3.1. XRD Analysis
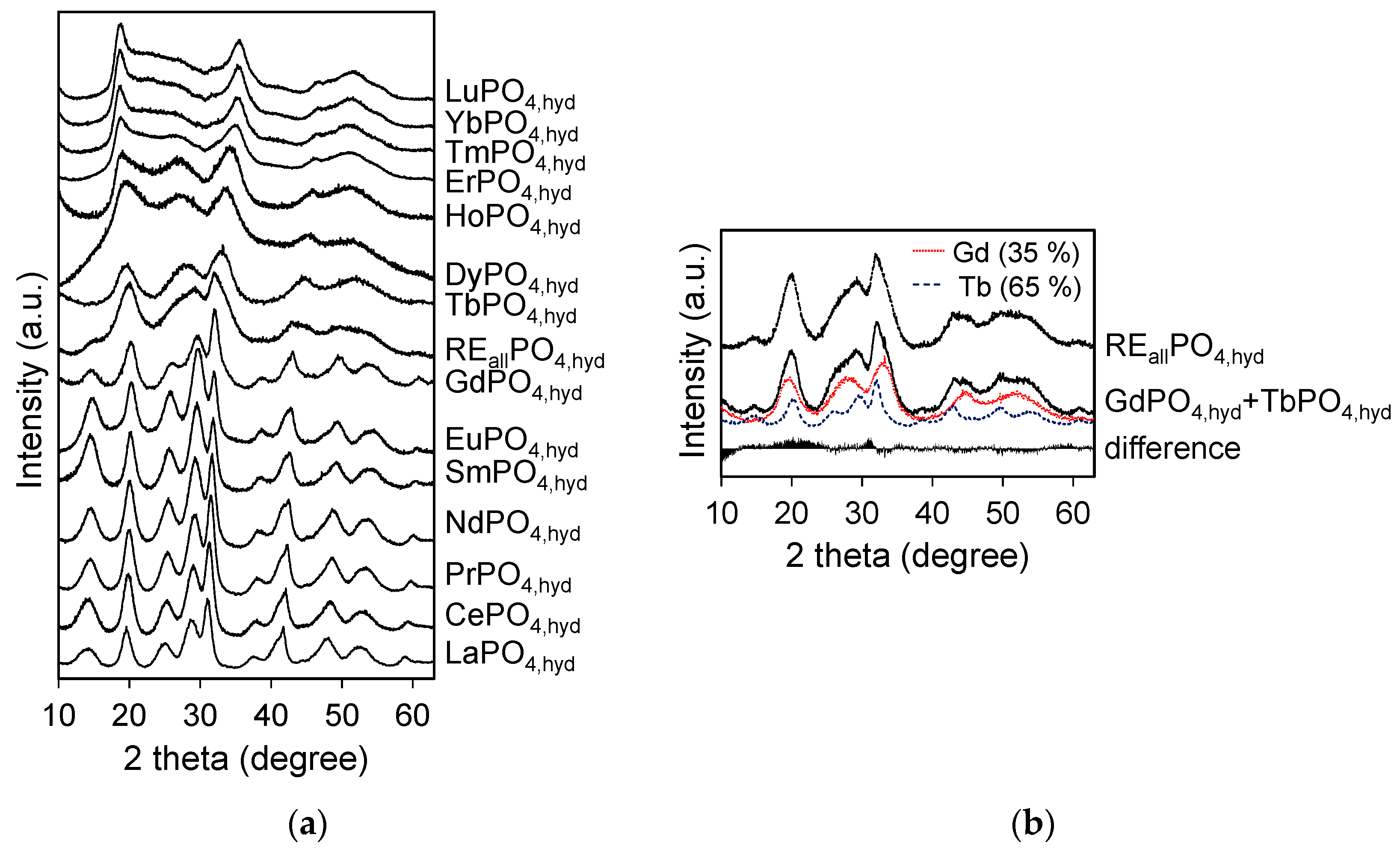
XRD patterns for RE
iPO
4,hyd (RE = La-Lu except Pm) and RE
allPO
4,hyd reacted at pH 3 for 3 days are shown in
Figure 1a. The peak positions of RE
iPO
4,hyd (RE = La-Gd except Pm) correspond well, with a slight peak shift, to the structure of rhabdophane [
8]. The patterns for RE
iPO
4,hyd (RE = Tb-Lu) and RE
allPO
4,hyd could not be identified due to the broadened peaks and lack of matching structures. Although a few of the RE
iPO
4,hyd (RE = Er-Lu) peaks appear to be similar to those of xenotime [
5], the patterns lack a (200) peak and, thus, the structures were not determined convincingly. Furthermore, the patterns for RE
iPO
4,hyd (RE = Tb-Ho) display intermediate profiles between those of RE
iPO
4,hyd (RE = La-Gd except Pm) and RE
iPO
4,hyd (RE = Er-Lu). Based on the structural characteristics indicated by XRD analysis in this study, the structure of the RE
iPO
4,hyd precipitates are classified as LREPO
4,hyd for phases containing RE = La-Gd, MREPO
4,hyd containing RE = Tb-Ho, and HREPO
4,hyd containing RE = Er-Lu, respectively. In LREPO
4,hyd, which has a rhabdophane structure, the coordination numbers (CNs) of RE
3+ ions are one third eight-fold and two-thirds nine-fold [
8], resulting in an average CN of 8.7. RE
3+ in MREPO
4,hyd and HREPO
4,hyd can be considered to have eight-fold coordination because the CN of xenotime and churchite is eight-fold. Based on the CNs of all possible RE
iPO
4,hyd phases, the average ionic radius of RE
allPO
4,hyd containing all RE (RE = La-Lu except Pm) is calculated to be 1.07 Å, which lies just between that of Gd (CN = 8.7, 1.09 Å) and of Tb (CN = 8, 1.04 Å) [
32].
The XRD pattern of RE
allPO
4,hyd correlates to a combination of the GdPO
4,hyd and TbPO
4,hyd patterns. A linear combination of these two profiles indicates volume fractions of 35% GdPO
4,hyd structure and 65% TbPO
4,hyd structure (
Figure 1b).
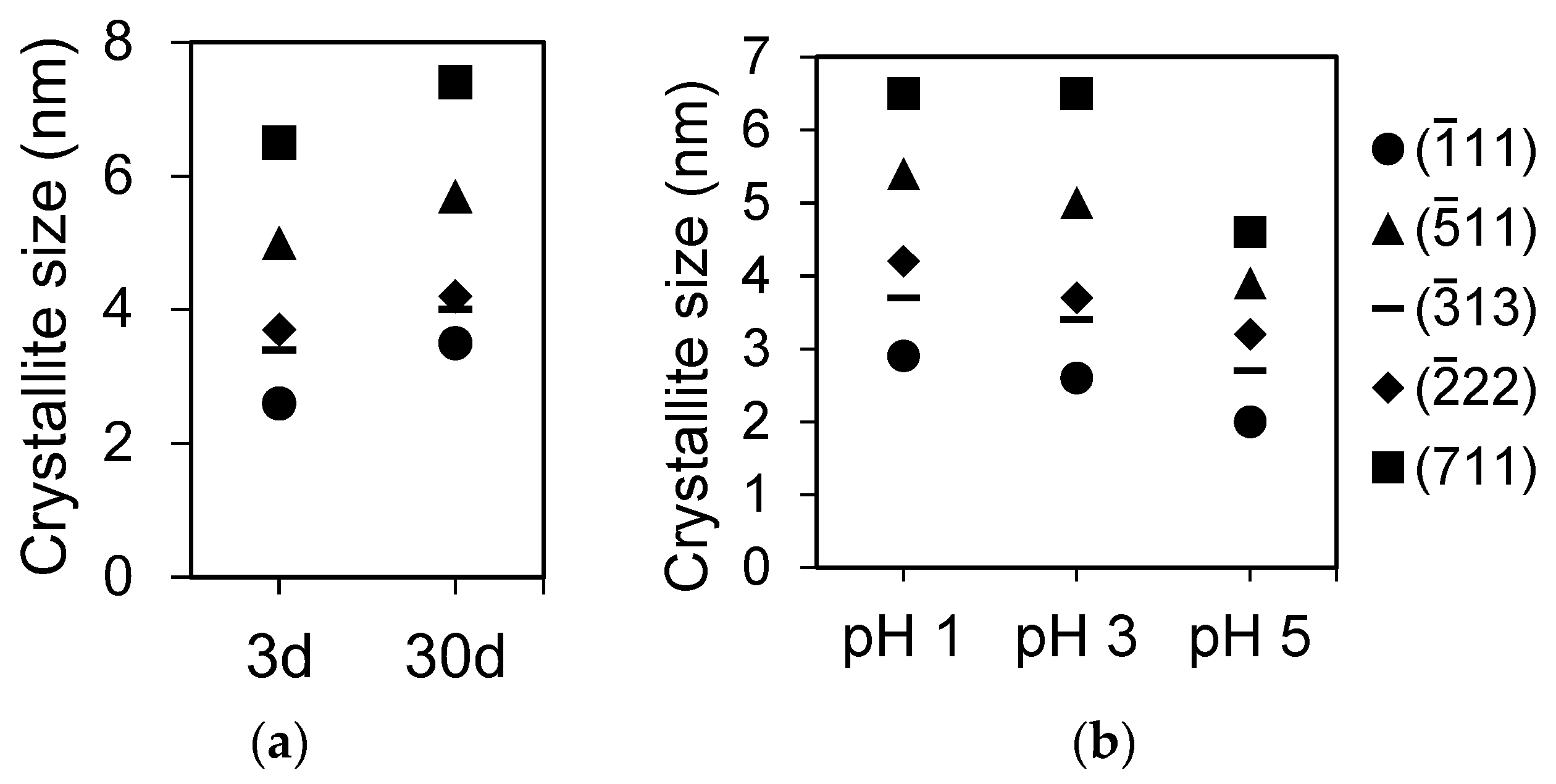
The effect of pH on LaPO
4,hyd precipitates are shown in
Figure 1a,c. The full width at half maximum (FWHM) of each peak becomes smaller as the pH lowers.
In the aging experiments, there was little difference in the XRD profiles between 3 and 30 days for RE
iPO
4,hyd (RE = La, Dy, Ho, and Yb); however, the structures of RE
allPO
4,hyd and TbPO
4,hyd changed significantly to become rhabdophane-structured after 30 days of aging (
Figure 1a,d). In the DyPO
4,hyd pattern, a small peak corresponding to the (
11) plane of rhabdophane appeared after 30 days of reaction, although the other peaks remained unchanged. The crystallite sizes of LaPO
4,hyd calculated on the basis of the XRD patterns in
Figure 1 using Scherrer equation are clearly a function of pH and the aging period (
Figure 2a,b) and are summarized in
Table 1.
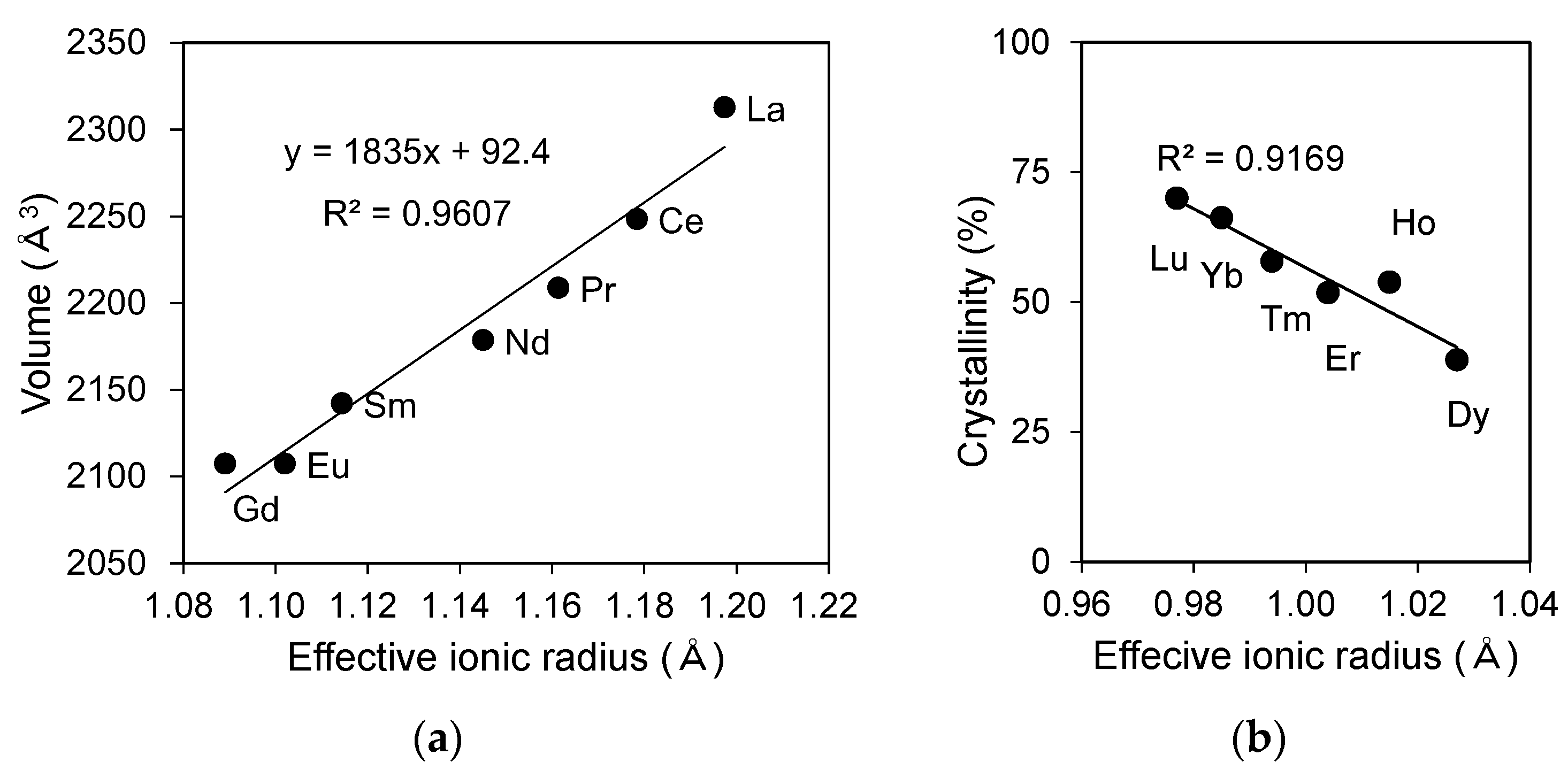
The calculated lattice volume of LREPO
4,hyd reacted at a pH of 3 for 3 days are plotted as a function of the effective ionic radius in
Figure 3a by fitting the (
11), (
11), (
13), (
22), and (711) peaks to the peaks of the rhabdophane structure using the JADE software. The calculated lattice parameters are summarized in
Table 2. The calculated lattice volume displays a positive linear correlation with the effective ionic radius, indicating that the crystal lattice shrinks uniformly as the ionic radius decreases (
Figure 3a). The lattice volumes calculated in the present experiment are larger than that of well-crystalline rhabdophane structure reported in [
8], most likely owing to the presence of more water molecules in the structure of LREPO
4,hyd.
The crystallinity of RE
iPO
4,hyd (RE = Dy-Lu) was calculated based on the profile fitting of amorphous halos in the XRD patterns (
Figure S2,
Table 3) and is plotted in
Figure 3b as a function of effective ionic radius. The results indicate that DyPO
4,hyd has the largest amorphous volume and that the amorphous fraction linearly decreases towards LuPO
4,hyd.
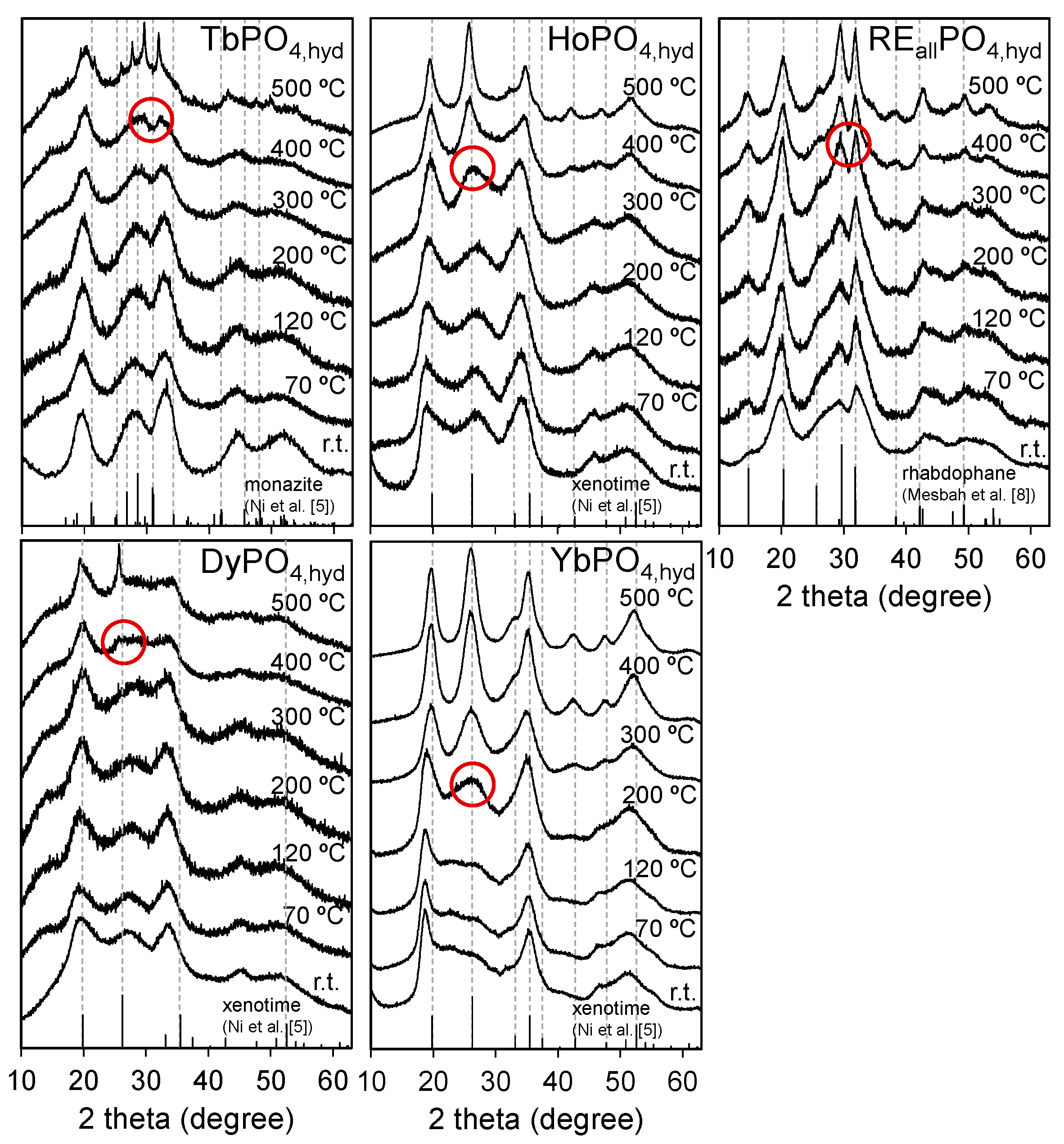
The XRD patterns of RE
iPO
4,hyd (RE = Tb, Ho, Dy, and Yb) and RE
allPO
4,hyd obtained from the annealing experiments are summarized in
Figure 4. TbPO
4,hyd transformed to a monazite structure at 400 °C with small peaks occurring at a 2
θ value around 30°, as indicated by the circle. DyPO
4,hyd appeared to transform to a xenotime structure at 400 °C, as indicated by the appearance of small peaks at a 2
θ value around 25.8°. HoPO
4,hyd and YbPO
4,hyd also transformed to a xenotime structure at around 300 and 200 °C, respectively. RE
allPO
4,hyd appeared to retain its initial structure up to 200 °C and then transformed to a rhabdophane structure, which was retained up to 500 °C. In contrast, TbPO
4,hyd did not become rhabdophane-structured but directly transformed to a monazite structure when annealed in air. The thermal behavior of TbPO
4,hyd is different from that observed in the aging experiments, in which the structure simply changed to that of rhabdophane.
3.2. FT-IR Analysis
Infrared spectra for RE
iPO
4,hyd (RE = La-Lu except Pm) and RE
allPO
4,hyd reacted at pH 3 for 3 days are shown in
Figure 5a. Two bands at 520–650 cm
−1 (labeled 1 and 2) correspond to the asymmetric P–O bending vibration,
ν3, while the bands at 1000–1100 cm
−1 (labeled 3 and 4) correspond to the antisymmetric P–O stretching vibration,
ν4 [
33,
34,
35]. Bands 3 and 4 are not clearly separated for LREPO
4,hyd. The symmetric stretching mode
ν1, which typically appears as a small absorption band at ~966–985 cm
−1 [
36] and the bending vibration
ν2 were not observed, possibly because those bands were superimposed in the
ν3 and
ν4 regions for the hydrated RE phosphates [
35]. The wavenumbers of band 4 in the
ν3 region plotted as a function of the effective ionic radius (
Figure 5b and
Table 4) exhibit a linear decrease with increasing ionic radius for LREPO
4,hyd and HREPO
4,hyd, while MREPO
4,hyd and RE
allPO
4,hyd did not exhibit such trends. The broad bands at ~1600 cm
−1 (band 5) and at ~3000–3500 cm
−1 (band 6) are derived from coordinated H
2O [
34]. Nitrate impurities derived from the initial REE reagents produce the band at ~1380 cm
−1.
FT-IR spectrum of the aged TbPO
4,hyd and RE
allPO
4,hyd appeared to be similar to that of LaPO
4,hyd, especially at bands 3 and 4 (
Figure 5c). These FT-IR results concur with the XRD analyses, i.e., they indicate a change in the structures of RE
allPO
4,hyd and TbPO
4,hyd to a rhabdophane structure after 30 days of aging in solution.
3.3. TG-DTA Analysis
Derivatives of the TG (DTG) and DTA spectra for RE
iPO
4,hyd (RE = La-Lu) and RE
allPO
4,hyd reacted at a pH of 3 for 3 days are shown in
Figure 6. LREPO
4,hyd and HREPO
4,hyd display a two-step dehydration, which is indicated by the presence of a DTG upper peak with an endothermic DTA peak. Assuming that the material remaining after heating to 500 °C entirely comprise anhydrous RE phosphates and the weight loss is derived solely from the dissociation of H
2O in REPO
4,hyd, the amount of dissociated water was calculated for the total heating duration (
Figure 7a) and for each dehydration step (
Figure 7b,c), and compiled in
Table 5. The starting temperature of the second dehydration step was determined by selecting the local minimum of the DTG spectrum or the peak of the DTA spectrum between the first and second dehydration steps. A diagram of the dehydration extent in the first step in HREPO
4,hyd indicates that the amount of water loss increases as a function of ionic radius (
Figure 7b). The amount of water loss in the second dehydration step also appears to exhibit a linear decrease as the ionic radii decrease in LREPO
4,hyd and HREPO
4,hyd (
Figure 7c). MREPO
4,hyd and RE
allPO
4,hyd each exhibited only one dehydration peak. For these phases, a DTG upper peak and a DTA exothermic peak are present, indicating that crystallization occurred during dehydration.
In addition, the DTG and DTA spectra of RE
iPO
4,hyd (RE = La, Tb, and Yb) and RE
allPO
4,hyd after 30 days of reaction time are shown in
Figure 6 for comparison with samples measured after 3 days of reaction time. Although LaPO
4,hyd and YbPO
4,hyd did not appear significantly different from those samples that had reacted for 3 days, additional dehydration peaks appeared in the spectra of TbPO
4,hyd and RE
allPO
4,hyd reacted for 30 days.
3.4. ICP-AES Analysis
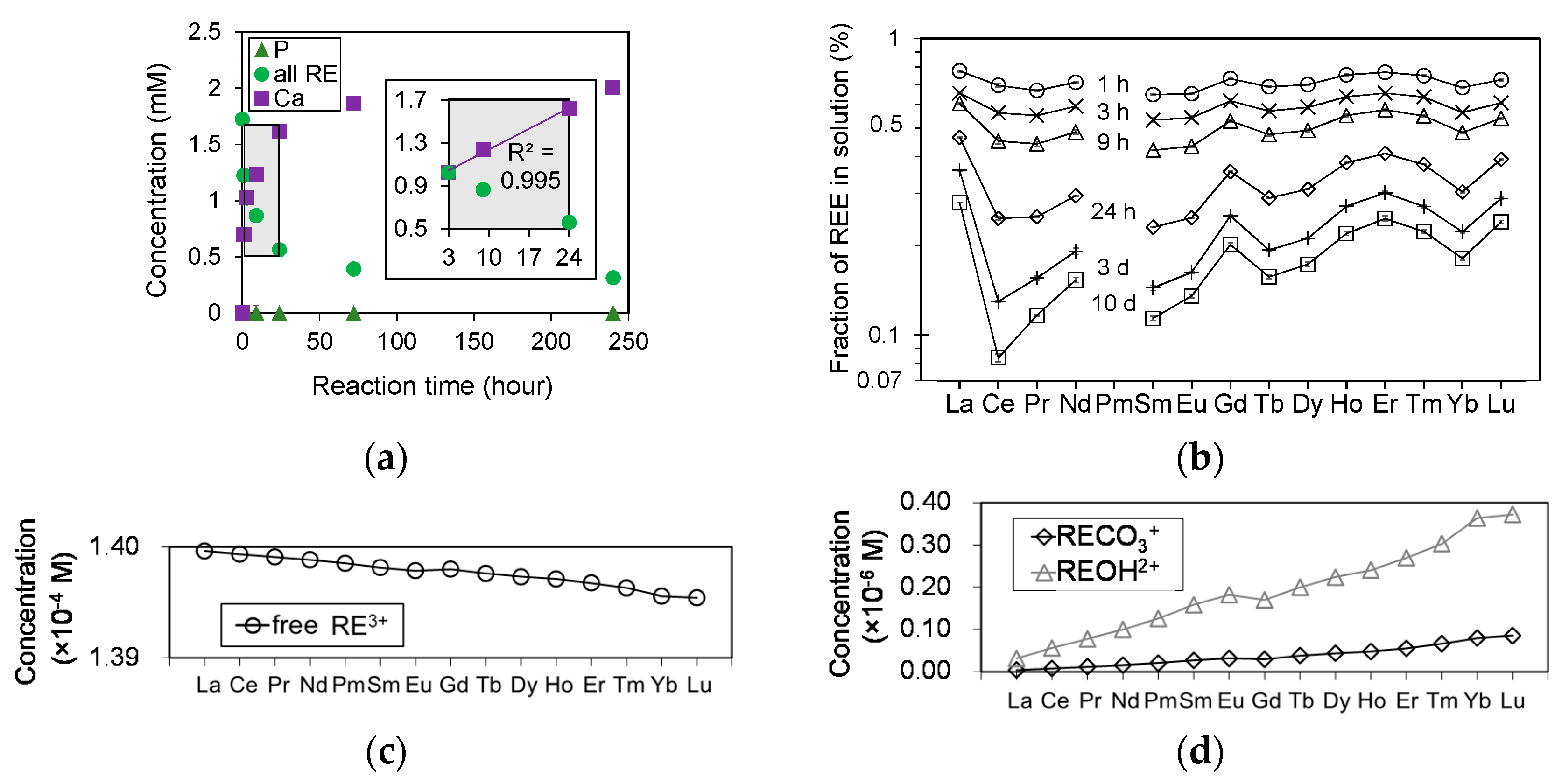
In experiments of HAP dissolution in a solution containing all REEs, the concentrations of RE (RE = La-Lu except Pm), Ca, and P were measured for each duration using ICP-AES. The results are summarized in
Table 6 and the total REE concentrations are plotted in
Figure 8a as a function of reaction time. The P concentration is under the detection limit for all durations. The Ca concentration increased linearly for periods from 3 to 24 h, whereas the total concentration of all REEs decreased inversely with the Ca concentration. The reaction did not reach equilibrium within the duration of this dissolution experiment (
Figure 8a); however, more than 80% of the total initial REE concentration was removed from solution after 240 h.
Figure 8b shows the transition in the patterns of REEs in solution reacted with HAP. The four curves at La-Nd, Sm-Gd, Gd-Ho, and Er-Lu exhibit the lanthanide tetrad effect [
37]. Furthermore, more heavy REEs remained in solution compared with light REEs, and this trend becomes more pronounced with reaction time. The possible concentrations of free RE
3+, RECO
3+, and REOH
2+ at these experimental conditions were calculated using Geochemist’s Workbench using their formation constants [
38], and input data of P
CO2 = 10
−3.5 bar [
39] and phosphate free solution. The calculation results clearly demonstrate that the concentration of free RE
3+ decreases as the ionic radius decreases because the amount of other REE complexes such as RECO
3+ and REOH
2+ increase with decreasing ionic radius (
Figure 8c,d).
3.5. TEM Analysis
The grain shapes of REPO
4,hyd gradually change with ionic radius. HRTEM images show that RE
iPO
4,hyd (RE = La, Nd, Tb) and RE
allPO
4,hyd form rod-shaped particles of ~50 nm in length. TmPO
4,hyd and LuPO
4,hyd are spherically shaped with diameters of 10 nm (
Figure 9). DyPO
4,hyd and HoPO
4,hyd precipitates do not exhibit any specific shape due to their amorphous characteristics. The SAED patterns of LREPO
4,hyd and RE
allPO
4,hyd display the same ring pattern as that of the rhabdophane structure. Although the SAED patterns of other REPO
4,hyd are indistinct due to the large amorphous fractions, the RE
iPO
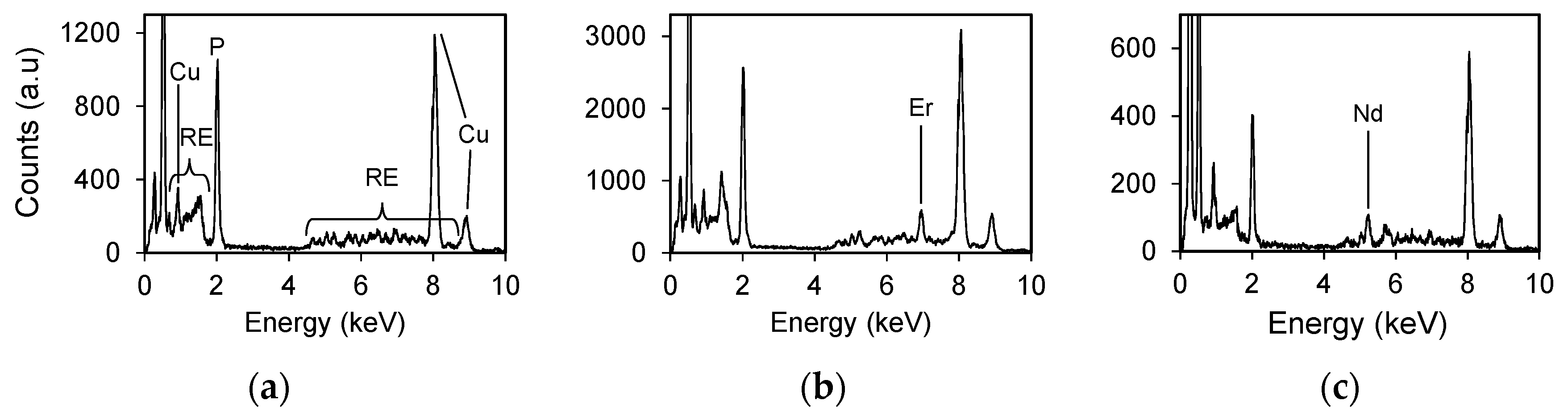
4,hyd (RE = Tb-Lu) structure can be distinguished from the rhabdophane structure by the lack of an innermost diffraction ring. In EDS analyses, most analytical points from the RE
allPO
4 crystals contain uniform collective peaks at 4–8 keV corresponding to the L lines of REEs (
Figure 10a). However, a few points display non-uniform spectrums, as shown in
Figure 10b,c, in which enhanced peaks for Er and Nd occur, respectively.
RE
iPO
4,hyd (RE = La, Tb, and Yb) and RE
allPO
4,hyd formed on HAP crystals appear to differ slightly from those formed in mixed solutions. For example, LaPO
4 precipitated as fibrous crystals on HAP (
Figure 11a–c), in contrast to the rod-shaped crystals precipitated from the mixed solutions. RE
allPO
4,hyd precipitates that formed on HAP exhibit shorter fibrous shaped crystals than the LaPO
4,hyd precipitates on HAP (
Figure 11d–i). The crystal shapes were not significantly affected by the reaction time. YbPO
4,hyd precipitated at the rim of HAP crystals with a non-fibrous morphology (
Figure 11j–k), reflecting the rapid precipitation of YbPO
4,hyd and consumption of phosphate upon the release of P from the surface of HAP crystals.