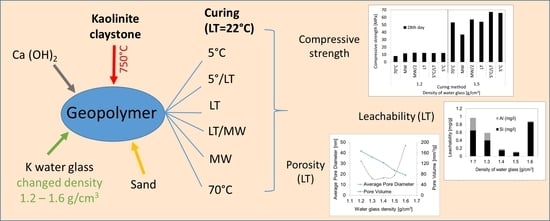
Kaolinite Claystone-Based Geopolymer Materials: Effect of Chemical Composition and Curing Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
2.2. Characterization
3. Results and Discussion
3.1. Compressive Strength
3.2. Leachability Tests
3.3. Porosity
4. Conclusions
- The correlation between pore volume and compressive strength was confirmed
- The low temperature (5 °C) during solidification has a positive effect on the GP—it increases compressive strength, decreases total pore volume and the leachability of Al.
- The higher density of the water glass used for the sample preparation resulted in lowering the total pore volume and increasing the compressive strength of the samples.
- Sudden increase of the Si and alkali metals leachability was proven in case of the GPs prepared from water glass of the highest density, i.e., 1.6 g·cm−3 compared to the GP made from water glass with lower densities.
- The most suitable GPs were prepared from water glass with density 1.5 g·cm−3 followed by solidification at a lowered temperature (5 °C). Such GPs then had the highest compressive strength, low total pore volume, and average pore diameter together with the lowest leachability of the structural elements and ions.
- Although the MW radiation shortened the solidification time to a minimum (26 min), it caused local overheating resulting in higher total pore volume (mainly in case of higher water glass density samples). This resulted in a lowered compressive strength of the MW treated samples.
Funding
Conflicts of Interest
References
- Davidovits, J. Geopolymers and geopolymeric materials. J. Therm. Anal. Calorim. 1989, 35, 429–441. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D. Geopolymerisation: A review and prospects for the minerals industry. Miner. Eng. 2007, 20, 1261–1277. [Google Scholar] [CrossRef]
- Liew, Y.M.; Kamarudin, H.; Al Bakri, A.M.; Bnhussain, M.; Luqman, M.; Nizar, I.K.; Ruzaidi, C.M.; Heah, C.Y. Optimization of solids-to-liquid and alkali activator ratios of calcined kaolin geopolymeric powder. Constr. Build. Mater. 2012, 37, 440–451. [Google Scholar] [CrossRef]
- Singh, N.B. Fly ash-based geopolymer binder: A future construction material. Minerals 2018, 8, 299. [Google Scholar] [CrossRef]
- Rosas-Casarez, C.A.; Arredondo-Rea, S.P.; Cruz-Enríquez, A.; Corral-Higuera, R.; Gómez-Soberón, J.M.; Medina-Serna, T.D.J. Influence of size reduction of fly ash particles by grinding on the chemical properties of geopolymers. Appl. Sci. 2018, 8, 365. [Google Scholar] [CrossRef]
- Van Jaarsveld, J.G.S. The Physical and Chemical Characterisation of Fly Ash Based Geopolymers. Ph.D. Thesis, The University of Melbourne, Melbourne, Australia, 2000. [Google Scholar]
- Temuujin, J.; Riessen, A.V.; MacKenzie, K.J.D. Preparation and characterisation of fly ash based geopolymer mortars. Constr. Build. Mater. 2010, 24, 1906–1910. [Google Scholar] [CrossRef]
- Medina-Serna, T.D.J.; Arredondo-Rea, S.P.; Gómez-Soberón, J.M.; Rosas-Casarez, C.A.; Corral-Higuera, R. Effect of curing temperaturein the alkali-activated blast-furnace slag paste and their structural influence of porosity. Adv. Sci. Technol. Res. J. 2016, 10, 74–79. [Google Scholar] [CrossRef]
- Yunsheng, Z.; Wei, S.; Qianli, C.; Lin, C. Synthesis and heavy metal immobilization behaviours of slag based geopolymer. J. Hazard. Mater. 2007, 143, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.J. A study on the setting characteristics of sodium silicate-activated slag pastes. Cem. Concr. Res. 2003, 33, 1005–1011. [Google Scholar] [CrossRef]
- Yunsheng, Z.; Wei, S.; Zongjin, L. Composition design and microstructural characterization of calcined kaolin-based geopolymer cement. Appl. Clay Sci. 2010, 47, 271–275. [Google Scholar] [CrossRef]
- Granizo, M.L.; Varela, M.T.B.; Martinez-Ramirez, S. Alkali activation of metakaolins: Parameters affecting mechanical, structural and microstructural properties. J. Mater. Sci. 2007, 42, 2934–2943. [Google Scholar] [CrossRef]
- Heah, C.Y.; Kamarudin, H.; Mustafa Al Bakri, A.M.; Bnhussain, M.; Luqman, M.; Khairul Nizar, I.; Ruzaidi, C.M.; Liew, Y.M. Study on solids-to-liquid and alkaline activator ratios on kaolin based geopolymers. Constr. Build. Mater. 2012, 35, 912–992. [Google Scholar] [CrossRef]
- Perná, I.; Hanzlíček, T.; Šupová, M. The identification of geopolymer affinity in specific cases of clay materials. Appl. Clay Sci. 2014, 102, 213–219. [Google Scholar] [CrossRef]
- Xie, J.; Kayali, O. Effect of water content on the development of fly ash-based geopolymers in heat and ambient curing conditions. In Proceedings of the Third International Conference on Sustainable Construction Materials and Technologies, Kyoto, Japan, 18–21 August 2013. [Google Scholar]
- Mu, S.; Liu, J.; Liu, J.; Wang, Y.; Shi, L.; Jiang, Q. Property and microstructure of waterborne self-setting geopolymer coating: Optimization effect of SiO2/Na2O molar ratio. Minerals 2018, 8, 162. [Google Scholar] [CrossRef]
- Subhash, V.P.; Yuwaraj, M.G.; Sanjay, S.J. Effect of concentration of sodium hydroxide and degree of heat curing on fly ash-based geopolymer mortar. Indian J. Mater. Sci. 2014, 2014, 938789. [Google Scholar] [CrossRef]
- Thakur, R.N.; Ghosh, S. Effect of mix composition on compressive strength and microstructure of fly ash based geopolymer composites. ARPN J. Eng. Appl. Sci. 2009, 4, 68–74. [Google Scholar]
- Bortnovsky, O.; Sobalik, S.; Tvaruzkova, Z.; Dedecek, J.; Roubicek, P.; Prudkova, Z.; Svoboda, M. Structure and stability of geopolymer synthesized from kaolinitic and shale clay residues. In Geopolymer, Green Chemistry and Sustainable Development Solutions: Proceedings of the World Congress Geopolymer; Davidovits, J., Ed.; Geopolymer Institute: Saint-Quentin, France, 2005; pp. 81–84. [Google Scholar]
- Somaratna, J.; Ravikumar, D.; Neithalath, N. Response of alkali activated fly ash mortars to microwave curing. Cem. Concr. Res. 2010, 40, 1688–1696. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Rattanasak, U.; Taebuanhuad, S. Role of microwave radiation in curing the fly ash geopolymer. Adv. Powder Technol. 2013, 24, 703–707. [Google Scholar] [CrossRef]
- Rovnaník, P. Effect of curing temperature on the developmentof hard structure of metakaolin-based geopolymer. Constr. Build. Mater. 2010, 24, 1176–1183. [Google Scholar] [CrossRef]
- Patil, S.G.; ManojKumar. Factors Influencing compressive strength of geopolymer concrete. Int. J. Res. Eng. Technol. 2013, 372–375. [Google Scholar]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Mallicoat, S.W.; Kriven, W.M.; van Deventer, J.S.J. Understanding the relationship between geopolymer composition, microstructure and mechanical properties. Colloids Surf. A Physicochem. Eng. Aspects 2005, 269, 47–58. [Google Scholar] [CrossRef]
- Duxson, P.; Mallicoat, S.W.; Lukey, G.C.; Kriven, W.M.; van Deventer, J.S.J. The effect of alkali and Si/Al ratio on the development of mechanical properties of metakaolin-based geopolymers. Colloids Surf. A Physicochem. Eng. Aspects 2007, 292, 8–20. [Google Scholar] [CrossRef]
- Xu, H. Geopolymerisation of Aluminosilicate Minerals. Ph.D. Thesis, University of Melbourne, Melbourne, Australia, 2001. [Google Scholar]
- Van Jaarsveld, J.G.S.; Van Deventer, J.S.J.; Lukey, G.C. The characterisation of source materials in fly ash-based geopolymers. Mater. Lett. 2003, 57, 1272–1280. [Google Scholar] [CrossRef]
- Shen, Y.J.; Zhang, Y.L.; Gao, F.; Yang, G.S.; Lai, X.P. Influence of Temperature on the Microstructure Deterioration of Sandstone. Energies 2018, 11, 1753. [Google Scholar] [CrossRef]
- Joseph, B.; Mathew, G. Influence of aggregate content on the behavior of fly ash based geopolymer concrete. Sci. Iranica 2012, 19, 1188–1194. [Google Scholar] [CrossRef]
- Kuenzel, C.; Li, L.; Vandeperre, L.; Boccaccini, A.R.; Cheeseman, C.R. Influence of sand on the mechanical properties of metakaolin geopolymers. Constr. Build. Mater. 2014, 66, 442–446. [Google Scholar] [CrossRef]
- Latella, B.A.; Perera, D.S.; Durce, D.; Mehrtens, E.G.; Davis, J. Mechanical properties of metakaolin-based geopolymers with molar ratios of Si/Al ≈ 2 and Na/Al ≈ 1. J. Mater. Sci. 2008, 43, 2693–2699. [Google Scholar] [CrossRef]
- Granizo, N.; Palomo, A.; Fernandez-Jiménez, A. Effect of temperature and alkaline concentration on metakaolin leaching kinetics. Ceram. Int. 2014, 40, 8975–8985. [Google Scholar] [CrossRef]
- Panagiotopoulou, C.; Kontori, E.; Perraki, T.; Kakali, G. Dissolution of aluminosilicate minerals and by-products in alkaline media. J. Mater. Sci. 2007, 42, 2967–2973. [Google Scholar] [CrossRef]
- Rouyer, J.; Benavent, V.; Frizon, F.; Poulesquen, A. Influence of geopolymer formulation parameters on the elastic and porous properties over a one-year monitoring. Mater. Lett. 2017, 207, 121–124. [Google Scholar] [CrossRef]








| Chemical Composition (wt %) | MgO | Al2O3 | SiO2 | P2O5 | SO3 | K2O | Na2O | CaO | TiO2 | Fe2O3 | LOI H2O |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Calcined kaolinite claystone | 0.13 | 41.45 | 52.03 | 0.06 | 0.20 | 0.79 | 0.15 | 1.62 | 1.05 | 2.52 | |
| Water glass | 27.75 | 26.17 | 1.11 | 44.97 |
| Geopolymer | Water Glass Density (g·cm−3) | Total Molar Ratio | ||||
|---|---|---|---|---|---|---|
| Si:Al | M:Al | H2O:Al | K:Na | Ca:Al | ||
| GP1.2 | 1.2 | 1.40 | 0.48 | 8.61 | 16.12 | 0.12 |
| GP1.3 | 1.3 | 1.54 | 0.66 | 7.71 | 15.95 | 0.12 |
| GP1.4 | 1.4 | 1.65 | 0.82 | 6.93 | 15.87 | 0.12 |
| GP1.5 | 1.5 | 1.76 | 0.95 | 6.25 | 15.82 | 0.12 |
| GP1.6 | 1.6 | 1.85 | 1.06 | 5.66 | 15.78 | 0.12 |
| Label | Curing Methods | Time to Hardening |
|---|---|---|
| LT | solidify at ambient temperature in laboratory (22 °C) | 12 h |
| 70 °C | solidify at 70 °C during 3 h | 3 h |
| MW/2 | solidify at ambient temperature and 2nd day 26 min at microwave radiation | 12 h |
| MW | solidify 26 min at microwave radiation | 26 min |
| 5 °C/LT | solidify at 5 °C during 12 h and then at ambient temperature in laboratory (22 °C) | 18 h |
| 5 °C | solidify at 5 °C | 24 h |
| Water Glass Density (g·cm−3) | 1.2 | 1.3 | 1.4 | 1.5 | 1.6 |
|---|---|---|---|---|---|
| Pore Volume (mm3·g−1) | 166.9 | 143.5 | 122.7 | 94.2 | 75.8 |
| Average Pore Diameter (nm) | 32.4 | 16 | 16.5 | 19 | 47.2 |
| Curing Method | 70 °C | MW | MW/2 | LT | 5 °C/LT | 5 °C |
|---|---|---|---|---|---|---|
| Pore Volume (mm3·g−1) | 104.1 | 110.7 | 100.5 | 94.2 | 81.4 | 73.3 |
| Average Pore Diameter (nm) | 15.4 | 16.9 | 14.5 | 19.0 | 17.6 | 19.2 |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hájková, P. Kaolinite Claystone-Based Geopolymer Materials: Effect of Chemical Composition and Curing Conditions. Minerals 2018, 8, 444. https://doi.org/10.3390/min8100444
Hájková P. Kaolinite Claystone-Based Geopolymer Materials: Effect of Chemical Composition and Curing Conditions. Minerals. 2018; 8(10):444. https://doi.org/10.3390/min8100444
Chicago/Turabian StyleHájková, Pavlína. 2018. "Kaolinite Claystone-Based Geopolymer Materials: Effect of Chemical Composition and Curing Conditions" Minerals 8, no. 10: 444. https://doi.org/10.3390/min8100444
APA StyleHájková, P. (2018). Kaolinite Claystone-Based Geopolymer Materials: Effect of Chemical Composition and Curing Conditions. Minerals, 8(10), 444. https://doi.org/10.3390/min8100444