Mineralogical Characteristics and Preliminary Beneficiation of Nickel Slag from Reduction Roasting-Ammonia Leaching
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
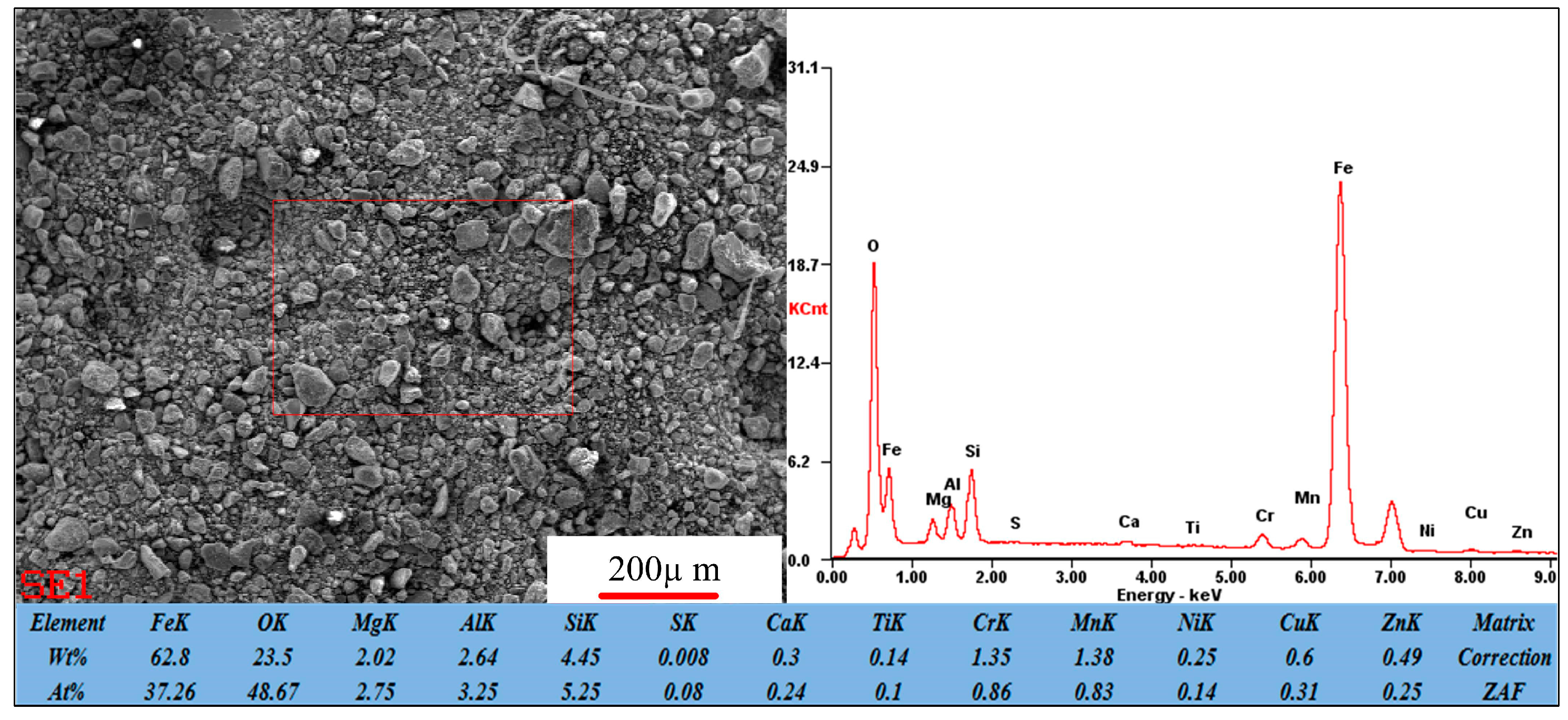
2.2.1. Methodology of Mineralogy Characteristics
2.2.2. Magnetic Separation
2.2.3. Phase Characterization
2.2.4. Thermomechanical Analysis
3. Results and Discussion
3.1. Phase Compositions of Nickel Slag
3.2. Occurrence of Valuable Metals
3.2.1. Iron Minerals
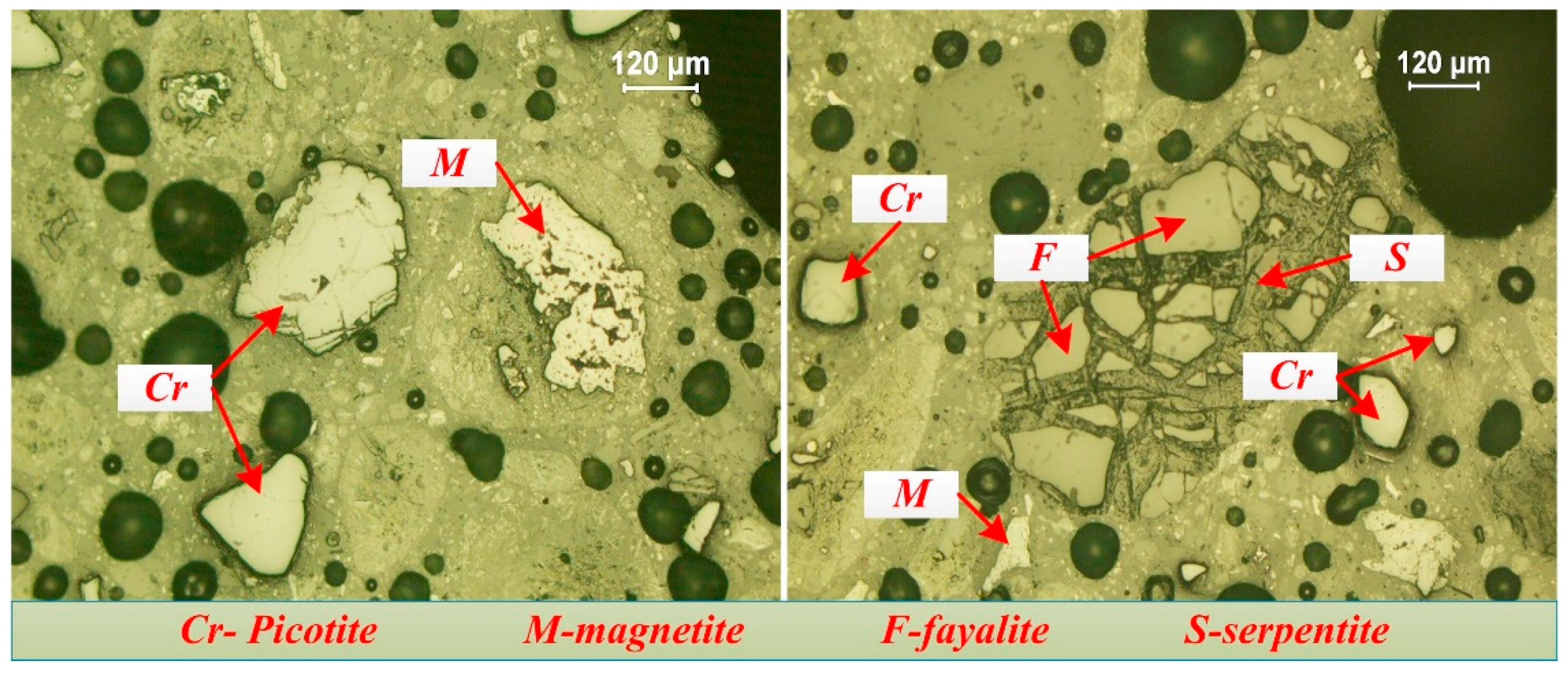
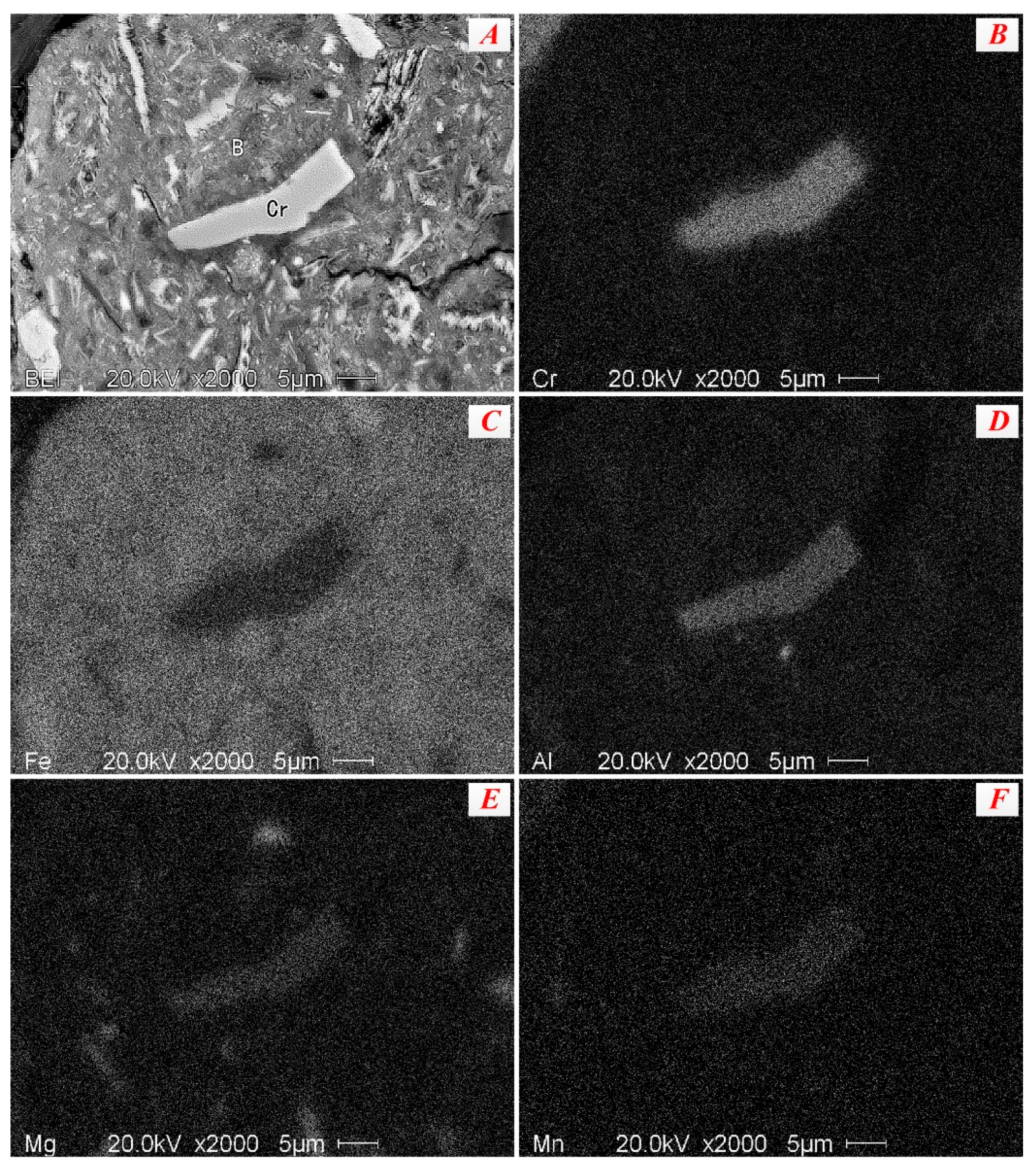
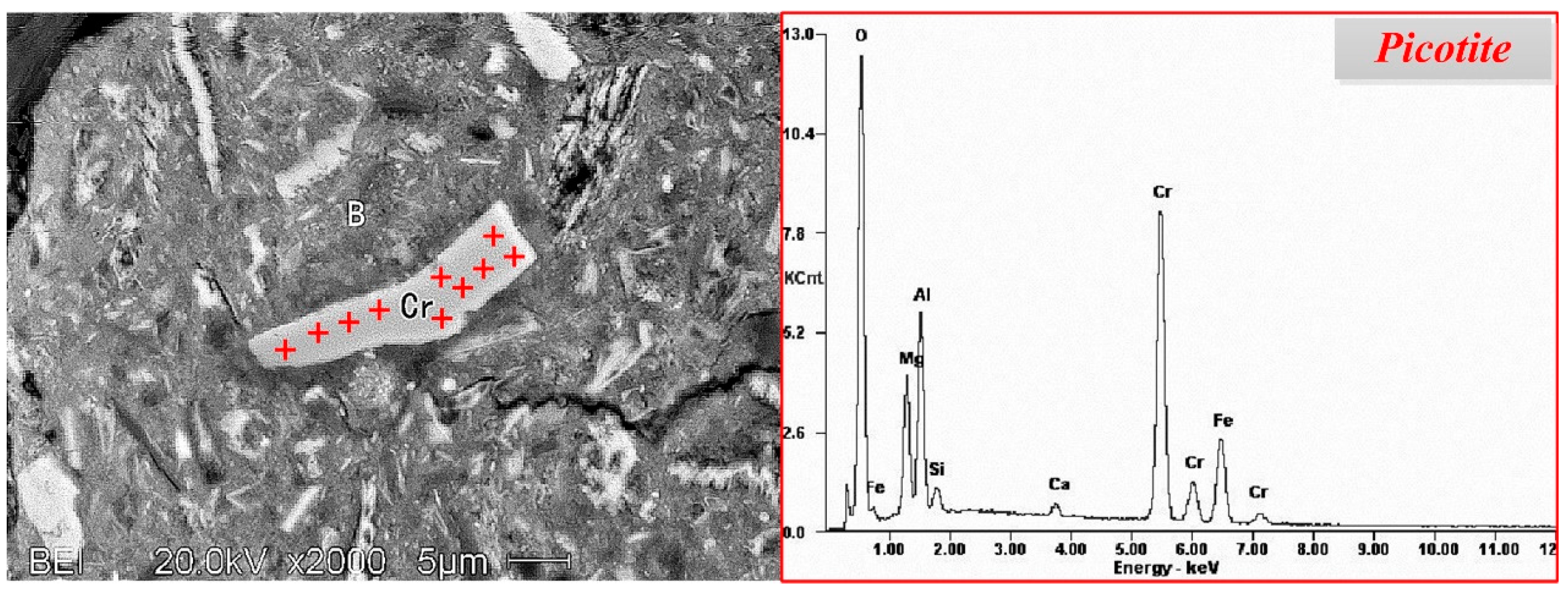
3.2.2. Chromium Minerals
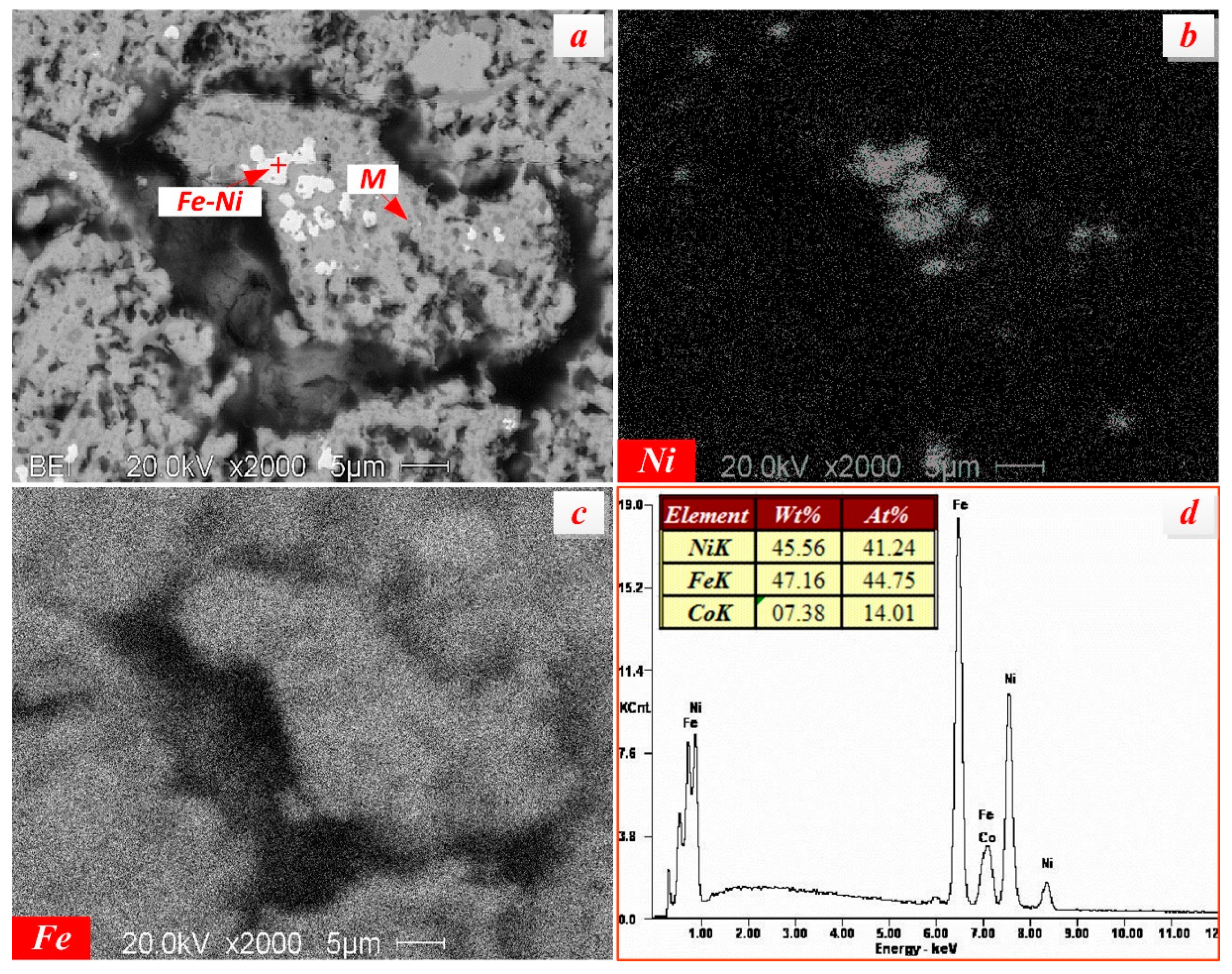
3.2.3. Nickel Minerals
3.3. Preliminary Upgrading of the Nickel Slag
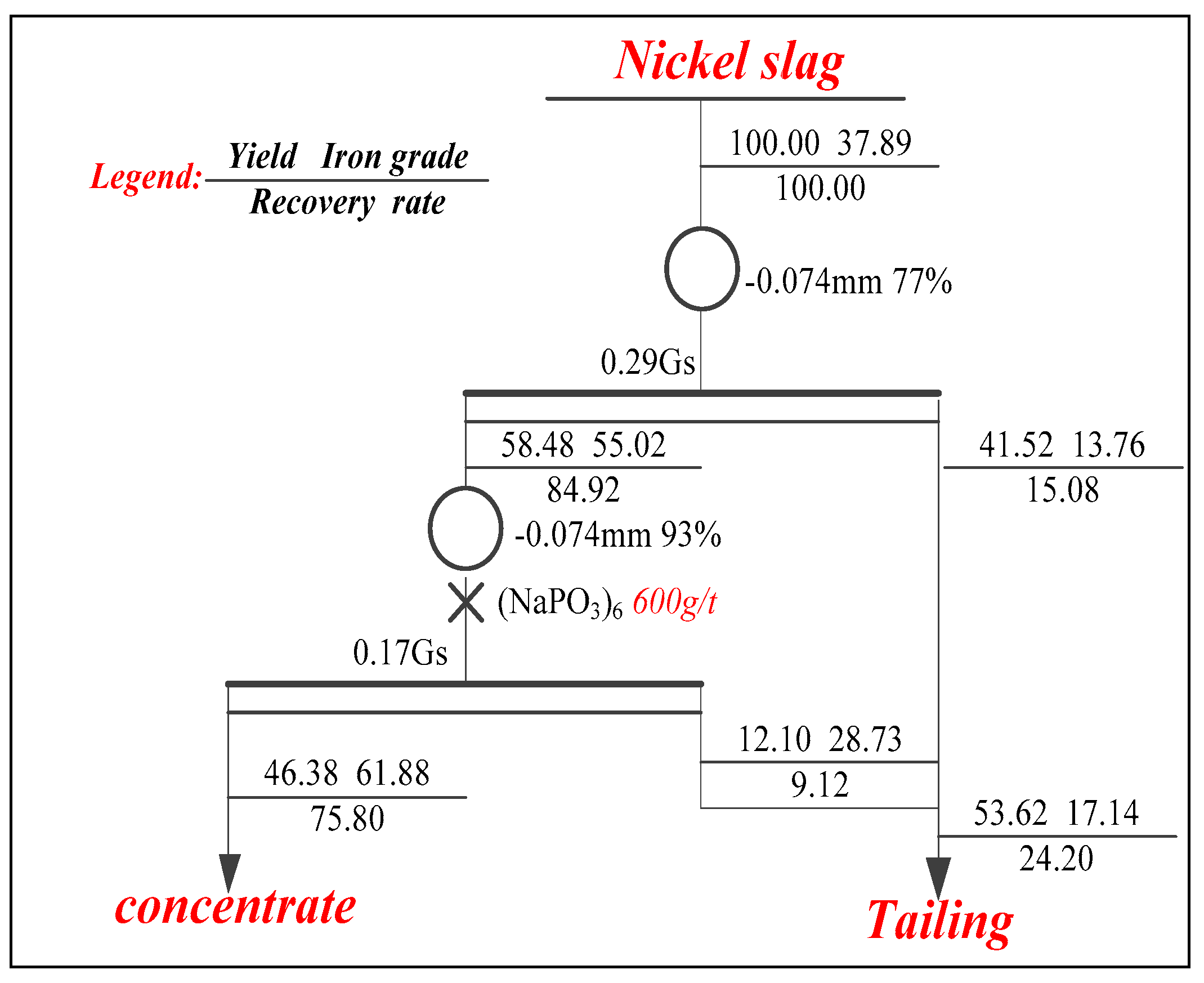
3.3.1. First Stage
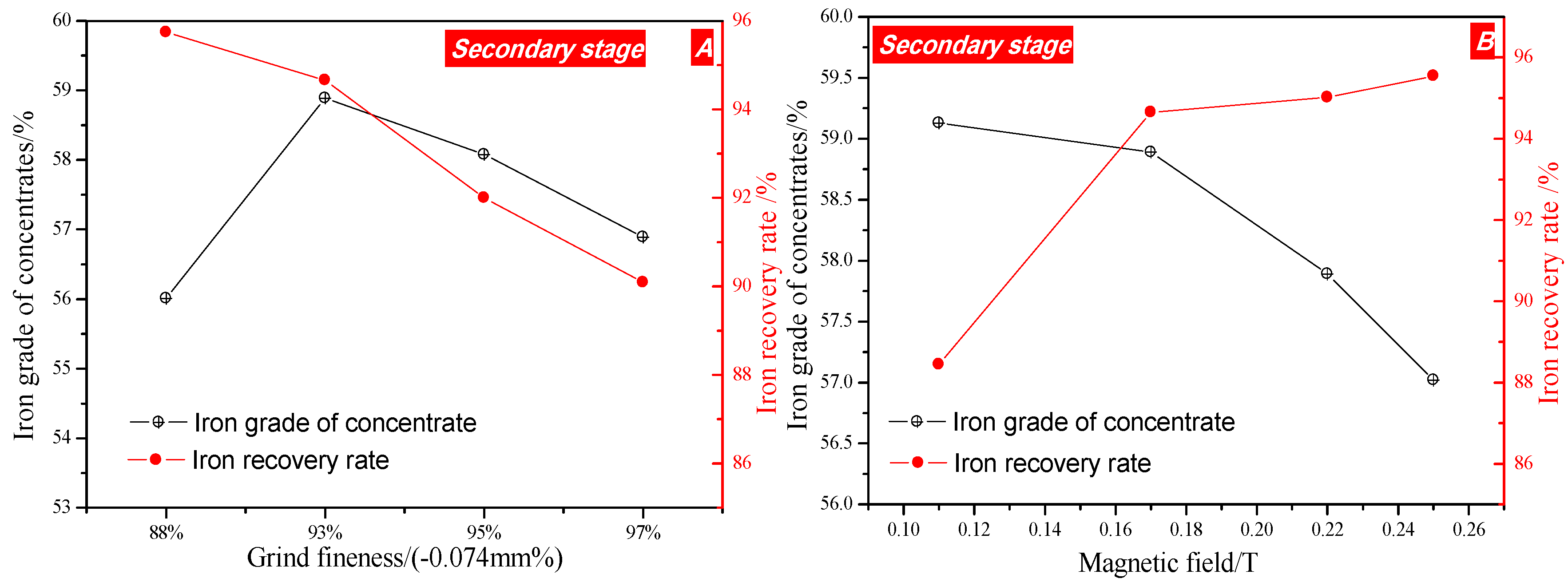
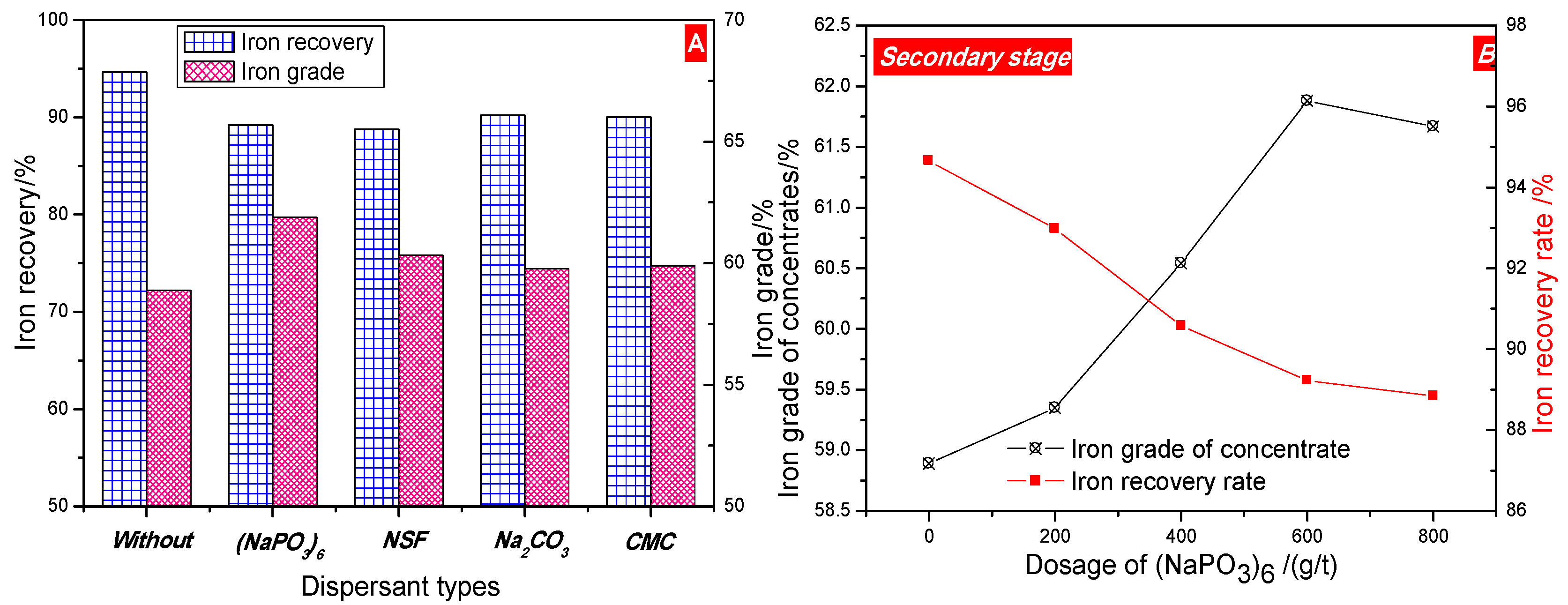
3.3.2. Second Stage
4. Conclusions
- (1)
- The occurrences of valuable metals, such as iron, chromium, and nickel, in the nickel slag are relatively complicated. Iron mainly exists in the form magnetite and maghemite with a coarse size over 50 μm, which are the main recoverable metal minerals. Chromium exists in the form of picotite, and some Al3+ and Mg2+ cations substitute Cr3+ cations in its lattice. The complicated chemical composition of picotite leads to poor recovery value due to difficult separation by the conventional physical processes. Fe-Ni alloy, as the predominate nickel-bearing phase, disperse in the slag and is closely surrounded by magnetite. Due to their fine and variable distribution, upgrading of nickel minerals by traditional beneficiation processes would be a great challenge.
- (2)
- The process of two stage grinding and two stage low intensity magnetic separation was developed to recover the iron metal. The magnetic concentrate, assaying total iron grade of 61.88% was obtained at 75.80% overall iron recovery under the optimal conditions of the grinding fineness of −0.074 mm 77% and 0.29 T intensity of magnetic field in the first magnetic separation, and the grinding fineness of −0.074 mm 93% and 0.17 T intensity of magnetic field in secondary magnetic separation, which can be used as raw material for subsequently sintering or pelletizing process in ironmaking industry. The (NaPO3)6 addition had a positive effect on the increase in grade of iron concentration.
- (3)
- These process allows for the preliminary utilization of nickel slag. However, further studies are needed to deal with tailings generated from that.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dalvi, A.D.; Bacon, W.G.; Osborne, R.C. The Past and the Future of Nickel Laterites. In Proceedings of the PDAC 2004 International Convention, Trade Show and Investors Exchange, Mississauga, ON, Canada, 7–10 March 2004. [Google Scholar]
- Wang, B.; Guo, Q.; Wei, G.; Zhang, P.; Qu, J.; Qi, T. Characterization and atmospheric hydrochloric acid leaching of a limonitic laterite from Indonesia. Hydrometallurgy 2012, 71, 1–6. [Google Scholar] [CrossRef]
- Norgate, T.; Jahanshahi, S. Low grade ores—Smelt, leach or concentrate? Miner. Eng. 2010, 2, 65–69. [Google Scholar] [CrossRef]
- Mudd, G.M. Global trends and environmental issues in nickel mining: Sulfides versus laterites. Ore Geol. Rev. 2010, 38, 9–26. [Google Scholar] [CrossRef]
- McDonald, R.G.; Whittington, B.I. Atmospheric acid leaching of nickel laterites review: Part I. Sulphuric acid technologies. Hydrometallurgy 2008, 91, 35–55. [Google Scholar] [CrossRef]
- Senanayake, G.; Das, G.K. A comparative study of leaching kinetics of limonitic laterite and synthetic iron oxides in sulfuric acid containing sulfur dioxide. Hydrometallurgy 2002, 72, 59–72. [Google Scholar] [CrossRef]
- Kaya, Ş.; Topkaya, Y.A. High pressure acid leaching of a refractory lateritic nickel ore. Miner. Eng. 2011, 24, 1188–1197. [Google Scholar] [CrossRef]
- Liu, K.; Chen, Q.; Hu, H. Comparative leaching of minerals by sulphuric acid in a Chinese ferruginous nickel laterite ore. Hydrometallurgy 2009, 98, 281–286. [Google Scholar] [CrossRef]
- Ma, B.; Wang, C.; Yang, W.; Yin, F.; Chen, Y. Screening and reduction roasting of limonitic laterite and ammonia-carbonate leaching of nickel–cobalt to produce a high-grade iron concentrate. Miner. Eng. 2013, 51, 106–111. [Google Scholar] [CrossRef]
- Caron, M.H. Fundamental and practical factors in ammonia leaching of nickel and cobalt ores. Trans. AIME 1950, 188, 67–90. [Google Scholar]
- Chander, S.; Sharma, V.N. Reduction roasting/ammonia leaching of nickeliferous laterites. Hydrometallurgy 1981, 7, 198–201. [Google Scholar] [CrossRef]
- Chen, S.L.; Guo, X.Y.; Shi, W.T.; Li, D. Extraction of valuable metals from low grade nickeliferous laterite ore by reduction roasting-ammonia leaching method. J Cent. South Univ. Technol. 2010, 17, 765–769. [Google Scholar] [CrossRef]
- Li, Y.; Papangselakis, V.G. High pressure oxidative acid leaching of nickel smelter slag: Characterization of feed and residue. Hydrometallurgy 2009, 97, 185–193. [Google Scholar] [CrossRef]
- Zhang, Y.; Man, R.; Ni, W.; Wan, H. Selective leaching of base metals from copper smelter slag. Hydrometallurgy 2010, 103, 25–29. [Google Scholar]
- Rudnik, E.; Burzysaka, L.; Gumowaska, W. Hydrometallurgical recovery of copper and cobalt from reduction roasted copper converter slag. Miner. Eng. 2009, 22, 85–89. [Google Scholar] [CrossRef]
- Yang, H.; Jing, L.; Dang, C. Iron recovery from copper-slag with lignite-based direct reduction followed by magnetic separation. Chin. J. Nonferr. Met. 2011, 21, 1165–1168. (In Chinese) [Google Scholar]
- Yucel, O.; Sahin, F.; Sirin, B.; Addemir, O.A. Reduction study of copper slag in DC arc furnace. Scand. J. Metall. 1999, 28, 93–97. [Google Scholar]
- Zhou, X.L.; Zhu, D.Q.; Pan, J.; Wu, T.J. Utilization of waste copper slag to produce directly reduced Iron for weathering resistant steel. Int. ISIJ 2015, 55, 1347–1352. [Google Scholar]
- Haselhuhn, H.J.; Kawatra, S.K. The Role of Surface Chemistry in Iron Ore Beneficiation. Miner. Metall. Process. 2017. [Google Scholar]
- Guo, Z.Q.; Zhu, D.Q.; Pan, J.; Wu, T.J.; Zhang, F. Improving beneficiation of copper and Iron from copper slag by modifying the molten copper slag. Metals 2016, 6, 86–96. [Google Scholar] [CrossRef]
- Zhu, D.Q.; Guo, Z.Q.; Pan, J.; Zhang, F. Mechanism of mineral phase reconstruction for improving the beneficiation of copper and iron from copper slag. JOM 2016, 68, 2341–2348. [Google Scholar]
- Batchelor, A.R.; Jones, D.A.; Plint, S.; Kingman, S.W. Increasing the grind size for effective liberation and flotation of a porphyry copper ore by microwave treatment. Miner. Eng. 2016, 94, 61–75. [Google Scholar] [CrossRef]
- Meng, X.L. Phase analysis and research of the iron ore. Chin. Chem. Trade 2013, 8, 231–258. [Google Scholar]
- Zhao, S.B. Chemical Phase Analysis of iron ore. Mater. High Speed Anal. 1996, 23, 60–65. (In Chinese) [Google Scholar]
- Zhang, H.B. Chemical Phase Analysis of Ores and Industrial Products; Metallurgical Industry Press: Beijing, China, 1992. (In Chinese) [Google Scholar]
- Powell, M.; Bye, A. Beyond mine-to-mill: Circuit design for energy efficient resource utilization. In Proceedings of the Tenth Mill Operators Conference, Adelaide, Australia, 12–14 October 2009; AusIMM: Carlton South, Australia, 2009; pp. 357–364. [Google Scholar]
- Mohanan, S.; Bhoja, S.K.; Kumar, C.R.; Kumar, A.; Venugopalan, T. Estimation of ore mineralogy from analytical analysis of iron ore. Miner. Metall. Process. 2015, 32, 97–101. [Google Scholar]
- Chen, W. Experimental research of recovering easy-to-slime limonite by flocculation-high intensity magnetic separation technology. Met. Mine 2003, 6, 32–34. [Google Scholar]
- Jun, B.S.; Wen, L.D.; Wen, S.M. Test research on mineral dressing-metallurgy combination processing of a refractory limonite ore. Mine Metall. 2009, 18, 16–20. [Google Scholar]
- Graca, L.M.; Lagoeiro, L.E.; Lima, R.M.F.; Barbosa, P.F.; Machado, M.M. Effect of the morphological types in grinding of iron-ore products. Miner. Process. Extr. Metall. Rev. 2015, 36, 324–331. [Google Scholar] [CrossRef]
- Miao, L.; He, Y.S.; Kang, J.T.; Kumar, A.; Venugopalan, T. Why was iron lost without significant isotope fractionation during the lateritic process in tropical environments? Geoderma 2017, 290, 1–9. [Google Scholar]
- Li, B.; Wang, H.; Wei, Y. The reduction of nickel from low-grade nickel laterite ore using a solid-state deoxidization method. Miner. Eng. 2011, 24, 1556–1562. [Google Scholar] [CrossRef]
- Sun, Y.S.; Han, Y.X.; Gao, P.; Yu, J.W. Size distribution behavior of metallic iron particles in coal-based reduction products of an oolitic iron ore. Miner. Process. Extr. Metall. Rev. 2015, 36, 249–257. [Google Scholar] [CrossRef]
- Haselhuhn, H.J.; Kawatra, S.K. Flocculation and Dispersion Studies of Iron Ore using Laser Scattering Particle Size Analysis. Miner. Metall. Process. 2015, 32, 191–196. [Google Scholar]
- Liu, S.; Wang, W.; Zhang, M.; Wen, S. Beneficiation of a low grade hematite-magnetite ore in China. Miner Metall. Process. 2014, 31, 136–142. [Google Scholar]
- Fu, J.Y.; Jiang, T.; Zhu, D.Q. Principle of Sintering and Pelletizing; Central South University of Technology Press: Changsha, China, 1996. [Google Scholar]
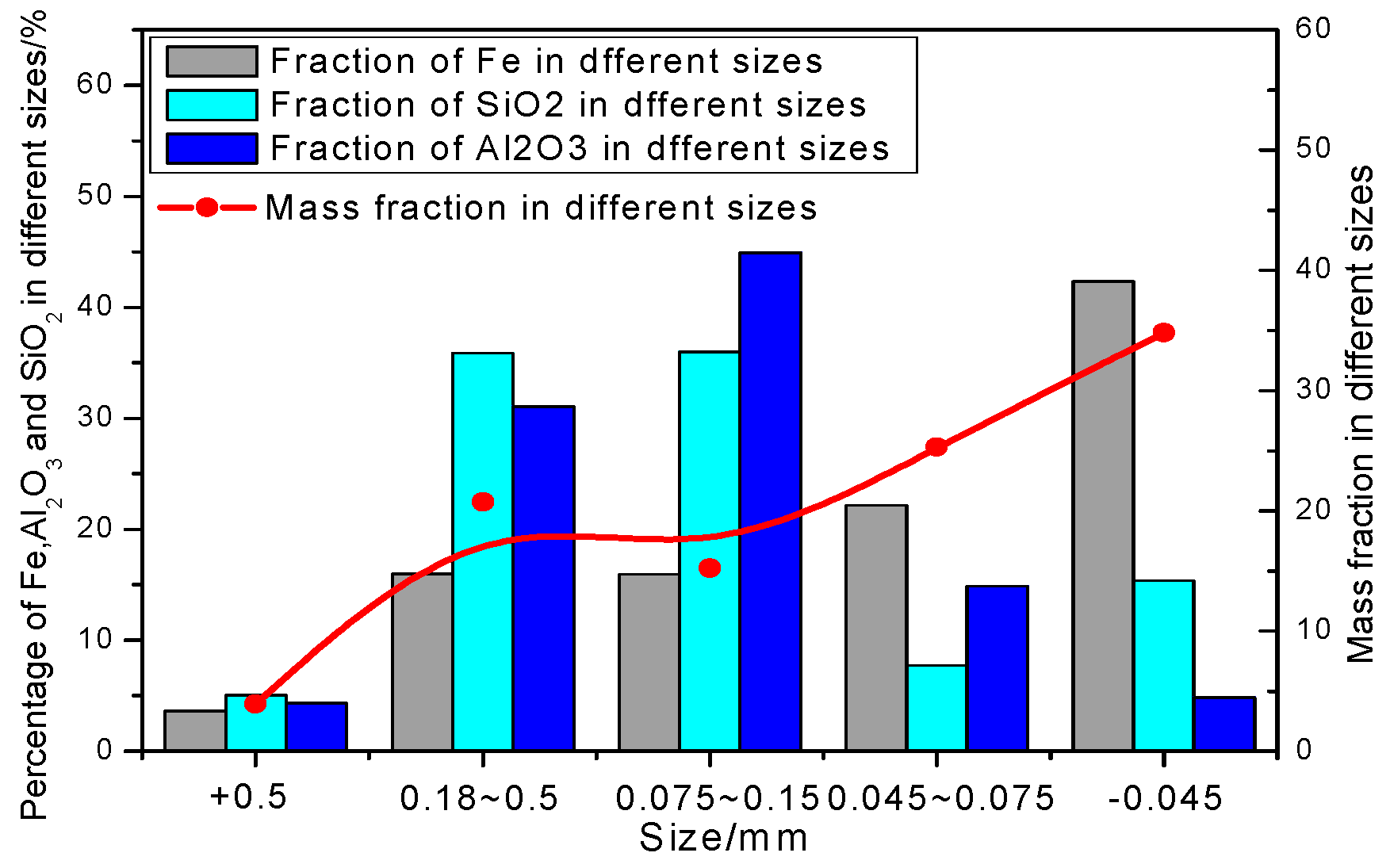
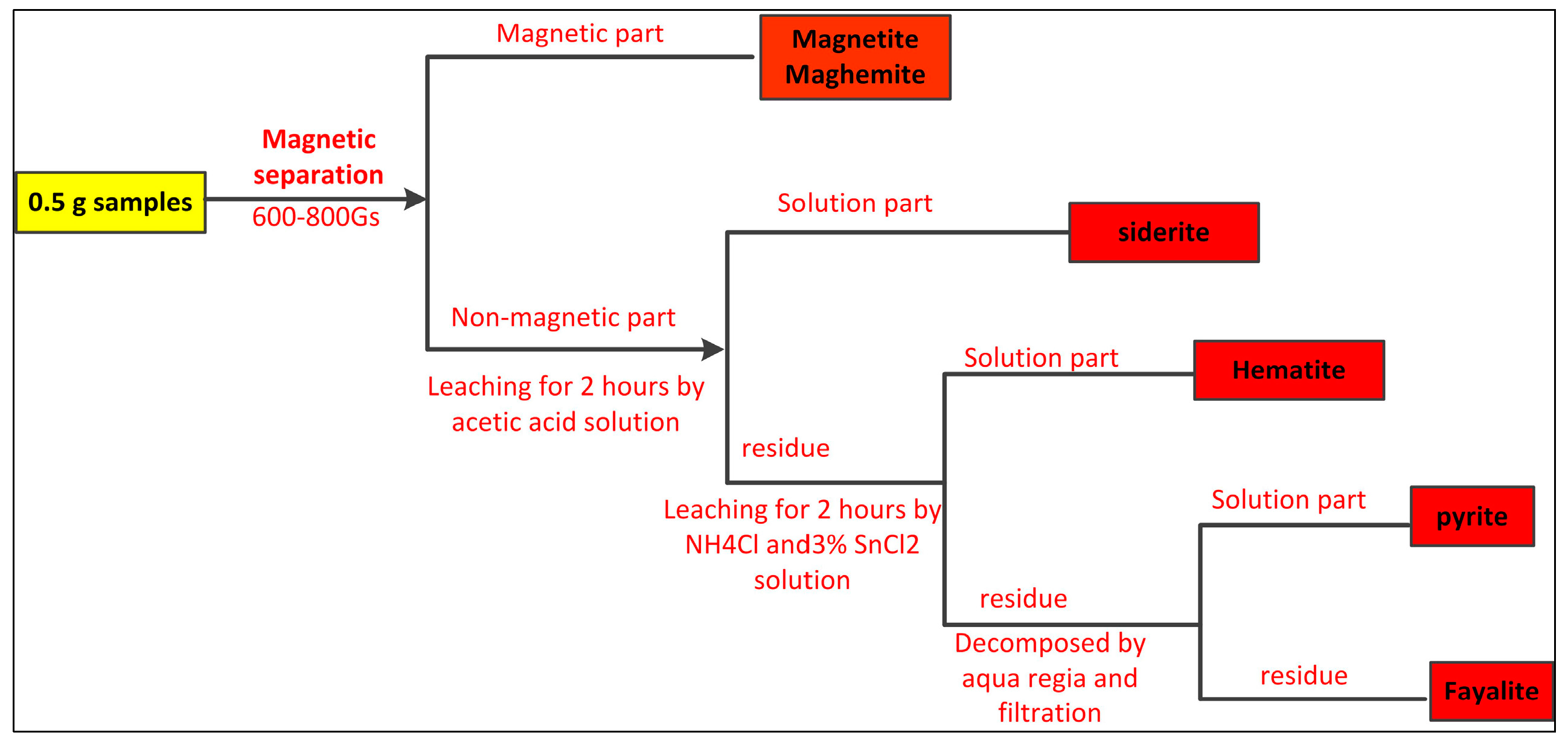
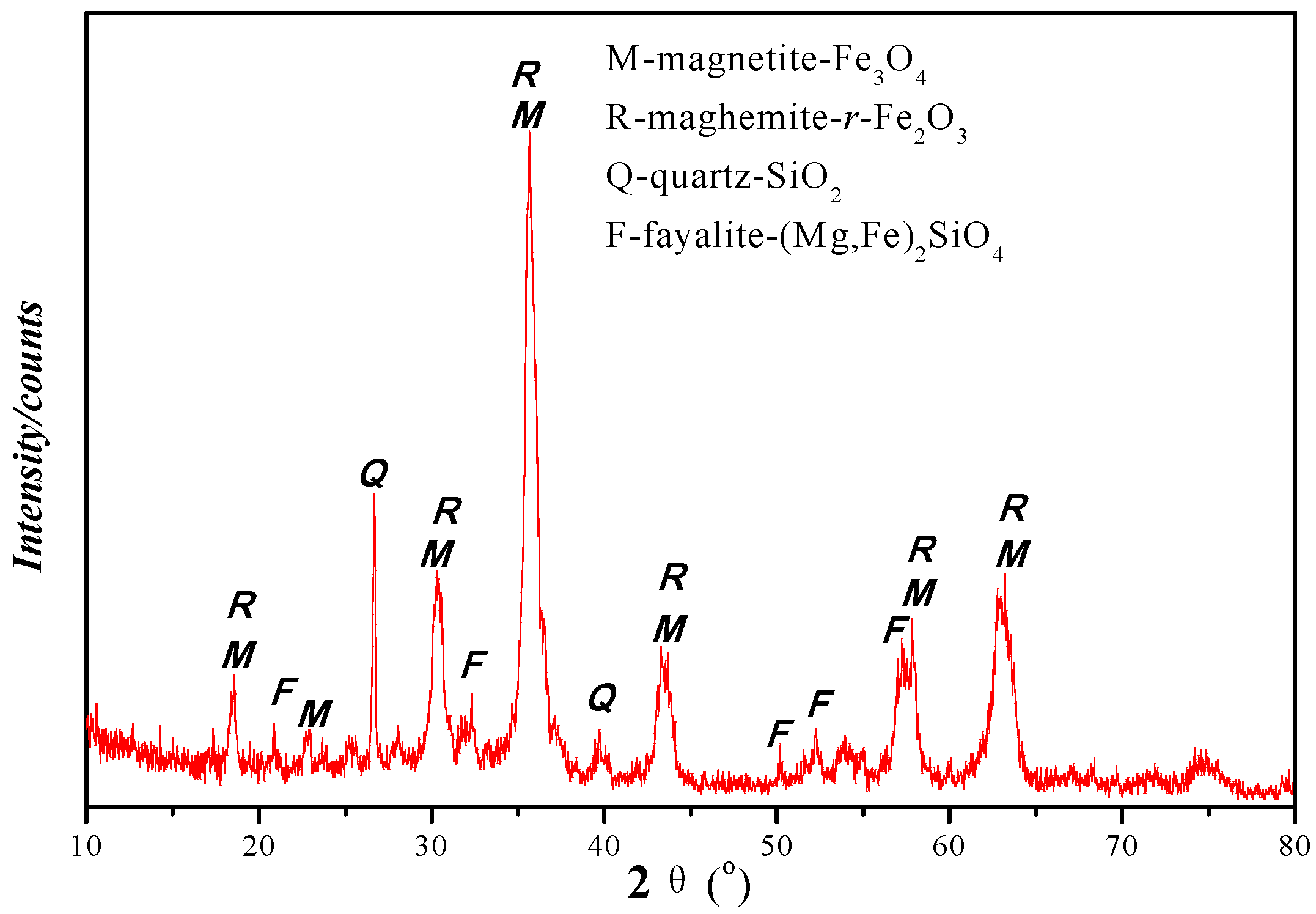

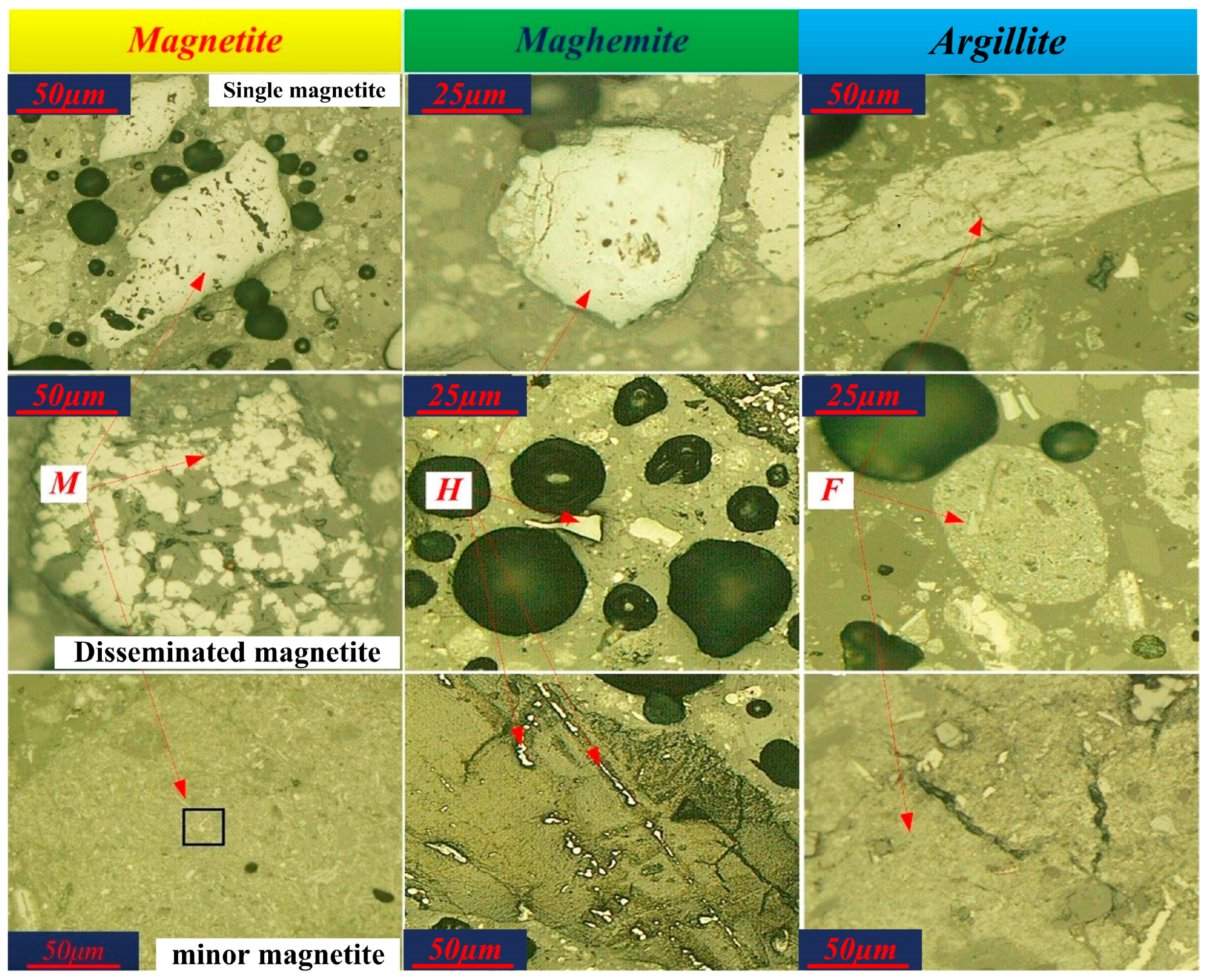



| Elements | TFe | Ni | Cr2O3 | SiO2 | CaO | MgO | Al2O3 | Pb | Zn | S | P | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Content | 37.86 | 0.32 | 5.57 | 19.76 | 0.17 | 9.31 | 5.31 | 0.03 | 0.05 | 0.43 | 0.02 | 0.18 |
| Mineral | Magnetite | Maghemite | Hematite | Fayalite | Others | Fetotal |
|---|---|---|---|---|---|---|
| Content | 28.68 | 4.73 | 0.97 | 2.08 | 1.40 | 37.86 |
| Fraction | 75.75 | 12.49 | 2.56 | 5.49 | 3.69 | 100.00 |
| Positions | Chemical Compositions | |||||
|---|---|---|---|---|---|---|
| Cr2O3 | FeO | CaO | MgO | SiO2 | Al2O3 | |
| 1 | 50.43 | 17.85 | 0.80 | 10.34 | 1.56 | 19.02 |
| 2 | 45.69 | 15.97 | 0.90 | 14.76 | 1.70 | 20.98 |
| 3 | 48.84 | 17.50 | 0.75 | 10.08 | 1.67 | 21.16 |
| 4 | 44.98 | 15.82 | 1.21 | 14.24 | 2.33 | 21.41 |
| 5 | 52.62 | 20.96 | 0.72 | 9.26 | 1.67 | 14.77 |
| 6 | 48.29 | 20.48 | 0.82 | 10.78 | 1.29 | 18.34 |
| 7 | 38.83 | 15.19 | 1.04 | 14.70 | 1.73 | 28.51 |
| 8 | 51.70 | 17.56 | 1.15 | 11.03 | 0.80 | 18.06 |
| 9 | 45.78 | 16.48 | 1.40 | 13.41 | 1.04 | 21.89 |
| 10 | 46.62 | 17.23 | 1.20 | 13.51 | 1.71 | 19.74 |
| Average | 47.35 | 17.50 | 1.00 | 12.21 | 1.55 | 20.39 |
| Elements | TFe | SiO2 | CaO | MgO | Al2O3 | S | P | Ni | Mn | Cr |
|---|---|---|---|---|---|---|---|---|---|---|
| Concentrates | 61.88 | 4.21 | 0.15 | 1.34 | 2.33 | 0.06 | 0.004 | 0.21 | 0.82 | 2.08 |
| Tailings | 17.88 | 34.21 | 0.19 | 17.15 | 7.09 | 0.68 | 0.04 | 0.40 | 1.12 | 5.01 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Z.; Zhu, D.; Pan, J.; Zhang, F. Mineralogical Characteristics and Preliminary Beneficiation of Nickel Slag from Reduction Roasting-Ammonia Leaching. Minerals 2017, 7, 98. https://doi.org/10.3390/min7060098
Guo Z, Zhu D, Pan J, Zhang F. Mineralogical Characteristics and Preliminary Beneficiation of Nickel Slag from Reduction Roasting-Ammonia Leaching. Minerals. 2017; 7(6):98. https://doi.org/10.3390/min7060098
Chicago/Turabian StyleGuo, Zhengqi, Deqing Zhu, Jian Pan, and Feng Zhang. 2017. "Mineralogical Characteristics and Preliminary Beneficiation of Nickel Slag from Reduction Roasting-Ammonia Leaching" Minerals 7, no. 6: 98. https://doi.org/10.3390/min7060098






