Influence of the Interaction between Sphalerite and Pyrite on the Copper Activation of Sphalerite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Minerals
2.2. Procedure
2.2.1. Flotation Tests
2.2.2. Copper Adsorption
2.2.3. Analysis Techniques
3. Results and Discussion
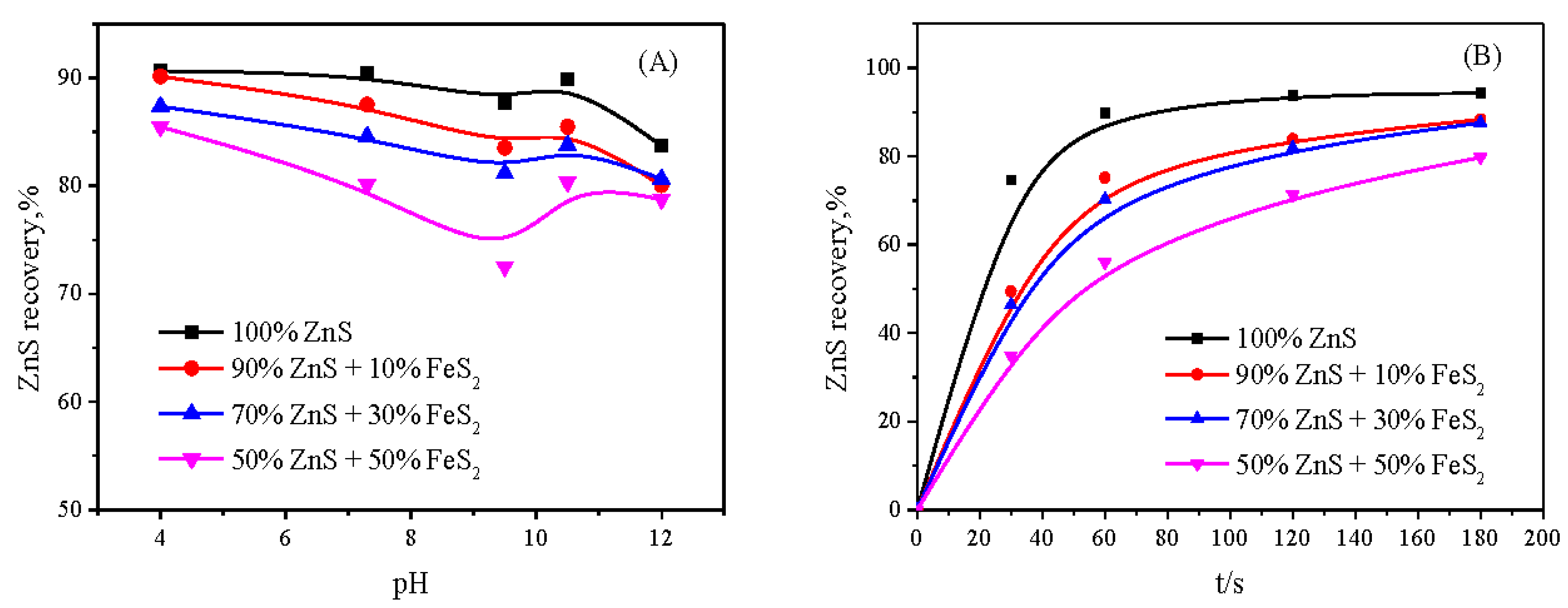
3.1. Flotation Behavior of Sphalerite with and without the Presence of Pyrite
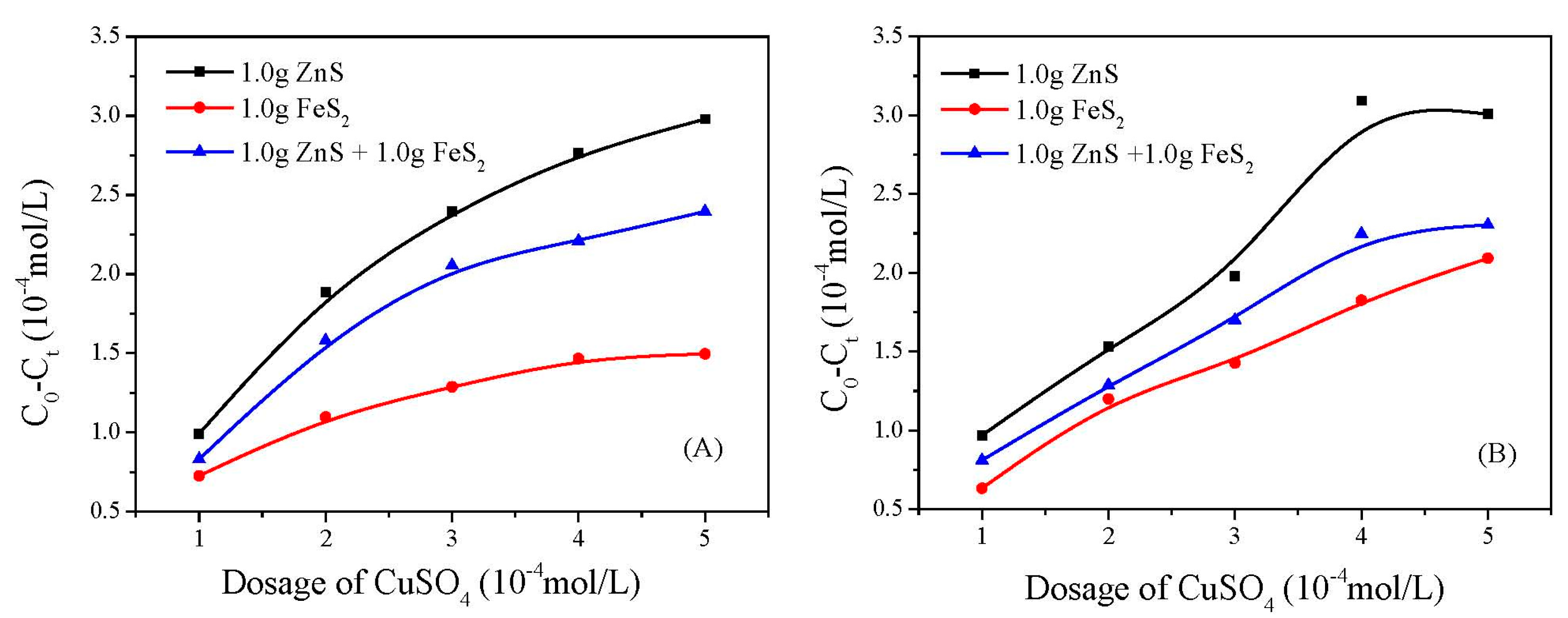
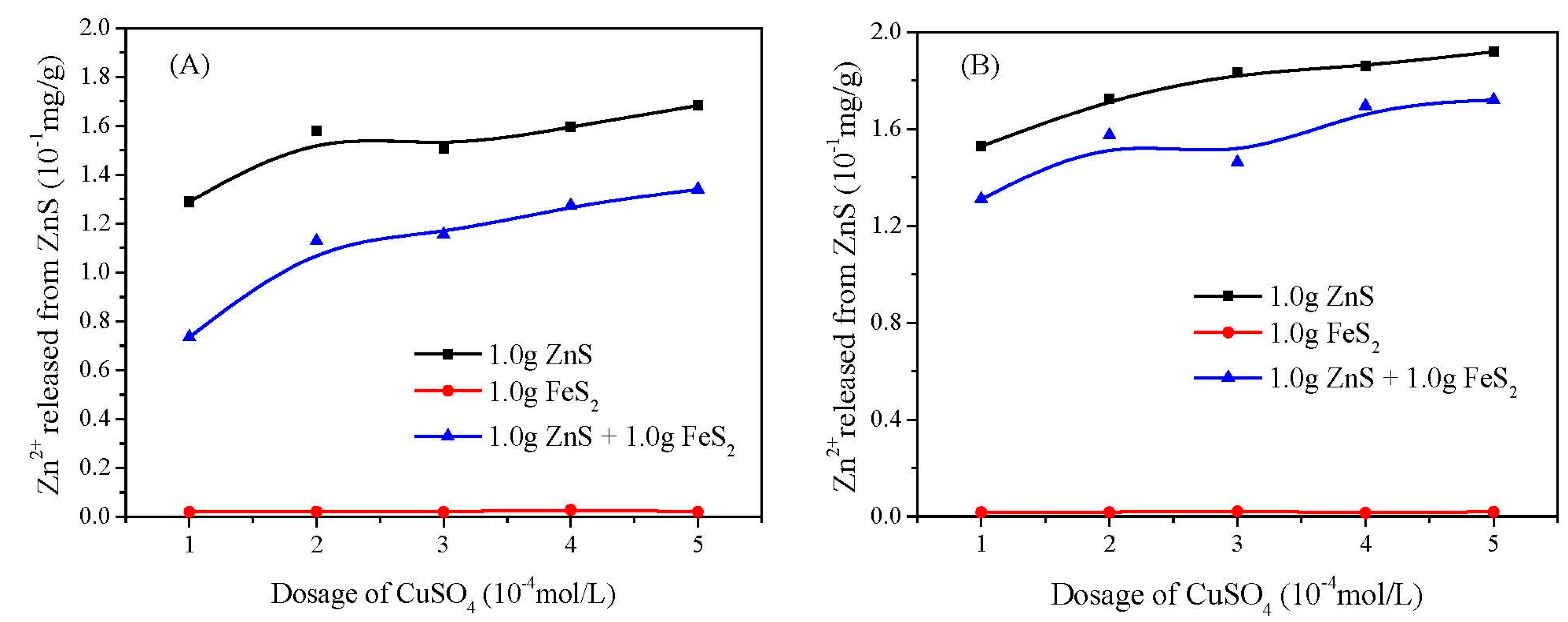
3.2. Copper Adsorption
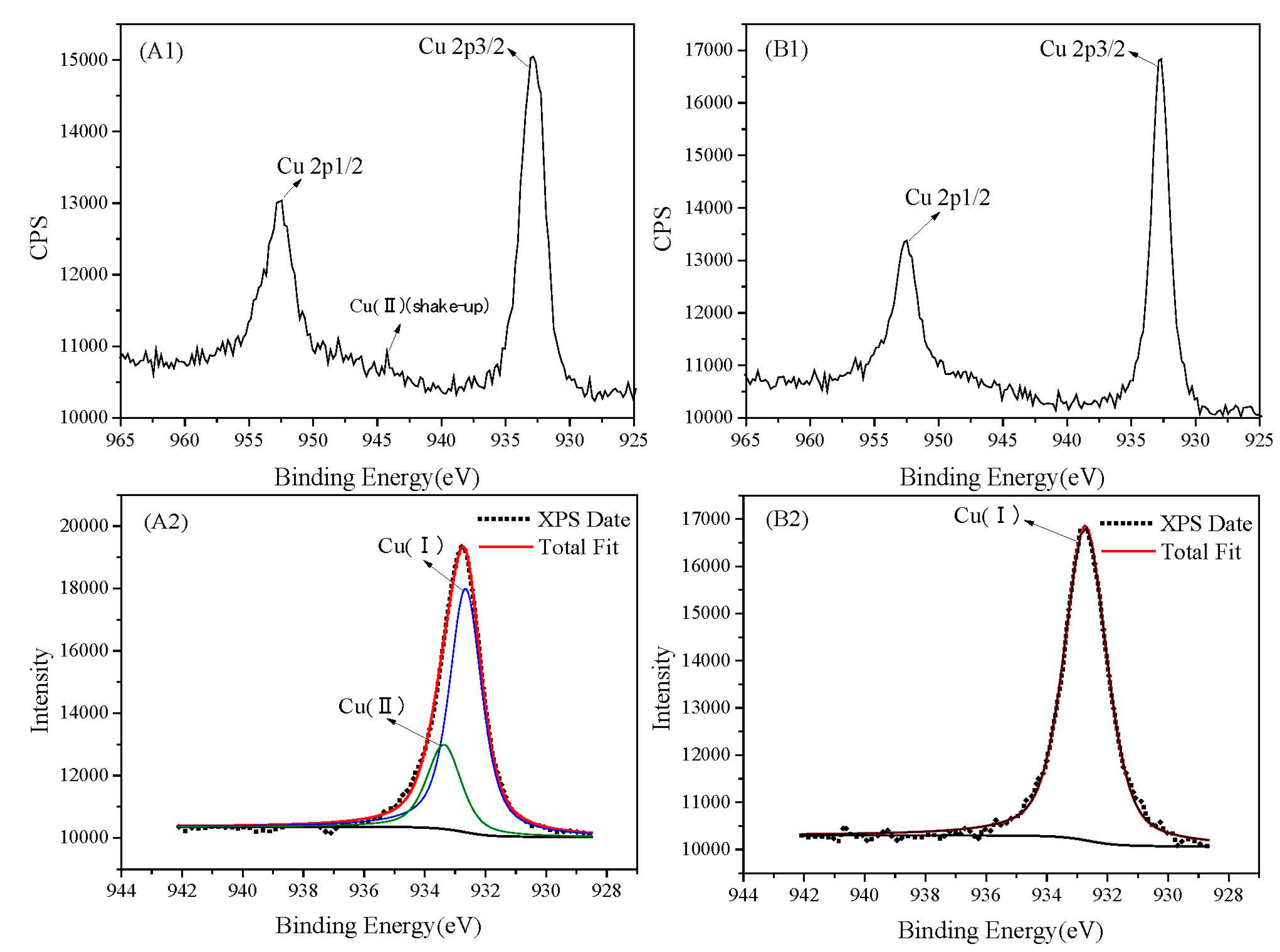
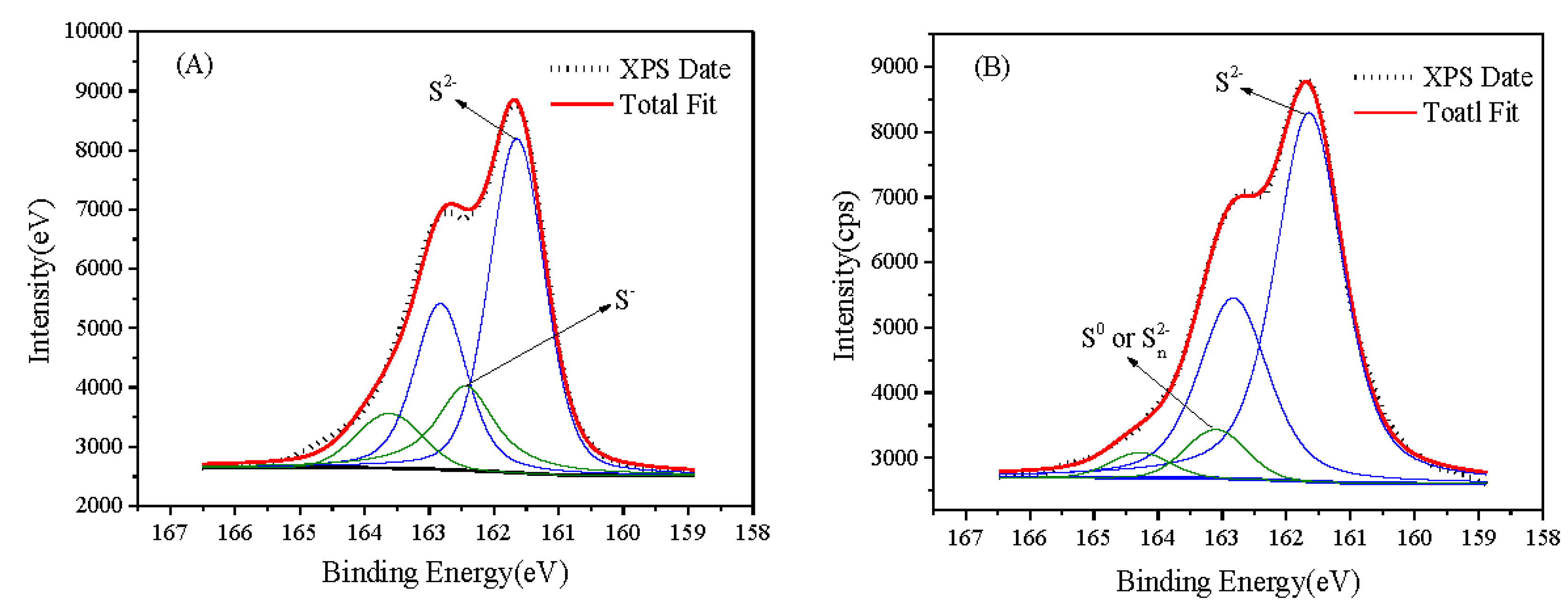
3.3. XPS Study
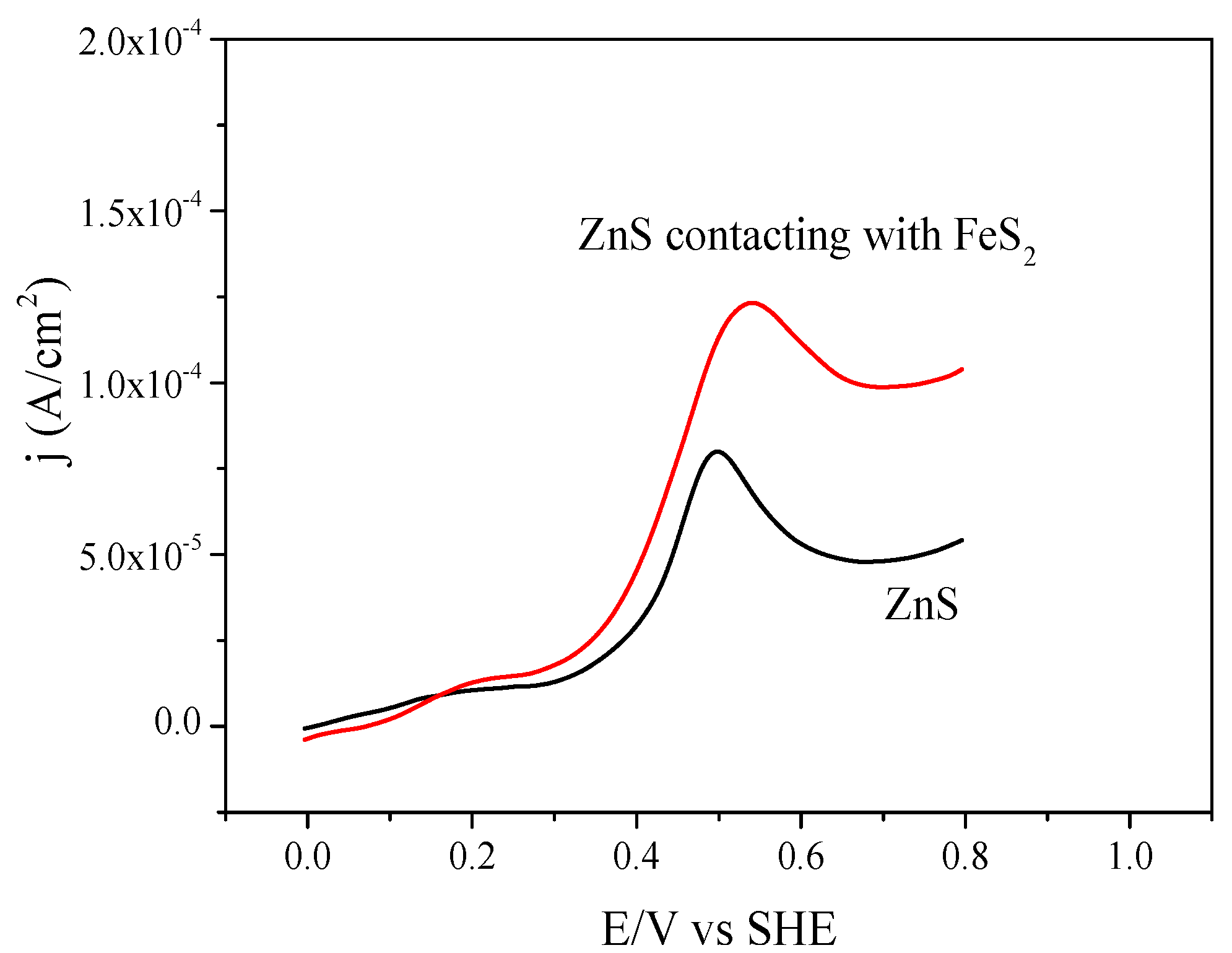
3.4. Voltammetry
4. Conclusions
- (1)
- The interaction between sphalerite and pyrite creates competitive adsorption for Cu2+, and also affects the flotation behavior of sphalerite and Cu activation products on the sphalerite surface.
- (2)
- The flotation recovery and rate of sphalerite decrease with increasing pyrite content in pulp. The adsorption capacity of Cu2+ on the sphalerite surface decreases, while the Zn2+ released from the sphalerite surface or lattice is reduced due to the interaction between sphalerite and pyrite.
- (3)
- The interaction between sphalerite and pyrite promotes the surface oxidation of sphalerite, which causes more Cu2+ species to be reduced to Cu+ species on a sphalerite surface. In addition, the oxidation of S2− on a sphalerite surface increases, leading to the presence of S0 and on the sphalerite surface after this interaction.
- (4)
- The interaction between sphalerite and pyrite has an important effect on the Cu activation of sphalerite, which may be another important factor affecting the selective separation of sphalerite from the associated pyrite.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, Y.; Chen, J.; Guo, J. A DFT study on the effect of lattice impurities on the electronic structures and floatability of sphalerite. Miner. Eng. 2010, 23, 1120–1130. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, J.; Lan, L.; Yang, M. The influence of the impurities on the flotation behaviors of synthetic ZnS. Miner. Eng. 2012, 27, 65–71. [Google Scholar] [CrossRef]
- Tong, X.; Song, S.; He, J.; Rao, F.; Lopez-Valdivieso, A. Activation of high-iron marmatite in froth flotation by ammoniacal copper(II) solution. Miner. Eng. 2007, 20, 259–263. [Google Scholar] [CrossRef]
- Albrecht, T.W.J.; Addai-Mensah, J.; Fornasiero, D. Critical copper concentration in sphalerite flotation: Effect of temperature and collector. Int. J. Miner. Process. 2016, 146, 15–22. [Google Scholar] [CrossRef]
- Chen, Z.; Yoon, R.-H. Electrochemistry of copper activation of sphalerite at pH 9.2. Int. J. Miner. Process. 2000, 58, 57–66. [Google Scholar] [CrossRef]
- Ejtemaei, M.; Nguyen, A.V. Characterisation of sphalerite and pyrite surfaces activated by copper sulphate. Miner. Eng. 2017, 100, 223–232. [Google Scholar] [CrossRef]
- Chandra, A.P.; Gerson, A.R. A review of the fundamental studies of the copper activation mechanisms for selective flotation of the sulfide minerals, sphalerite and pyrite. Adv. Colloid Interface Sci. 2009, 145, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Chandra, A.P.; Puskar, L.; Simpson, D.J.; Gerson, A.R. Copper and xanthate adsorption onto pyrite surfaces: Implications for mineral separation through flotation. Int. J. Miner. Process. 2012, 114–117, 16–26. [Google Scholar] [CrossRef]
- He, M.-F.; Qin, W.-Q.; Li, W.-Z.; Zeng, K. Pyrite depression in marmatite flotation by sodium glycerine-xanthate. Trans. Nonferr. Met. Soc. China 2011, 21, 1161–1165. [Google Scholar] [CrossRef]
- Moslemi, H.; Gharabaghi, M. A review on electrochemical behavior of pyrite in the froth flotation process. J. Ind. Eng. Chem. 2017, 47, 1–18. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, Z.; Bozkurt, V.; Finch, J.A. Pyrite flotation in the presence of metal ions and sphalerite. Int. J. Miner. Process. 1997, 52, 187–201. [Google Scholar] [CrossRef]
- Dichmann, T.K.; Finch, J.A. The role of copper ions in sphalerite-pyrite flotation selectivity. Miner. Eng. 2001, 14, 217–225. [Google Scholar] [CrossRef]
- Rao, S.R.; Finch, J.A. Galvanic interaction studies on sulfide minerals. Can. Metall. Q. 1988, 27, 253–259. [Google Scholar] [CrossRef]
- Rao, M.K.Y.; Natarajan, K.A. Electrochemical effects of mineral-mineral interactions on the flotation of chalcopyrite and sphalerite. Int. J. Miner. Process. 1989, 27, 279–293. [Google Scholar]
- Mehrabani, J.V.; Noaparast, M.; Mousavi, S.M.; Dehghan, R.; Rasooli, E.; Hajizadeh, H. Depression of pyrite in the flotation of high pyrite low-grade lead–zinc ore using Acidithiobacillus ferrooxidans. Miner. Eng. 2010, 23, 10–16. [Google Scholar] [CrossRef]
- Owusu, C.; Brito e Abreu, S.; Skinner, W.; Addai-Mensah, J.; Zanin, M. The influence of pyrite content on the flotation of chalcopyrite/pyrite mixtures. Miner. Eng. 2014, 55, 87–95. [Google Scholar] [CrossRef]
- Owusu, C.; Fornasiero, D.; Addai-Mensah, J.; Zanin, M. Influence of pulp aeration on the flotation of chalcopyrite with xanthate in chalcopyrite/pyrite mixtures. Int. J. Miner. Process. 2015, 134, 50–57. [Google Scholar] [CrossRef]
- Qin, W.; Wang, X.; Ma, L.; Jiao, F.; Liu, R.; Yang, C.; Gao, K. Electrochemical characteristics and collectorless flotation behavior of gelena: With and without the presence of pyrite. Miner. Eng. 2015, 74, 99–104. [Google Scholar] [CrossRef]
- Ejtemaei, M.; Nguyen, A.V. Kinetic studies of amyl xanthate adsorption and bubble attachment to Cu-activated sphalerite and pyrite surfaces. Miner. Eng. 2017, 112 (Suppl. C), 36–42. [Google Scholar] [CrossRef]
- Ejtemaei, M.; Nguyen, A.V. A comparative study of the attachment of air bubbles onto sphalerite and pyrite surfaces activated by copper sulphate. Miner. Eng. 2017, 109 (Suppl. C), 14–20. [Google Scholar] [CrossRef]
- Ekmekçi, Z.; Demirel, H. Effects of galvanic interaction on collectorless flotation behaviour of chalcopyrite and pyrite. Int. J. Miner. Process. 1997, 52, 31–48. [Google Scholar] [CrossRef]
- Eslami Andargoli, M.B.; Jannesar Malakooti, S.; Doulati Ardejani, F.; Abdollahi, H. Effect of galvanic contact on the flotability of galena in the presence and absence of a collector. Int. J. Min. Sci. Technol. 2012, 22, 629–632. [Google Scholar] [CrossRef]
- Santos, F.E.-D.L.; Rivera-Santillán, R.E.; Talavera-Ortega, M.; Bautista, F. Catalytic and galvanic effects of pyrite on ferric leaching of sphalerite. Hydrometallurgy 2016, 163, 167–175. [Google Scholar] [CrossRef]
- Urbano, G.; Meléndez, A.M.; Reyes, V.E.; Veloz, M.A.; González, I. Galvanic interactions between galena–sphalerite and their reactivity. Int. J. Miner. Process. 2007, 82, 148–155. [Google Scholar] [CrossRef]
- Qin, W.; Wang, X.; Ma, L. Effects of galvanic interaction galena and pyrite on their flotation in the presence of butyl xanthate. Trans. Nonferr. Met. Soc. China 2015, 25, 3111–3118. [Google Scholar] [CrossRef]
- Boulton, A.; Fornasiero, D.; Ralston, J. Characterisation of sphalerite and pyrite flotation samples by XPS and ToF-SIMS. Int. J. Miner. Process. 2003, 70, 205–219. [Google Scholar] [CrossRef]
- Chen, X.; Seaman, D.; Peng, Y.; Bradshaw, D. Importance of oxidation during regrinding of rougher flotation concentrates with a high content of sulfides. Miner. Eng. 2014, 66, 165–172. [Google Scholar] [CrossRef]
- Peng, Y.; Grano, S.; Fornasiero, D.; Ralston, J. Control of grinding conditions in the flotation of chalcopyrite and its separation from pyrite. Int. J. Miner. Process. 2003, 69, 87–100. [Google Scholar] [CrossRef]
- Gerson, A.R.; Lange, A.G.; Prince, K.E.; Smart, R.S.C. The mechanism of copper activation of sphalerite. Appl. Surf. Sci. 1999, 137, 207–223. [Google Scholar] [CrossRef]
| Elements | Zn | Fe | S | Pb | SiO2 |
|---|---|---|---|---|---|
| Sphalerite | 63.26 | 1.04 | 33.25 | 0.43 | 0.93 |
| Pyrite | 0.006 | 53.26 | 46.16 | 0.21 | 0.22 |
| Samples | Elements | B.E. (eV) | Species | Atom (%) |
|---|---|---|---|---|
| ZnS alone | Cu 2p3/2 | 932.3 | Cu(Ⅰ) | 4.58 |
| Cu 2p3/2 | 933.4 | Cu(Ⅱ) | 1.99 | |
| S 2p3/2 | 161.6 | S2− | 18.23 | |
| S 2p3/2 | 162.5 | S− | 2.05 | |
| S 2p1/2 | 162.8 | S2− | 9.1 | |
| S 2p1/2 | 163.7 | S− | 1.03 | |
| Zn 2p3/2 | 1022.09 | Zn2+ | 18.14 | |
| O 1s | 532.7 | C=O | 5.62 | |
| O 1s | 533.6 | OH– | 3.26 | |
| C 1s | 284.8 | C–C (Ref) | 30.55 | |
| C 1s | 286.3 | O–C–O | 3.91 | |
| C 1s | 288.2 | O–C=O | 1.54 | |
| ZnS with the presence of FeS2 | Cu 2p3/2 | 932.4 | Cu(Ⅰ) | 5.15 |
| S 2p3/2 | 161.6 | S2− | 19.08 | |
| S 2p1/2 | 162.8 | S2− | 9.54 | |
| S 2p3/2 | 163.2 | or S0 | 1.96 | |
| S 2p1/2 | 164.4 | or S0 | 0.98 | |
| Zn 2p3/2 | 1022.0 | Zn2+ | 17.76 | |
| O 1s | 532.7 | C=O | 5.73 | |
| O 1s | 533.6 | OH− | 3.51 | |
| C 1s | 284.8 | C–C (Ref) | 30.83 | |
| C 1s | 286.2 | O–C–O | 4.06 | |
| C 1s | 288.8 | O–C=O | 1.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Tong, X.; Lan, Z.; Cui, Y.; Xie, X. Influence of the Interaction between Sphalerite and Pyrite on the Copper Activation of Sphalerite. Minerals 2018, 8, 16. https://doi.org/10.3390/min8010016
Yang B, Tong X, Lan Z, Cui Y, Xie X. Influence of the Interaction between Sphalerite and Pyrite on the Copper Activation of Sphalerite. Minerals. 2018; 8(1):16. https://doi.org/10.3390/min8010016
Chicago/Turabian StyleYang, Bo, Xiong Tong, Zhuoyue Lan, Yiqi Cui, and Xian Xie. 2018. "Influence of the Interaction between Sphalerite and Pyrite on the Copper Activation of Sphalerite" Minerals 8, no. 1: 16. https://doi.org/10.3390/min8010016
APA StyleYang, B., Tong, X., Lan, Z., Cui, Y., & Xie, X. (2018). Influence of the Interaction between Sphalerite and Pyrite on the Copper Activation of Sphalerite. Minerals, 8(1), 16. https://doi.org/10.3390/min8010016




