1. Introduction
Vanadium-containing titanomagnetite ores are among the most promising types of unconventional ores, and an important source of titanium, vanadium and iron [
1,
2,
3]. The content of these components in the ore is sufficient for industrial extraction, though it can vary widely.
About 6.5% of the proven ferrous ore reserves, more than 90% of V
2O
5 reserves and around 60% of TiO
2 reserves are related to these industrial types of ores. In Russia, stocks of V
2O
5 and TiO
2 account for 80% and 18.5%, respectively, in these ores. The world’s total reserves of titanomagnetites amount to tens of billions of tons [
1,
4,
5,
6] and about 50% of iron ore reserves of this type belong to the Russian Federation. More than 40 deposits in Russia have been discovered and estimated to varying degrees. The Chineisk vanadium-containing titanomagnetites field is located in the Northern Transbaikal area of the Baikal-Amur railway zone. It is one of the largest deposits in the world in terms of ore reserves (about 30 billion tons) [
1,
4]. The use of titanomagnetite ores for metallurgical processing is especially important due to the depletion of rich magnetite stocks. Besides, titanomagnetite ore processing can alleviate the shortage of the titanium raw materials.
Titanomagnetite ores have been actively used as a raw material for cast iron, steels and vanadium salt production for centuries [
1,
2]. However, their processing technologies are continuously developing [
1,
2] in order to increase the recoveries of titanium and vanadium as well as the complex utilization of these raw materials. Titanomagnetite ore processing is a sophisticated large-scale production, which poses technological challenges and causes a number of ecological problems such as environmental pollution, land usage for mill tailing storage, the creation of sludge tanks for vanadium milling slimes, etc. [
6].
There are two main methods for processing titanomagnetites—pyrometallurgical methods (reduction melting to form metallic iron and titanium sludge) [
7,
8,
9] and high-temperature hydrometallurgical methods (autoclave leaching at elevated temperatures up to 200 °C and pressures up to 25–40 atm.) [
10,
11,
12].
The disadvantages of the pyrometallurgical method include the large material and energy expenditures for titanomagnetite processing; titanium contained in the raw material is not extracted and is lost with the waste at different stages of the process. Moreover, one of the most important disadvantages of this method is that titanium remains in the processed materials and significantly reduces their quality. This is one of the reasons why the concentration of titanium in raw materials should be less than 4% [
4]. Also, the chloride method of material processing based on the treatment of the ore by hydrochloric acid can be used. Disadvantages of this method include high costs for processing one ton of ore and negative impact on the environment. In addition, the usage of the chloride method as a technology for titanomagnetite ore processing is economically impractical due to the low content of titanium in such ores [
13,
14,
15,
16]. Consequently, the purpose of this research is to develop more cost-effective and efficient ways for complex hydrometallurgical processing of titanomagnetite with the possibility of obtaining iron and titanium-vanadium concentrates.
One of the most appealing hydrometallurgical processing technologies is fluoride technology, which is due to the fact that the chemicals used are environmentally safe and relatively cheap [
17]. Moreover, ammonium fluoride has good solubility in water; it is more reactive than many other inorganic fluorides. At room temperature, it is a relatively inert crystalline powder, and as a result poses no special environmental danger [
18].
The basis of the ore processing by ammonium fluoride is that the oxygenated compounds of transition and non-transition elements in contact with NH
4F form fluorometallates or oxofluorometallates favorable for further processing [
18,
19]. For instance, in [
20] the possibility of TiO
2 formation from ilmenite concentrate with the use of ammonium fluoride is presented. The processing of ilmenite concentrate in this work was performed with aqueous solutions of fluoride and ammonium bifluoride at 100–108 °C and pyro-hydrolysis of fluorine-containing titanium salts at 800–900 °C. Thus, the urgent task is to develop an effective, low-cost and environmentally friendly ammonium-fluoride-based hydrometallurgical technology, to obtain iron and titanium-vanadium concentrates from titanomagnetite ores.
2. Materials and Methods
In the current work, we used a titanomagnetite ore from the Chineisk deposit (Chita region). This ore is granular with size about 2 mm (the ore was provided by public corporation Zabaikalstalinvest, Novaya Chara, Russia).
The leaching of the titanium from the ore was simulated in agitators. It was performed using aqueous solutions of ammonium fluoride in a wide range of concentrations (from 0.08 to 4.2 mol/L). The mass of each ore sample leached was 150 g. The ratio of solid:liquid was 1:3. The interaction between titanomagnetite ore and ammonium fluoride occurred according the following equation:
Another way of titanium extraction involved adding hydrofluoric acid to the ammonium fluoride solutions. The HF concentration in ammonium fluoride solutions varied from 0.86 to 4.07 mol/L, while the concentration of ammonium fluoride solutions was held constant 0.42 mol/L. The mass of each ore sample was 150 g with the solid:liquid ratio of 1:3. The interaction between titanomagnetite ore, ammonium fluoride, and hydrofluoric acid occurred according to the reaction:
The experiments were conducted in 1000-mL polyethylene agitators with constant stirring for 20 h at room temperature. Solid and liquid fractions were separated using a Nutsche filter. The resulting solid residue was washed with distilled water and dried at 120 °C.
The determination of phase composition and structural parameters for the samples was carried out at the Center of Materials Science for Collective Use of Tomsk State University, Tomsk, Russia. The study was conducted using a XRD-6000 diffractometer (Shimadzu, Japan) with CuKα-radiation = 1.54056 Å, and 2θ in the range of 10°–80°. The phase composition was analyzed with the use of PDF databases 4+ and the full-profile analysis software PowderCell 2.4 (Germany) [
21].
The titanium and iron concentration in the solution, and the elemental composition of the ore were determined by inductively coupled plasma atomic emission spectrometry using an ICAP 6200 DUO spectrometer (Thermo Scientific, Waltham, MA, USA).
We then calculated the weight percentage of iron and titanium in the solid phases of the ore and the Fe/Ti ratio (Fe content, wt %, Ti content, wt %, at the solid phase), using the X-ray diffraction data. Thus, the extraction efficiency (ω %) of titanium and iron was calculated as the ratio of the mass of the component in the liquid phase to the mass of this component in the initial ore expressed in percentage terms:
3. Results and Discussion
3.1. Titanomagnetite Composition
After crushing the ore, the granulometric composition was determined. The results are presented in
Table 1.
As given in
Table 1, particles with size range from 0.5 to 1 mm dominated in the ore.
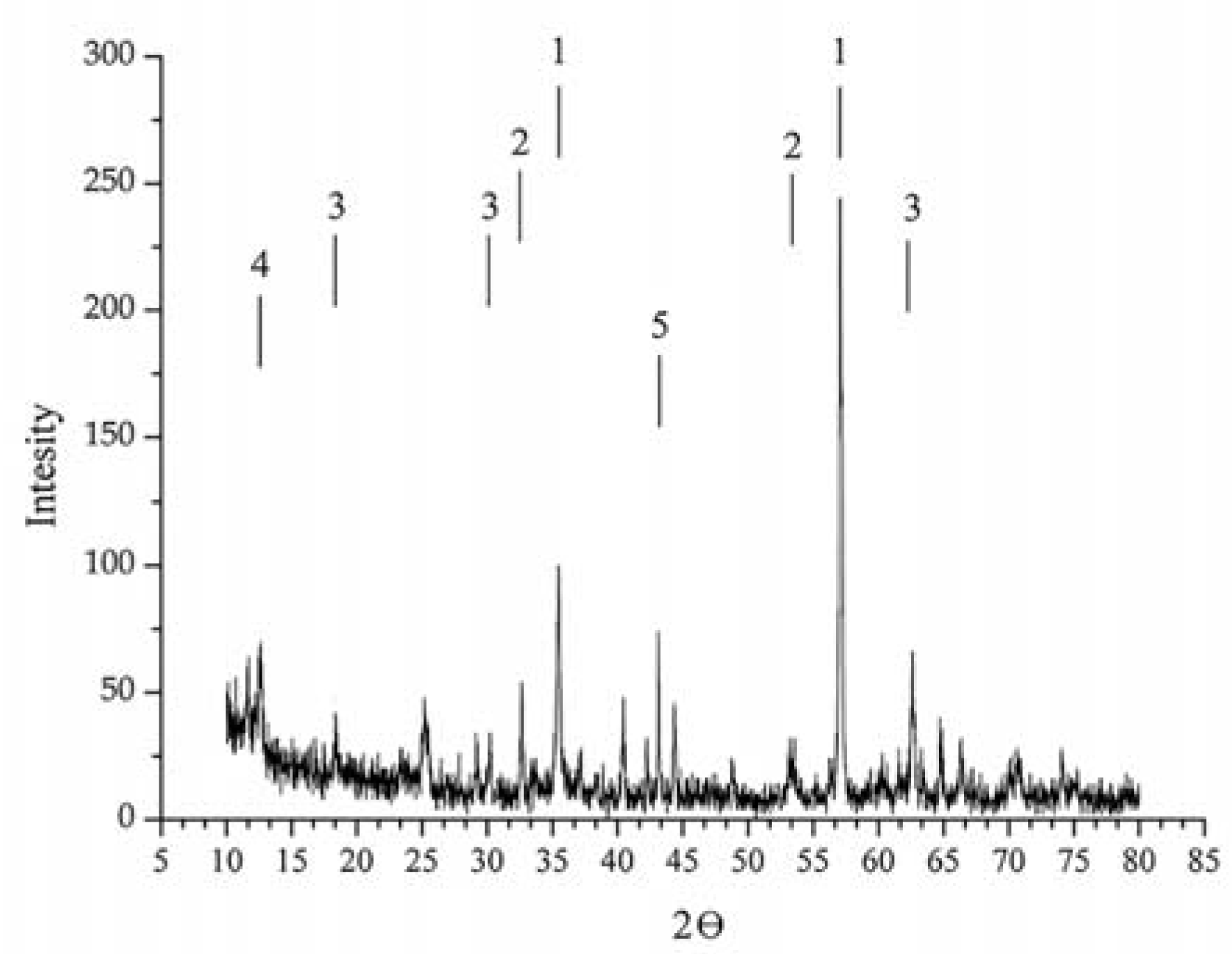
Figure 1 shows the X-ray diffraction (XRD) data on a sample of titanomagnetite ore from the Chineisk deposit in Chita region.
According to the XRD analyses, the samples consisted of several mineral phases.
Table 2 and
Table 3 illustrate the mineralogical composition of titanomagnetite ore from the Chineisk deposit and the elemental composition of the ore, respectively.
Table 2 reveals that magnetite (Fe
3O
4) and ilmenite (FeTiO
3) dominate in the ore. The ore also included such impurities as magnesioferrite (MgFe
2O
4), cronstedtite Fe
3((FeSi)O
5)(OH)
4 and epidote-monoclinic (AlFe). The magnetite and magnesioferrite was characterized by cubic crystal lattice, the ilmenite and cronstedtite had hexagonal crystal lattice, and the epidote had monoclinic crystal lattice.
As shown in
Table 3, the iron content was 53.0 wt %, the titanium content was 8.82 wt %, and the Fe/Ti ratio was 6.01. Thus, the titanomagnetite оrе of the Chineisk deposit is a promising raw material and can be considered as a huge resource for the production of iron and titanium-vanadium concentrates.
3.2. Concentration of Ammonium Fluoride and Processing Efficiency
This section presents the results of studying the possibility for titanomagnetite ore processing through the selective recovery of titanium by solutions that contain ammonium and fluoride ions. The interaction of the titanomagnetite with ammonium fluoride in the presence of oxygen as an oxidizing agent proceeds according to the reaction 1. In the experiments, we varied the concentration of ammonium fluoride from 0.08 to 4.2 mol/L, the temperature was 20–25 °C, the leaching duration equaled 20 h, and the solid:liquid ratio was 1:3.
The results of the experiments are presented in the form of diagrams: titanium content in solid and liquid phases vs. concentration of ammonium fluoride (
Figure 2); iron content in solid and liquid phases vs. concentration of ammonium fluoride (
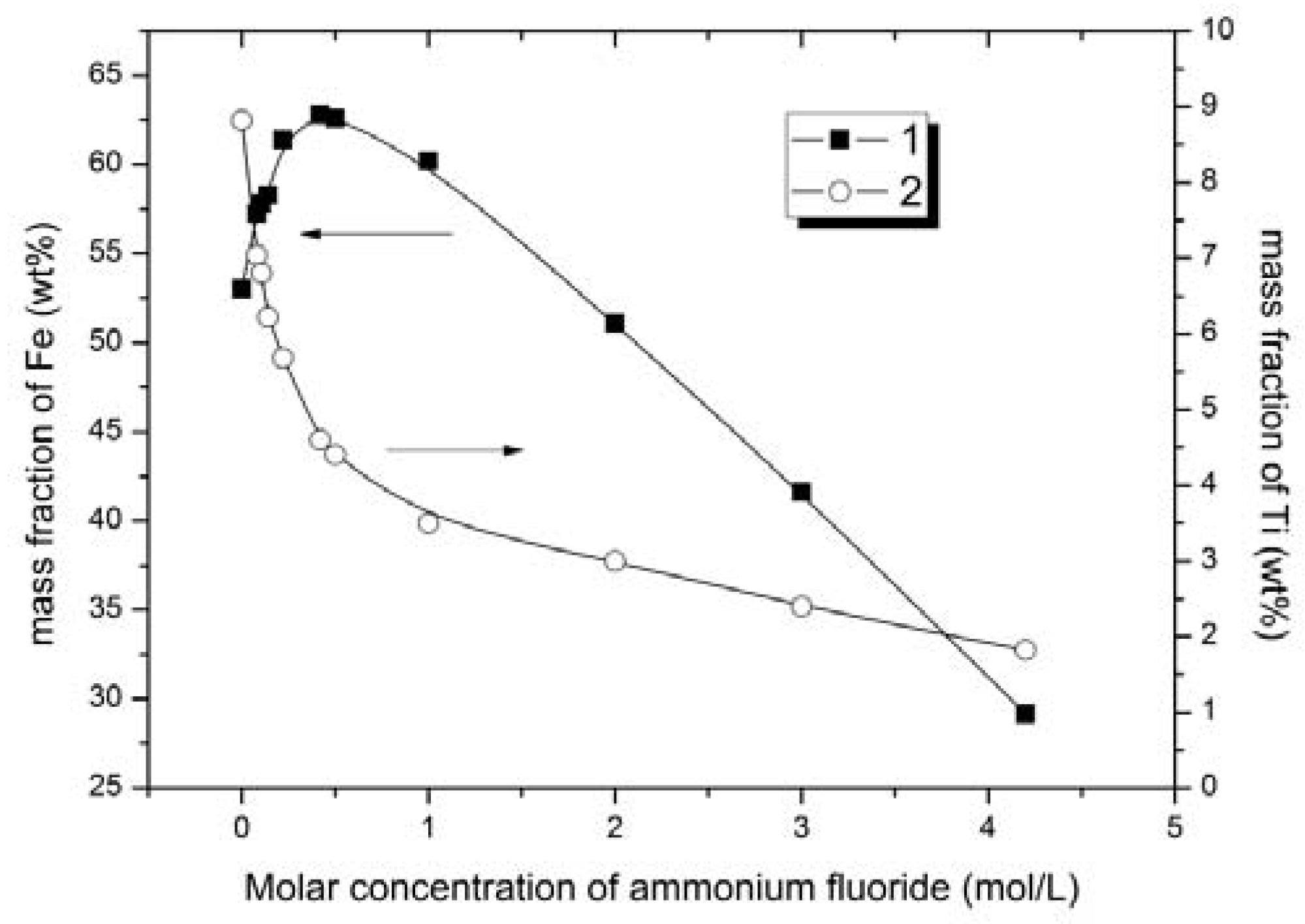
Figure 3); the iron and titanium content in solid phase vs. concentration of ammonium fluoride (
Figure 4).
Table 4 illustrates content of the phases in the solid residue obtained during the processing of titanomagnetite ore by the ammonium fluoride with concentration of 4.2 mol/L.
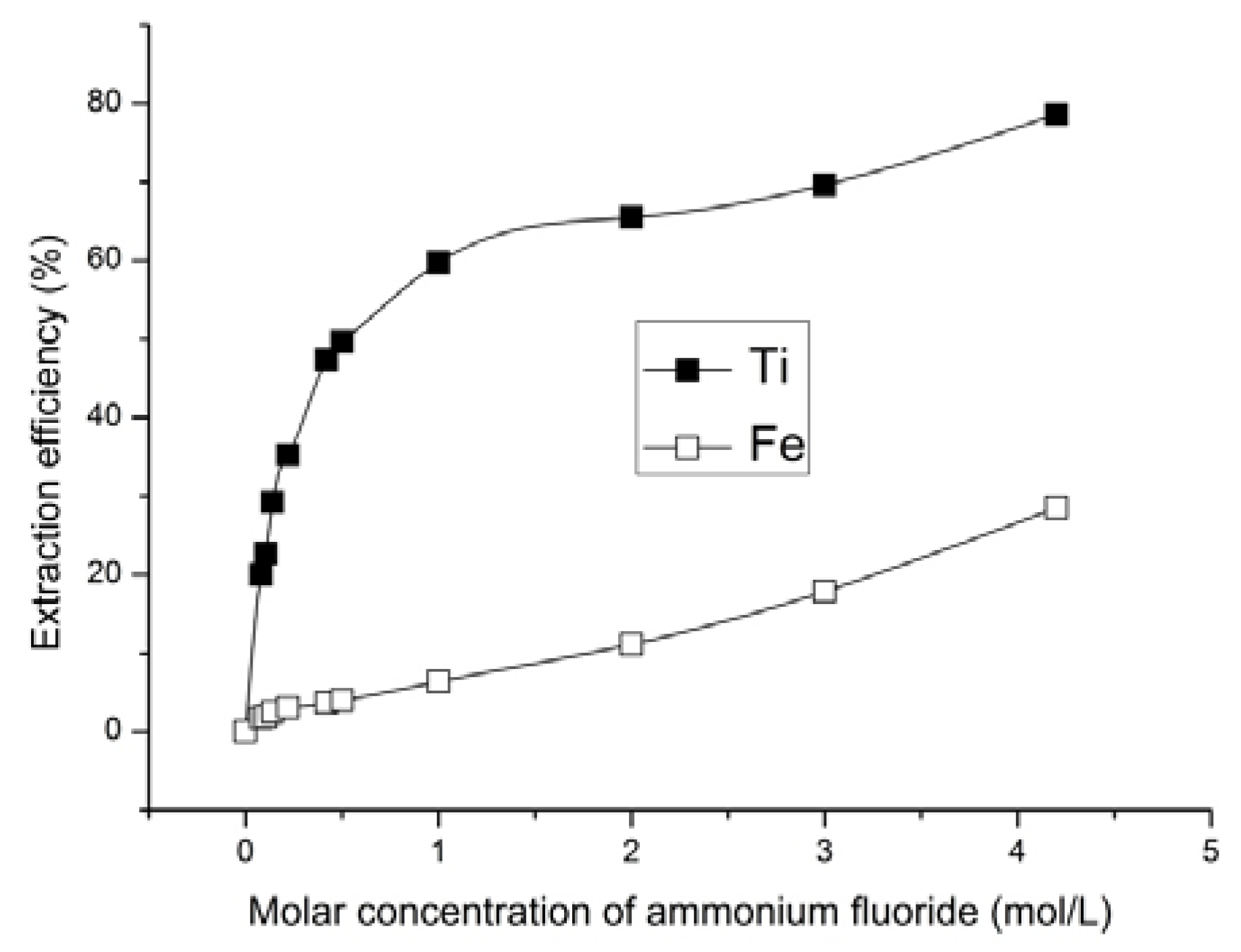
The experimental data analysis showed that the extent of ore leaching increased with the increase in the ammonium fluoride solution concentration. According to the results of X-ray diffraction and atomic emission spectrometry, the extraction rate of titanium from solid phase to the solution increased with the increase in ammonium fluoride concentration in the leaching solution. The lowest content of titanium in solid phase of 1.9 wt % was achieved in ore products that were treated with 4.2 mol/L ammonium fluoride solution (
Figure 2). Titanium extraction at this ammonium fluoride concentration was 78.5% (
Figure 5) and this was the maximum achieved. However, selective titanium extraction was only found with ammonium fluoride concentration up to 1 mol/L.
The separation of iron and titanium occurred due to the formation of water-soluble (NH4)2TiF6 and partially soluble in water (NH4)3FeF6. The effective separation of these elements was achieved by varying the concentration of the reagents in the leaching solution.
The ion content in the solid phase increased with ammonium fluoride concentration from 0.08 to 0.42 mol/L (
Figure 3,
Figure 4 and
Figure 5). With further increase in ammonium fluoride concentration in the leaching solution, iron dissolved to a greater extent, and its mass fraction in the solid phase decreased affecting the quality of ferrous concentrate (
Figure 4) and leading to an increased consumption of the ammonium fluoride. This is due to the formation of (NH
4)
3FeF
6 ammonium hexafluoroferrate (III) in which iron mass fraction was 25 wt % of the residue. Insoluble magnesium fluoride MgF
2 and calcium fluoride CaF
2 were also produced by reaction of magnesium and calcium with the ammonium fluoride. These fluorides are used as fluxes in steelmaking [
22]. The water-soluble complexes (NH
4)
3[AlF
6], (NH
4)
2SiF
6 and (NH
4)
3VF
6 were formed from reaction of the leaching solution with aluminum, silicon and vanadium. As a result, aluminum, silicon and vanadium were extracted to the solution, while calcium and magnesium remained in the iron concentrate.
Based upon the iron content of the solid phase (iron concentrate) the optimum ammonium fluoride concentrations to generate an iron concentrate are from 0.42 to 1 mol/L. Having varied ammonium fluoride concentrations in this range, the iron content was increased from 53.0 to 60.2–62.8 wt % and the titanium content decreased from 8.8 to 3.5–4.6 wt % in solid phase (iron concentrate). Titanium extraction was 47.8–60.3%.
3.3. HF Concentration and Processing Efficiency
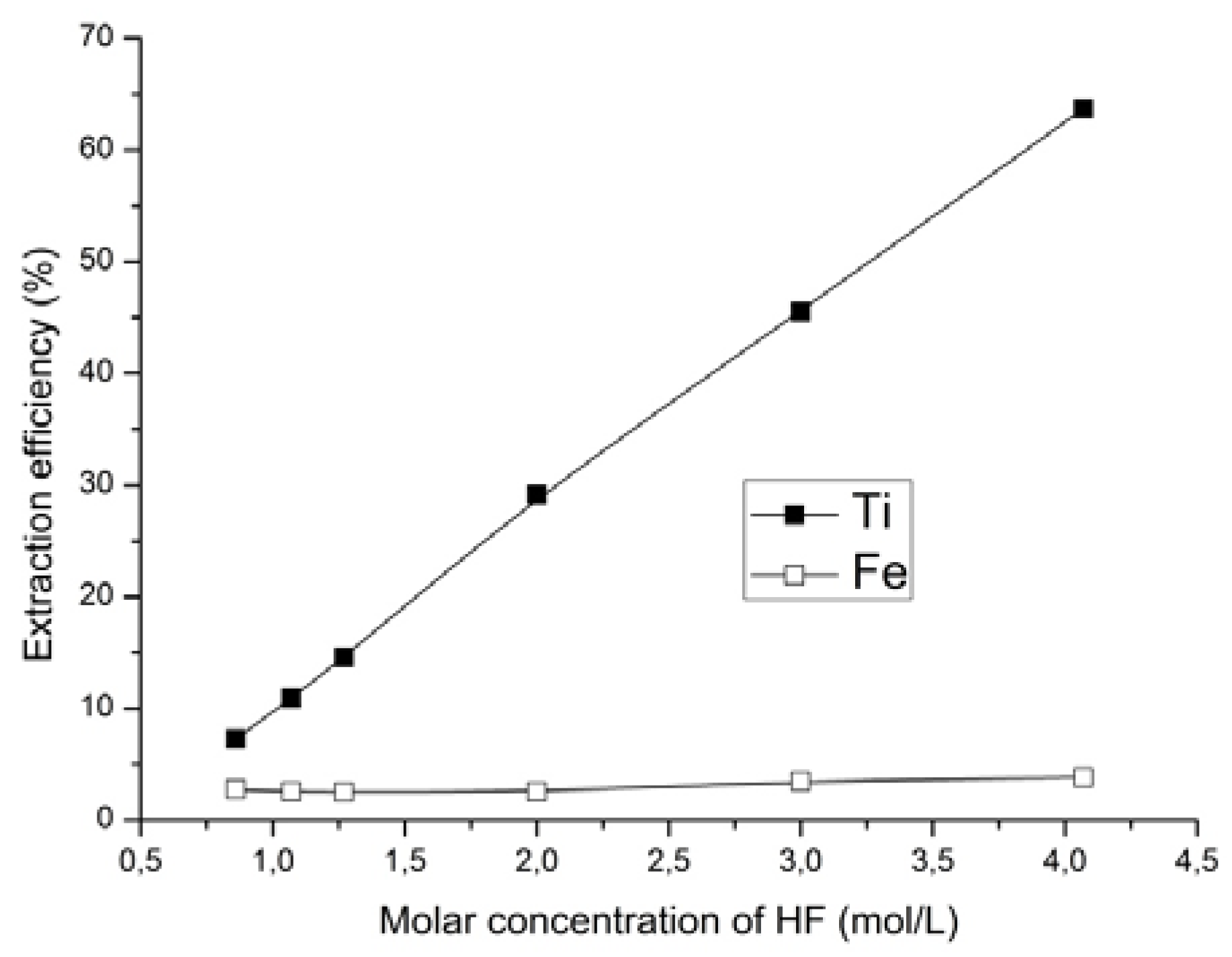
The influence of acidity on the production of concentrates was studied. The acidity of the leaching solution was changed by adding hydrofluoric acid to ammonium fluoride solution. As presented in
Section 2, the interaction of titanomagnetite ore with ammonium fluoride after introducing the hydrofluoric acid and in the presence of oxygen as an oxidizing agent occurred according to reaction (2). Hydrofluoric acid concentration was varied from 0.86 to 4.07 mol/L with the other parameters fixed and using optimum concentration of ammonium fluoride of 0.42 mol/L (
Figure 3 and
Figure 4); 20–25 °C, leaching duration—20 h, (solid:liquid was 1:3). The results of the experiments are presented in
Figure 6 and
Figure 7.
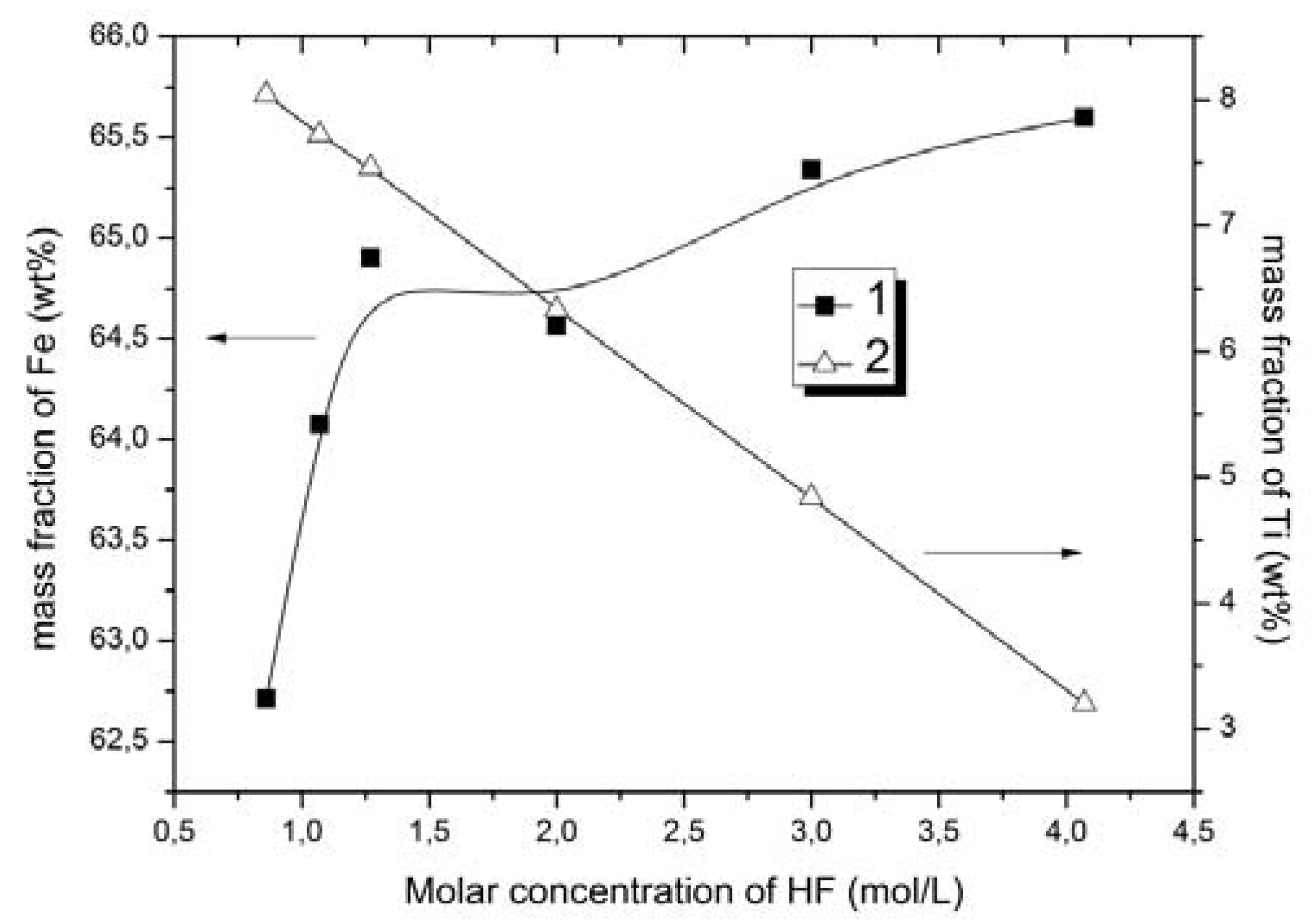
It was found that when increasing hydrofluoric acid concentration, the titanium content decreased while the iron content simultaneously increased in the solid phase. Using a solution of hydrofluoric acid with concentration of 4.07 mol/L and ammonium fluoride with concentration of 0.42 mol/L, the titanium content in solid phase decreased from 8.82 to 3.2 wt %, and the iron content increased from 53.0 to 65.6 wt %. The extraction of titanium in this case was increased from 47.8 to 63.7%. Thus, the intensification of the process after the addition of hydrofluoric acid may be due to the fact that the increase in F− ions concentration as well as the unchanged concentration of (NH4)+ ions in the solution lead to the increase in the yield of ammonium hexafluorotitanate (IV).
As can be seen from
Figure 7, the change in the concentration of hydrofluoric acid at a constant concentration of ammonium fluoride had no significant effect on the extraction of iron. At the same time, the increase of concentration in the hydrofluoric acid substantially increased the extraction of the titanium.
Table 5 demonstrates that most of the iron remained in the oxide form, which positively affected the quality of the iron concentrate and the consumption of the leaching reagents.
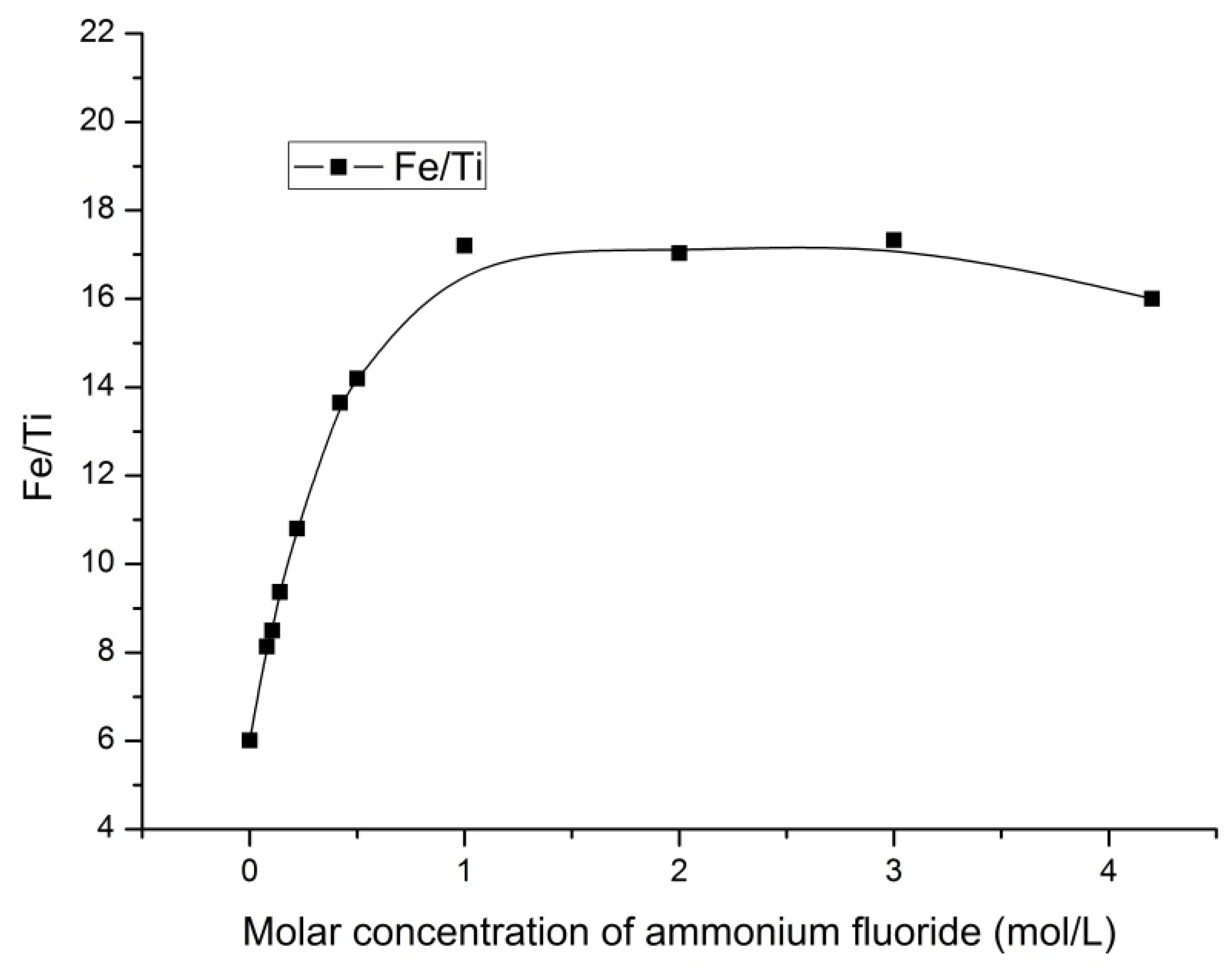
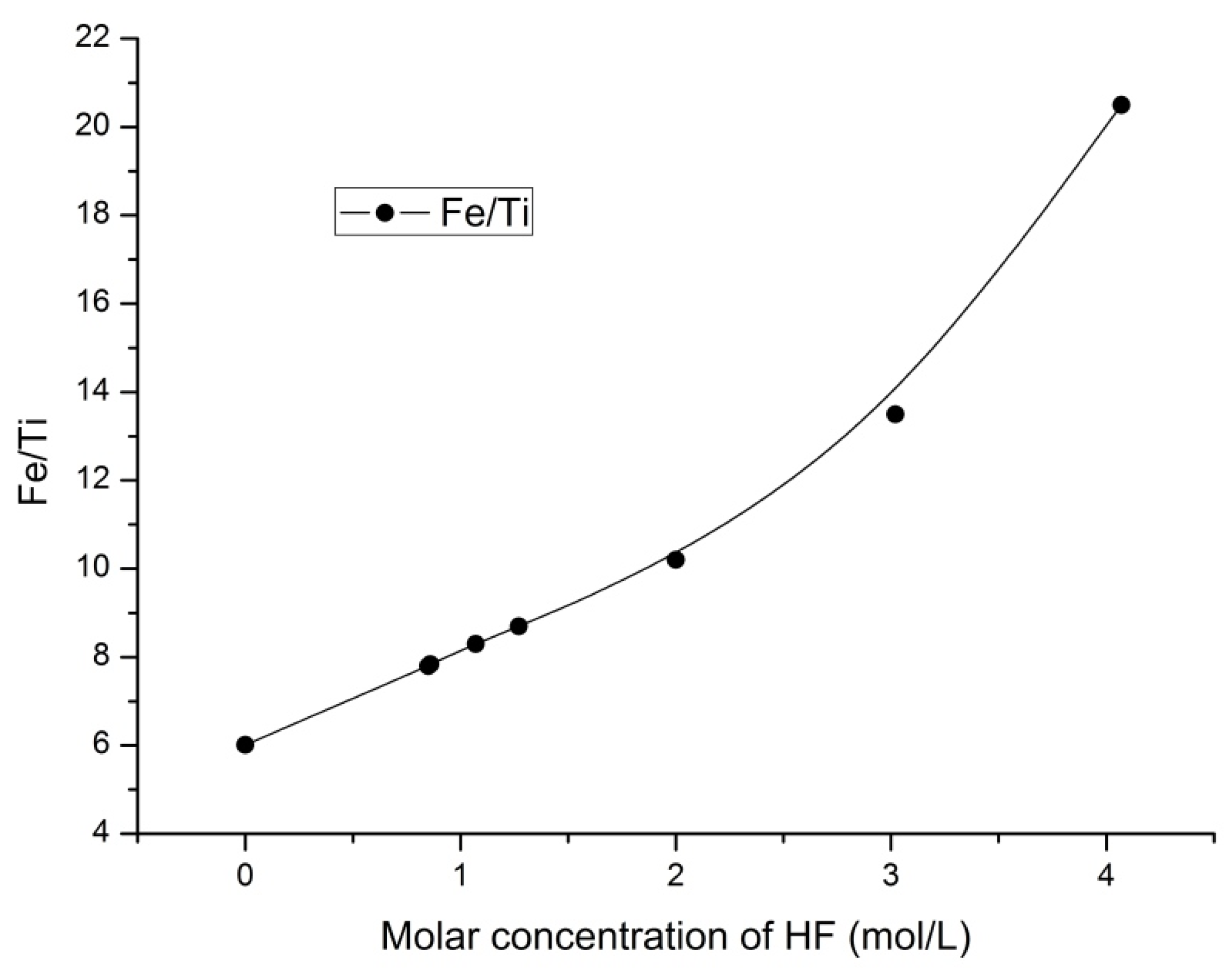
3.4. The Fe/Ti Ratio
The changes in the ratio of iron to titanium in the solid products were studied.
Figure 8 and
Figure 9 present the influence of the ammonium fluoride concentration and HF concentration on the Fe/Ti ratio, respectively.
The data analysis (
Figure 8) indicates the increase in the Fe/Ti ratio from 6.011 to 17.2 when leaching with ammonium fluoride solution with concentration up to 1 mol/L. The Fe/Ti ratio decreased slightly with the further increase in concentration.
Hydrofluoric acid addition (
Figure 9) enabled the Fe/Ti ratio to increase to 20.5 (i.e., 3.4 times) thus confirming the synergistic effect of acidity and ammonium fluoride on upgrading the iron content of the solid phase. The best results for the hydrometallurgical processing of titanomagnetite ore are presented in
Table 6.
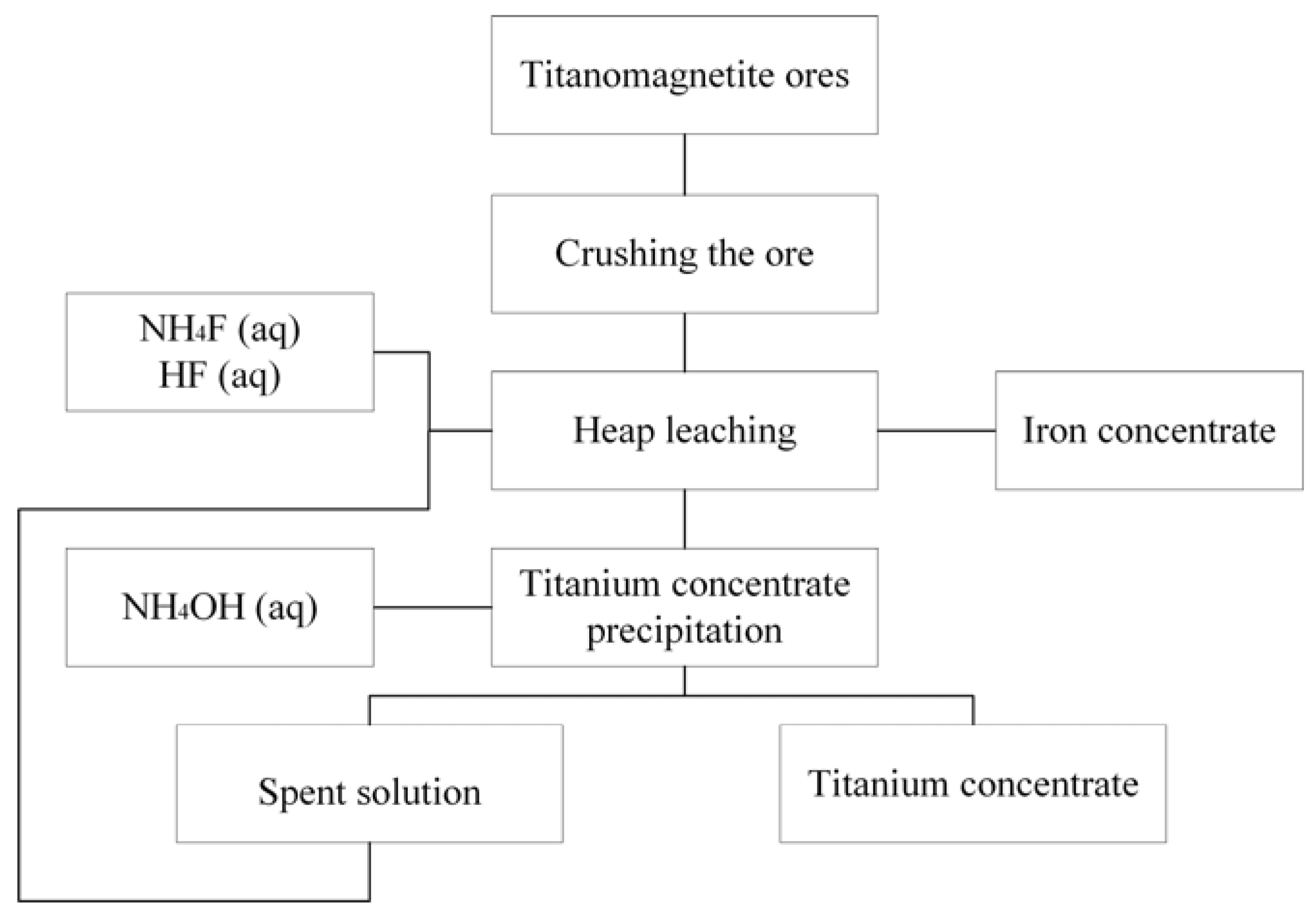
Thus, the presented results can be used for processing titanomagnetite ores in technological scale and the heap leaching on this technology is an optimal method of ore processing due to an involvement in the process of significant volumes of the processed ore. Processing schematic diagram is presented in
Figure 10.
As follows from the diagram (
Figure 10), the ore is treated by the leaching solution of the required composition. Precipitation of the titanium concentrate by ammonium hydroxide is the next stage after a partial extraction of the titanium. The usage of ammonium hydroxide allows using the solutions as cycling solutions that, after adjusting the concentration, are returned to the leaching stage. After the extraction of a predetermined amount of titanium from the ore, the heap is an iron concentrate that is suitable for further metallurgical processing.
The usage of ammonium hydroxide enables the recycling of the spent solution to the leaching step after the concentration has been adjusted. Varying the concentration of reagents in the leaching solution makes it possible to obtain the iron concentrate-containing fluorides in an amount that does not interfere with the pyrometallurgical reduction of iron. Also, the fluorides of magnesium and calcium formed during leaching can be used as fluxes. Their use allows better and faster separate metals and slags, and contributes to the removal of impurities, especially sulfur and phosphorus [
22].
4. Conclusions
The development of hydrometallurgical processing technology for titanomagnetite ores such as from the Chineisk deposit of Chita region has been considered. It was shown that ore which contains Fe (53.0%), Ti (8.8%), and with the Fe/Ti ratio of 6.011 is a prospective raw material for the production of titanium-vanadium and iron concentrates.
The possibility for titanomagnetite ore processing through the selective recovery of titanium using solutions that contain ammonium and fluorine ions has been demonstrated. We achieved the 78.5% titanium content in the solution by treating the ore with ammonium fluoride solution of concentration 4.2 mol/L. However, it was shown that optimum ammonium fluoride concentrations for ore processing with iron concentrate formation are from 0.42 to 1 mol/L. At these concentrations, the iron content increased from 53.0 to 62.8 wt % and the titanium content decreased from 8.8 to 3.5 wt % in solid phase.
The impact of acidity on the process to obtain concentrates has also been investigated. It was found that increasing HF concentration (from 0.86 mol/L to 4.07 mol/L) leads to an increase in Fe contained in the solid phase. The condition for increasing the iron content in the ore was obtained using a concentration for hydrofluoric acid of 4.07 mol/L in 0.42 mol/L ammonium fluoride solution. The content of titanium in the solid phase was reduced to 3.2 wt %, and the iron content was increased to 65.6 wt %. In addition, the extraction of titanium was 63.7%, which significantly increased the Fe/Ti ratio to 20.5, i.e., 3.4 times higher than for the original ore.
The results indicate that acidity and ammonium fluoride concentration of the leaching solution are the most important factors for efficiently and selectively processing titanomagnetite ores. This manuscript has shown that these ores can be effectively upgraded by a hydrometallurgical method, which is based on the interaction of titanomagnetite and ammonium fluoride.