Flotation and Adsorption of a New Polysaccharide Depressant on Pyrite and Talc in the Presence of a Pre-Adsorbed Xanthate Collector
Abstract
:1. Introduction
2. Experimental
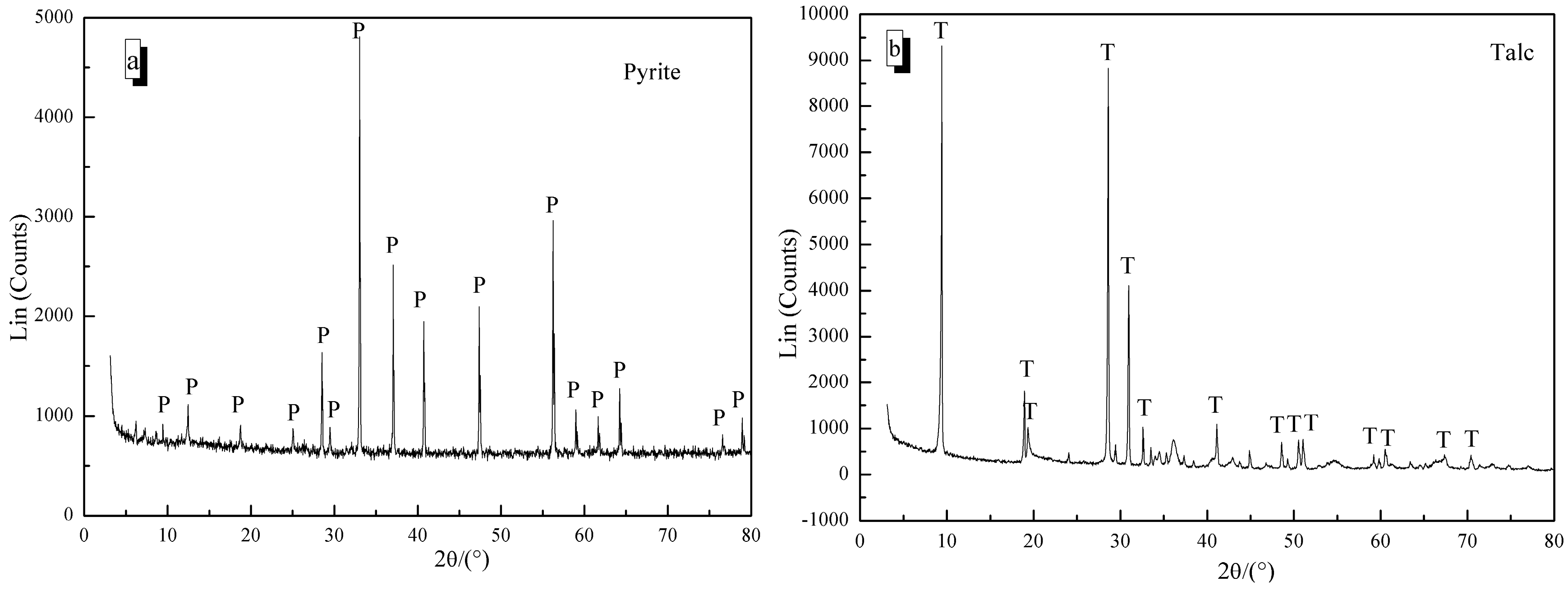
2.1. Materials and Reagents
2.2. Flotation Tests
2.3. Contact Angle Measurements
2.4. Adsorption Measurements
2.5. FTIR Measurements
3. Results and Discussion
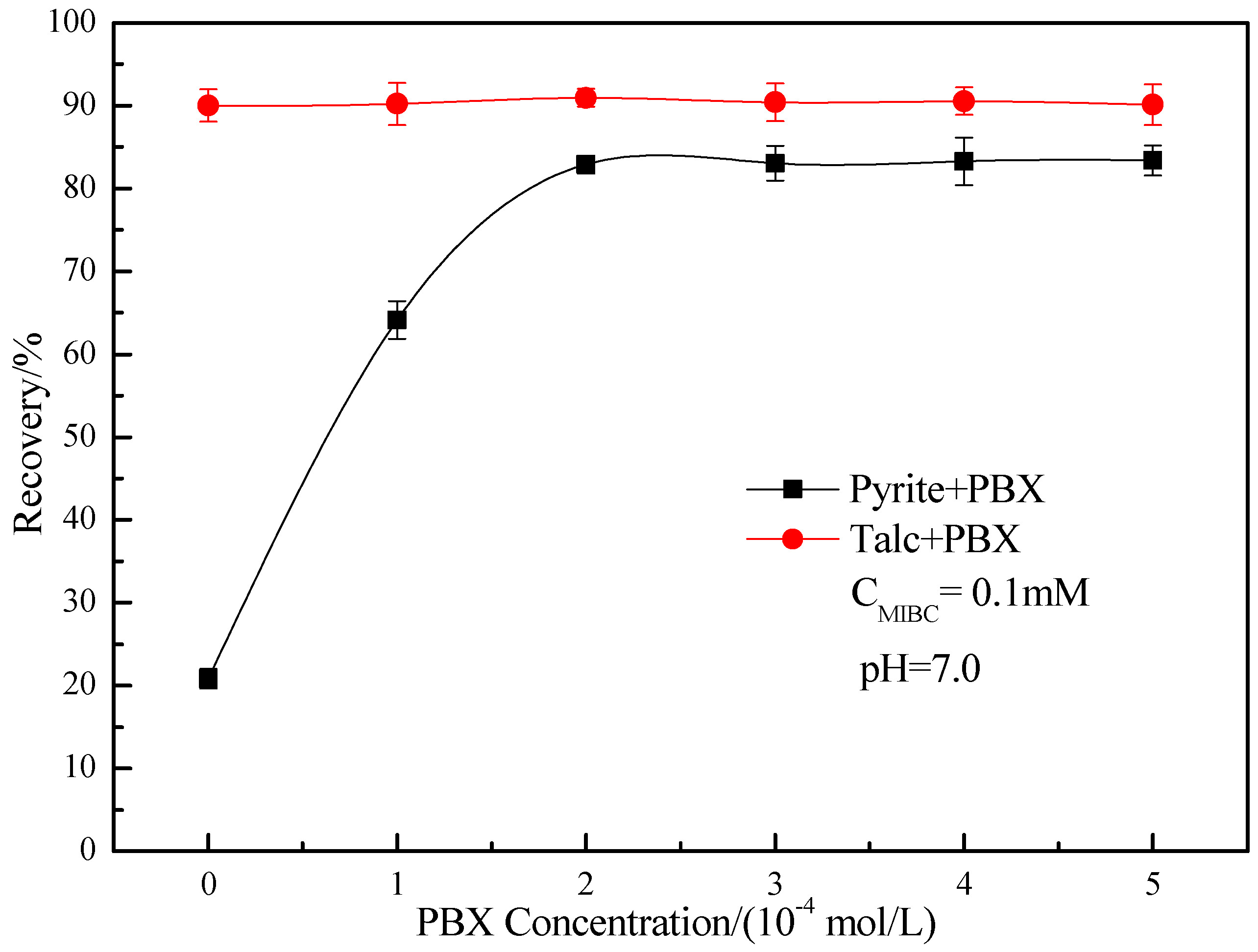
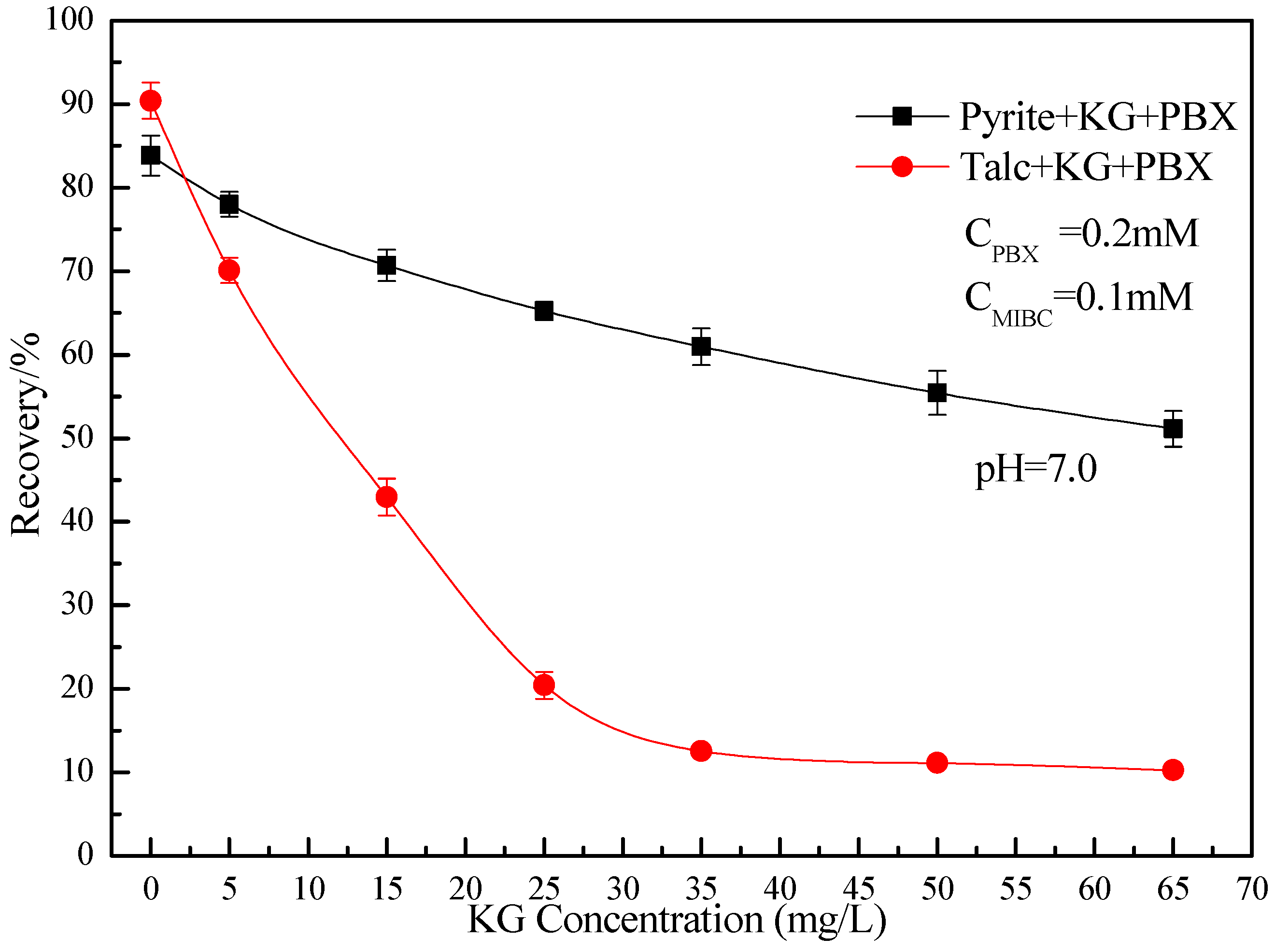
3.1. Microflotation Results
3.2. Artificially Mixed Minerals Results
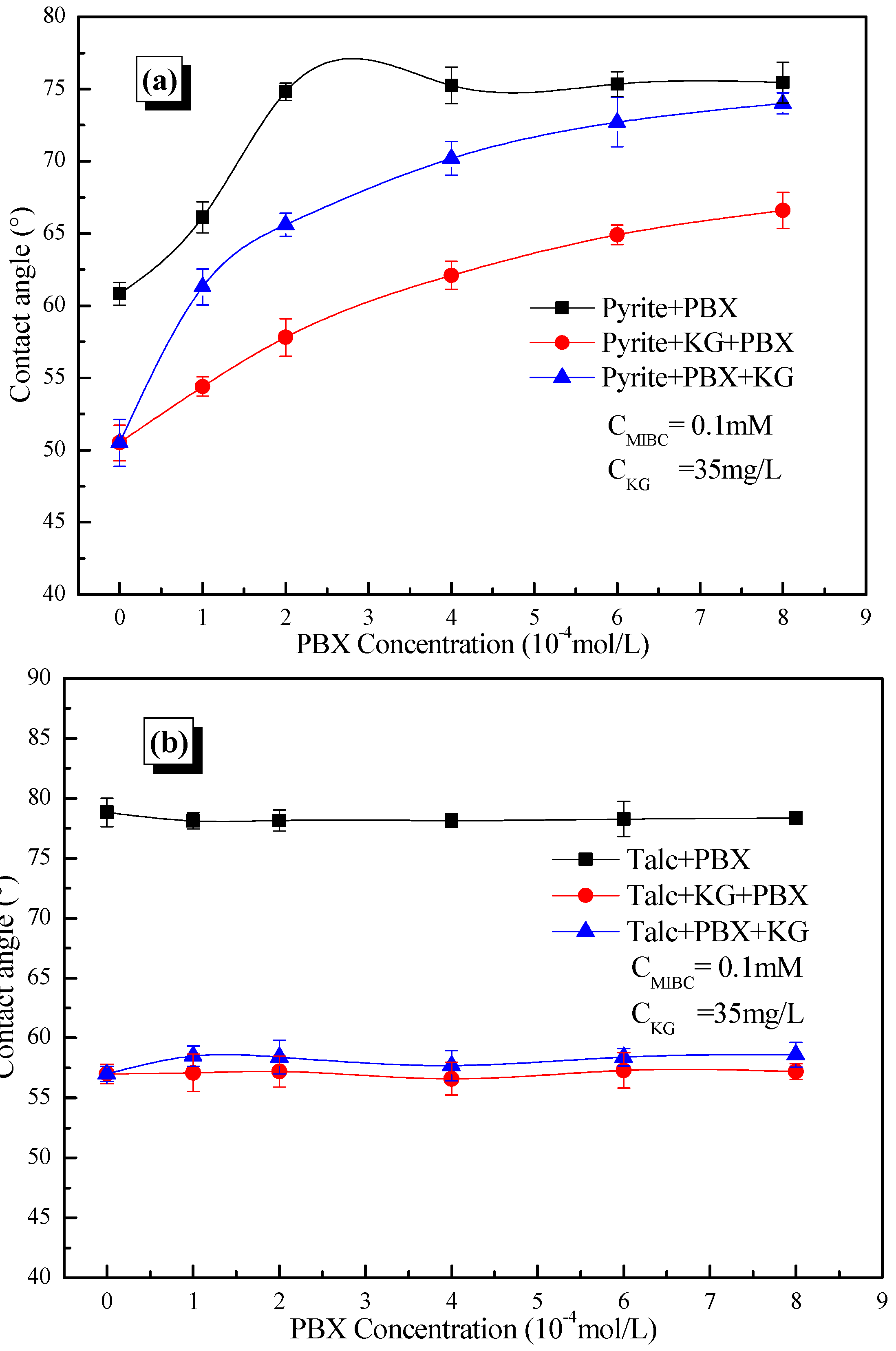
3.3. Contact Angle Results
3.4. Adsorption Test Results
3.5. FTIR Spectra Analysis
4. Conclusions
- (1)
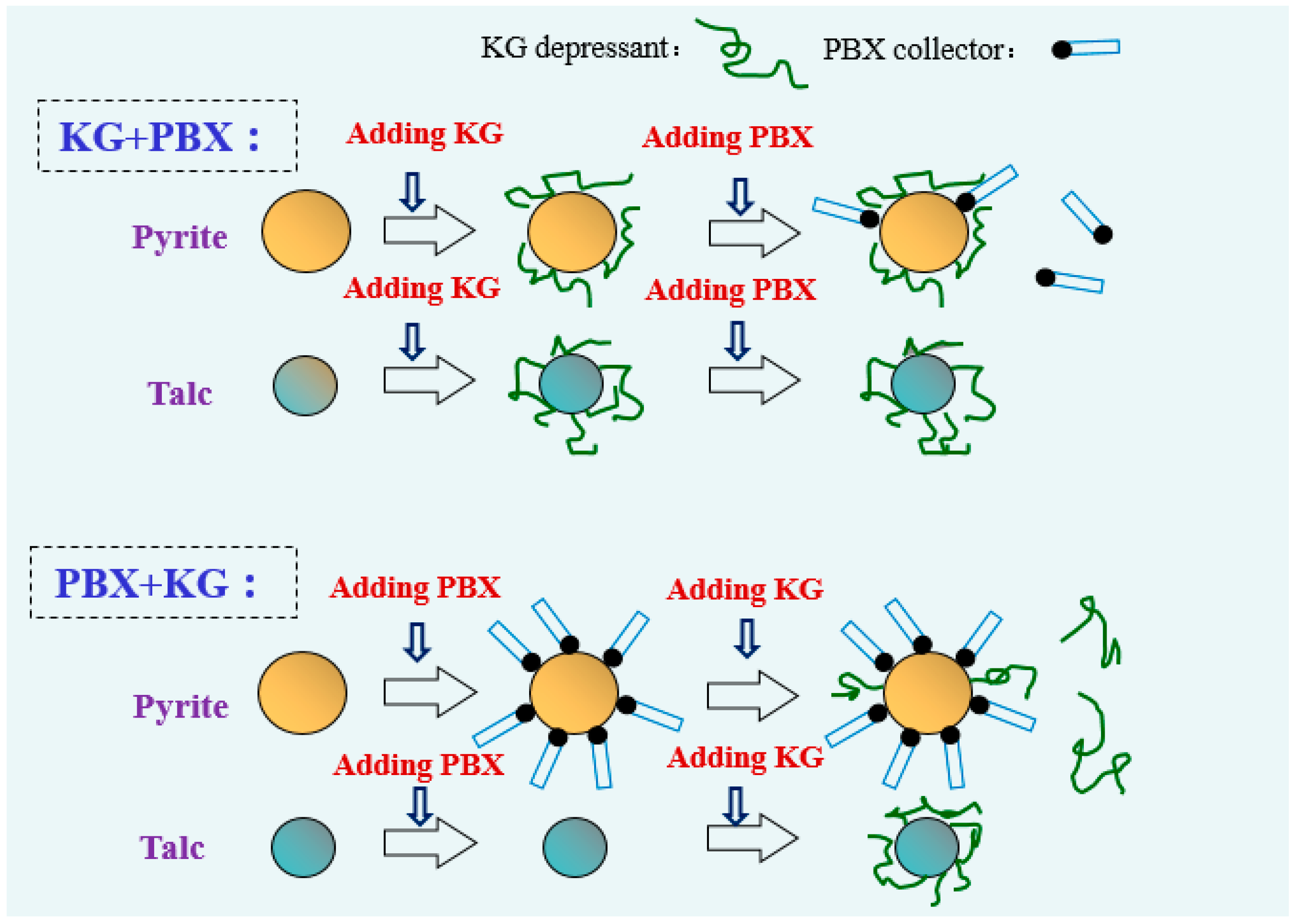
- KG is a quality depressant of talc. The addition of KG increases the microflotation recovery difference between pyrite and talc. KG and PBX can adsorb on the surface of pyrite, while only KG can adsorb on the surface of talc.
- (2)
- The addition order of KG and PBX significantly influences the recovery, wettability and reagent adsorption of pyrite, but has little influence on these parameters on talc.
- (3)
- KG adsorbs on pyrite and talc surfaces via a chemical reaction, while PBX only adsorbs on the pyrite surface via a chemical reaction. Competitive adsorption between KG and PBX exists on the surface of pyrite, and pre-adsorbing pyrite with PBX prevents pyrite from continuously adsorbing KG.
- (4)
- By controlling the addition order of KG/PBX, the flotation difference between pyrite and talc increases and the flotation separation of pyrite from talc becomes feasible.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Douillard, J.M.; Salles, F.; Henry, M.; Malandrini, H.; Clauss, F. Surface energy of talc and chlorite: Comparison between electronegativity calculation and immersion results. J. Colloid Interface Sci. 2007, 305, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Farrokhpay, S.; Filippov, L. Challenges in processing nickel laterite ores by flotation. Int. J. Miner. Process. 2016, 151, 59–67. [Google Scholar] [CrossRef]
- Liu, G.; Feng, Q.; Ou, L.; Lu, Y.; Zhang, G. Adsorption of polysaccharide onto talc. Miner. Eng. 2006, 19, 147–153. [Google Scholar] [CrossRef]
- Xu, L.; Wu, H.; Dong, F.; Wang, L.; Wang, Z.; Xiao, J. Flotation and adsorption of mixed cationic/anionic collectors on muscovite mica. Miner. Eng. 2013, 41, 41–45. [Google Scholar] [CrossRef]
- Zhao, K.L.; Gu, G.H.; Wang, H.; Wang, C.L.; Wang, X.H.; Luo, C. Influence of depressant foenum-graecum on the flotation of a sulfide ore which contains hydrophobic gangue. Int. J. Miner. Process. 2015, 141, 68–76. [Google Scholar] [CrossRef]
- Beattie, D.A.; Huynh, L.; Kaggwa, G.B.; Ralston, J. Influence of adsorbed polysaccharides and polyacrylamides on talc flotation. Int. J. Miner. Process. 2006, 78, 238–249. [Google Scholar] [CrossRef]
- Beattie, D.A.; Huynh, L.; Kaggwa, G.B.; Ralston, J. The effect of polysaccharides and polyacrylamides on the depression of talc and the flotation of sulphide minerals. Miner. Eng. 2006, 19, 598–608. [Google Scholar] [CrossRef]
- Leung, A.; Wiltshire, J.; Blencowe, A.; Fu, Q.; Solomon, D.H.; Qiao, G.G. The effect of acrylamide-co-vinylpyrrolidinone copolymer on the depression of talc in mixed nickel mineral flotation. Miner. Eng. 2011, 24, 449–454. [Google Scholar] [CrossRef]
- Bagci, E.; Ekmekci, Z.; Bradshaw, D. Adsorption behaviour of xanthate and dithiophosphinate from their mixtures on chalcopyrite. Miner. Eng. 2007, 20, 1047–1053. [Google Scholar] [CrossRef]
- Bicak, O.; Ekmekci, Z.; Bradshaw, D.J.; Harris, P.J. Adsorption of guar gum and CMC on pyrite. Miner. Eng. 2007, 20, 996–1002. [Google Scholar] [CrossRef]
- López Valdivieso, A.; Celedón Cervantes, T.; Song, S.; Robledo Cabrera, A.; Laskowski, J.S. Dextrin as a non-toxic depressant for pyrite in flotation with xanthates as collector. Miner. Eng. 2004, 17, 1001–1006. [Google Scholar] [CrossRef]
- Garcia Vidal, C.A.; Pawlik, M. Molecular weight effects in interactions of guar gum with talc. Int. J. Miner. Process. 2015, 138, 38–43. [Google Scholar] [CrossRef]
- Khraisheh, M.; Holland, C.; Creany, C.; Harris, P.; Parolis, L. Effect of molecular weight and concentration on the adsorption of CMC onto talc at different ionic strengths. Int. J. Miner. Process. 2005, 75, 197–206. [Google Scholar] [CrossRef]
- McFadzean, B.; Dicks, P.; Groenmeyer, G.; Harris, P.; O’Connor, C. The effect of molecular weight on the adsorption and efficacy of polysaccharide depressants. Miner. Eng. 2011, 24, 463–469. [Google Scholar] [CrossRef]
- McFadzean, B.; Groenmeyer, G. Selective molecular weight adsorption from polydisperse polysaccharide depressants. Miner. Eng. 2015, 77, 172–178. [Google Scholar] [CrossRef]
- Zhao, K.; Gu, G.; Wang, C.; Rao, X.; Wang, X.; Xiong, X. The effect of a new polysaccharide on the depression of talc and the flotation of a nickel–copper sulfide ore. Miner. Eng. 2015, 77, 99–106. [Google Scholar] [CrossRef]
- Wiese, J.; Harris, P.; Bradshaw, D. The response of sulphide and gangue minerals in selected Merensky ores to increased depressant dosages. Miner. Eng. 2007, 20, 986–995. [Google Scholar] [CrossRef]
- Feng, B.; Feng, Q.; Lu, Y.; Gu, Y. The effect of PAX/CMC addition order on chlorite/pyrite separation. Miner. Eng. 2013, 42, 9–12. [Google Scholar] [CrossRef]
- Feng, B.; Lu, Y.; Luo, X. The effect of quartz on the flotation of pyrite depressed by serpentine. J. Mater. Res. Technol. 2015, 4, 8–13. [Google Scholar] [CrossRef]
- Mu, Y.; Peng, Y.; Lauten, R.A. The depression of pyrite in selective flotation by different reagent systems—A Literature review. Miner. Eng. 2016, 96, 143–156. [Google Scholar] [CrossRef]
- Xu, L.; Hu, Y.; Tian, J.; Wu, H.; Wang, L.; Yang, Y.; Wang, Z. Synergistic effect of mixed cationic/anionic collectors on flotation and adsorption of muscovite. Colloids Surf. A 2016, 492, 181–189. [Google Scholar] [CrossRef]
- Wang, J.; Somasundaran, P.; Nagaraj, D.R. Adsorption mechanism of guar gum at solid–liquid interfaces. Miner. Eng. 2005, 18, 77–81. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Cao, Z.; Cao, Y.D.; Sun, C.Y. FTIR studies of xanthate adsorption on chalcopyrite, pentlandite and pyrite surfaces. J. Mol. Struct. 2013, 1048, 434–440. [Google Scholar] [CrossRef]
- Chiem, L.T.; Huynh, L.; Ralston, J.; Beattie, D.A. An in situ ATR-FTIR study of polyacrylamide adsorption at the talc surface. J. Colloid Interface Sci. 2006, 297, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Qian, Y.; Xu, L.H.; Dai, B.; Xiao, J.H.; Fu, K. Selective chalcopyrite flotation from pyrite with glycerine-xanthate as depressant. Miner. Eng. 2015, 74, 86–90. [Google Scholar] [CrossRef]







| Sample | SiO2 | MgO | CaO | Al2O3 | Fe2O3 | S |
|---|---|---|---|---|---|---|
| Talc | 61.42 | 31.40 | 5.12 | - | 1.06 | - |
| Pyrite | 4.67 | 0.67 | 0.62 | 2.05 | 30.73 | 53.00 |
| Reagent System | Product | Yield/% | Grade/% | Recovery/% | ||
|---|---|---|---|---|---|---|
| S | MgO | S | MgO | |||
| PBX | Concentrates | 80.36 | 23.72 | 17.97 | 89.91 | 76.65 |
| Tailing | 19.64 | 10.89 | 22.40 | 10.09 | 23.35 | |
| Feed | 100.00 | 21.20 | 18.84 | 100.00 | 100.00 | |
| KG + PBX | Concentrates | 50.81 | 33.79 | 4.30 | 80.98 | 11.59 |
| Tailing | 49.19 | 8.20 | 33.86 | 19.02 | 88.41 | |
| Feed | 100.00 | 21.20 | 18.84 | 100.00 | 100.00 | |
| PBX + KG | Concentrates | 51.25 | 35.75 | 3.52 | 86.42 | 9.56 |
| Tailing | 48.75 | 5.90 | 34.95 | 13.58 | 90.44 | |
| Feed | 100.00 | 21.20 | 18.84 | 100.00 | 100.00 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, W.; Xu, L.; Tian, J.; Hu, Y.; Han, Y. Flotation and Adsorption of a New Polysaccharide Depressant on Pyrite and Talc in the Presence of a Pre-Adsorbed Xanthate Collector. Minerals 2017, 7, 40. https://doi.org/10.3390/min7030040
Deng W, Xu L, Tian J, Hu Y, Han Y. Flotation and Adsorption of a New Polysaccharide Depressant on Pyrite and Talc in the Presence of a Pre-Adsorbed Xanthate Collector. Minerals. 2017; 7(3):40. https://doi.org/10.3390/min7030040
Chicago/Turabian StyleDeng, Wei, Longhua Xu, Jia Tian, Yuehua Hu, and Yuexin Han. 2017. "Flotation and Adsorption of a New Polysaccharide Depressant on Pyrite and Talc in the Presence of a Pre-Adsorbed Xanthate Collector" Minerals 7, no. 3: 40. https://doi.org/10.3390/min7030040






