1. Introduction
Climate change and abnormal weather phenomena have been worsening, owing to increased CO
2 emissions around the world. Typically, the Korean peninsula is not a region that is known to suffer from water shortages [
1]. However, since in the last few years there have been severe annual droughts, especially in west-coast areas, with increased CO
2 emissions being blamed. Several methods of preserving water resources have been examined, and seawater desalination has been found to be a successful candidate [
2,
3]. Seawater desalination processes remove the salts from seawater by distillation, membrane separation, and/or reverse osmosis (RO) to produce useful water that is suitable for drinking and industrial purposes [
3,
4,
5]. One result of these processes is that concentrated seawater (i.e., brine) is also produced, in which the rejected salts in seawater have been concentrated. Desalination consumes large amounts of energy, meaning that large-scale desalination plants are often placed near power stations [
2], and also leads to the emission of massive amounts of CO
2, which will eventually exacerbate the problems that desalination is attempting to solve. Methods of limiting CO
2 emissions or removing CO
2 from desalination plants are, therefore, of great interest.
CO
2 mineralization is one such method of sequestering CO
2. In this method, CO
2 reacts with calcium and/or magnesium to form calcium carbonate and/or magnesium carbonate [
6]. The rate of CO
2 mineralization can be accelerated when the reaction takes place in the aqueous phase, as happens in typical chemical reactions. Ionization of CO
2 to CO
32− takes place under alkaline conditions. This is essential for CO
2 mineralization, as it allows ionic reactions between CO
32− and Ca
2+ and/or Mg
2+. For this reason, alkaline chemicals such as NaOH or NH
4OH are applied to increase the pH of the aqueous phase in CO
2 mineralization [
7,
8].
Meanwhile, using Ca and Mg from industrial waste such as waste concrete, slags from steel production, and liquid wastes is preferable when attempting to design feasible CO
2 mineralization processes [
9,
10,
11,
12,
13]. Detailed principles and applications of CO
2 mineralization using alkaline waste have been summarized by Pan et al. [
14]. Some pretreatment steps, such as crushing, sieving, activation, and/or digestion are required to extract Ca and Mg from solid waste [
13,
15,
16]. When using liquid waste, such pretreatment procedures are not required because the Ca and/or Mg are already present as ions. For this reason, the use of brine for CO
2 mineralization is a promising route for investigation [
6,
8,
17]. In the implementation of this technology, both engineering and economic factors are important, as is environmentally balanced performance [
18].
It is reported that the concentrations of the rejected salts in brine from desalination plants using reverse osmosis (RO) systems are 2–2.5 times higher than those in influent seawater [
19], suggesting that brine could be a promising material for CO
2 mineralization. If Mg alone is present in a solution, it readily reacts with CO
2 to precipitate Mg carbonates; the reaction between MgCl
2·6H
2O solution and CO
2 produced nesquehonite (MgCO
3·3H
2O), dypingite (Mg
5(CO
3)
4(OH)
2·5H
2O), dypingite-like (Mg
5(CO
3)
4(OH)
2·8H
2O), and hydromagnesite (Mg
5(CO
3)
4(OH)
2·4H
2O) crystal phases, depending on the reaction conditions [
20]. When considering brine for CO
2 mineralization, it is worth noting that in previously reported studies, materials in a replica brine solution with coexisting Mg and Ca were not readily carbonated. Liu and Maroto-Valer [
21] synthesized oil-field brine and conducted CO
2 mineralization experiments under mimicked underground conditions to precipitate calcite. An artificial brine containing metal ions such as Na
+, Mg
2+, Ca
2+, K
+, and Sr
2+, and anions including Cl
−, SO
42−, Br
−, BO
33−, F
−, and HCO
3− was unfavorable for the precipitation of Mg carbonates, but was favorable for CaCO
3 precipitation [
22]. In the same manner, simulated brine composed of NaCl, MgCl
2, CaCl
2, Na
2SO
4, and KCl precipitated CaCO
3 by the primary separation of Mg(OH)
2 from the Ca
2+ solution [
23]. Owing to the significantly higher concentration of Mg than Ca in seawater and brine, Mg would allow much more CO
2 mineralization than Ca does. However, this does not necessarily mean that more precipitating Mg carbonates than Ca carbonates will be produced, because Ca carbonates are more sparingly soluble than Mg carbonates (for example, K
sp (hydromagnesite) = 1.26 × 10
−5 [
24], and K
sp (calcite) = 3.36 × 10
−9 at 25 °C each [
25]).
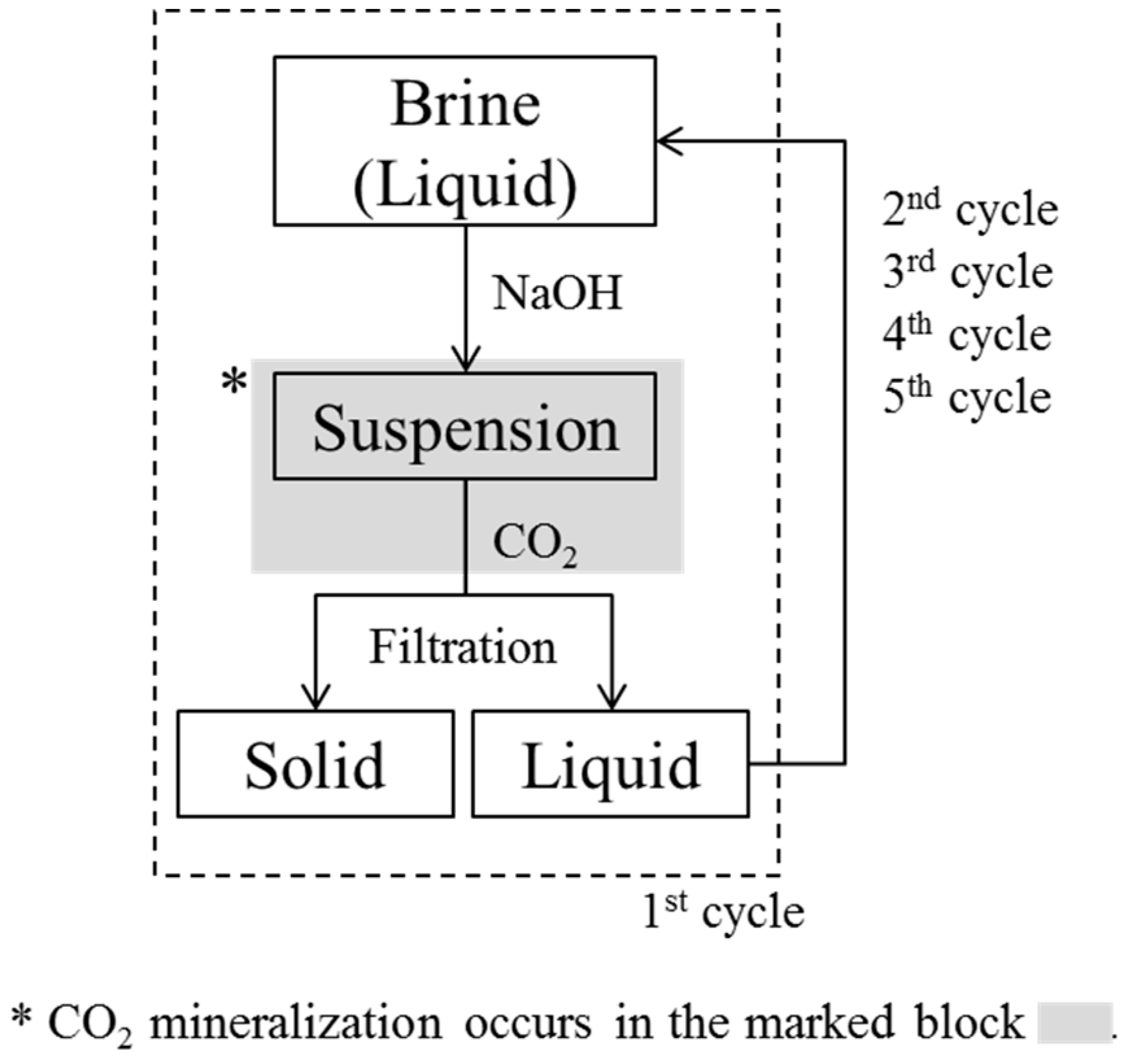
This paper introduces the first use of non-synthesized brine discharged from a functional seawater desalination plant for CO2 mineralization; we have particularly focused on the precipitation of Mg carbonates. Repeated cycles involved the pH adjustment of brine, and the injection of CO2 microbubbles showed that Mg carbonation could be achieved alongside Ca. Various analytical techniques were used for the identification of the precipitates, including X-ray diffraction (XRD) analysis. We have empirically proved that CO2 mineralization using brine for CO2 sequestration.
2. Experimental
The brine used in this study was collected from an ongoing seawater RO process for industrial water supplementation in Korea. The major cations and anions in this brine and their concentrations as measured by inductively coupled plasma atomic emission spectroscopy (ICP-OES; Optima 8300, PerkinElmer, Shelton, CT, USA) are listed in
Table 1. The concentrations of Sr, Si, Li, Cu, and rare earth elements such as W, Mo, and Rb were also measured, but the values obtained were not significant. Anion concentrations were measured by ion chromatography (IC; Dionex ICS-5000+, Thermo Fisher Scientific, Sunnyvale, CA, USA), and the SO
42− concentration was measured using IC equipped with an Ag cartridge (Dionex OnGuard II Ag, Thermo Fisher Scientific).
NaOH solution (1 M) was prepared for use as an alkaline chemical by dilution of NaOH (96%, Junsei Chemicals, Tokyo, Japan) with deionized water (Milli-Q Gradient-A10, Millipore, Billerica, MA, USA). The pH of 0.25 L of brine was increased from an initial value of 8.2 to 10–13.5 (measured by Orion Star A215, Thermo Scientific, Singapore) by adding the 1 M NaOH solution to the brine in a beaker with a lid at a rate of 4 mL/min using a peristaltic pump (EMP-2000W, EMS-tech, GyeongGi, Korea) at ambient temperature and pressure. The brine was stirred while the NaOH solution was added, and as the pH of the brine increased, a suspension of white precipitates was formed. Once the target pH was reached, the suspension was filtered with a 0.45 μm pore nylon membrane (Whatman, Brentford, UK) to allow the concentrations of the metal ions listed in
Table 1 to be measured by ICP-OES. However, only the concentrations of Mg, Ca, and Na changed with pH and, as such, only the concentrations of these three elements are reported in the results section.
In the cyclic CO
2 mineralization experiments, as 1 L of brine reached pH 10.5, we reacted the suspension with CO
2 (99%, Gasco) instantly, and monitored for further pH changes at atmospheric pressure. A jacket reactor was used for CO
2 mineralization, with a coolant at 1 °C being circulated between the walls by a chiller, and a lid being placed on the upper part of the reactor. As CO
2 microbubbles were injected into the suspension using a microbubble generator, the pH decreased rapidly, as has already been shown in the literature [
7,
26,
27]. CO
2 injection was stopped once the pH of the brine had decreased to 8. This was because we found that we were unable to collect the CO
2-reacted precipitates when CO
2 injection was stopped at pH 7, which was the value we had used in previous studies [
7,
26]. The final products, which consisted of precipitates and transparent solutions, were obtained by filtering the brine through a 0.45 μm nylon filter. The experimental cycle was then begun again, with the pH of the solution being increased to 10.5, CO
2 bubbling taking place, and the precipitate formed being filtered off. This cycle was repeated until no precipitates were obtained on addition of NaOH, which occurred during the 5th cycle. This indicated that there were no divalent ions such as Mg or Ca that were able to form hydroxides; thus, CO
2 bubbling did not lead to the formation of particles. Therefore, in our experiment, four cycles were sufficient to remove all divalent ions from the brine through the formation of particles. The experimental cycle is shown in
Figure 1. We decided not to consider the effect of dissolved CO
2 from atmosphere during the experiments, because we believed that Mg
2+, which was 3 times more abundant than Ca
2+ in the brine, would not be carbonated by atmospheric CO
2 under the experimental conditions.
After the reaction with CO2, the suspension was filtered, the concentrations of Mg, Ca, and Na in the filtered liquid samples were analyzed, and the filter cake was dried in an oven at 80 °C for 24 h, then characterized using XRD (X’pert MPD, Philips, EA Almelo, The Netherlands), a field emission scanning electron microscope (FESEM; JSM-7800 FPRIME, JEOL, Tokyo, Japan) equipped with an energy dispersive spectrometer (EDS; X-MaxN, Oxford), thermogravimetric analysis (TGA; DTG-60H, Shimadzu, Kyoto, Japan) at argon environment (10 °C/min heating rate), and Fourier transform infrared spectroscopy (FTIR; NICOLET 380, Thermo Fisher Scientific Inc., Costa Mesa, CA, USA).
3. Results and discussion
3.1. Concentrations of Mg, Ca, and Na in Brine with Different pH Values
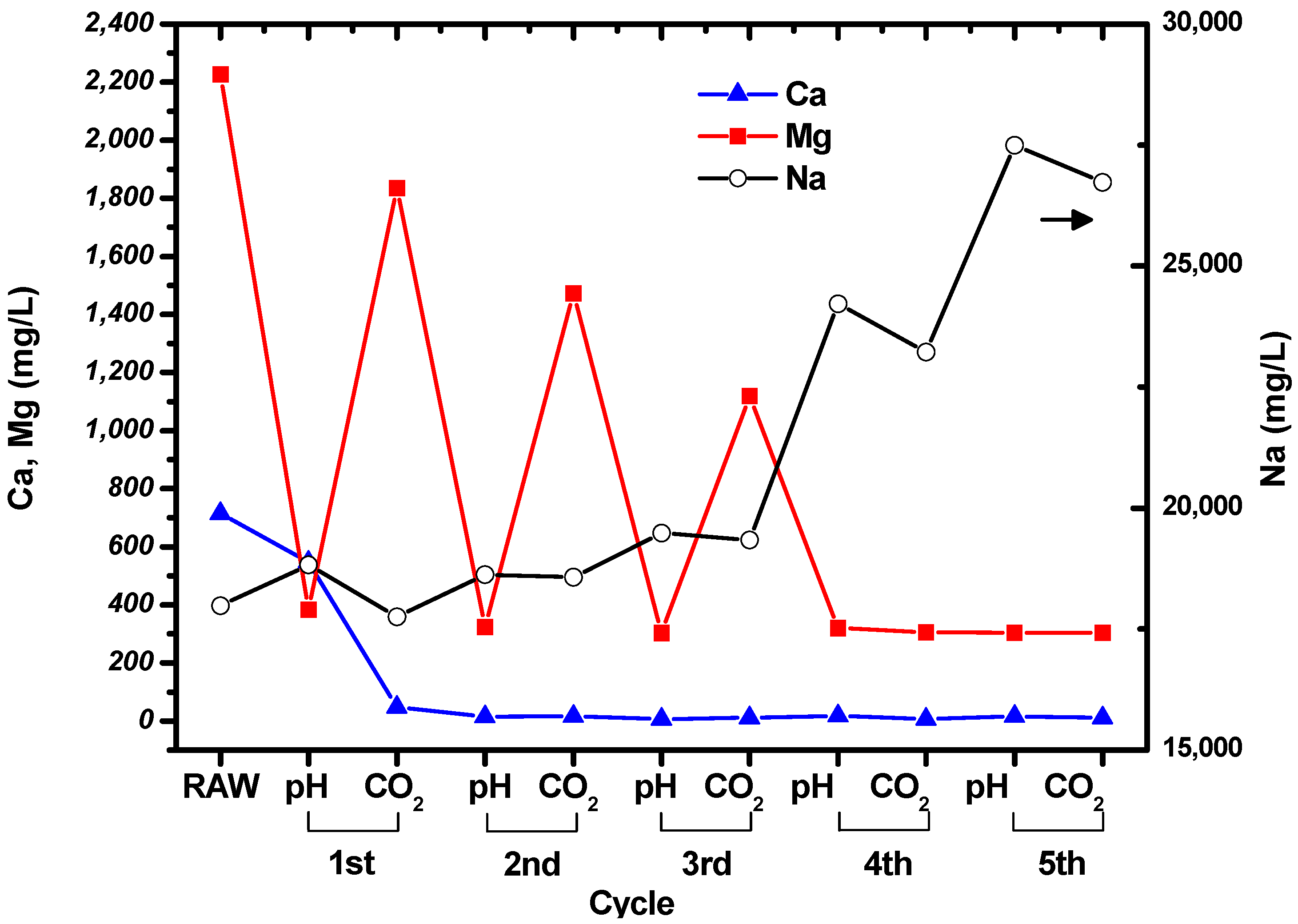
Initial experiments aimed to obtain information on the changes in the concentrations of Mg and Ca as the pH was varied from 10–13.5 by adding NaOH solution to brine. This resulted in the formation of a white suspension. The suspension thickened as the amount of NaOH solution added increased. ICP-OES was used to measure the concentrations of the ions; the results are shown in
Figure 2. The label “raw” on the horizontal axis represents the untreated brine (i.e., no NaOH solution had been added). The left vertical axis shows the concentrations of Mg and Ca, while the right vertical axis shows the concentration of Na. It can be seen that the concentrations of Mg and Ca were reproducible; however, the concentration of Na showed large standard deviations because the solutions were prepared for ICP-OES measurement by diluting them by a factor of 1000. It was expected that the concentration of Na would increase as the pH increased, owing to the addition of NaOH solution to the brine.
It is likely that as the measured concentrations of Mg and Ca decreased as NaOH was added to the brine, the Mg and Ca ions had formed their hydroxides. If a brine suspension with a specific pH resulted in a substantial reduction in the concentration of one ion, it would strongly suggest that this was the optimal pH for isolating Mg and Ca from each other.
CO
2 mineralization is an ionic reaction between CO
32− and (divalent) metal ions. CO
2 will change its form depending on the pH environment it is exposed to, as shown in the middle column of
Table 2. Therefore, increasing the pH is essential when attempting to enhance the transformation of injected gaseous CO
2 into its ionic form, CO
32−. The pH can be increased by the addition of alkaline agents, which will dissolve and yield OH
− in aqueous solutions. If a reactant contains metal ions, metal hydroxide particles will precipitate after reaction with existing OH
−. Consequently, there is an increase in pH results in the formation of metal hydroxides and ionization of CO
2. This is the reason that addition of NaOH is required.
When the pH was increased from 10 to 10.5, the concentration of Mg decreased by approximately 2000 mg/L, and when the pH was increased to 11, the concentration decreased by a further 180 mg/L. After this, the Mg concentration remained relatively constant up to pH 13.5. The concentration of Ca did not change significantly as the pH was varied. Consequently, we chose pH 10.5, to allow Mg from brine to form Mg(OH)2 without significantly altering the concentration of Ca. Relatively pure Mg(OH)2 can facilitate the production of pure Mg-carbonated particles by CO2 injection. This is the reason for the separation or isolation of Mg and Ca from each other by pH adjustment before CO2 injection.
We used the equations below by calculating the ion selectivity of Mg and Ca in hydroxide salts at pH 10.5 and 11.
According to Equation (1), at pH 10.5, 90% of Mg and 23% of Ca in brine precipitated as their hydroxides. As the pH was increased to 11, 99% of Mg and 29% of Ca had formed their hydroxides. Equation (1) seems to indicate that pH 11 would be more favorable for the recovery of Mg and Ca from brine. However, the precipitated ratio does not give enough information to allow a useful pH for the separation of Mg and Ca to be selected: in this study, CO
2 mineralization requires relatively pure Mg- and Ca-hydroxides to ensure that pure carbonated particles are obtained.
Equation (2) shows that at pH 10.5, the precipitated particles were composed of 92.3% Mg(OH)2 and 7.70% Ca(OH)2. Meanwhile, at pH 11, the precipitated particles were composed of 91.5% Mg(OH)2 and 8.50% Ca(OH)2. Therefore, the Mg(OH)2 obtained was purer at pH 10.5. In addition, adjusting the pH to 10.5 required 36.1 ± 0.5 mL of 1 M NaOH solution for 0.25 L brine, while adjusting to pH 11 required 42.0 ± 0.6 mL of 1 M NaOH solution. Therefore, increasing the pH from 10.5 to 11 required an additional 5.90 mL of NaOH solution: this would increase the operational cost for the process.
3.2. Bubbling CO2 through a Suspension at pH 10.5
CO
2 mineralization was performed by bubbling CO
2 microbubbles through a brine suspension at pH 10.5. In our previous studies on the precipitation of CaCO
3 by CO
2 mineralization, we found that as the pH of the suspension of Ca(OH)
2 into which CO
2 was injected approached neutral, all the particles had formed CaCO
3, leading us to regard this as the end of the reaction. We therefore discontinued CO
2 injection at this point [
7,
26]. On the basis of this experience, we initially intended to stop CO
2 injection when the pH of the brine approached neutral. However, in contrast to our previous experiments, we found that no particles were suspended at this neutral pH. We therefore decided to stop CO
2 injection at a higher pH of 8. CO
2 at 600 mL/min was injected for 4.5, 5, 7, 9 mins for four experimental cycles.
The concentrations of Mg, Ca, and Na in the suspension were measured at the end of each step in the cycle shown in
Figure 1. The measured results are shown in
Figure 3, and the concentrations of the ions in the raw, untreated brine are given for comparison. It can be seen that the concentrations of Mg and Ca decreased during the cycles, while the concentration of Na increased owing to the addition of NaOH solution. Filtration was used to collect the particles after the CO
2 bubbling step in each cycle.
The concentration of Ca decreased to tens of mg/L after the first cycle, and then remained constant. This indicates that all the Ca in the brine had precipitated. Interestingly, the concentrations of Mg fluctuated during the cycles. The concentration of Mg in the suspension decreased when the pH was increased to 10.5, and then increased as CO2 was bubbled through the brine. The Mg concentration at pH 10.5 remained constant at around ~300 mg/L, while the concentration after CO2 bubbling decreased as the number of cycles increased. It seems that precipitation of carbonated Mg particles was less favorable than precipitation of carbonated Ca particles. However, it can be seen from the Mg and Ca concentrations measured after CO2 bubbling that Mg carbonate precipitated during the cycles. No particles were formed after increasing the pH and bubbling CO2 through the solution during the fifth cycle.
Based on the brine that we used, 10 million tons of brine contains 22,260 tons of Mg and 7140 tons of Ca. If assume that Mg and Ca precipitate as MgCO3 and CaCO3, each ion ideally reacts with 40,306 and 7854 tons of CO2. According to the measured concentration of remained Mg and Ca in brine after the fourth cycle, 19,260 and 7080 tons of Mg and Ca react with 34,874 and 7788 tons of CO2, respectively. The estimated amount of MgCO3 and CaCO3 are 66,816 and 17,700 tons by CO2 mineralization.
3.3. Characterization of Suspended Particles from CO2 Mineralization
After each pH increasing/CO2-bubbling cycle, the particles in the suspensions were collected via filtration and characterized. It should be noted that too few particles were formed during the first cycle for full characterization to be carried out.
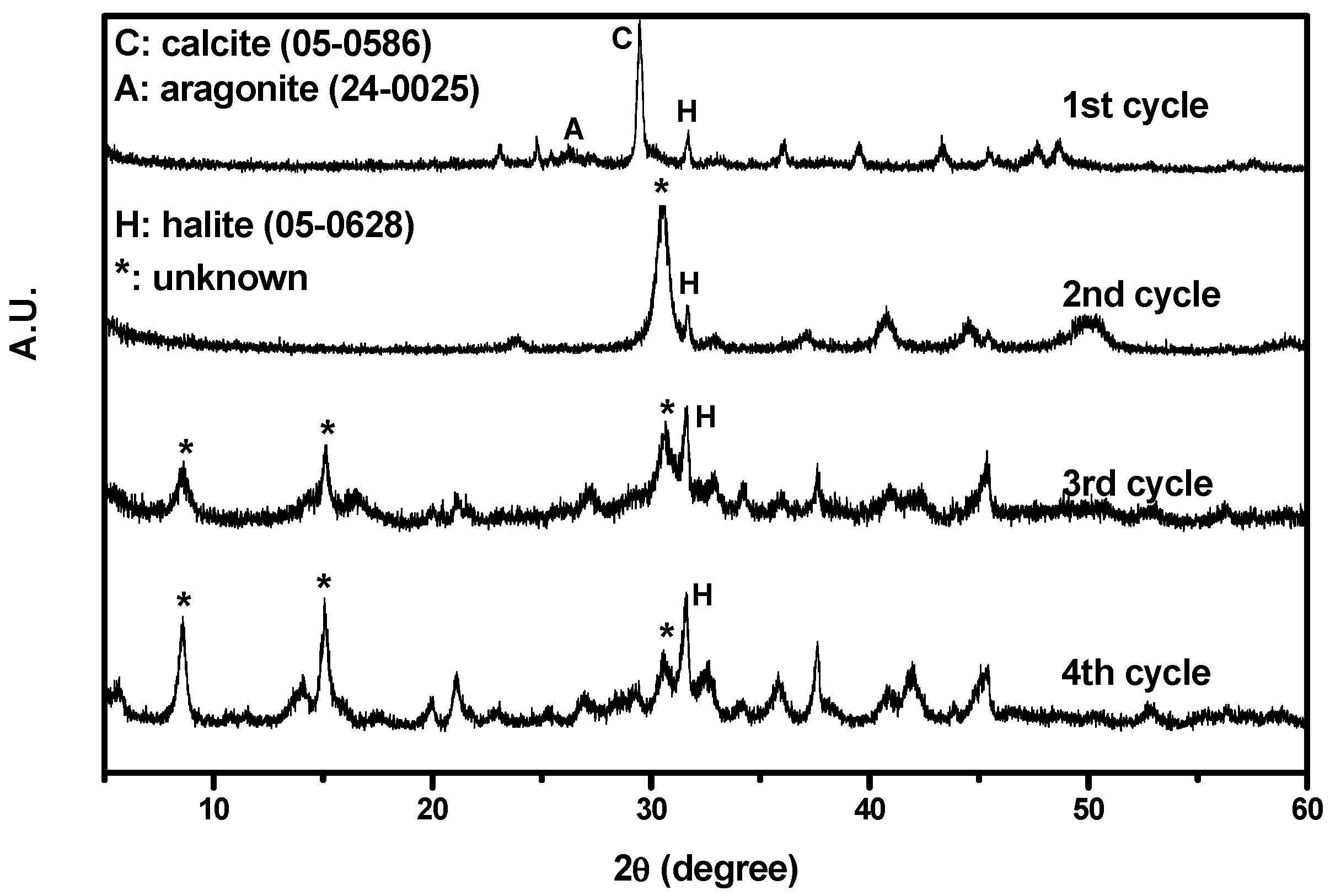
Figure 4 shows the XRD results. As depicted in the figure, the particles collected from the first cycle consisted of calcite and halite. In addition, some aragonite had crystallized owing to the coexistence of Mg and Ca ions [
28]. The CaCO
3 successfully precipitated from the non-synthesized brine during CO
2 mineralization. However, particles collected from other cycles could not be identified positively by standard Joint Committee on Powder Diffraction Standards (JCPDS). Only halite phase was identified. As the number of cycles increased, the halite signal became more intense.
Mg carbonates have been reported to form many crystal phases, depending on the conditions such as pressure, temperature, water content, and agitation of the reactant liquid phase [
29,
30,
31,
32]. We utilized other analytical methods that would provide information regarding the presence of Mg carbonates in the particles.
Elemental mapping by SEM-EDS revealed that the particles from the second, third, and fourth cycles consisted of Ca and Mg carbonates (
Figure 5). This shows that the Mg formed carbonates, despite the fact that the precipitation of Mg carbonates is less favorable than the precipitation of Ca carbonates because Mg carbonates are more soluble than Ca carbonates and hydrated Mg ions possess more energy [
33].
Figure 5 shows that the elemental distributions of Ca, Mg, C, and O were broadly similar. The second cycle produced Ca carbonate, while the third and fourth cycles produced Mg carbonate. The samples were prepared on silicon wafers to prevent the appearance of the carbon signal from the commonly used carbon adhesive tape.
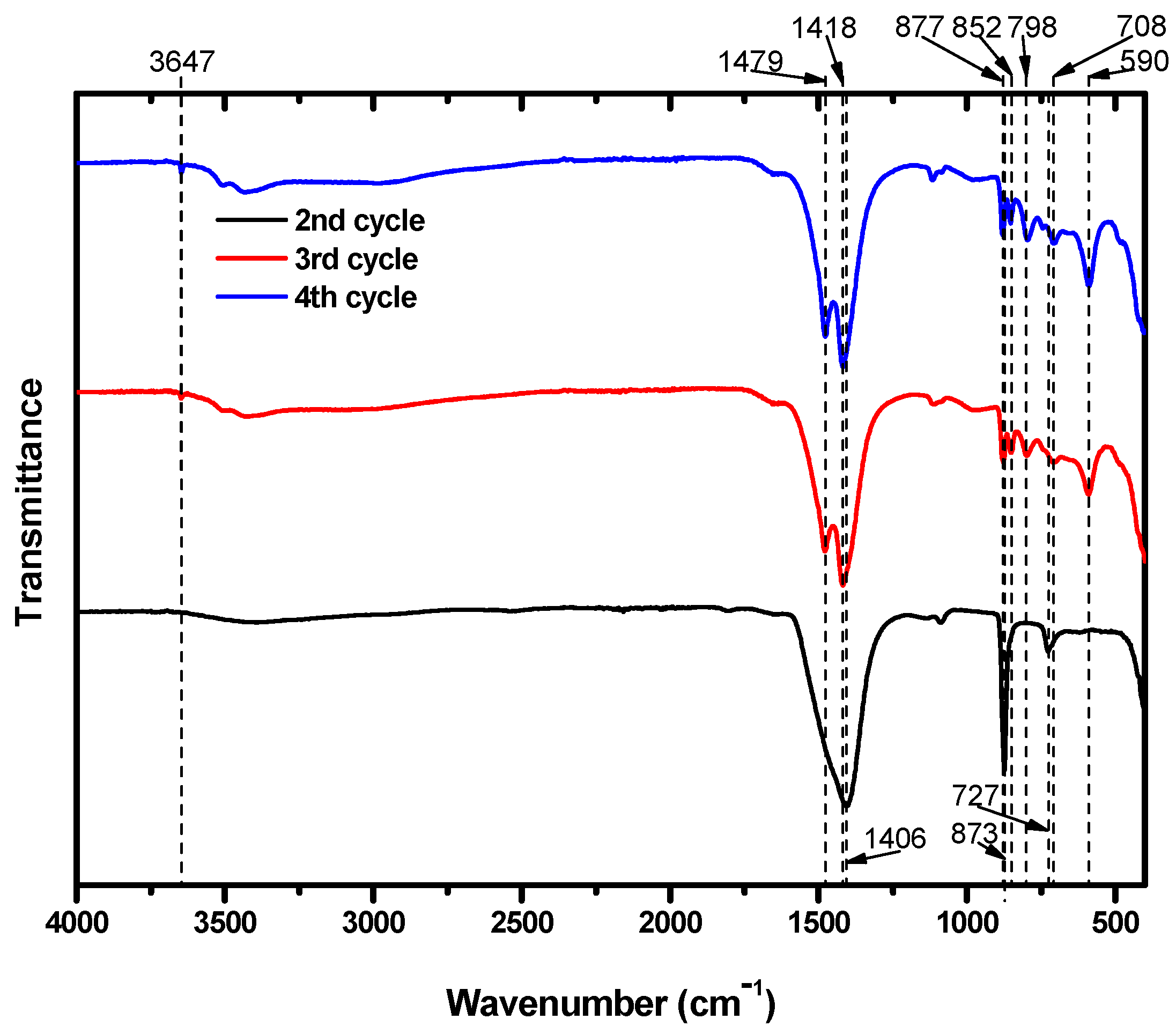
The particles were also subjected to FTIR analysis; the results are shown in
Figure 6. The particles that precipitated during the second cycle showed FTIR absorption bands at 727, 873, and 1406 cm
−1. Several research papers that identified Mg carbonates using FTIR analysis suggest that the peak at 727 cm
−1 was likely caused by the presence CaCO
3 in the structure. Long et al. [
34] reported that peaks at 724, 726, and 730 cm
−1 were obtained from calcite particles including 30 mol% Mg. Similarly, Gunasekaran et al. [
34,
35] and Xu and Poduska [
36] reported dolomite peaks at 729 and 730 cm
−1, respectively. These peaks arose from CO
32−. Consequently, it is possible to assign the IR peak at 727 cm
−1 to Mg carbonates. In addition, the peaks at 873 and 1406 cm
−1 were from the CO
32− ions in CaCO
3 [
35,
37], which was already known to be present due to XRD analysis.
The particles obtained after the third and fourth cycles both showed peaks at 852 and 877 cm
−1. In these cycles, both Ca and Mg carbonates formed. The peak at 852 cm
−1 was due to calcite that included Mg. Long et al. [
34] reported that calcite including Mg at 20 and 39 mol% absorbed IR at 852 cm
−1. CO
32− ions in dolomite also absorbed IR near this position, at 853 cm
−1. Meanwhile, the peak at 877 cm
−1 indicates the presence of CaCO
3 [
35,
36,
38,
39,
40]. Although we expected that the third and fourth cycles would produce Mg carbonates that excluded Ca, some of the Ca remaining in the suspension after the second cycle managed to participate in the carbonation of Mg. The peaks at 1418 and 1479 cm
−1 are typical absorption peaks of hydromagnesite. In addition, the peak at 3647 cm
−1 provides further evidence of the presence of hydromagnesite. Mg from brine successfully precipitated as hydromagnesite, indicating that brine could be a useful resource for CO
2 mineralization.
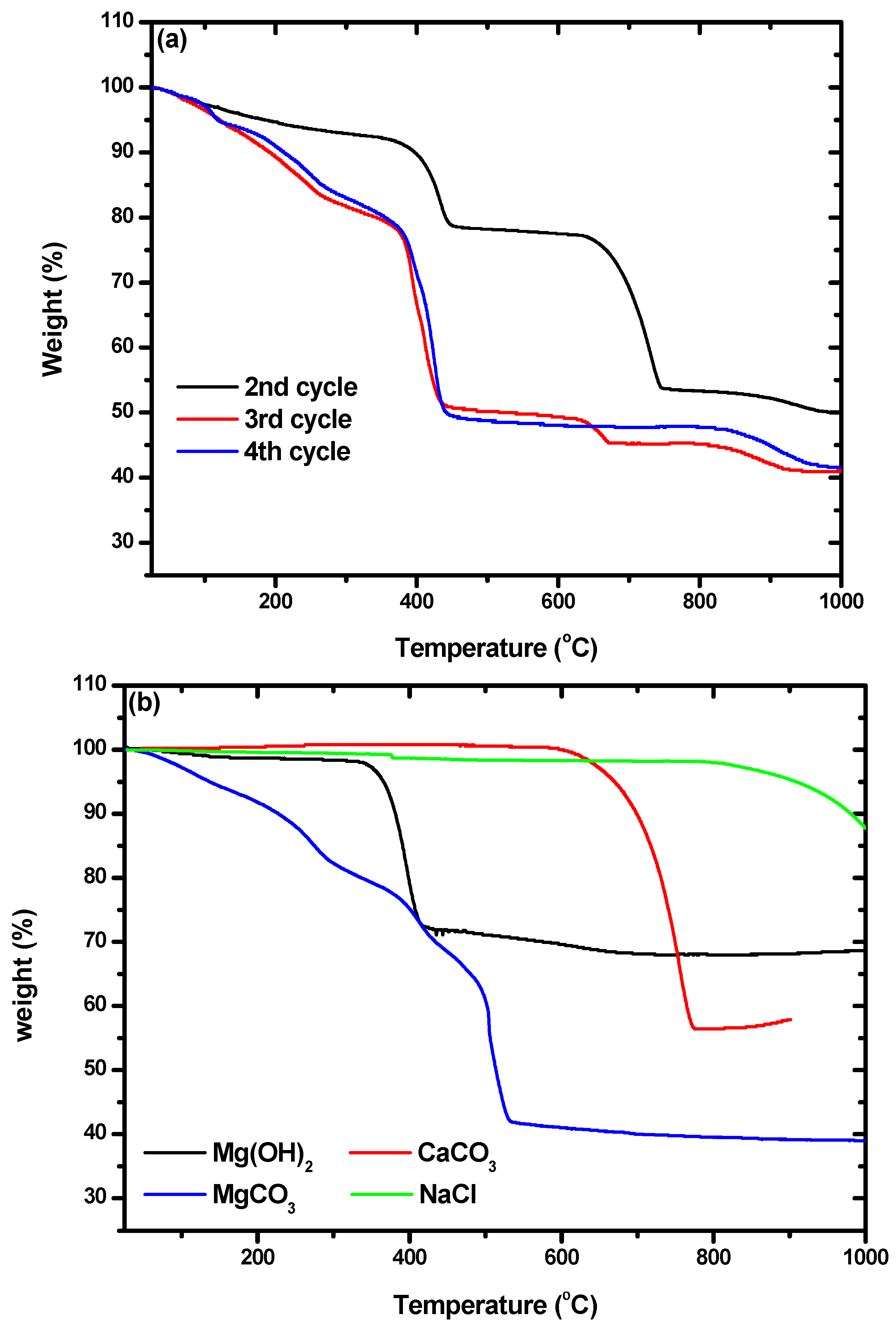
In addition, characterization by TGA was used to confirm the presence of hydromagnesite. The weight losses of the particles we obtained, shown in
Figure 7a, were compared with those of commercial Mg(OH)
2, CaCO
3, MgCO
3, and NaCl, which are shown in
Figure 7b. Weight loss due to Mg(OH)
2 occurred between 328 and 422 °C. Losses at 589–778 °C and 180–524 °C were due to CaCO
3 and hydromagnesite, respectively. By comparing
Figure 7a,b, it can be seen that the particles from the second cycle were composed of Mg(OH)
2 and CaCO
3. A small amount of NaCl was included in all the particles, as shown by the weight losses that began at 810 °C. The TGA curves of the particles from the third and fourth cycles were quite different from that obtained for the particles from the second cycle. The weight losses at ~450 °C in the curves of the particles from the third and fourth cycles did not quite match with what would be expected for the loss of hydromagnesite. Montes-Hermandez et al. [
41] synthesized hydromagnesite and characterized particles of it using TGA. They found that hydromagnesite exhibited weight loss at 450 °C with some fluctuation at around 375 °C, which precisely matched the results shown for the particles from the third and fourth cycles in
Figure 7a. There were also some differences between the TGA curves obtained for the particles from the third and fourth cycles. The curve for cycle three indicated an additional weight loss of 4.5% in the range of 635–665 °C, which indicates the coexistence of calcite and hydromagnesite. As the number of cycles increased, the purity of the hydromagnesite that precipitated increased.
4. Conclusions
Non-synthesized brine from a functional RO seawater desalination plant was used for CO2 mineralization to fix CO2 under ambient temperature and pressure. To the best of our knowledge, this is the first time that non-synthesized brine has been used for CO2 mineralization. Cycles consisting of an increase in pH followed by CO2 injection and finally filtration precipitated 99% of Ca and 86% of Mg from the brine, using them to fix CO2 in the form of carbonated minerals. CO2 was successfully mineralized with Ca to yield CaCO3 and with Mg to produce hydromagnesite. This was shown by the characterization of the precipitates obtained from the cycles by XRD, SEM-EDS, FTIR, and TGA-DTA.
It was thought that co-presence of Ca with Mg was not favorable for the precipitation of Mg carbonates. In this study, we precipitated hydromagnesite followed by precipitation of calcite from brine. This means that the concentrations of Mg and Ca in brine are twice those in influent seawater, making it a good source of divalent ions for CO2 mineralization. According to our experiments, 42,662 tons of CO2 could be sequestered from 10 million tons of the brine used for a year. Moreover, 17,700 tons of CaCO3 and 66,816 tons of MgCO3 would be obtained.