Adsorption of Scandium and Neodymium on Biochar Derived after Low-Temperature Pyrolysis of Sawdust
Abstract
:1. Introduction
2. Materials and Methods
2.1. Adsorbents
2.2. Geochemical Modelling
2.3. Kinetic and Equilibrium Experiments
3. Results and Discussion
3.1. Characterization of Biochar
3.1.1. Properties
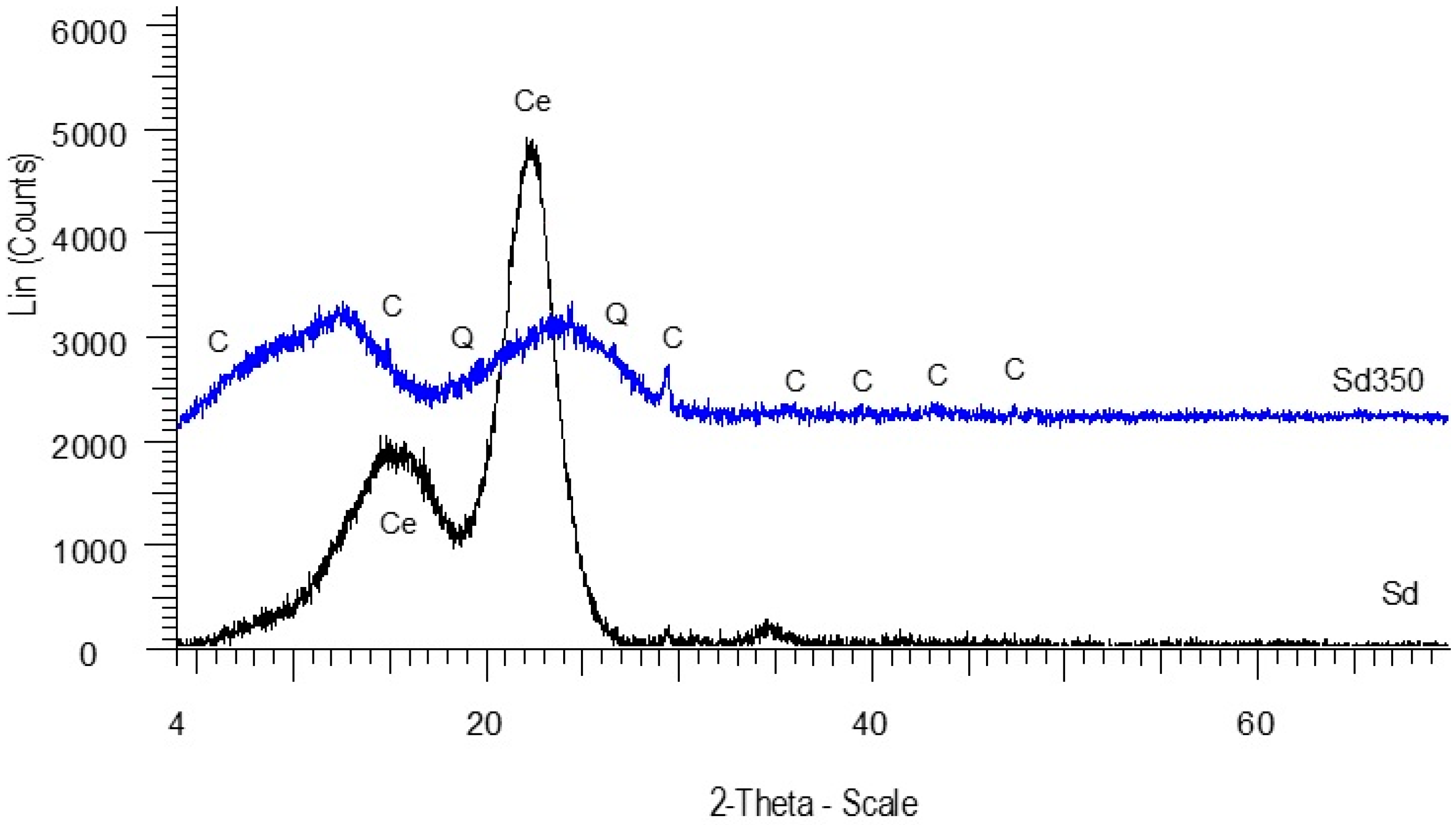
3.1.2. Mineralogical Analysis
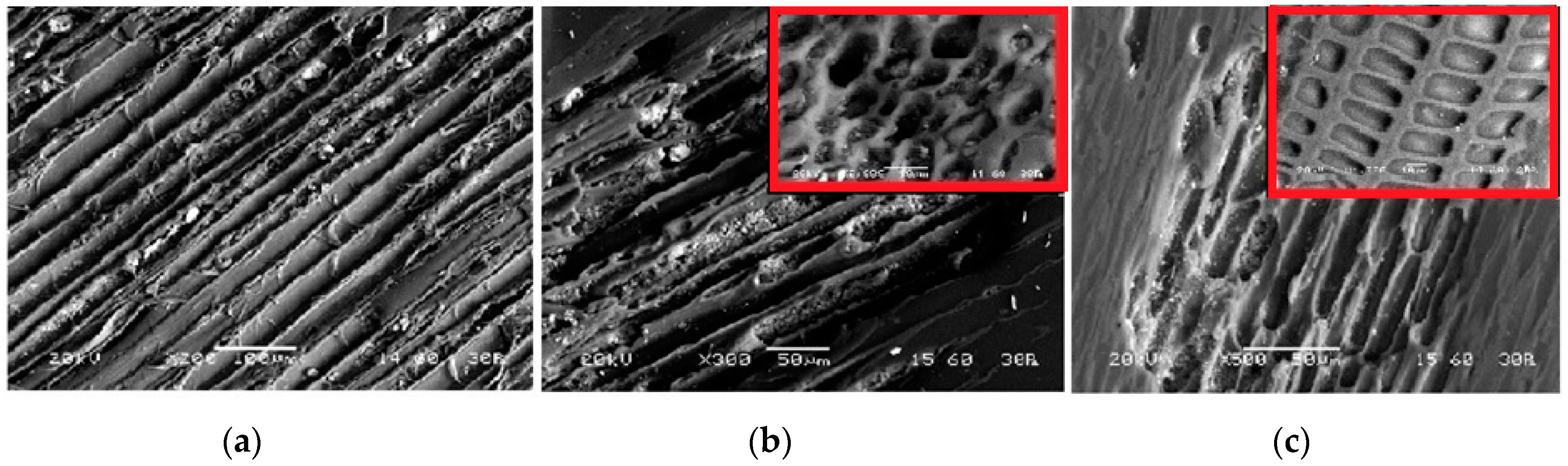
3.1.3. Morphology
3.1.4. Thermogravimetric Analysis
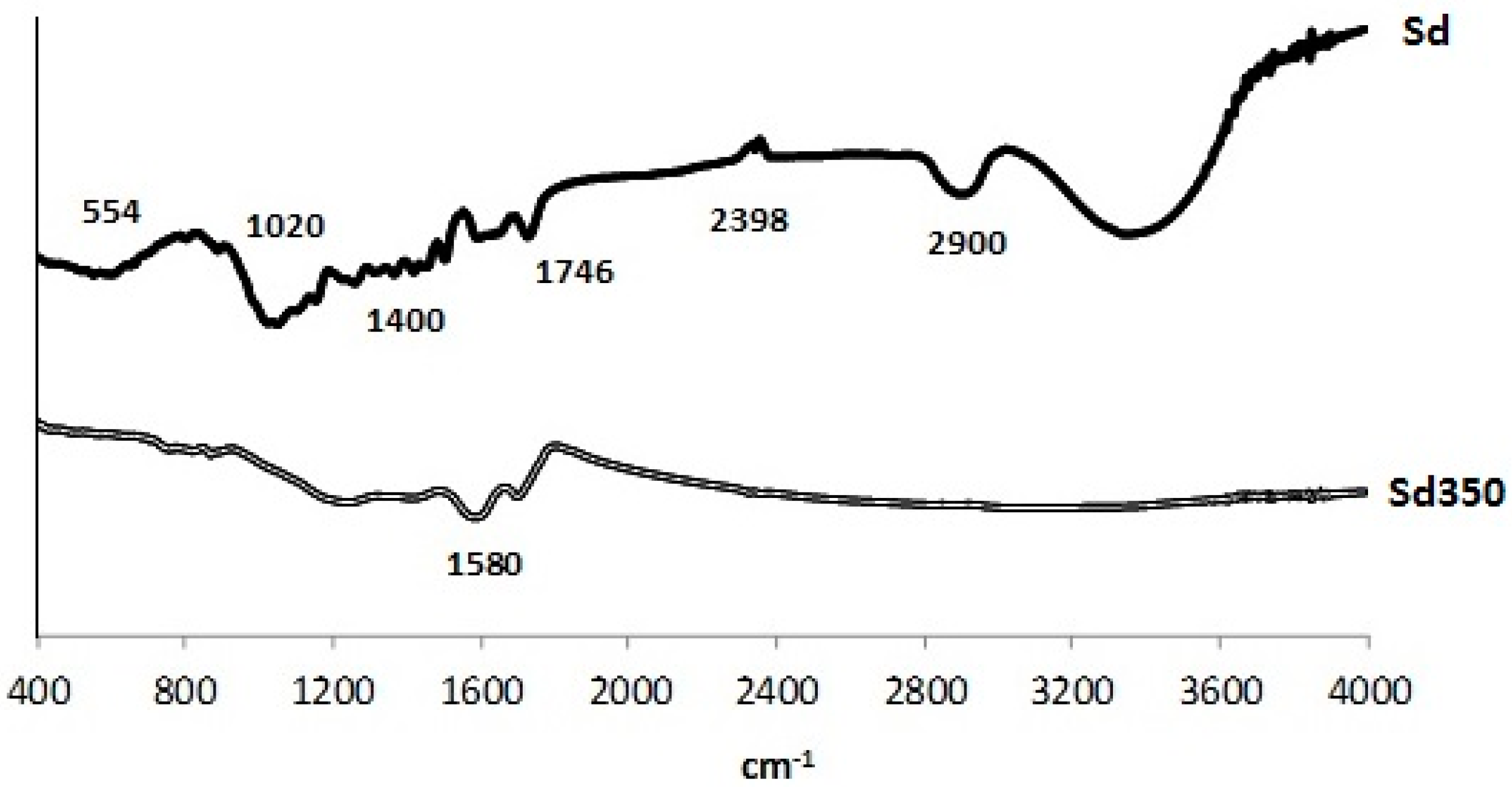
3.1.5. FTIR Analysis
3.1.6. pHPZC
3.2. Speciation Studies: Identification of Potential Solubility-Controlling Phases
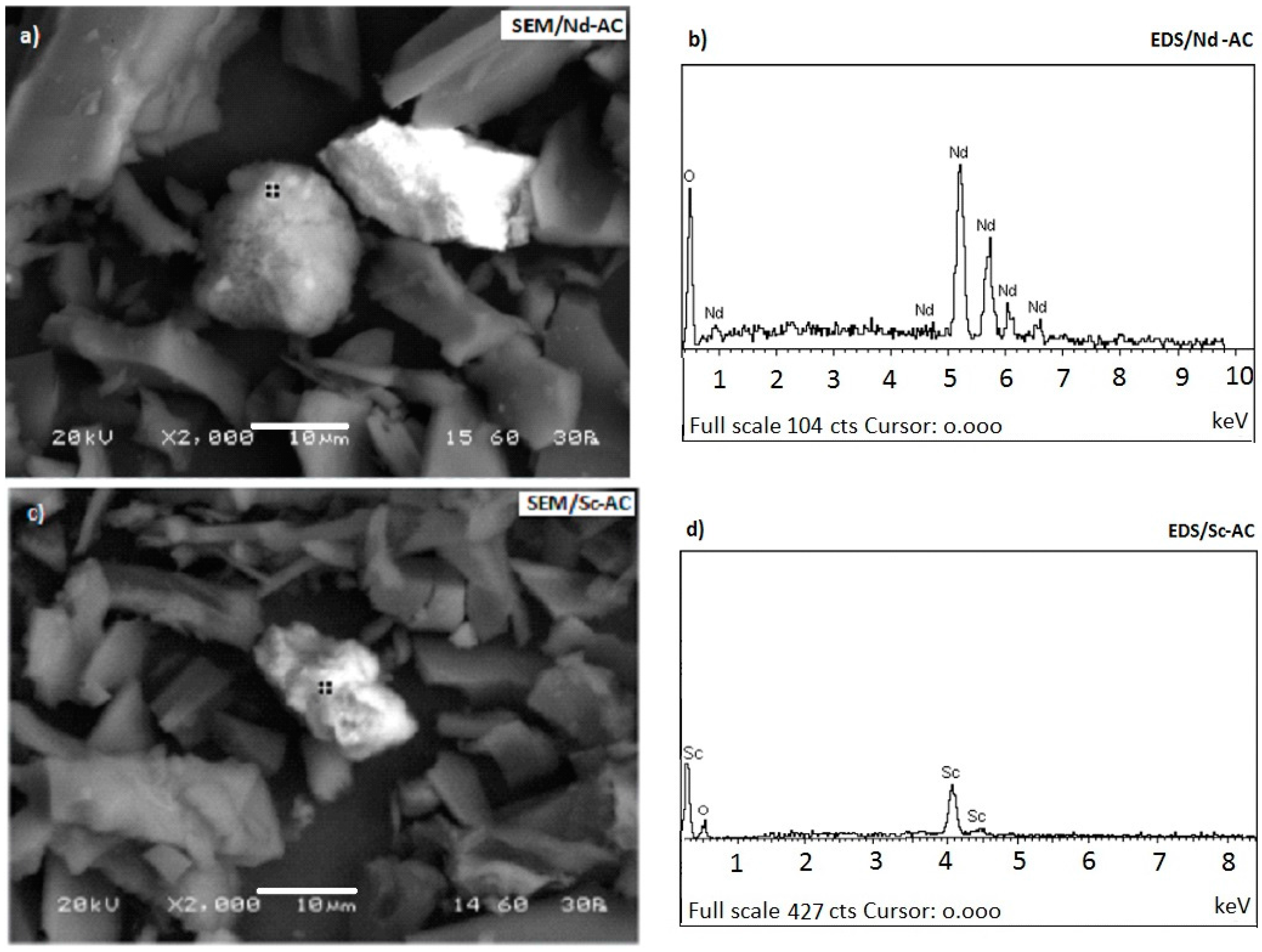
3.3. Morphology of the Adsorbents
3.4. Determination of Adsorbents Efficiency
3.4.1. Kinetic Studies
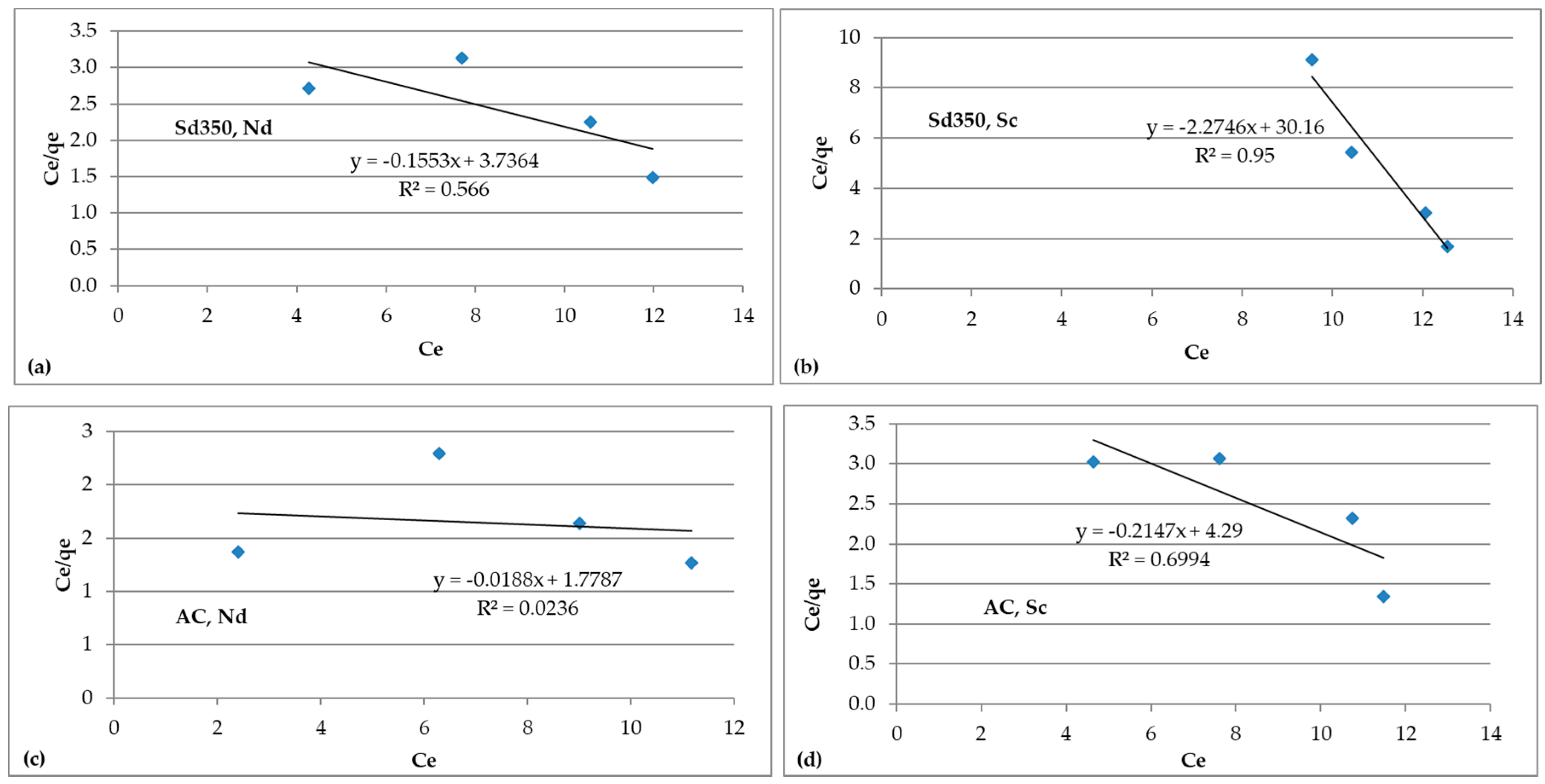
3.4.2. Isotherm Models
3.4.3. Adsorption Mechanism
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Massari, S.; Ruberti, M. Rare earth elements as critical raw materials: Focus on international markets and future strategies. Resour. Policy 2013, 38, 36–43. [Google Scholar] [CrossRef]
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions on the 2017 List of Critical Raw Materials for the EU, Brussels, 13.9.2017, COM(2017) 490 Final. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52017DC0490&from=EN (accessed on 10 October 2017).
- Kennedy, J. Rare earth production, regulatory USA/International constraints and Chinese dominance; The economic viability is bounded by geochemistry and value chain integration. In Rare Earths Industry: Technological, Economic, and Environmental Implications; De Lima, I.B., Leal, W., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 37–55. [Google Scholar]
- Kamenopoulos, S.; Agioutantis, Z.; Komnitsas, K. A new Hybrid Decision Support Tool for evaluating the sustainability of mining projects. Int. J. Min. Sci. Technol. 2017. [Google Scholar] [CrossRef]
- ERECON (European Rear Earth Competency Network). Strengthening the European Rare Earths Supply Chain: Challenges and Policy Options. Kooroshy, J., Tiess, G., Tukker, A., Walton, A., Eds.; 2015. Available online: http://reinhardbuetikofer.eu/wp-content/uploads/2015/03/ERECON_Report_v05.pdf (accessed on 14 August 2017).
- Haque, N.; Hughes, A.; Lim, S.; Vernon, C. Rare earth elements: Overview of mining, mineralogy, uses, sustainability and environmental impact. Resources 2014, 3, 614–635. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Van Acker, K.; Blanpain, B.; Mishra, B.; Apelian, D. Rare-earth economics: The balance problem. JOM 2013, 65, 846–848. [Google Scholar] [CrossRef]
- Kiggins, R.D. The Strategic and Security Implications of Rare Earths. In The Political Economy of Rare Earth Elements: Rising Powers and Technological Change; Kiggins, R.D., Ed.; Palgrave Macmillan: Basingstoke, UK, 2015; pp. 1–19. ISBN 978-1-137-36424-1. [Google Scholar]
- Manyà, J. Pyrolysis for Biochar Purposes: A Review to Establish Current Knowledge Gaps and Research Needs. Environ. Sci. Technol. 2012, 46, 7939–7954. [Google Scholar] [CrossRef] [PubMed]
- Moussavi, G.; Khosravi, R. Preparation and characterization of a biochar from pistachio hull biomass and its catalytic potential for ozonation of water recalcitrant contaminants. Bioresour. Technol. 2012, 119, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Agrafioti, E.; Kalderis, D.; Diamadopoulos, E. Arsenic and chromium removal from water using biochars derived from rice husk, organic solid wastes and sewage sludge. J. Environ. Manag. 2014, 133, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Kung, C.-C.; Kong, F.; Choi, Y. Pyrolysis and biochar potential using crop residues and agricultural wastes in China. Ecol. Indic. 2015, 51, 139–145. [Google Scholar] [CrossRef]
- Cole, A.J.; Paul, N.A.; de Nys, R.; Roberts, D.A. Good for Sewage Treatment and Good for Agriculture: Algal Based Compost and Biochar. J. Environ. Manag. 2017, 200, 105–113. [Google Scholar] [CrossRef] [PubMed]
- McHenry, M.P. Agricultural bio-char production, renewable energy generation and farm carbon sequestration in Western Australia: Certainty, uncertainty and risk. Agr. Ecosyst. Environ. 2009, 129, 1–7. [Google Scholar] [CrossRef]
- Ding, W.; Dong, X.; Ime, I.M.; Gao, B.; Ma, L.Q. Pyrolytic temperatures impact lead sorption mechanisms by bagasse biochars. Chemosphere 2014, 105, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.; Gan, J. Comparing black carbon types in sequestering polybrominated diphenyl ethers (PBDEs) in sediments. Environ. Pollut. 2014, 184, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Rajapaksha, A.U.; Chen, S.S.; Tsang, D.C.W.; Zhang, M.; Vithanage, M.; Mandal, S.; Gao, B.; Bolan, N.S.; Ok, Y.S. Engineered/designer biochar for contaminant removal/immobilization from soil and water: Potential and implication of biochar modification. Chemosphere 2016, 148, 276–291. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.A.; Di Rauso Simeone, G.; Scelza, R.; Pellegrino Conte, P. Biochar based remediation of water and soil contaminated by phenanthrene and pentachlorophenol. Chemosphere 2017, 186, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Liu, D.; Gao, F.; Li, M.; Luo, X. Effects of biochar-derived sewage sludge on heavy metal adsorption and immobilization in soils. Int. J. Environ. Res. Public Health 2017, 14, 681. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Babu, J.N.; Kumar, R.; Srivastava, P.; Singh, P.; Raghubanshi, A.S. Multifaceted application of crop residue biochar as a tool for sustainable agriculture: An ecological perspective. Ecol. Eng. 2015, 77, 324–347. [Google Scholar] [CrossRef]
- Komnitsas, K.; Doula, M. Framework to improve sustainability of agriculture in small islands: The case of Pistacia vera L. cultivation in Aegina, Greece. Environ. Forensics 2017, 18, 1–12. [Google Scholar] [CrossRef]
- Qambrani, N.A.; Rahman, M.M.; Won, S.; Shim, S.; Ra, C. Biochar properties and eco-friendly applications for climate change mitigation, waste management, and wastewater treatment: A review. Renew. Sustain. Energy Rev. 2017, 79, 255–273. [Google Scholar] [CrossRef]
- Bartzas, G.; Komnitsas, K. Life cycle assessment of FeNi production in Greece: A case study. Resour. Conserv. Recycl. 2015, 105, 113–122. [Google Scholar] [CrossRef]
- Mousa, E.; Wang, C.; Riesbeck, J.; Larsson, M. Biomass applications in iron and steel industry: An overview of challenges and opportunities. Renew. Sustain. Energy Rev. 2016, 65, 1247–1266. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D.; Pyliotis, I.; Vamvuka, D.; Bartzas, G. Assessment of pistachio shell biochar quality and its potential for adsorption of heavy metals. Waste Biomass Valor. 2015, 6, 805–816. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D.; Bartzas, G.; Kaliakatsou, G.; Kritikaki, A. Efficiency of pecan shells and sawdust biochar on Pb and Cu adsorption. Desalin. Water Treat. 2016, 57, 3237–3246. [Google Scholar] [CrossRef]
- Higashikawa, F.S.; Conz, R.F.; Colzato, M.; Cerri, C.E.P.; Alleoni, L.R.F. Effects of feedstock type and slow pyrolysis temperature in the production of biochars on the removal of cadmium and nickel from water. J. Clean. Prod. 2016, 137, 965–972. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Inyang, M.; Zimmerman, A.R.; Cao, X.; Pullammanappallil, P.; Yang, L. Removal of phosphate from aqueous solution by biochar derived from anaerobically digested sugar beet tailings. J. Hazard. Mater. 2011, 190, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Dong, H.; Yang, C.; Zhang, Q.; Liao, C.; Zha, F.; Gao, L. Application of goethite modified biochar for tylosin removal from aqueous solution. Colloids Surf. A 2016, 502, 81–88. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D. Morphology of modified biochar and its potential for phenol removal from aqueous solutions. Front. Environ. Sci. 2016, 4, 26. [Google Scholar] [CrossRef]
- Shimabuku, K.K.; Kearns, J.P.; Martinez, J.E.; Mahoney, R.B.; Moreno-Vasquez, L.; Summers, R.S. Biochar sorbents for sulfamethoxazole removal from surface water, stormwater, and wastewater effluent. Water Res. 2016, 96, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Pittman, C.U.; Bricka, M.; Smith, F.; Yancey, B.; Mohammad, J.; Steele, P.H.; Alexandre-Franco, M.F.; Gómez-Serrano, V.; Gong, H. Sorption of arsenic, cadmium, and lead by biochars produced from fast pyrolysis of wood and bark during bio-oil production. J. Colloid Interface Sci. 2007, 310, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Baral, N.R.; Shah, A. Techno-economic analysis of utilization of stillage from a cellulosic biorefinery. Fuel Process. Technol. 2017, 166, 59–68. [Google Scholar] [CrossRef]
- Smebye, A.B.; Sparrevik, M.; Schmidt, H.P.; Cornelissen, G. Life-cycle assessment of biochar production systems in tropical rural areas: Comparing flame curtain kilns to other production methods. Biomass Bioenerg. 2017, 101, 35–43. [Google Scholar] [CrossRef]
- Ogata, T.; Narita, H.; Tanaka, M. Adsorption behavior of rare earth elements on silica gel modified with diglycol amic acid. Hydrometallurgy 2015, 152, 178–182. [Google Scholar] [CrossRef]
- Smith, Y.R.; Bhattacharyya, D.; Willhard, T.; Misra, M. Adsorption of aqueous rare earth elements using carbon black derived from recycled tires. Chem. Eng. J. 2016, 296, 102–111. [Google Scholar] [CrossRef]
- Sun, X.; Luo, H.; Mahurin, S.M.; Liu, R.; Hou, X.; Dai, S. Adsorption of rare earth ions using carbonized polydopamine nano carbon shells. J. Rare Earths 2016, 34, 77–82. [Google Scholar] [CrossRef]
- Zhao, F.; Repo, E.; Meng, Y.; Wang, X.; Yin, D.; Sillanpää, M. An EDTA-β-cyclodextrin material for the adsorption of rare earth elements and its application in preconcentration of rare earth elements in seawater. J. Colloid Interface Sci. 2016, 465, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Atrees, M.S.; Metwally, E.; Demerdash, M.; Salem, H. Sorption behavior of Pr and Nd upon chitosan benzoyl thiourea derivatives. J. Radiat. Res. Appl. Sci. 2016, 9, 207–216. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, S.; Zhao, K.; Wang, Z.; Xu, S.; Liang, Z.; Wu, K. Adsorption of La3+ and Ce3+ by poly-γ-glutamic acid crosslinked with polyvinyl alcohol. J. Rare Earths 2015, 33, 884–891. [Google Scholar] [CrossRef]
- Park, D.M.; Reed, D.W.; Yung, M.C.; Eslamimanesh, A.; Lencka, M.M.; Anderko, A.; Fujita, Y.; Riman, R.E.; Navrotsky, A.; Jiao, Y. Bioadsorption of rare earth elements through cell surface display of lanthanide binding tags. Environ. Sci. Technol. 2016, 50, 2735–2742. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhan, F.; Li, L.; Zhang, K. Adsorption rare earth metal ions from aqueous solution by polyamidoamine dendrimer functionalized soy hull. Waste Biomass Valor. 2016, 7, 1211–1219. [Google Scholar] [CrossRef]
- Liu, C.; Yan, C.; Zhou, S.; Ge, W. Fabrication of sponge biomass adsorbent through UV-induced surface-initiated polymerization for the adsorption of Ce(III) from wastewater. Water Sci. Technol. 2017, 75, 2755–2764. [Google Scholar] [CrossRef] [PubMed]
- Anastopoulos, I.; Bhatnagar, A.; Lima, E.C. Adsorption of rare earth metals: A review of recent literature. J. Mol. Liq. 2016, 221, 954–962. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Lu, H.-H.; Liu, Y.-X.; Yang, S.-M. Ammonium citrate-modified biochar: An adsorbent for La(III) ions from aqueous solution. Colloids Surf. A 2016, 509, 550–563. [Google Scholar] [CrossRef]
- Hadjittofi, L.; Charalambous, S.; Pashalidis, I. Removal of trivalent samarium from aqueous solutions by activated biochar derived from cactus fibres. J. Rare Earths 2016, 34, 99–104. [Google Scholar] [CrossRef]
- Mahmood, T.; Saddique, M.T.; Naeem, A.; Westerhoff, P.; Mustafa, S.; Alum, A. Comparison of Different Methods for the Point of Zero Charge Determination of NiO. Ind. Eng. Chem. Res. 2011, 50, 10017–10023. [Google Scholar] [CrossRef]
- Suliman, W.; Harsh, J.B.; Abu-Lail, N.I.; Fortuna, A.-M.; Dallmeyer, I.; Garcia-Perez, M. Modification of biochar surface by air oxidation: Role of pyrolysis temperature. Biomass Bioenerg. 2016, 85, 1–11. [Google Scholar] [CrossRef]
- Parkhurst, D.L.; Appelo, C.A.J. Description of Input and Examples for PHREEQC Version 3-A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations; U.S. Geological Survey Techniques and Methods; U.S. Geological Survey: Reston, WV, USA, 2013; Book 6, Chapter A43; p. 497. Available online: https://pubs.usgs.gov/tm/06/a43/ (accessed on 12 April 2017).
- Haynes, W.M.; Bruno, T.J.; Lide, D.R. Section 4: Properties of the Elements and Inorganic Compounds. In CRC Handbook of Chemistry and Physics, 96th ed.; Haynes, W.M., Ed.; CRC Press/Taylor and Francis: New York, NY, USA, 2015; pp. 4–145. ISBN 1-4822-6096-4. [Google Scholar]
- Horovitz, C.T. Biochemistry of Scandium and Yttrium, Part 1: Physical and Chemical Fundamentals; Springer Science: New York, USA, 1999; p. 313. ISBN 978-1-4615-4313-8. [Google Scholar]
- Jordan, D.S.; Saslow, S.A.; Geiger, F.M. Exponential sensitivity and speciation of Al(III), Sc(III), Y(III), La(III), and Gd(III) at fused silica/water interfaces. J. Phys. Chem. A 2011, 115, 14438–14445. [Google Scholar] [CrossRef] [PubMed]
- Martell, A.E.; Smith, R.M.; Motekaitis, R.J. NIST Critically Selected Stability Constants of Metal Complexes Database 46; Version 8; Standard Reference Data Program; National Institute of Standards and Technology/U.S. Department of Commerce: Gaithersburg, MD, USA, 2004.
- Komnitsas, K.; Bartzas, G.; Paspaliaris, I. Modeling of Reaction Front Progress in Fly Ash Permeable Reactive Barriers. Environ. Forensics 2006, 7, 219–231. [Google Scholar] [CrossRef]
- Ho, Y.-S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Ho, Y.-S. Review of second-order models for adsorption systems. J. Hazard. Mater. 2006, B136, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Gaskin, J.W.; Steiner, C.; Harris, K.; Das, K.C.; Bibens, B. Effect of low-temperature pyrolysis conditions on biochar for agricultural use. Trans. ASABE 2008, 51, 2061–2069. [Google Scholar] [CrossRef]
- Dai, Z.; Meng, J.; Muhammad, N.; Liu, X.; Wang, H.; He, Y.; Brookes, P.C.; Xu, J. The potential feasibility for soil improvement, based on the properties of biochars pyrolyzed from different feedstocks. J. Soils Sediments 2013, 13, 989–1000. [Google Scholar] [CrossRef]
- Gray, M.; Johnson, M.G.; Dragila, M.I.; Kleber, M. Water uptake in biochars: The roles of porosity and hydrophobicity. Biomass Bioenerg. 2014, 61, 196–205. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies, 3rd ed.; John Wiley & Sons Ltd.: Chistester, UK, 2001; ISBN 978-0-470-09307-8. [Google Scholar]
- Kosmulski, M. pH-dependent surface charging and points of zero charge. IV. Update and new approach. J. Colloid Interface Sci. 2009, 337, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Charrua, A.B.; Weng, C.-H.; Yuan, X.; Ding, F. Characterization of biochars derived from agriculture wastes and their adsorptive removal of atrazine from aqueous solution: A comparative study. Bioresour. Technol. 2015, 198, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Kołodyńska, D.; Krukowska, J.; Thomas, P. Comparison of sorption and desorption studies of heavy metal ions from biochar and commercial active carbon. Chem. Eng. J. 2017, 307, 353–363. [Google Scholar] [CrossRef]
- Mondal, S.; Bobde, K.; Aikat, K.; Halder, G. Biosorptive uptake of ibuprofen by steam activated biochar derived from mung bean husk: Equilibrium, kinetics, thermodynamics, modeling and eco-toxicological studies. J. Environ. Manag. 2016, 182, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Bentouhami, E.; Bouet, G.M.; Meullemeestre, J.; Vierling, F.; Khan, M.A. Physicochemical study of the hydrolysis of rare-earth elements (III) and thorium (IV). C. R. Chim. 2004, 7, 537–545. [Google Scholar] [CrossRef]
- Bruque, S.; Mozas, T.; Rodriguez, A. Factors influencing retention of lanthanide ions by montmorillonite. Clay Miner. 1980, 15, 413–420. [Google Scholar] [CrossRef]
- Moldoveanu, G.A.; Papangelakis, V.G. Recovery of rare earth elements adsorbed on clay minerals: II. Leaching with ammonium sulfate. Hydrometallurgy 2013, 131, 158–166. [Google Scholar] [CrossRef]
- Mohan, D.; Sarswat, A.; Ok, Y.S.; Pittman, C.U., Jr. Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent—A critical review. Bioresour. Technol. 2014, 160, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Du, Q.; Wang, X.; Xia, Y. Removal of lead from aqueous solution by activated carbon prepared from Entermorpha prolifera by zinc chloride activation. J. Hazard. Mater. 2010, 183, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Hui, C.-W.; Chen, B.; McKay, G. Pore-Surface Diffusion Model for Batch Adsorption Processes. Langmuir 2003, 19, 4188–4196. [Google Scholar] [CrossRef]
- Lin, L.-C.; Thirumavalavan, M.; Wang, Y.-T.; Jiunn-Fwu Lee, J.-F. Effect of Preparation Conditions on the Adsorption of Heavy Metal Ions from Aqueous Solution by Mesoporous Silica Materials Prepared Using Organic Template (HDTMAB). J. Chem. Eng. Data 2010, 55, 3667–3673. [Google Scholar] [CrossRef]
- Girods, P.; Dufour, A.; Fierro, V.; Rogaume, Y.; Rogaume, C.; Zoulalian, A.; Celzard, A. Activated carbons prepared from wood particleboard wastes: Characterisation and phenol adsorption capacities. J. Hazard. Mater. 2009, 166, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.-Y.; Seo, Y.-D. Sorption of halogenated phenols and pharmaceuticals to biochar: Affecting factors and mechanisms. Environ. Sci. Pollut. Res. 2016, 23, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Iriarte-Velasco, U.; Zudaire, L.; Ayastuy, J.L. Preparation of a porous biochar from the acid activation of pork bones. Food Bioprod. Process. 2016, 98, 341–353. [Google Scholar] [CrossRef]
- Kong, M.; Liu, Q.; Guo, F.; Jiang, L.; Yao, L.; Ren, S.; Yang, J. Physicochemical properties of pine-derived bio-chars modified by metal oxides and their performance in the removal of NO. J. Energy Inst. 2017. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, J.P.; Wen, C.; Zhang, L. An enhanced approach for biochar preparation using fluidized bed and its application for H2S removal. Chem. Eng. Process. 2016, 104, 1–12. [Google Scholar] [CrossRef]
- Xiong, C.; He, R.; Pi, L.; Li, J.; Yao, C.; Jiang, J.; Zheng, X. Adsorption of Neodymium(III) on Acrylic Resin (110 Resin) from Aqueous Solutions. Sep. Sci. Technol. 2015, 50, 564–572. [Google Scholar] [CrossRef]
- Kilian, K.; Pyrzyńska, K.; Pęgier, M. Comparative Study of Sc(III) Sorption onto Carbon-based Materials. Solv. Extr. Ion Exch. 2017. [Google Scholar] [CrossRef]
- Guo, Z.; Li, Y.; Pan, S.; Xu, J. Fabrication of Fe3O4@cyclodextrin magnetic composite for the high-efficient removal of Eu(III). J. Mol. Liq. 2015, 206, 272–277. [Google Scholar] [CrossRef]





| Parameter | Sd | Sd350 | AC |
|---|---|---|---|
| yP, % | - | 30.20 | - |
| pH | 5.4 | 3.7 | 10.2 |
| EC, mS·cm−1 | 0.34 | 0.24 | - |
| Surface area, m2·g−1 | - | 4.71 | 912.10 |
| Porosity, % | 10.4 | 20.41 | - |
| VM, % | 87.9 | 42.11 | - |
| Char, % | 12.1 | 54.17 | - |
| FC, % | 10.5 | 52.46 | - |
| Ash, % | 1.6 | 1.71 | - |
| % C | 46.7 | 62.23 | 78.91 |
| % H | 5.8 | 2.24 | - |
| % N | 0.51 | 0.34 | - |
| % O | 47 | 35.19 | - |
| Adsorbent-REE | Pseudo-First Order | Pseudo-Second Order | ||||
|---|---|---|---|---|---|---|
| k1 (min−1) | qe (mg·g−1) | R2 | k2 (g·mg−1·min−1) | qe (mg·g−1) | R2 | |
| Sd350-Sc | 0.287 | 0.361 | 0.989 | 2.723 | 1.059 | 0.999 |
| AC-Sc | 0.519 | 0.166 | 0.774 | 13.799 | 1.539 | 0.999 |
| Sd350-Nd | 0.187 | 0.688 | 0.991 | 0.875 | 1.611 | 0.999 |
| AC-Nd | 0.179 | 0.331 | 0.719 | 2.258 | 1.772 | 1.000 |
| Adsorbent | Adsorbent Conc. (g·L−1) | % Nd Adsorbed | Nd Adsorption Rate (mg·g−1) | % Sc Adsorbed | Sc Adsorption Rate (mg·g−1) |
|---|---|---|---|---|---|
| Sd350 | 1 | 40.2 | 8.0 | 37.9 | 7.5 |
| 2 | 47.1 | 4.7 | 39.7 | 4.0 | |
| 5 | 61.5 | 2.5 | 47.9 | 1.9 | |
| 10 | 78.6 | 1.6 | 52.3 | 1.1 | |
| AC | 1 | 44.2 | 8.8 | 42.6 | 8.5 |
| 2 | 55.0 | 5.5 | 46.3 | 4.6 | |
| 5 | 68.5 | 2.7 | 62.0 | 2.5 | |
| 10 | 87.9 | 1.8 | 76.8 | 1.5 |
| Adsorbent | Nd | Sc |
|---|---|---|
| Sd350 | −3.23 | −0.23 |
| AC | −4.92 | −2.96 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komnitsas, K.; Zaharaki, D.; Bartzas, G.; Alevizos, G. Adsorption of Scandium and Neodymium on Biochar Derived after Low-Temperature Pyrolysis of Sawdust. Minerals 2017, 7, 200. https://doi.org/10.3390/min7100200
Komnitsas K, Zaharaki D, Bartzas G, Alevizos G. Adsorption of Scandium and Neodymium on Biochar Derived after Low-Temperature Pyrolysis of Sawdust. Minerals. 2017; 7(10):200. https://doi.org/10.3390/min7100200
Chicago/Turabian StyleKomnitsas, Konstantinos, Dimitra Zaharaki, Georgios Bartzas, and Georgios Alevizos. 2017. "Adsorption of Scandium and Neodymium on Biochar Derived after Low-Temperature Pyrolysis of Sawdust" Minerals 7, no. 10: 200. https://doi.org/10.3390/min7100200





