Heating Changes Bio-Schwertmannite Microstructure and Arsenic(III) Removal Efficiency
Abstract
:1. Introduction
2. Materials and Methods
2.1. A. Ferrooxidans LX5 and Its Culture
2.2. Preparation of Bio-Synthesized Schwertmannite
2.3. Bio-Schwertmannite Heating
2.4. Arsenic(III) Adsorption Efficiency of Heated Bio-Schwertmannite
2.5. Analytical Procedures
3. Results and Discussion
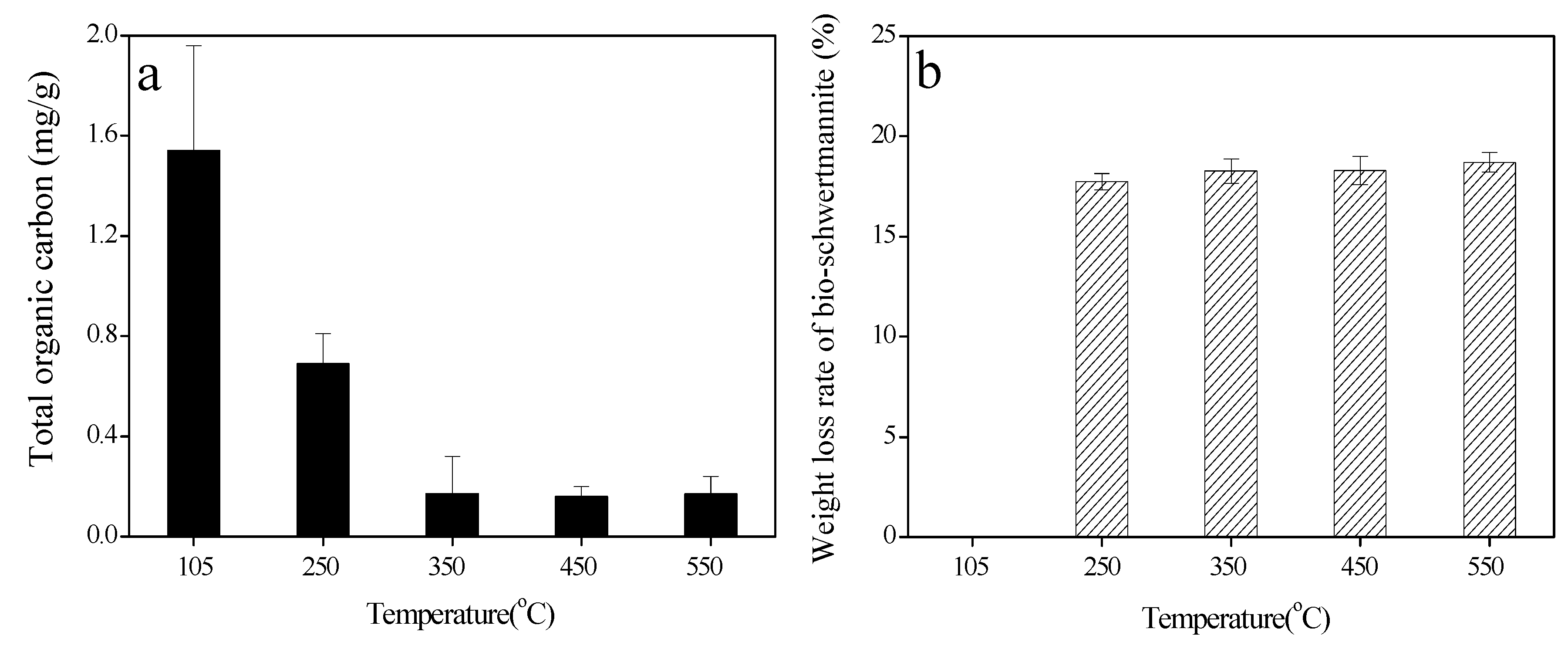
3.1. Color, Total Organic Carbon (TOC) Content, and Weight Loss of Bio-Schwertmannite
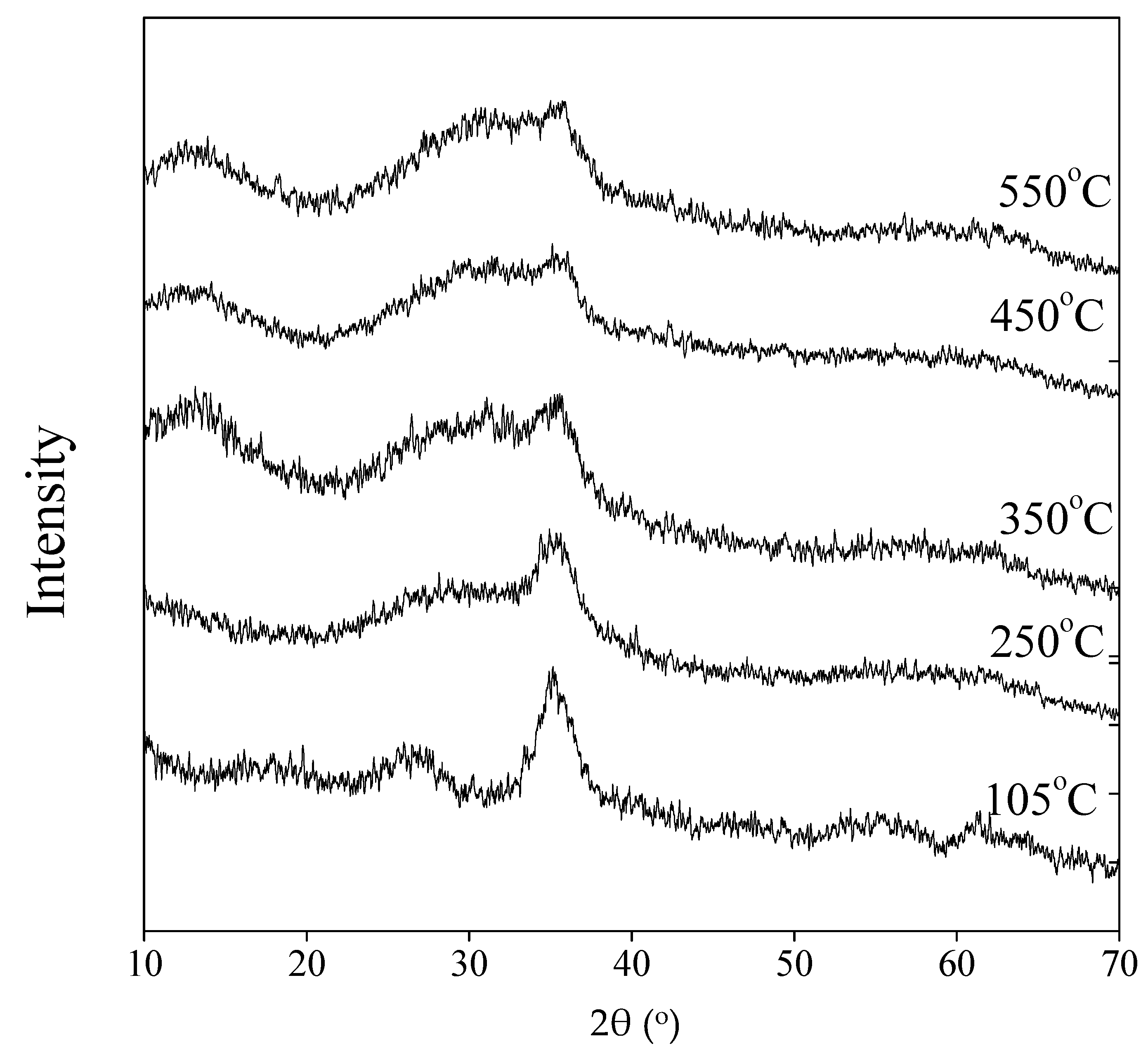
3.2. Chemical Functional Groups and Phase of Bio-Schwertmannite
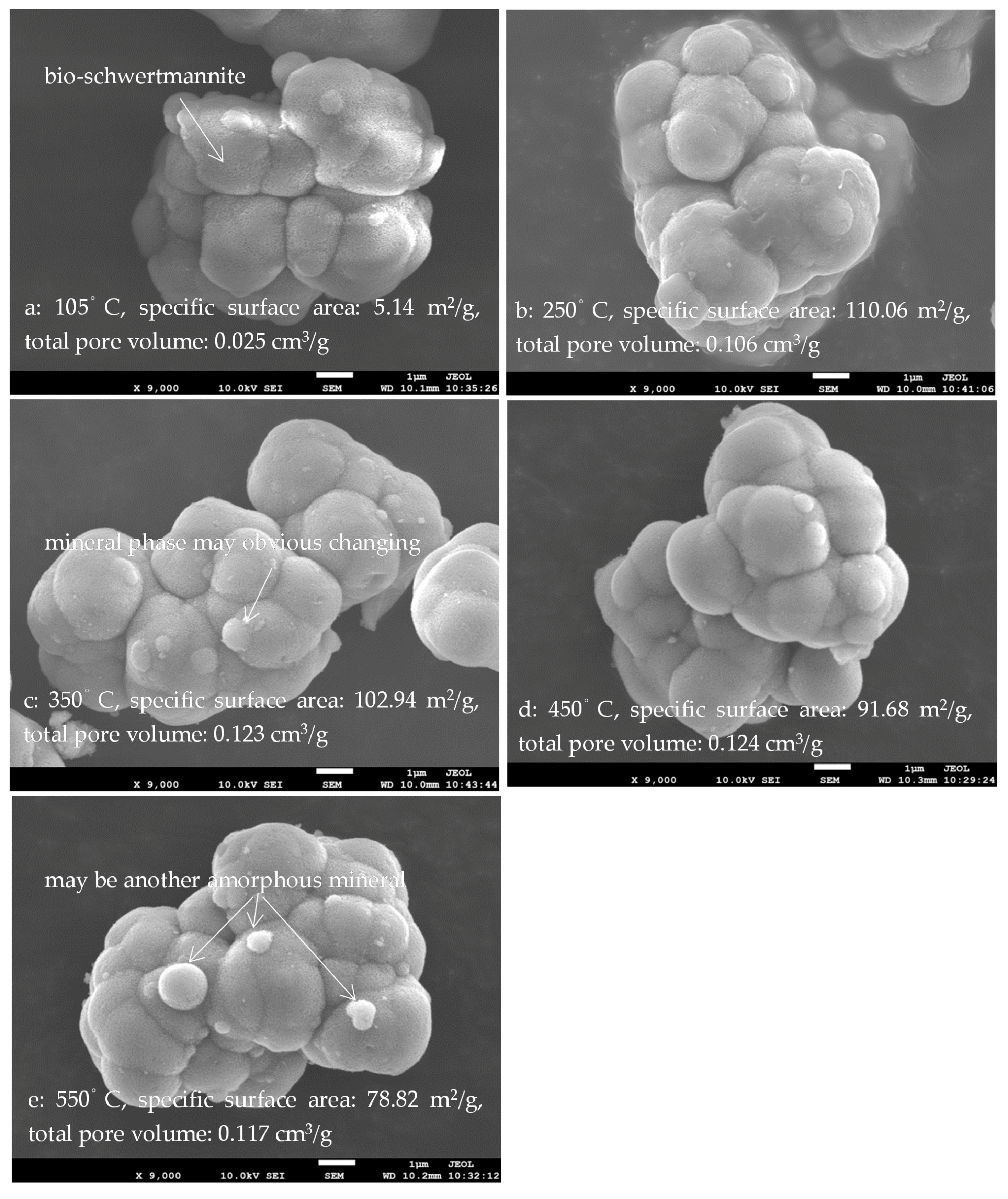
3.3. Morphology, Specific Surface Area, and Total Pore Volume of Bio-Schwertmannite
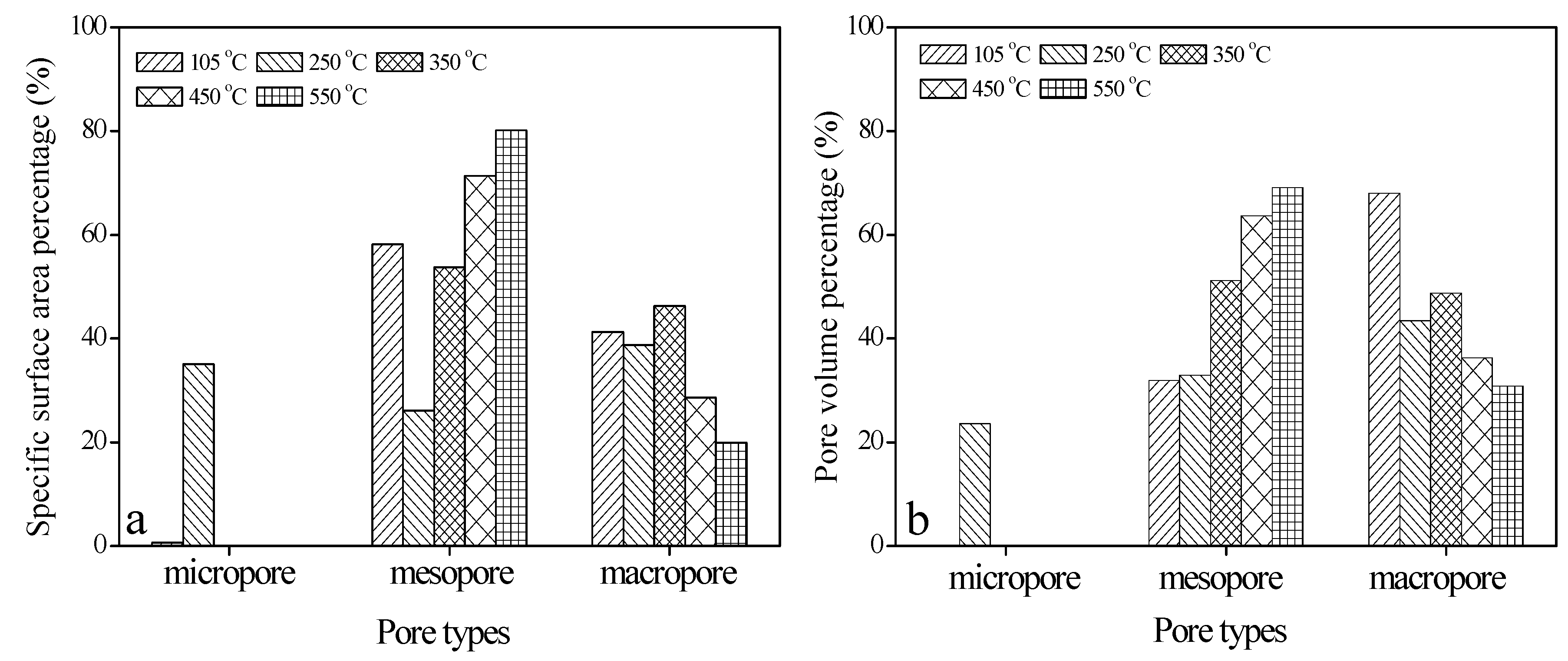
3.4. Specific Surface Area and Pore Volume Distribution among Micropores, Mesopores, and Macropores in Bio-Schwertmannite
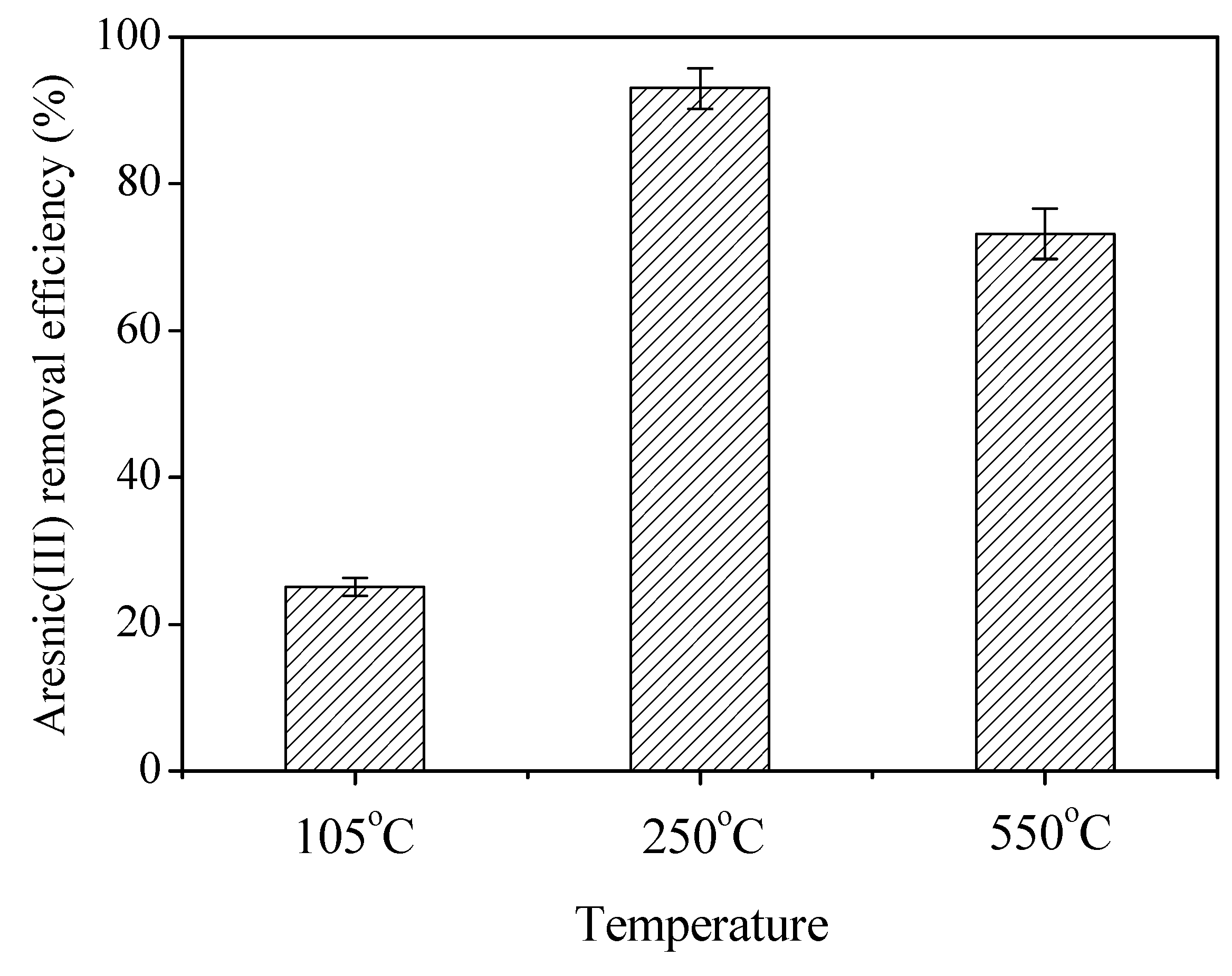
3.5. Arsenic(III) Removal Efficiency of Bio-Schwertmannite
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, P.; Wu, J.; Qian, H. Hydrochemical appraisal of groundwater quality for drinking and irrigation purposes and the major influencing factors: A case study in and around Hua County, China. Arab. J. Geosci. 2016, 9, 1–17. [Google Scholar] [CrossRef]
- Das, S.; Sudipta, B.S.; Lahan, P.; Jyoti, B.; Mridul, C.; Barooah, M. Groundwater arsenic contamination in north eastern states of India. J. Environ. Res. Dev. 2015, 9, 621–632. [Google Scholar]
- Shrestha, S.M.; Rijal, K.; Pokhrel, M.R. Assessment of Arsenic Contamination in Deep Groundwater Resources of the Kathmandu Valley, Nepal. J. Geosci. Environ. Prot. 2015, 3, 79–89. [Google Scholar] [CrossRef]
- Bulka, C.M.; Jones, R.M.; Turyk, M.E.; Stayner, L.T.; Argos, M. Arsenic in drinking water and prostate cancer in Illinois counties: An ecologic study. Environ. Res. 2016, 148, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Pittman, C.U., Jr. Arsenic removal from water/wastewater using adsorbents-A critical review. J. Hazard. Mater. 2007, 142, 1–53. [Google Scholar] [CrossRef] [PubMed]
- Liaw, J.; Marshall, G.; Yuan, Y.; Ferreccio, C.; Steinmaus, C.; Smith, A.H. Increased childhood liver cancer mortality and arsenic in drinking water in Northern Chile. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1982–1987. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, S.; Sengupta, M.K.; Mukherjee, A.; Hossain, M.A.; Das, B.; Nayak, B.; Pal, A.; Mukherjee, S.C.; Pati, S.; Dutta, R.N.; et al. Arsenic groundwater contamination and its health effects in the state of Uttar Pradesh (UP) in upper and middle Ganga plain, India: A severe danger. Sci. Total Environ. 2006, 370, 310–322. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Ma, T.; Deng, Y.M.; Yang, H.; Wang, Y.X. Environmental geochemistry of high arsenic groundwater at western Hetao plain, Inner Mongolia. Front. Earth Sci. China 2008, 3, 63–72. [Google Scholar] [CrossRef]
- George, C.M.; Zheng, Y.; Graziano, J.H.; Rasul, S.B.; Hossain, Z.; Mey, J.L.; Geen, A.V. Evaluation of an arsenic test kit for rapid well screening in Bangladesh. Environ. Sci. Technol. 2012, 46, 11213–11219. [Google Scholar] [CrossRef] [PubMed]
- Chakraborti, D.; Rahman, M.M.; Ahamed, S.; Dutta, R.N.; Pati, S.; Mukherjee, S.C. Arsenic groundwater contamination and its health effects in Patna district (capital of Bihar) in the middle Ganga plain, India. Chemosphere 2016, 152, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.J.; Wang, Y.X.; Su, C.L.; Liu, H.Q.; Duan, M.Y.; Xie, Z.M. Arsenic mobilization in shallow aquifers of Datong Basin: Hydrochemical and mineralogical evidences. J. Geochem. Explor. 2008, 98, 107–115. [Google Scholar] [CrossRef]
- Sarkar, A.; Paul, B. The global menace of arsenic and its conventional remediation-A critical review. Chemosphere 2006, 158, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.H.; Liang, J.R.; Zhou, L.X. Adsorptive removal of As(III) by biogenic schwertmannite from simulated As-contaminated groundwater. Chemosphere 2011, 83, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.K.; Choudhury, M.R.; Ali, M.A. As(III) and As(V) Adsorption on Magnetite Nanoparticles: Adsorption Isotherms: Effect of pH and phosphate, and Adsorption Kinetics. Int. J. Chem. Environ. Eng. 2013, 4, 55–63. [Google Scholar]
- Song, J.; Jia, S.Y.; Ren, H.T.; Wu, S.H.; Han, X. Application of a high-surface-area schwertmannite in the removal of arsenate and arsenite. Int. J. Environ. Sci. Technol. 2015, 12, 1559–1568. [Google Scholar] [CrossRef]
- Lin, T.F.; Wu, J.K. Adsorption of arsenite and arsenate within activated alumina grains: Equilibrium and kinetics. Water Res. 2001, 35, 2049–2057. [Google Scholar] [CrossRef]
- Dodbiba, G.; NuKaya, T.; Kamioka, Y.; Tanimura, Y.; Fujita, T. Removal of arsenic from wastewater using iron compound: Comparing two different types of adsorbents in the context of LCA. Resour. Conserv. Recycl. 2009, 53, 688–697. [Google Scholar] [CrossRef]
- Kumar, A.S.K.; Jiang, S.J. Chitosan-functionalized graphene oxide: A novel adsorbent an efficient adsorption of arsenic from aqueous solution. J. Environ. Chem. Eng. 2016, 4, 1698–1713. [Google Scholar] [CrossRef]
- Zhu, N.Y.; Yan, T.M.; Qiao, J.; Cao, H.L. Adsorption of arsenic, phosphorus and chromium by bismuth impregnated biochar: Adsorption mechanism and depleted adsorbent utilization. Chemosphere 2016, 164, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Namor, A.F.D.; Hakawati, N.; Hamdan, W.A.; Soualhi, R.; Korfali, S.; Valiente, L. Calix[4] pyrrole for the removal of arsenic(III) and arsenic(V) from water. J. Hazard. Mater. 2017, 326, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Strosnider, W.H.J.; Winfrey, B.K.; Peer, R.A.M.; Nairn, R.W. Passive co-treatment of acid mine drainage and sewage: Anaerobic incubation reveals a regeneration technique and further treatment possibilities. Ecol. Eng. 2013, 61, 268–273. [Google Scholar] [CrossRef]
- Bigham, J.M.; Schwertmann, U.; Carlson, L.; Murad, E. A poorly crystallized oxyhydroxysulfate of iron formed by bacterial oxidation of Fe(II) in acid mine waters. Geochim. Cosmochim. Acta 1990, 54, 2743–2758. [Google Scholar] [CrossRef]
- Gan, M.; Sun, S.J.; Zheng, Z.H.; Tang, H.J.; Sheng, J.R.; Zhu, J.Y.; Liu, X.X. Adsorption of Cr(VI) and Cu(II) by AlPO4 modified biosynthetic schwertmannite. Appl. Surf. Sci. 2015, 356, 986–997. [Google Scholar] [CrossRef]
- Nordstrom, D.K.; Alpers, C.N. Geochemistry of acid mine waters. Soc. Econ. Geol. 1999, 6A, 133–160. [Google Scholar]
- Fernandez-Martinez, A.; Timon, V.; Román-Ross, G.; Cuello, G.J.; Daniels, J.E.; Ayora, C. The structure of schwertmannite, a nanocrystalline iron oxyhydroxysulfate. Am. Mineral. 2010, 95, 1312–1322. [Google Scholar] [CrossRef]
- Antelo, J.; Fiol, S.; Gondar, D.; López, R.; Arce, F. Comparison of arsenate, chromate and molybdate binding on schwertmannite: Surface adsorption vs. anion-exchange. J. Colloid Interface Sci. 2012, 386, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.W.; Zhou, J.; Zhang, S.S.; Liu, L.L.; Zhou, L.X.; Fan, W.H. Schwertmannite synthesis through ferrous ion chemical oxidation under different H2O2 supply rates and its removal efficiency for arsenic from contaminated groundwater. PLoS ONE 2015, 10, e0138891. [Google Scholar] [CrossRef] [PubMed]
- Bigham, J.M.; Carlson, L.; Murad, E. Schwertmannite, a new iron oxyhydroxysulfate from pyhäsalmi, Finland, and other localities. Mineral. Mag. 1994, 58, 641–648. [Google Scholar] [CrossRef]
- Wang, X.; Gu, C.; Feng, X.; Zhu, M. Sulfate local coordination environment in schwertmannite. Environ. Sci. Technol. 2015, 49, 10440–10448. [Google Scholar] [CrossRef] [PubMed]
- Paikaray, S.; Göttlicher, J.; Peiffer, S. As(III) retention kinetics, equilibrium and redox stability on biosynthesized schwertmannite and its fate and control on schwertmannite stability on acidic (pH 3.0) aqueous exposure. Chemosphere 2012, 86, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.H.; Zhou, L.X.; Liang, J.R.; Xiong, H.X. Biosynthesis of schwertmannite by Acidithiobacillus ferrooxidans cell suspensions under different pH condition. Mater. Sci. Eng. C 2009, 29, 211–215. [Google Scholar] [CrossRef]
- Liang, J.R.; Li, Z.Y.; Liu, F.W.; Zhou, L.X. Mineralogical characteristics of biogenic schwertmannite amended with different pretreatment methods and the effects on As(III) adsorption. Environ. Sci. 2012, 33, 3605–3612. [Google Scholar]
- Paikaray, S.; Göttlicher, J.; Peiffer, S. Removal of As(III) from acidic waters using schwertmannite: Surface speciation and effect of synthesis pathway. Chem. Geol. 2011, 283, 134–142. [Google Scholar] [CrossRef]
- Yu, R.L.; Ou, Y.; Tan, J.X.; Wu, F.D.; Sun, J.; Miao, L.; Zhong, D.L. Effect of EPS on adhesion of Acidithiobacillus ferrooxidans on chalcopyrite and pyrite mineral surfaces. Chin. J. Nonferr. Met. 2011, 21, 407–412. [Google Scholar] [CrossRef]
- Liu, F.W.; Zhou, J.; Zhou, L.X.; Zhang, S.S.; Liu, L.L.; Wang, M. Effect of neutralized solid waste generated in lime neutralization on the ferrous ion bio-oxidation process during acid mine drainage treatment. J. Hazard. Mater. 2015, 299, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.N.; Nair, A.S.; Yang, S.Y.; Peng, S.J.; Elumalai, N.K.; Ramakrishna, S. Rice grain-shaped TiO2-MWCNT composite-A functional material with a novel morphology for dye-sensitized solar cells. J. Photochem. Photobiol. A 2012, 231, 9–18. [Google Scholar]
- Zhou, K.; Zhang, W.Y.; Pan, H.M.; Li, J.H.; Yue, H.D.; Wu, L.F. Adaptation of spherical multicellular magnetotactic prokaryotes to the geochemically variable habitat of an intertidal zone. Environ. Microbiol. 2013, 15, 1595–1605. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Duan, T.; Han, Y.; Jia, X.; Chen, H. On-line solid phase extraction-hydride generation atomic fluorescence spectrometric determination of trace arsenic in high purity antimony(III) oxide. J. Anal. At. Spectrom. 2009, 25, 206–209. [Google Scholar] [CrossRef]
- Wang, H.M.; Bigham, J.M.; Tuovinen, O.H. Formation of schwertmannite and its transformation to jarosite in the presence of acidophilic iron-oxidizing microorganisms. Mater. Sci. Eng. C 2006, 26, 588–592. [Google Scholar] [CrossRef]
- Sun, H.F.; Zhao, F.H.; Cong, Z.Y.; Yue, M.; Ren, D.Y. The mineral Schwertmannite found in China and its characteristics. Acta Mineral. Sin. 2006, 26, 38–42. [Google Scholar]
- Zhang, S.L.; Jia, S.Y.; Yu, B.; Liu, Y.; Wu, S.H.; Han, X. Sulfidization of As(V)-containing schwertmannite and its impact on arsenic mobilization. Chem. Geol. 2016, 420, 270–279. [Google Scholar] [CrossRef]
- Goswami, A.; Purkait, M.K. Removal of fluoride from drinking water using nanomagnetite aggregated schwertmannite. J. Water Process Eng. 2014, 1, 91–100. [Google Scholar] [CrossRef]
- Schwertmann, U.; Bigham, J.M.; Murad, E. The first occurrence of schwertmannite in a natural stream environment. Eur. J. Mineral. 1995, 7, 547–552. [Google Scholar]
- Bigham, J.M.; Schwertmann, U.; Pfab, G. Influence of pH on mineral speciation in a bioreactor simulating acid mine drainage. Appl. Geochem. 1996, 11, 845–849. [Google Scholar] [CrossRef]
- Jiang, X.L.; Peng, C.J.; Fu, D.; Chen, Z.; Shen, L.; Li, Q.B.; Ouyang, T.; Wang, Y.P. Removal of arsenate by ferrihydrite via surface complexation and surface precipitation. Appl. Surf. Sci. 2015, 353, 1087–1094. [Google Scholar] [CrossRef]
- Zhu, J.; Pigna, M.; Cozzolino, V.; Caporale, A.G.; Violante, A. Sorption of arsenite and arsenate on ferrihydrite: Effect of organic and inorganic ligands. J. Hazard. Mater. 2011, 189, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Hsieh, T.Y.; Yang, P.Y.; Hwang, C.C.; Shye, D.C.; Lee, I.C. Oxygen annealing effect on field-emission characteristics of hydrothermally synthesized Al-doped ZnO nanowires. Surf. Coat. Technol. 2013, 231, 423–427. [Google Scholar] [CrossRef]
- Gagliano, W.B.; Brill, M.R.; Bigham, J.M.; Jones, F.S.; Traina, S.J. Chemistry and mineralogy of ochreous sediments in a constructed mine drainage wetland. Geochim. Cosmochim. Acta 2004, 68, 2119–2128. [Google Scholar] [CrossRef]
- Dou, X.M.; Mohan, D.; Pittman, C.U., Jr. Arsenate adsorption on three types of granular schwertmannite. Water Res. 2013, 47, 2938–2948. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, S.; Hir, Z.A.M.; Yousefi, A.T.; Hamid, S.B.A. Progress on mesoporous titanium dioxide: Synthesis, modification and applications. Microporous Mesoporous Mater. 2015, 218, 206–222. [Google Scholar] [CrossRef]
- Chen, Y.H.; Wu, Q.L.; Ning, P.; Gong, J.H.; Ding, P. Rayon-based activated carbon fibers treated with both alkali metal salt and Lewis acid. Microporous Mesoporous Mater. 2008, 109, 138–146. [Google Scholar] [CrossRef]
- Xie, Y.; Zhou, L.X. Arsenite removal from simulated groundwater by biogenic schwertmannite: A column trial. Pedosphere 2013, 23, 402–408. [Google Scholar] [CrossRef]

© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, X.; Liu, L.; Shi, J.; Zhou, L.; Guo, Y.; Ge, Y.; Fan, W.; Liu, F. Heating Changes Bio-Schwertmannite Microstructure and Arsenic(III) Removal Efficiency. Minerals 2017, 7, 9. https://doi.org/10.3390/min7010009
Qiao X, Liu L, Shi J, Zhou L, Guo Y, Ge Y, Fan W, Liu F. Heating Changes Bio-Schwertmannite Microstructure and Arsenic(III) Removal Efficiency. Minerals. 2017; 7(1):9. https://doi.org/10.3390/min7010009
Chicago/Turabian StyleQiao, Xingxing, Lanlan Liu, Jing Shi, Lixiang Zhou, Yanhan Guo, Yuanying Ge, Wenhua Fan, and Fenwu Liu. 2017. "Heating Changes Bio-Schwertmannite Microstructure and Arsenic(III) Removal Efficiency" Minerals 7, no. 1: 9. https://doi.org/10.3390/min7010009






