Factors to Enable Crystallization of Environmentally Stable Bioscorodite from Dilute As(III)-Contaminated Waters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganism
2.2. Preparation of Bio- and Chemical- Scorodite As Seed Crystals
2.3. Bioscorodite Crystallization Experiment
2.4. Toxicity Characteristic Leaching Procedure (TCLP) Test
2.5. Ion Activity Product (IAP) Calculation
2.6. Solid Analysis
3. Results and Discussion
3.1. Effect of Seed Feeding and pH
3.1.1. Characterization of Scorodite Seeds
3.1.2. Effect of Seed Feeding
3.1.3. Effect of Initial pH
3.1.4. Stability of Final Bioscorodite Products
3.2. Optimal Fe(II)/As(III) Molar Ratios for As Removal as Bioscorodite from a Range of Dilute As(III)-Bearing Solutions
4. Conclusions
- Morphological and structural characteristics of bio- and chemical- scorodite seed crystals were compared; bioscorodite (FeAsO4·1.55H2O; spherical aggregates; 36 µm; 2.0 m2/g), chemical scorodite (FeAsO4·1.91H2O; orthorhombic crystals; 98 µm; 0.7 m2/g). The former seeds showed greater stability (As; 0.33 ± 0.08 mg/L) than the latter (As; 5.49 ± 0.03 mg/L) by TCLP test.
- At pH 1.5, feeding bioscorodite seeds (0.15%) enabled effective As removal from dilute As(III) solution ([As(III)ini] = 4.7 mM; [Fe(II)ini] = 9.5 mM), by inducing a two-stage As and Fe precipitation (98% final As removal at day 21).
- Contrasting bioscorodite crystallization processes on bio- and chemical- scorodite seeds were found. Hollow bioscorodite seed particles became increasingly filled with newly formed scorodite, whilst solid chemical seeds induced their surface to be thoroughly coated with new scorodite precipitates.
- Lowering the initial pH from 1.5 to 1.2 (using bioscorodite seeds) led to a steady and continuous formation of bioscorodite particles (instead of two-stage As precipitation at pH 1.5). However, As removal remained relatively incomplete at pH 1.2 (91%) compared to at pH 1.5 (98%).
- TCLP leachabilities of final bioscorodite products formed on bio- and chemical- scorodite seeds (at pH 1.5) were 0.59 ± 0.08 mg/L and 1.86 ± 0.05 mg/L, respectively.
- Under the favorable conditions above described, 94–99% of As were successfully removed as crystalline bioscorodite from 3.3–20 mM As(III) solutions by setting initial [Fe(II)]/[As(III)] molar ratios at 1.4–2.0.
- Optimal conditions found for the bioscorodite method enabling treatment of dilute As(III) concentrations did not necessarily match those found for chemical scorodite syntheses targeting high As(V) concentrations.
- For possible practical application using the continuous process (supposedly composed of an aeration tank followed by a settling tank), recycling microbiologically active bioscorodite sludge could maintain both high cell density and seed pulp density to support steady As(III) and Fe(II) oxidation and the resultant scorodite crystallization.
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Ion Species | Hydrolysis Reaction | Ki | pK (25 °C) [19] | pK (70 °C) |
|---|---|---|---|---|
| Fe(III) | Fe3+ + H2O = Fe(OH)2+ + H+ | KFe1 | 2.19 | 2.18 |
| Fe(OH)2+ + H2O = Fe(OH)2+ + H+ | KFe2 | 3.48 | 3.47 | |
| Fe(OH)2+ + H2O = Fe(OH)3 + H+ | KFe3 | 6.33 | 6.31 | |
| Fe(OH)3 + H2O = Fe(OH)4− + H+ | KFe4 | 9.6 | 9.57 | |
| As(V) | H3AsO4 = H2AsO4− + H+ | KAs1 | 2.24 | 2.27 |
| H2AsO4− = HAsO42− + H+ | KAs2 | 6.86 | 6.94 | |
| HAsO42− = AsO43− + H+ | KAs3 | 11.49 | 11.51 |
| Nature of Crystals | Size Crystals | Arsenic Leaching | Arsenic Leaching at Other Test Conditions |
|---|---|---|---|
| TCLP Test | |||
| (* pH 5, 20 h) | |||
| Mineral | |||
| Scorodite [29] | - | - | 0.32 mg/L pH 2.43, 14 d (H2SO4) |
| - | - | 1.49 mg/L pH 2.05, 14 d (H2SO4) | |
| Biogenic crystals | |||
| Bioscorodite 72 °C [12] | Flakes < 1 cm | 0.1 mg/L, 1 d | - |
| 0.5 mg/L, 100 d | |||
| Bioscorodite 72 °C [13] | 175 μm | 1.9 mg/L, 60 d | - |
| Chemical synthesized crystals (70–95 °C) | |||
| Nanocrystalline scorodite 70 °C [30] | 50 nm | 0.58 mg/L, 13 d | 20.8 mg/L pH 1, 13 d |
| 0.56 mg/L pH 2, 13 d | |||
| 5.38 mg/L pH 7, 13 d | |||
| Amorphous ferric arsenate 70 °C [30] | 50–100 nm | 60 mg/L, 13 d | 90 mg/L pH 2, 13 d |
| 30 mg/L pH 3, 13 d | |||
| 50 mg/L pH 4, 13 d | |||
| Scorodite 70 °C [31] | 0.77 μm | 5.13 mg/L, 7 h | 2.69 mg/L pH 3.00, 7 h |
| 1.01 mg/L pH 5.74, 7 h (water) | |||
| Scorodite 95 °C [16] | 1.6 μm | 13.6 mg/L, 18 h | - |
| 5.3 μm | 0.1 mg/L, 18 h | ||
| Scorodite 95 °C [10] | 16 μm | 0.37 mg/L, 6 h | - |
| Scorodite 95 °C [17] | 17 μm | 0.18 mg/L, 35 d | 0.4 mg/L pH 3, 35 d |
| 3.1 mg/L pH 7, 35 d | |||
| 351 mg/L pH 9, 35 d | |||
| Scorodite 95 °C [32] | - | 0.3 mg/L, 4 h | 0.45 mg/L pH 6, 4 h |
| Scorodite 95 °C [6] a | 10 μm | 4.8 mg/L * | - |
| Chemical synthesized crystals (150–175 °C) | |||
| Scorodite 150 °C [16] | 1.5 μm | 13 mg/L, 8 h | - |
| 2.5 μm | 5 mg/L, 8 h | ||
| Scorodite 160 °C [33] | - | 0.35 mg/L, 460 d | 0.61 mg/L pH 6, 460 d |
| 5.89 mg/L pH 7, 460 d | |||
| Scorodite 160 °C [34] | 0.3 μm | 0.35 mg/L, 460 d | 5.89 mg/L pH 7, 460 d |
| 386 mg/L pH 9, 460 d | |||
| Scorodite 160 °C [6] a | 5 μm | 0.8 mg/L * | - |
| Scorodite 160 °C [29] | 2.5 μm | - | 0.2 mg/L pH 5.57, 14 d (H2SO4) |
| 0.19 mg/L pH 3.45, 14 d (H2SO4) | |||
| 0.33 mg/L pH 2.85, 14 d (H2SO4) | |||
| Scorodite 175 °C [35] | - | 0.1 mg/L * | - |
References
- Riveros, P.A.; Dutrizac, J.E.; Spencer, P. Arsenic disposal practices in the metallurgical industry. Can. Metall. Quart. 2001, 40, 395–420. [Google Scholar] [CrossRef]
- Dutrizac, J.E.; Jambor, J.L. The synthesis of crystalline scorodite, FeAsO4·2H2O. Hydrometallurgy 1988, 19, 377–384. [Google Scholar] [CrossRef]
- Monhemius, A.J.; Swash, P.M. Removing and stabilizing as from copper refining circuits by hydrothermal processing. JOM 1999, 51, 30–33. [Google Scholar] [CrossRef]
- Demopoulos, G.P.; Droppert, D.J.; Van Weert, G. Precipitation of crystalline scorodite (FeAsO4·2H2O) from chloride solutions. Hydrometallurgy 1995, 38, 245–261. [Google Scholar] [CrossRef]
- Filippou, D.; Demopoulos, G.P. Arsenic immobilization by controlled scorodite precipitation. JOM 1997, 49, 52–55. [Google Scholar] [CrossRef]
- Singhania, S.; Wang, Q.; Filippou, D.; Demopoulos, G.P. Temperature and seeding effects on the precipitation of scorodite from sulfate solutions under atmospheric-pressure conditions. Metall. Mater. Trans. B 2005, 36, 327–333. [Google Scholar] [CrossRef]
- Singhania, S.; Wang, Q.; Filippou, D.; Demopoulos, G.P. Acidity, valency and third-ion effects on the precipitation of scorodite from mixed sulfate solutions under atmospheric-pressure conditions. Metall. Mater. Trans. B 2006, 37, 189–197. [Google Scholar] [CrossRef]
- Fujita, T.; Taguchi, R.; Abumiya, M.; Matsumoto, M.; Shibata, E.; Nakamura, T. Novel atmospheric scorodite synthesis by oxidation of ferrous sulfate solution. Part I. Hydrometallurgy 2008, 90, 92–102. [Google Scholar] [CrossRef]
- Fujita, T.; Taguchi, R.; Abumiya, M.; Matsumoto, M.; Shibata, E.; Nakamura, T. Novel atmospheric scorodite synthesis by oxidation of ferrous sulfate solution. Part II. Effect of temperature and air. Hydrometallurgy 2008, 90, 85–91. [Google Scholar] [CrossRef]
- Fujita, T.; Taguchi, R.; Kubo, H.; Shibata, E.; Nakamura, T. Immobilization of arsenic from novel synthesized scorodite—Analysis on solubility and stability. Mater. Trans. 2009, 50, 321–331. [Google Scholar] [CrossRef]
- Gonzalez-Contreras, P.; Weijma, J.; van der Weijden, R.; Buisman, C.J.N. Biogenic scorodite crystallization by Acidianus sulfidivorans for arsenic removal. Environ. Sci. Technol. 2010, 44, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Contreras, P.; Weijma, J.; Buisman, C.J.N. Bioscorodite crystallization in an airlift reactor for arsenic removal. Cryst. Growth Des. 2012, 12, 2699–2706. [Google Scholar] [CrossRef]
- Gonzalez-Contreras, P.; Weijma, J.; Buisman, C.J.N. Continuous bioscorodite crystallization in CSTRs for arsenic removal and disposal. Water Res. 2012, 46, 5883–5892. [Google Scholar] [CrossRef] [PubMed]
- Okibe, N.; Koga, M.; Morishita, S.; Tanaka, M.; Heguri, S.; Asano, S.; Sasaki, K.; Hirajima, T. Microbial formation of crystalline scorodite for treatment of As(III)-bearing copper refinery process solution using Acidianus brierleyi. Hydrometallurgy 2014, 143, 34–41. [Google Scholar] [CrossRef]
- Okibe, N.; Morishita, S.; Tanaka, M.; Sasaki, K.; Hirajima, T.; Hatano, K.; Ohata, A. Bioscorodite crystallization using Acidianus brierleyi: Effects caused by Cu(II) present in As(III)-bearing copper refinery wastewaters. Hydrometallurgy 2017, 168, 121–126. [Google Scholar] [CrossRef]
- Caetano, M.L.; Ciminelli, V.S.T.; Rocha, S.D.F.; Spitale, M.C.; Caldeira, C.L. Batch and continuous precipitation of scorodite from dilute industrial solutions. Hydrometallurgy 2009, 95, 44–52. [Google Scholar] [CrossRef]
- Fujita, T.; Taguchi, R.; Abumiya, M.; Matsumoto, M.; Shibata, E.; Nakamura, T. Effect of pH on atmospheric scorodite synthesis by oxidation of ferrous ions: Physical properties and stability of the scorodite. Hydrometallurgy 2009, 96, 189–198. [Google Scholar] [CrossRef]
- Johnson, D.B.; Joulian, C.; d’Hugues, P.; Hallberg, K.B. Sulfobacillus benefaciens sp. Nov., an acidophilic facultative anaerobic Firmicute isolated from mineral bioleaching operations. Extremophiles 2008, 12, 789–798. [Google Scholar] [CrossRef] [PubMed]
- US EPA. Method 1311: Toxicity Characteristic Leaching Procedure. Test Methods for Evaluating Solid Wastes Physical/Chemical Methods; United States Environmental Protection Agency: Washington, DC, USA, 1992.
- Dove, P.M.; Rimstidt, J.D. The solubility and stability of scorodite, FeAsO4·2H2O. Am. Mineral. 1985, 70, 838–844. [Google Scholar]
- Ondruš, P.; Skala, R.; Viti, C.; Veselovský, F.; Novák, F.; Jansa, J. Parascorodite, FeAsO4·2H2O—A new mineral from Kaňk near Kutná Hora, Czech Republic. Am. Mineral. 1999, 84, 1439–1444. [Google Scholar] [CrossRef]
- Baghurst, D.R.; Barret, J.; Coleyshaw, E.E.; Griffith, W.P.; Mingos, D.M.P. Microwave techniques for the synthesis and deuteration of minerals, with particular reference to scorodite, FeAsO4·2H2O. Mineral. Mag. 1996, 60, 821–828. [Google Scholar] [CrossRef]
- Legal, J.M.; Manfait, M.; Theophanides, T. Applications of FTIR spectroscopy in structural studies of cells and bacteria. J. Mol. Struct. 1991, 242, 397–407. [Google Scholar] [CrossRef]
- Langmuir, D.; Mahoney, J.; Rowson, J. Solubility products of amorphous ferric arsenate and crystalline scorodite (FeAsO4·2H2O) and their application to arsenic behavior in buried mine tailings. Geochim. Cosmochim. Acta 2006, 70, 2942–2956. [Google Scholar] [CrossRef]
- Gomez, M.A.; Becze, L.; Cutler, J.N.; Demopoulos, G.P. Hydrothermal reaction chemistry and characterization of ferric arsenate phases precipitated from Fe2(SO4)3–As2O5–H2SO4 solutions. Hydrometallurgy 2011, 107, 74–90. [Google Scholar] [CrossRef]
- Dutrizac, J.E.; Jambor, J.L. Characterization of the iron arsenate–sulphate compounds precipitated at elevated temperatures. Hydrometallurgy 2007, 86, 147–163. [Google Scholar] [CrossRef]
- Gomez, M.A.; Becze, L.; Celikin, M.; Demopoulos, G.P. The effect of copper on the precipitation of scorodite (FeAsO4·2H2O) under hydrothermal conditions: Evidence for a hydrated copper containing ferric arsenate sulfate-short lived intermediate. J. Colloid Interface Sci. 2011, 360, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Swash, P.M.; Monhemius, A.J. Hydrothermal precipitation from aqueous solutions containing iron(III), arsenate and sulphate. In Hydrometallurgy ’94; Springer: Dordrecht, The Netherlands, 1994; pp. 177–190. [Google Scholar]
- Krause, E.; Ettel, V.A. Solubility and stability of scorodite, FeAsO4·2H2O: New data and further discussion. Am. Mineral. 1988, 73, 850–854. [Google Scholar]
- Paktunc, D.; Bruggeman, K. Solubility of nanocrystalline scorodite and amorphous ferric arsenate: Implications for stabilization of arsenic in mine wastes. Appl. Geochem. 2010, 25, 674–683. [Google Scholar] [CrossRef]
- Fujita, T.; Fujieda, S.; Shinoda, K.; Suzuki, S. Environmental leaching characteristics of scorodite synthesized with Fe(II) ions. Hydrometallurgy 2012, 111, 87–102. [Google Scholar] [CrossRef]
- Harvey, M.C.; Schreiber, M.E.; Rimstidt, J.D.; Griffith, M.M. Scorodite dissolution kinetics: Implications for arsenic release. Environ. Sci. Technol. 2006, 40, 6709–6714. [Google Scholar] [CrossRef] [PubMed]
- Bluteau, M.-C.; Demopoulos, G.P. The incongruent dissolution of scorodite—Solubility, kinetics and mechanism. Hydrometallurgy 2007, 87, 163–177. [Google Scholar] [CrossRef]
- Bluteau, M.-C.; Becze, L.; Demopoulos, G.P. The dissolution of scorodite in gypsum-saturated waters: Evidence of Ca–Fe–AsO4 mineral formation and its impact on arsenic retention. Hydrometallurgy 2009, 97, 221–227. [Google Scholar] [CrossRef]
- Gomez, M.; Becze, L.; Bluteau, M.; Le Berre, J.; Cutler, J.; Demopoulos, G. Autoclave precipitation and characterization of Fe(III)–AsO4–SO4 phases. Hydrometallurgy 2008, 8, 1078–1085. [Google Scholar]
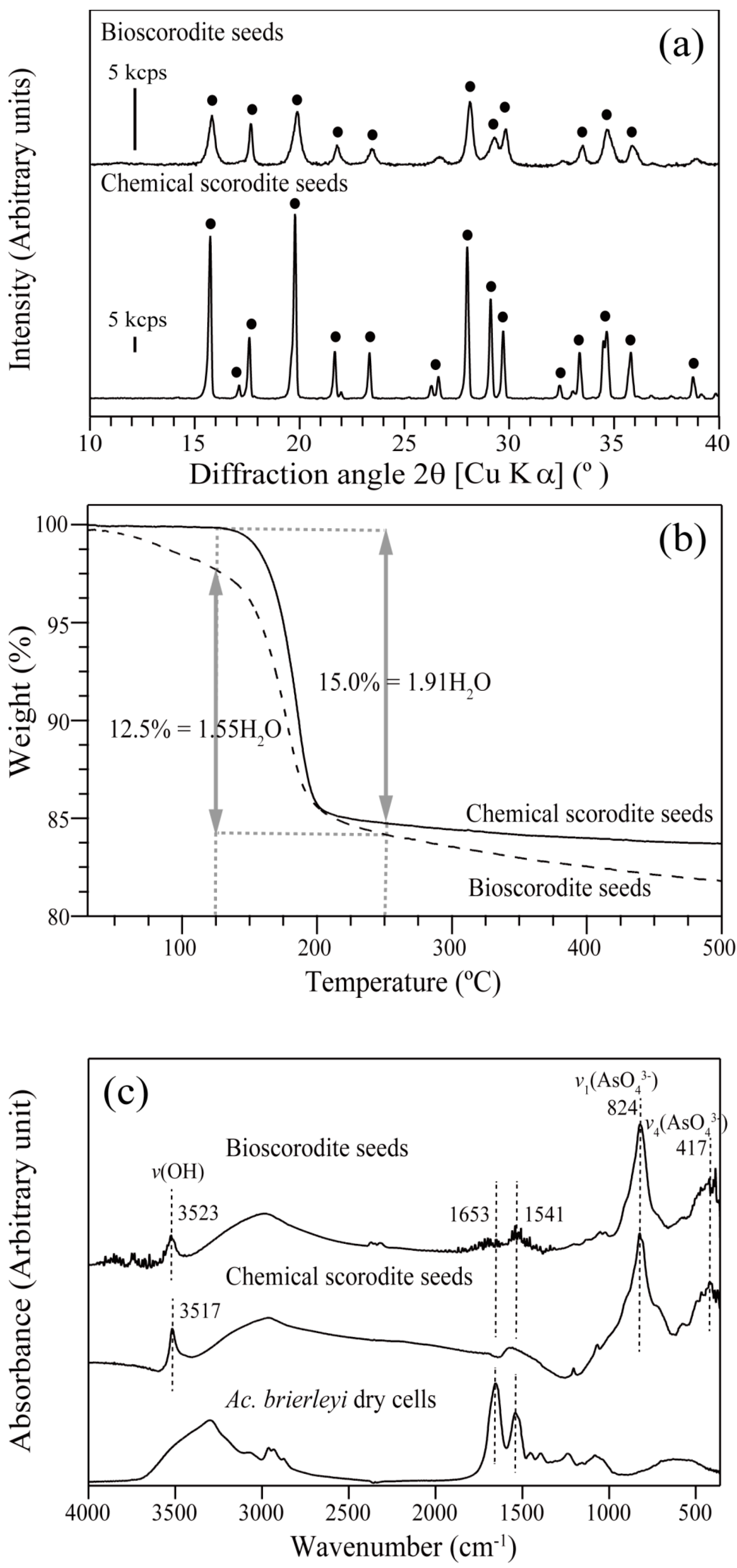
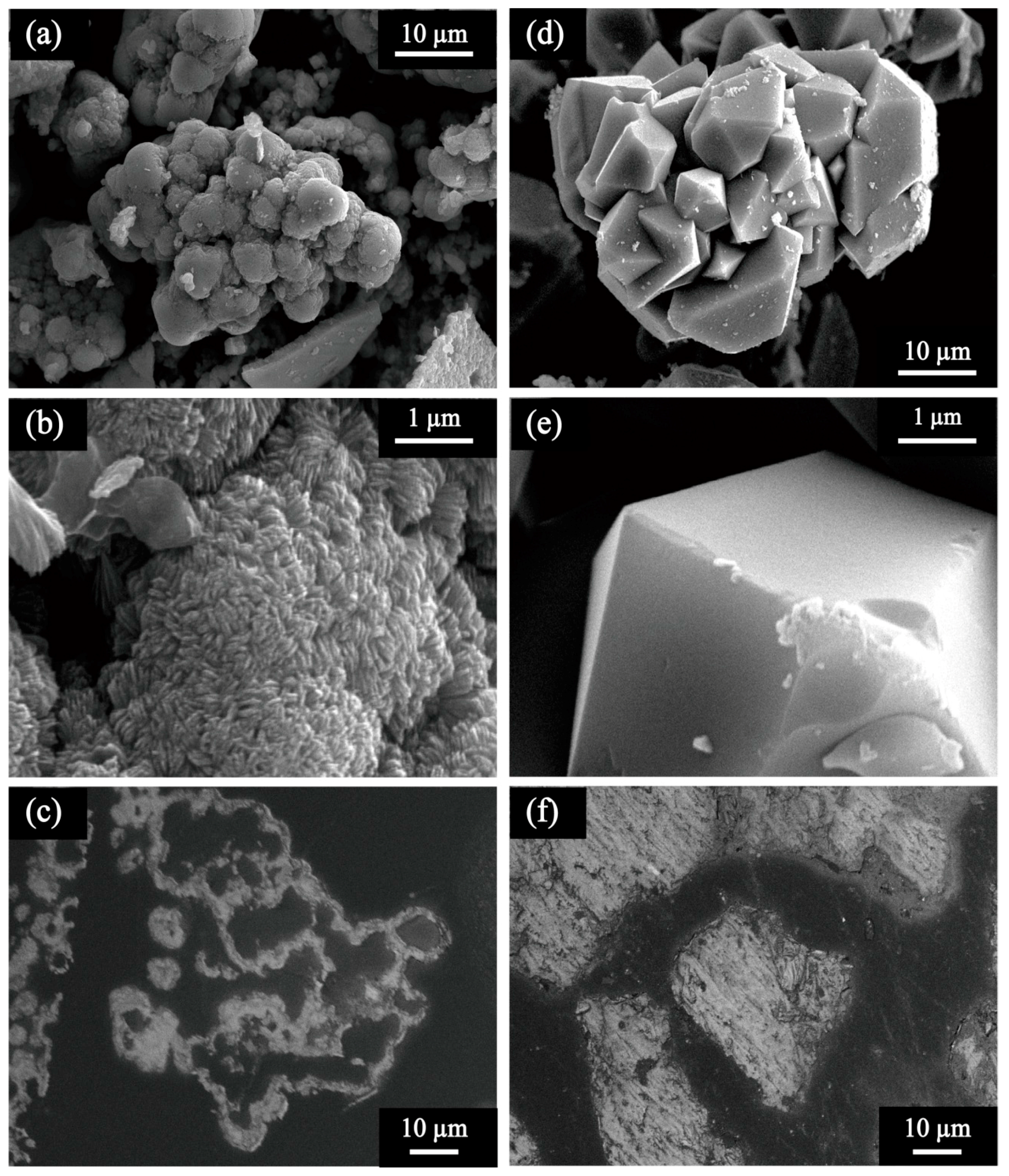

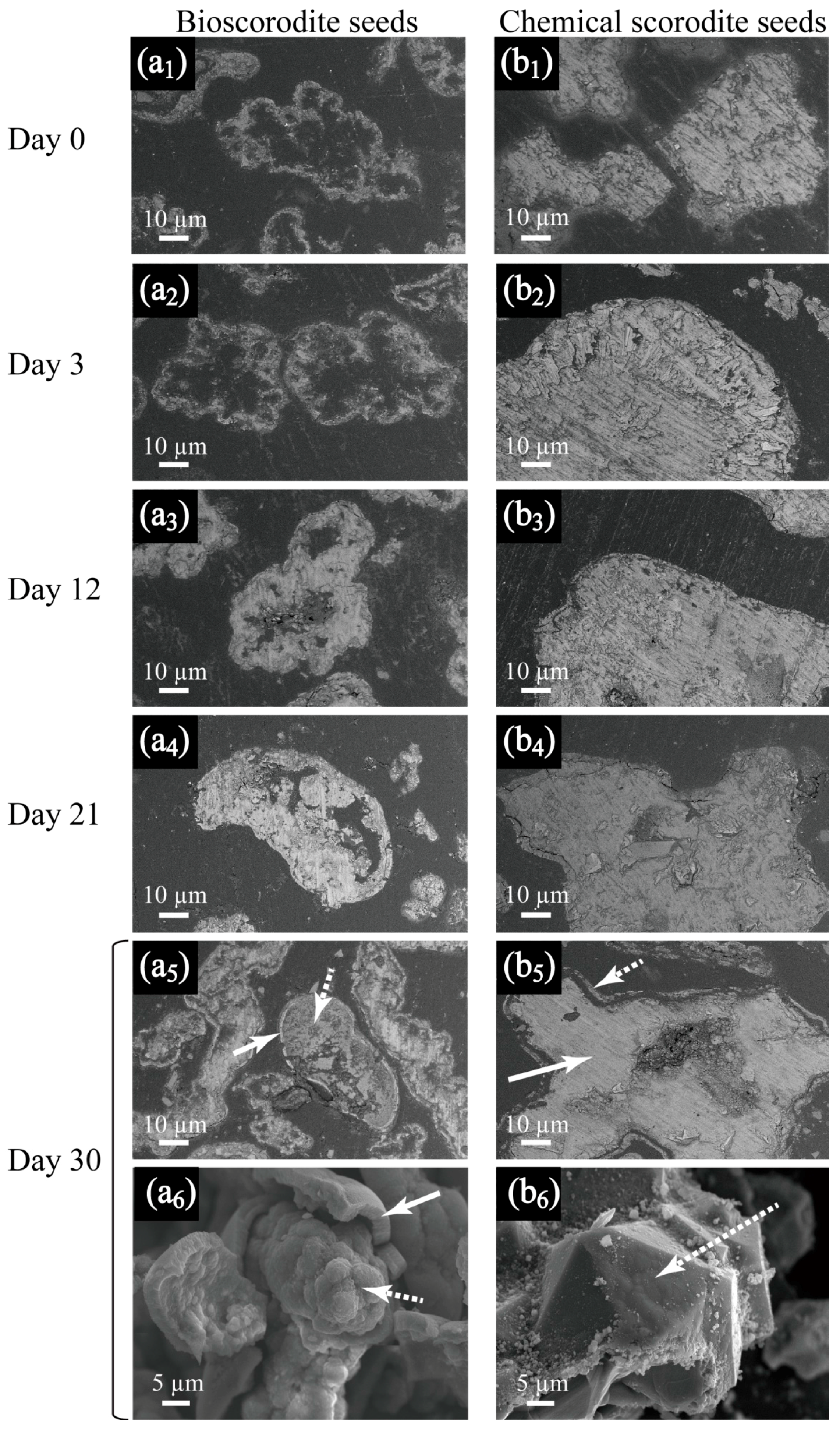
| Seed Crystal | Particle Size (µm) | Specific Surface Area (m2/g) | ||
|---|---|---|---|---|
| Average | Median | Mode | ||
| Bioscorodite Seeds | 36 | 35 | 36 | 2.0 |
| Chemical Scorodite Seeds | 98 | 91 | 90 | 0.7 |
| Conditions Used for Bioscorodite Crystallization Reaction | Original Seed Crystals a | Final Bioscorodite Products b | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TCLP Leachability | Chemical Composition (Weight in 50 mg Sample; mg) | TCLP Leachability | Chemical Composition (Weight in 50 mg Sample; mg) | ||||||||
| Seed Crystals Fed | Initial pH | [As] (mg/L) | [Fe] (mg/L) | Fe | As | Fe/As Molar Ratio | [As] (mg/L) | [Fe] (mg/L) | Fe | As | Fe/As Molar Ratio |
| Bioscorodite | 1.5 | 0.33 ± 0.08 | <0.004 | 11.7 (0.21 mmol) | 14.1 (0.19 mmol) | 1.1 | 0.59 ± 0.20 | <0.004 | 12.1 (0.22 mmol) | 12.0 (0.16 mmol) | 1.4 |
| 1.2 | 0.20 ± 0.01 | 0.11 ± 0.03 | 11.5 (0.21 mmol) | 11.8 (0.16 mmol) | 1.3 | ||||||
| Chemical scorodite | 1.5 | 5.49 ± 0.03 | 0.11 ± 0.01 | 9.12 (0.16 mmol) | 12.2 (0.16 mmol) | 1.0 | 1.86 ± 0.05 | 0.005 | 10.5 (0.19 mmol) | 11.2 (0.15 mmol) | 1.3 |
| Initial Media Condition | Final As Removal | Solid Analysis | Secondary Mineral Identified | References | ||||
|---|---|---|---|---|---|---|---|---|
| [As(III)]ini (mM) | [Fe(II)]ini (mM) | [Fe(II)]ini/[As(III)]ini Molar Ratio | Seed Feeding (Bioscorodite; %) | (%) | (Days) | [Fe]im/[As]im Molar Ratio | ||
| 3.3 | 4.5 | 1.4 | 0 | 11 | 24 | - | Amoprhous | This study |
| (250 ppm) | 0.15 | 95 | 1.2 | Scorodite | ||||
| 6.5 | 2.0 | 98 | 1.2 | Scorodite | ||||
| 13.5 | 4.0 | 59 | - | Jarosite | ||||
| 20.0 | 6.0 | 59 | - | Jarosite | ||||
| 4.7 | 6.3 | 1.4 | 0 | 15 | 21 | - | Amoprhous | This study |
| (350 ppm) | 4.7 | 1.0 | 0.15 | 80 | - | Scorodite | ||
| 6.5 | 1.4 | 94 | 1.5 | Scorodite | ||||
| 9.5 | 2.0 | 98 | 1.4 | Scorodite | ||||
| 14.0 | 3.0 | 53 | - | Amoprhous | ||||
| 19.0 | 4.0 | 65 | - | Jarosite | ||||
| 6.5 | 6.5 | 1.0 | 0.15 | 84 | 20 | 1.1 | Scorodite | This study |
| (500 ppm) | 9.0 | 1.4 | 97 | 1.1 | Scorodite | |||
| 10.5 | 1.6 | 98 | 1.1 | Scorodite | ||||
| 12.0 | 1.8 | 99 | 1.1 | Scorodite | ||||
| 13.0 | 2.0 | 99 | 1.1 | Scorodite | ||||
| 16.5 | 2.5 | 71 | - | Amoprhous | ||||
| 19.5 | 3.0 | 71 | - | Jarosite | ||||
| 13.0 | 18.0 | 1.4 | 0 | 99 | 14 | 1.1 | Scorodite | Okibe et al., 2014 [14] |
| (1000 ppm) | ||||||||
| 20.0 | 16.0 | 0.8 | 0.15 | 66 | 14 | 1.1 | Scorodite | This study |
| (1500 ppm) | 20.0 | 1.0 | 87 | 1.1 | Scorodite | |||
| 28.0 | 1.4 | 99 | 1.1 | Scorodite | ||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, M.; Okibe, N. Factors to Enable Crystallization of Environmentally Stable Bioscorodite from Dilute As(III)-Contaminated Waters. Minerals 2018, 8, 23. https://doi.org/10.3390/min8010023
Tanaka M, Okibe N. Factors to Enable Crystallization of Environmentally Stable Bioscorodite from Dilute As(III)-Contaminated Waters. Minerals. 2018; 8(1):23. https://doi.org/10.3390/min8010023
Chicago/Turabian StyleTanaka, Masahito, and Naoko Okibe. 2018. "Factors to Enable Crystallization of Environmentally Stable Bioscorodite from Dilute As(III)-Contaminated Waters" Minerals 8, no. 1: 23. https://doi.org/10.3390/min8010023




