 206 | |
| Lithotype/Sample Number | Paste pH | ARDI (/50) | Total Sulphur (%) | NAG pH | ANC (kg H2SO4/t) | NAPP (kg H2SO4/t) |
|---|---|---|---|---|---|---|
| Clastic sediment (n = 71) | 8.6 | 17.9 | 1.2 | 2.4 | 18.7 | 39.9 |
| Dacite (n = 1) | 8.4 | 19.5 | 0.7 | - | - | - |
| Volcaniclastite (n = 38) | 7 | 21.1 | 0.9 | 2.8 | 7.6 | 39.5 |
| Feldspar porphyry (n = 45) | 7.9 | 21.7 | 0.6 | 2.8 | 11.1 | 19.2 |
| Basalt (n = 17) | 8.2 | 21.5 | 0.4 | 2.9 | 13.7 | 9.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parbhakar-Fox, A.; Fox, N.; Jackson, L.; Cornelius, R. Forecasting Geoenvironmental Risks: Integrated Applications of Mineralogical and Chemical Data. Minerals 2018, 8, 541. https://doi.org/10.3390/min8120541
Parbhakar-Fox A, Fox N, Jackson L, Cornelius R. Forecasting Geoenvironmental Risks: Integrated Applications of Mineralogical and Chemical Data. Minerals. 2018; 8(12):541. https://doi.org/10.3390/min8120541
Chicago/Turabian StyleParbhakar-Fox, Anita, Nathan Fox, Laura Jackson, and Rebekah Cornelius. 2018. "Forecasting Geoenvironmental Risks: Integrated Applications of Mineralogical and Chemical Data" Minerals 8, no. 12: 541. https://doi.org/10.3390/min8120541