Influence of Sulfobacillus thermosulfidooxidans on Initial Attachment and Pyrite Leaching by Thermoacidophilic Archaeon Acidianus sp. DSM 29099
Abstract
:1. Introduction
2. Experimental Section
2.1. Strains and Media
2.2. Pyrite Preparation
2.3. Iron and pH Determination
2.4. Preparation of Pyrite Pre-Colonized by S. thermosulfidooxidansT or of S. thermosulfidooxidansT Exudates
2.5. Initial Attachment
2.6. Leaching Experiments
2.7. Visualization of Biofilm Formation by Confocal Laser Scanning Microscopy
3. Results and Discussion
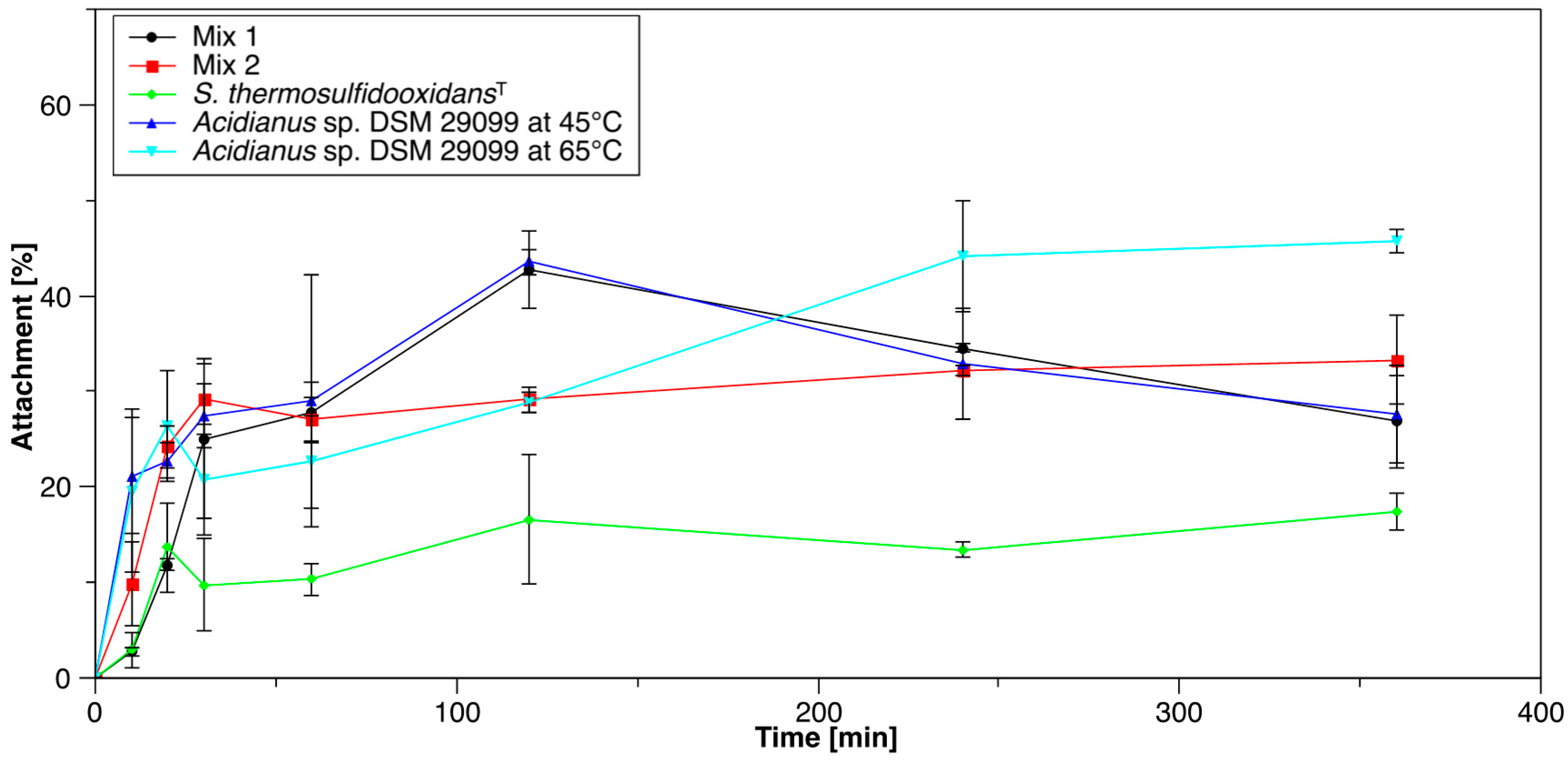
3.1. Initial Attachment of Cells to Pyrite
3.1.1. Pure and Mixed Cultures
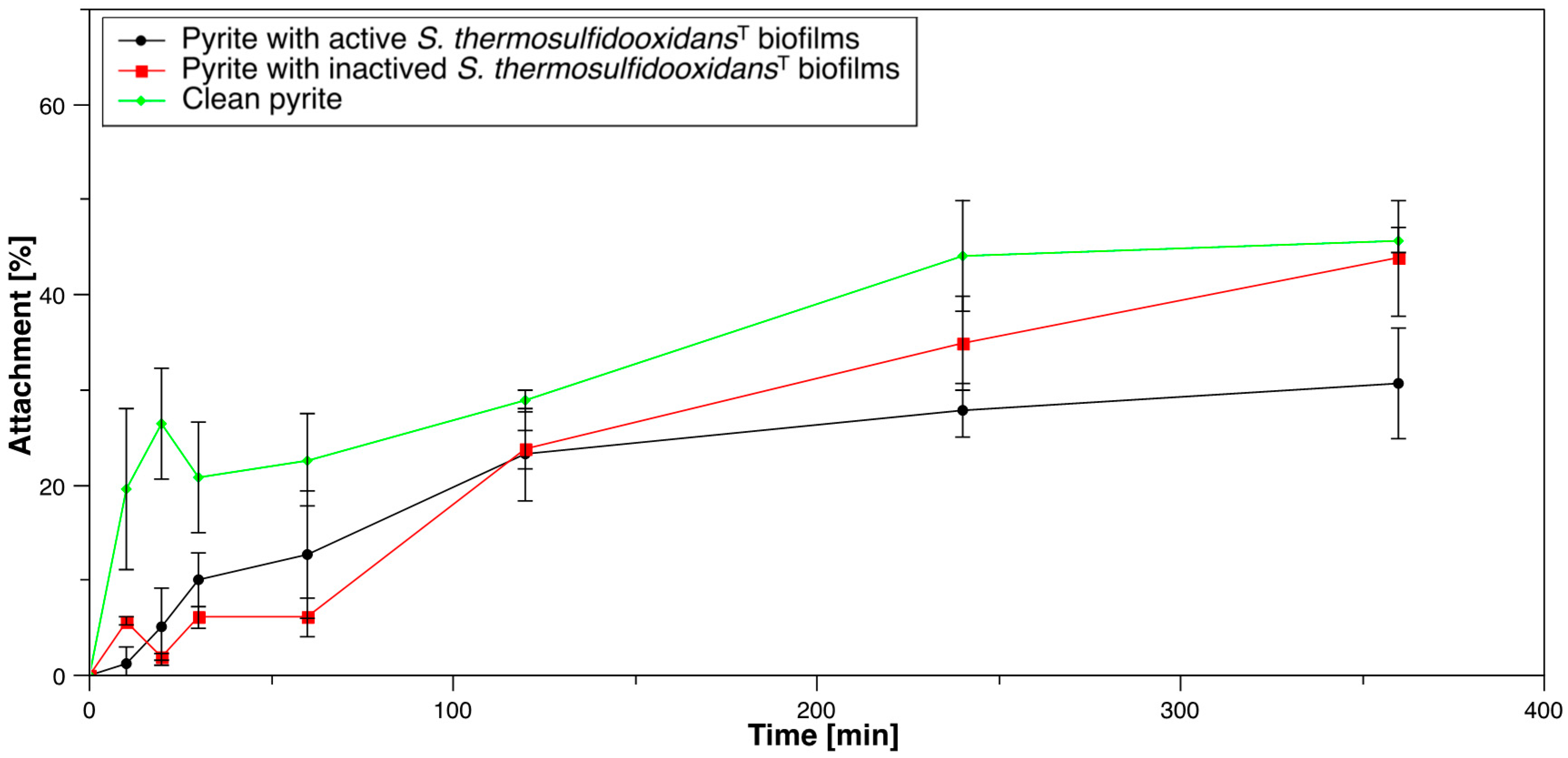
3.1.2. Acidianus sp. DSM 29099 to Pyrite Pre-Colonized with S. thermosulfidooxidansT
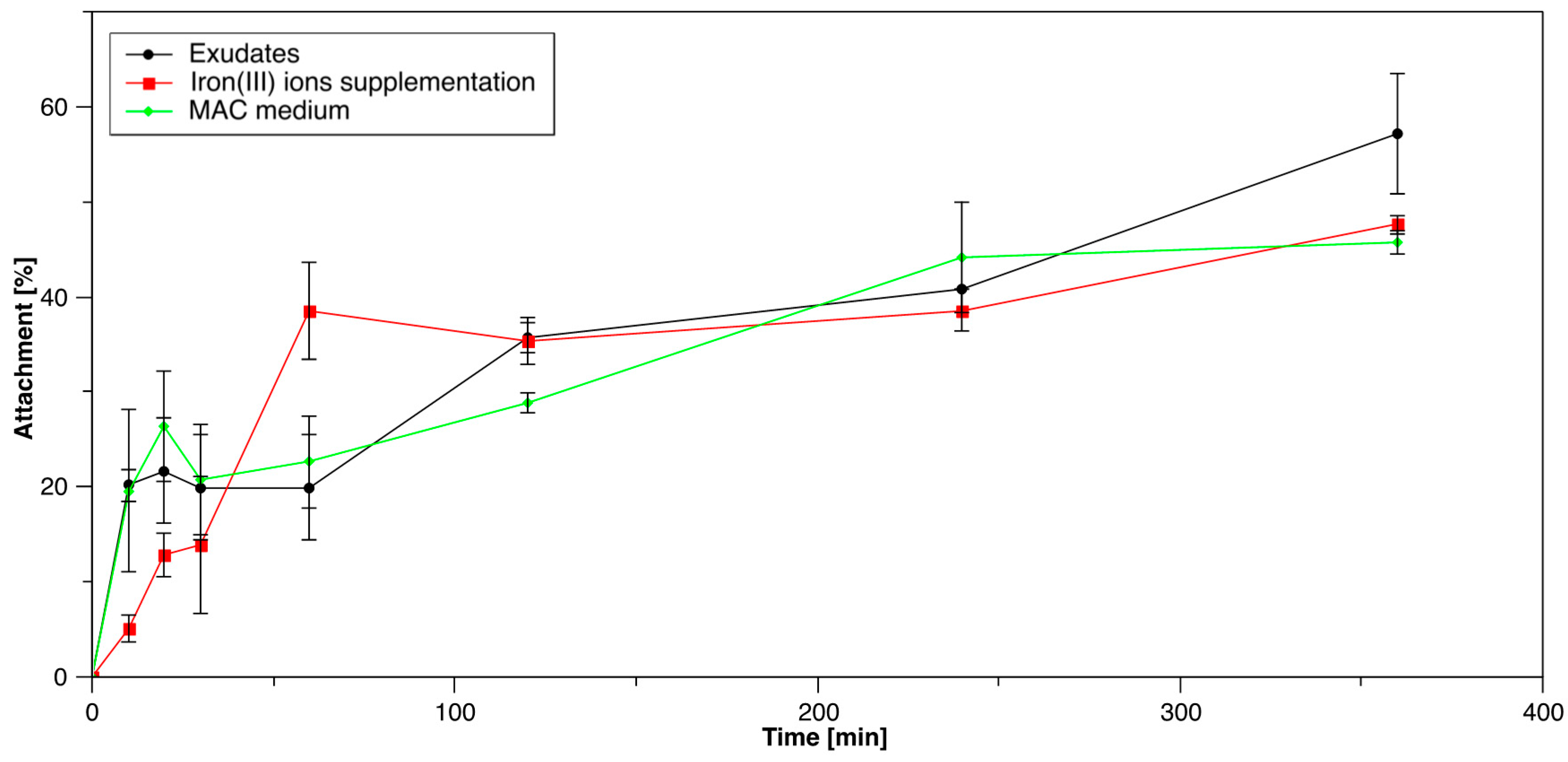
3.1.3. Influence of S. thermosulfidooxidansT Exudates on Initial Attachment of Acidianus sp. DSM 29099
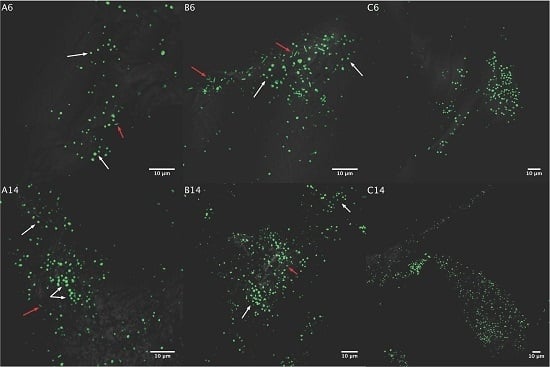
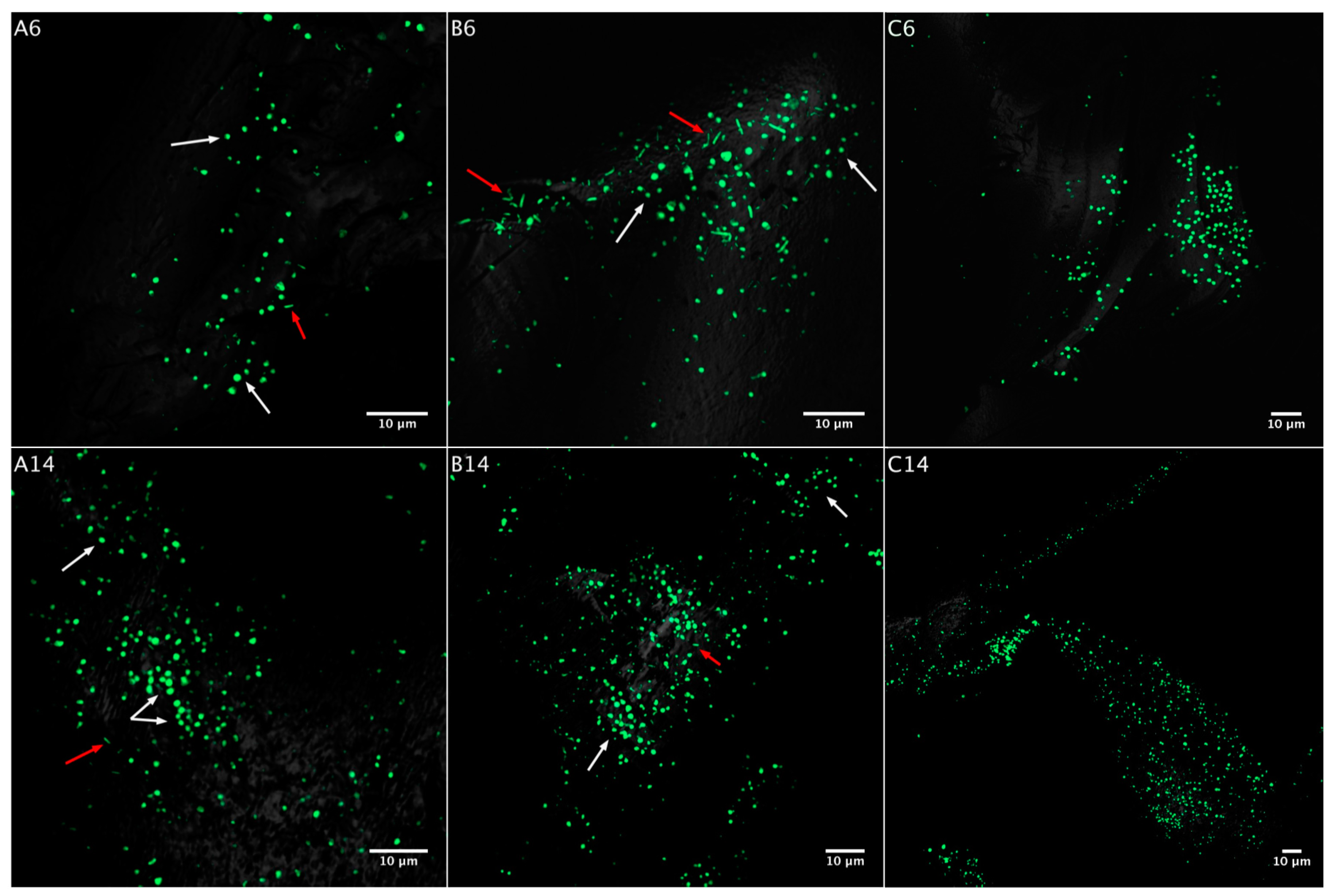
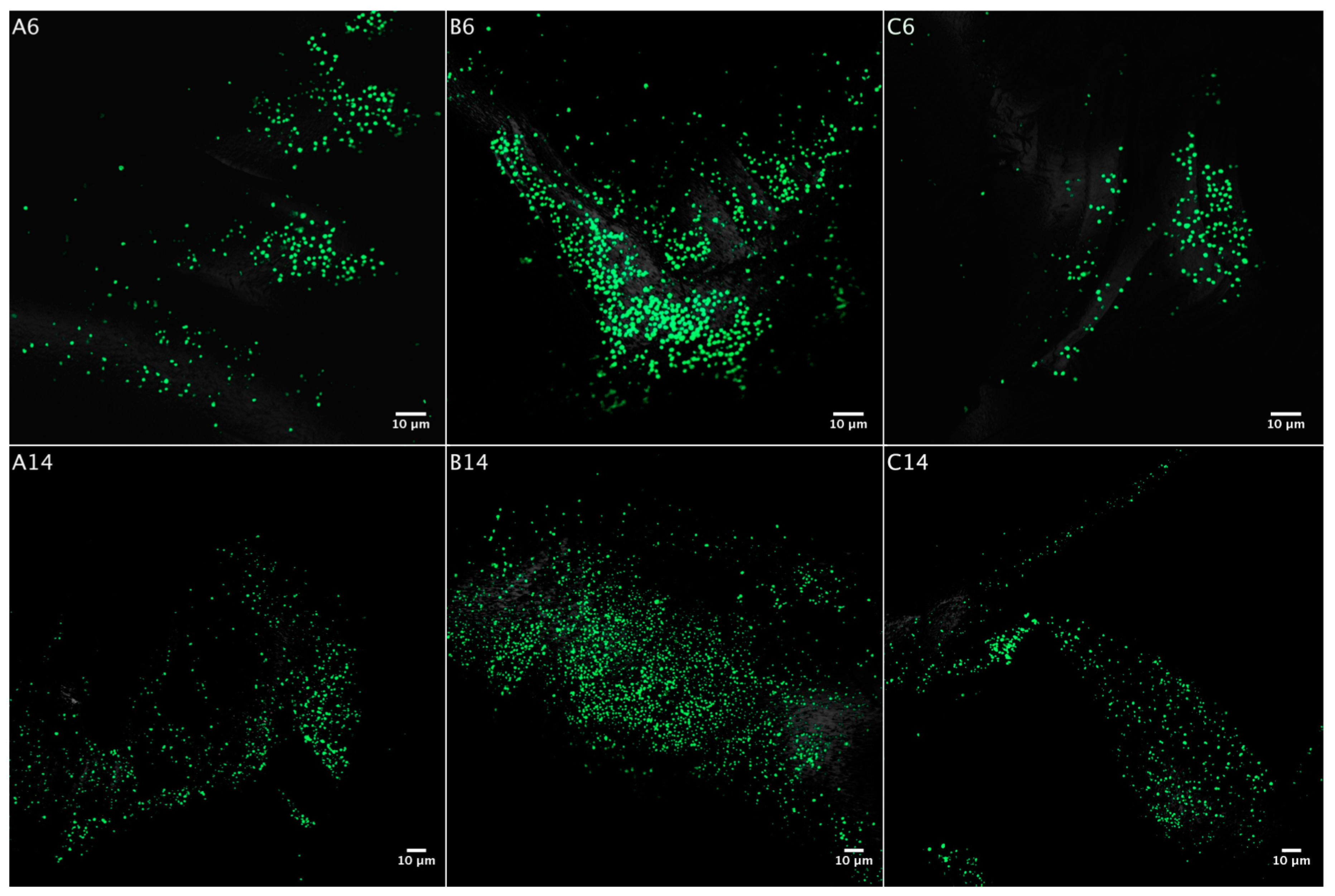
3.2. Biofilm Formation
3.2.1. Acidianus sp. DSM 29099 on Pyrite Pre-Colonized by S. thermosulfidooxidansT
3.2.2. Influence of S. thermosulfidooxidansT Exudates on Biofilm Formation by Acidianus sp. DSM 29099
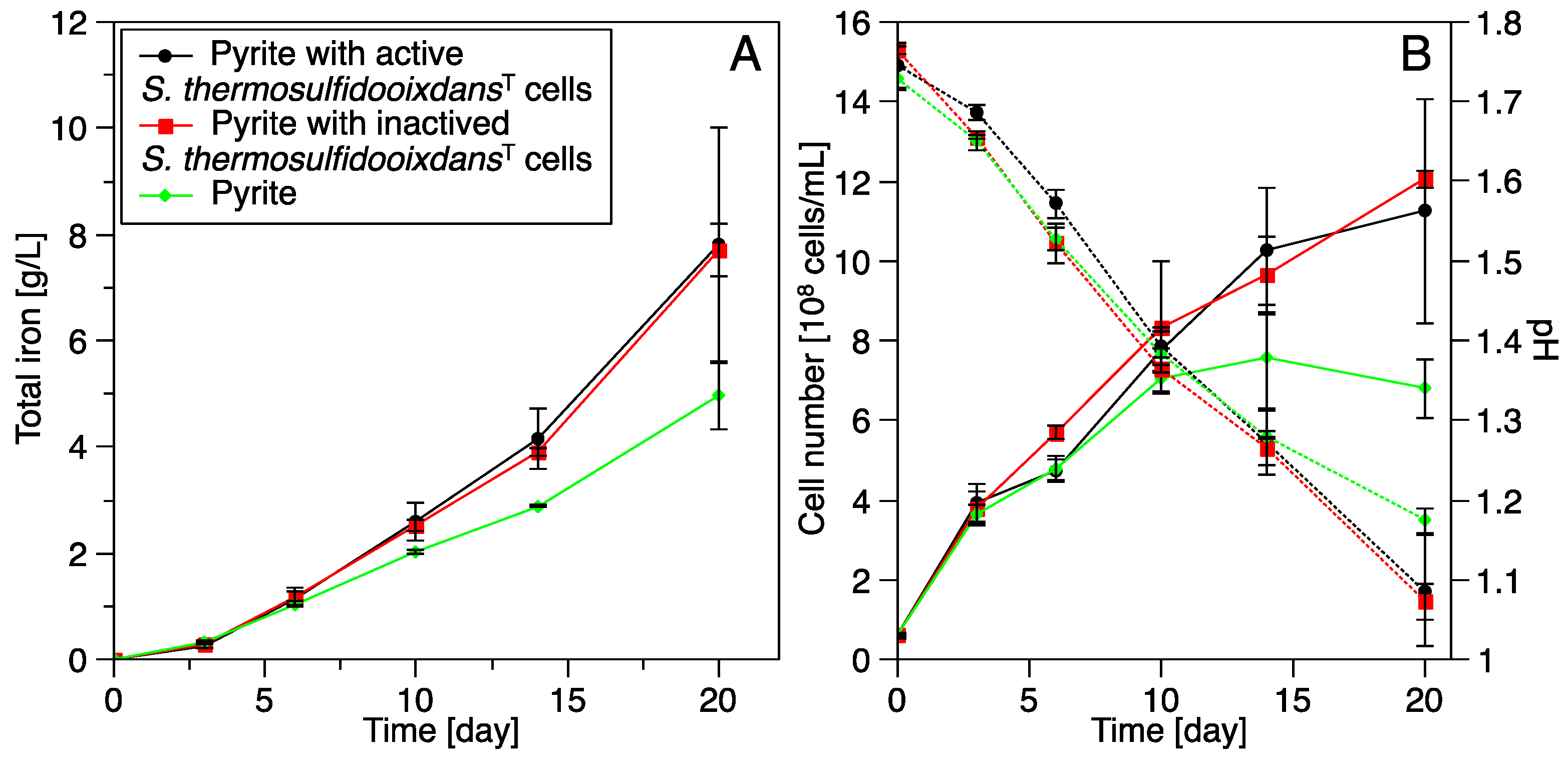
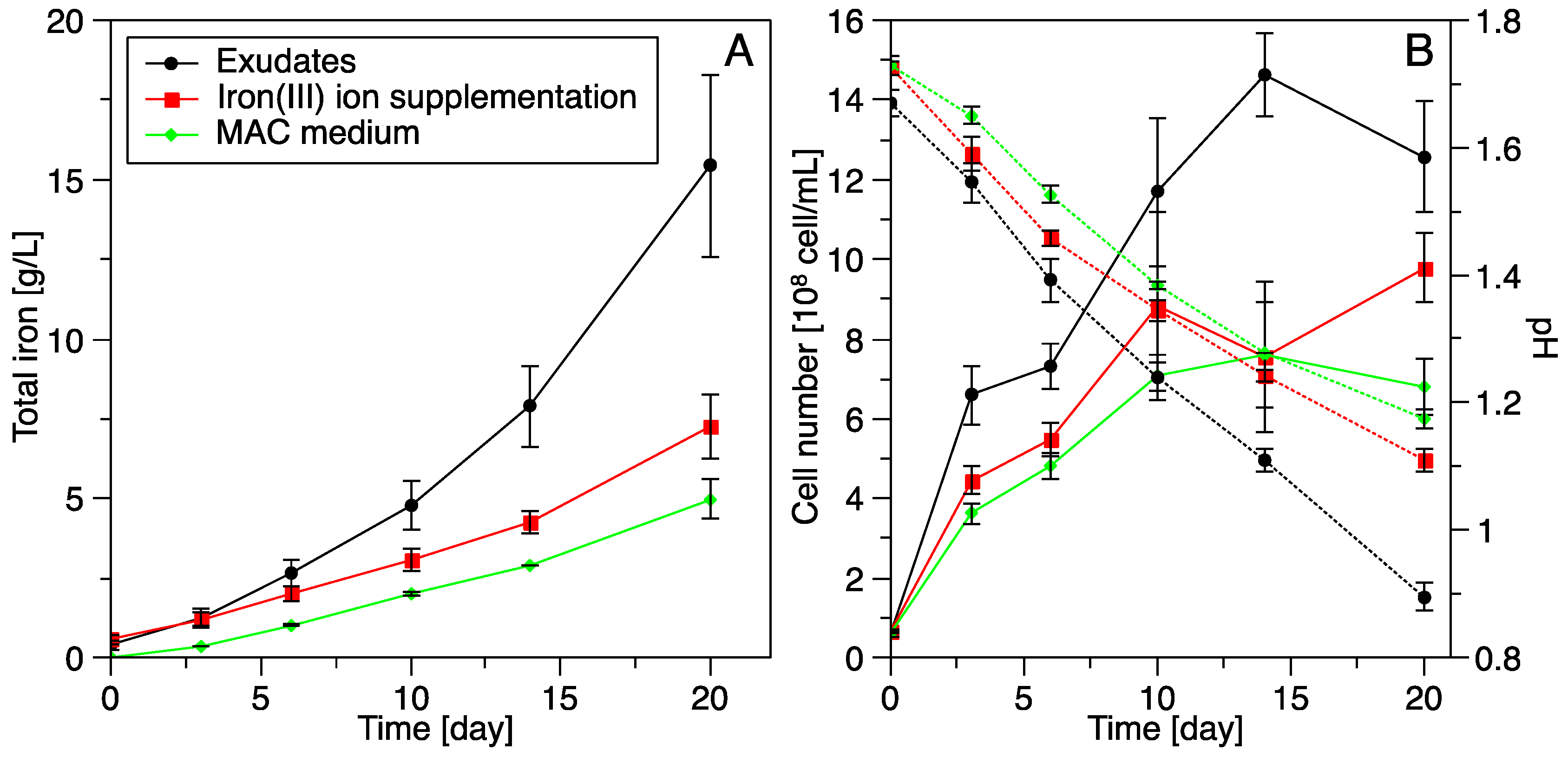
3.3. Pyrite Leaching
3.3.1. Pure and Mixed Cultures
3.3.2. Bioleaching by Acidianus sp. DSM 29099 with Pyrite Pre-Colonized by S. thermosulfidooxidansT
3.3.3. Influence of S. thermosulfidooxidansT Exudates on Pyrite Leaching by Acidianus sp. DSM 29099
4. Summary and Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brierley, C.L.; Brierley, J.A. Progress in bioleaching: Part B: Applications of microbial processes by the minerals industries. Appl. Microbiol. Biotechnol. 2013, 97, 7543–7552. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B. Biomining—Biotechnologies for extracting and recovering metals from ores and waste materials. Curr. Opin. Biotechnol. 2014, 30, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Watling, H. Review of biohydrometallurgical metals extraction from polymetallic mineral resources. Minerals 2014, 5, 1–60. [Google Scholar] [CrossRef]
- Gentina, J.C.; Acevedo, F. Copper bioleaching in Chile. Minerals 2016, 6, 23. [Google Scholar] [CrossRef]
- Johnson, D.B.; Hallberg, K.B. Acid mine drainage remediation options: A review. Sci. Total Environ. 2005, 338, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Sand, W.; Jozsa, P.-G.; Kovacs, Z.-M.; Sasaran, N.; Schippers, A. Long-term evaluation of acid rock drainage mitigation measures in large lysimeters. J. Geochem. Explor. 2007, 92, 205–211. [Google Scholar] [CrossRef]
- Schippers, A.; Breuker, A.; Blazejak, A.; Bosecker, K.; Kock, D.; Wright, T. The biogeochemistry and microbiology of sulfidic mine waste and bioleaching dumps and heaps, and novel Fe(II)-oxidizing bacteria. Hydrometallurgy 2010, 104, 342–350. [Google Scholar] [CrossRef]
- Vera, M.; Schippers, A.; Sand, W. Progress in bioleaching: Fundamentals and mechanisms of bacterial metal sulfide oxidation—Part A. Appl. Microbiol. Biotechnol. 2013, 97, 7529–7541. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, M.S.; Rainey, F.A.; Albuquerque, L. Sulfobacillus. In Bergey's Manual of Systematics of Archaea and Bacteria; Whitman, W.B., Ed.; John Wiley & Sons, Ltd: Hoboken, NJ, USA, 2015; pp. 1–6. [Google Scholar]
- Watling, H.R.; Perrot, F.A.; Shiers, D.W. Comparison of selected characteristics of Sulfobacillus species and review of their occurrence in acidic and bioleaching environments. Hydrometallurgy 2008, 93, 57–65. [Google Scholar] [CrossRef]
- Wheaton, G.; Counts, J.; Mukherjee, A.; Kruh, J.; Kelly, R. The confluence of heavy metal biooxidation and heavy metal resistance: Implications for bioleaching by extreme thermoacidophiles. Minerals 2015, 5, 397–451. [Google Scholar] [CrossRef]
- Norris, P.R.; Burton, N.P.; Clark, D.A. Mineral sulfide concentrate leaching in high temperature bioreactors. Miner. Eng. 2013, 48, 10–19. [Google Scholar] [CrossRef]
- Franzmann, P.D.; Hawkes, R.B.; Haddad, C.M.; Plumb, J.J. Mining with microbes. Microbiol. Aust. 2007, 28, 124–126. [Google Scholar]
- Plumb, J.J.; Hawkes, R.B.; Franzmann, P.D. The microbiology of moderately thermophilic and transiently thermophilic ore heaps. In Biomining; Springer: Berlin, Germany, 2007; pp. 217–235. [Google Scholar]
- Rawlings, D.E. Heavy metal mining using microbes. Annu. Rev. Microbiol. 2002, 56, 65–91. [Google Scholar] [CrossRef] [PubMed]
- Norris, P.R.; Johnson, D.B. Acidophilic microorganisms. In Extremophiles: Microbial Life in Extreme Environments; Horikoshi, K., Grant, W.D., Eds.; Wiley-Liss: New York, NY, USA; 1998. [Google Scholar]
- Dixon, D.G. Analysis of heat conservation during copper sulphide heap leaching. Hydrometallurgy 2000, 58, 27–41. [Google Scholar] [CrossRef]
- Zhang, R.; Bellenberg, S.; Neu, T.R.; Sand, W.; Vera, M. The biofilm lifestyle of acidophilic metal/sulfur-oxidizing microorganisms. In Biotechnology of Extremophiles: Advances and Challenges; Rampelotto, H.P., Ed.; Springer International Publishing: Cham, Germany, 2016; pp. 177–213. [Google Scholar]
- Li, Q.; Zhang, R.; Krok, B.A.; Vera, M.; Sand, W. Biofilm formation of Sulfobacillus thermosulfidooxidans on pyrite in the presence of Leptospirillum ferriphilum. Adv. Mater. Res. 2015, 1130, 141–144. [Google Scholar] [CrossRef]
- Noël, N.; Florian, B.; Sand, W. AFM & EFM study on attachment of acidophilic leaching organisms. Hydrometallurgy 2010, 104, 370–375. [Google Scholar]
- Bellenberg, S.; Diaz, M.; Noel, N.; Sand, W.; Poetsch, A.; Guiliani, N.; Vera, M. Biofilm formation, communication and interactions of leaching bacteria during colonization of pyrite and sulfur surfaces. Res. Microbiol. 2014, 165, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Neu, T.; Bellenberg, S.; Kuhlicke, U.; Sand, W.; Vera, M. Use of lectins to in situ visualize glycoconjugates of extracellular polymeric substances in acidophilic archaeal biofilms. Microb. Biotechnol. 2015, 8, 448–461. [Google Scholar] [CrossRef] [PubMed]
- Mackintosh, M. Nitrogen fixation by Thiobacillus ferrooxidans. J. Gen. Microbiol. 1978, 105, 215–218. [Google Scholar] [CrossRef]
- Schippers, A.; Jozsa, P.; Sand, W. Sulfur chemistry in bacterial leaching of pyrite. Appl. Environ. Microbiol. 1996, 62, 3424–3431. [Google Scholar] [PubMed]
- Florian, B.; Noël, N.; Thyssen, C.; Felschau, I.; Sand, W. Some quantitative data on bacterial attachment to pyrite. Miner. Eng. 2011, 24, 1132–1138. [Google Scholar] [CrossRef]
- Zhang, R.; Bellenberg, S.; Castro, L.; Neu, T.R.; Sand, W.; Vera, M. Colonization and biofilm formation of the extremely acidophilic archaeon Ferroplasma acidiphilum. Hydrometallurgy 2014, 150, 245–252. [Google Scholar] [CrossRef]
- González, R.; Gentina, J.; Acevedo, F. Attachment behaviour of Thiobacillus ferrooxidans cells to refractory gold concentrate particles. Biotechnol. Lett. 1999, 21, 715–718. [Google Scholar] [CrossRef]
- Maulani, N. Interaction of the Acidophilic Archaeon Ferroplasma acidiphilum with Leaching Bacteria during Initial Biofilm Formation on Pyrite. Master’s Thesis, Universität Duisburg-Essen, Essen, Germany, 2015. [Google Scholar]
- Zhang, R.; Zhang, Y.; Neu, T.R.; Li, Q.; Bellenberg, S.; Sand, W.; Vera, M. Initial attachment and biofilm formation of a novel crenarchaeote on mineral sulfides. Adv. Mater. Res. 2015, 1130, 127–130. [Google Scholar] [CrossRef]
- Castro, C.; Vera, M.; Donati, E.; Sand, W. Visualization of attachment and colonization of pyrite surfaces by a novel species of Acidianus. Adv. Mater. Res. 2013, 825, 70–73. [Google Scholar] [CrossRef]
- Castro, C.; Donati, E. Effects of different energy sources on cell adhesion and bioleaching of a chalcopyrite concentrate by extremophilic archaeon Acidianus copahuensis. Hydrometallurgy 2016, 162, 49–56. [Google Scholar] [CrossRef]
- Gehrke, T.; Hallmann, R.; Kinzler, K.; Sand, W. The EPS of Acidithiobacillus ferrooxidans—A model for structure-function relationships of attached bacteria and their physiology. Water Sci. Technol. 2001, 43, 159–167. [Google Scholar] [PubMed]
- Afzal Ghauri, M.; Okibe, N.; Barrie Johnson, D. Attachment of acidophilic bacteria to solid surfaces: The significance of species and strain variations. Hydrometallurgy 2007, 85, 72–80. [Google Scholar] [CrossRef]
- Africa, C.-J.; van Hille, R.P.; Sand, W.; Harrison, S.T.L. Investigation and in situ visualisation of interfacial interactions of thermophilic microorganisms with metal-sulphides in a simulated heap environment. Miner. Eng. 2013, 48, 100–107. [Google Scholar] [CrossRef]
- Bellenberg, S.; Barthen, R.; Boretska, M.; Zhang, R.; Sand, W.; Vera, M. Manipulation of pyrite colonization and leaching by iron-oxidizing Acidithiobacillus species. Appl. Microbiol. Biotechnol. 2015, 99, 1435–1449. [Google Scholar] [CrossRef] [PubMed]
- Aguirre Morales, M. Extracellular Polymeric Substances (EPS) Production in Sulfobacillus thermosulfidooxidans and Its Relevance on Attachment to Metal Sulfides. Master’s Thesis, Universidad Nacional de Colombia, Bogota, Colombia, 2013. [Google Scholar]
- Baier, R. Influence of the Initial Surface Condition of Materials on Bioadhesion. In Proceedings of the Third International Congress on Marine Corrosion and Fouling, Gaithersburg, MD, USA, 2–6 October 1973; pp. 633–639.
- Bhosle, N.B.; Garg, A.; Fernandes, L.; Citon, P. Dynamics of amino acids in the conditioning film developed on glass panels immersed in the surface seawaters of Dona Paula Bay. Biofouling 2005, 21, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Sand, W.; Gehrke, T. Analysis and function of the EPS from the strong acidophile Thiobacillus ferrooxidans. In Microbial Extracellular Polymeric Substances; Springer: Berlin, Germany, 1999; pp. 127–141. [Google Scholar]
- Neu, T.R. Significance of bacterial surface-active compounds in interaction of bacteria with interfaces. Microbiol. Rev. 1996, 60, 151. [Google Scholar] [PubMed]
- Starkey, R.; Jones, G.; Frederick, L. Effects of medium agitation and wetting agents on oxidation of sulphur by Thiobacillus thiooxidans. J. Gen. Microbiol. 1956, 15, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Neu, T.R.; Zhang, Y.; Bellenberg, S.; Kuhlicke, U.; Li, Q.; Sand, W.; Vera, M. Visualization and analysis of EPS glycoconjugates of the thermoacidophilic archaeon Sulfolobus metallicus. Appl. Microbiol. Biotechnol. 2015, 99, 7343–7356. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Bhosle, N.B. Biochemical composition of the marine conditioning film: Implications for bacterial adhesion. Biofouling 2009, 25, 13–19. [Google Scholar] [CrossRef] [PubMed]
- De Kerchove, A.J.; Elimelech, M. Impact of alginate conditioning film on deposition kinetics of motile and nonmotile Pseudomonas aeruginosa strains. Appl. Environ. Microbiol. 2007, 73, 5227–5234. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Sand, W.; Zhang, R. Enhancement of biofilm formation on pyrite by Sulfobacillus thermosulfidooxidans. Minerals 2016, 6, 71. [Google Scholar] [CrossRef]
- Gehrke, T.; Telegdi, J.; Thierry, D.; Sand, W. Importance of extracellular polymeric substances from Thiobacillus ferrooxidans for bioleaching. Appl. Environ. Microbiol. 1998, 64, 2743–2747. [Google Scholar] [PubMed]
- Norris, P.; Owen, J. Mineral sulphide oxidation by enrichment cultures of novel thermoacidophilic bacteria. FEMS Microbiol. Rev. 1993, 11, 51–56. [Google Scholar] [CrossRef]
- Norris, P. Factors affecting bacterial mineral oxidation: The example of carbon dioxide in the context of bacterial diversity. In Proceedings of the International Biohydrometallurgical Symposium, Jakson Hole, WY, USA, 13–18 August 1989; pp. 3–14.
- Giaveno, M.A.; Urbieta, M.S.; Ulloa, J.R.; Toril, E.G.; Donati, E.R. Physiologic versatility and growth flexibility as the main characteristics of a novel thermoacidophilic Acidianus strain isolated from Copahue geothermal area in Argentina. Microb. Ecol. 2013, 65, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Vardanyan, A.; Stepanyan, S.; Vardanyan, N.; Markosyan, L.; Sand, W.; Vera, M.; Zhang, R. Study and assessment of microbial communities in natural and commercial bioleaching systems. Miner. Eng. 2015, 81, 167–172. [Google Scholar] [CrossRef]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Li, Q.; Sand, W.; Zhang, R. Influence of Sulfobacillus thermosulfidooxidans on Initial Attachment and Pyrite Leaching by Thermoacidophilic Archaeon Acidianus sp. DSM 29099. Minerals 2016, 6, 76. https://doi.org/10.3390/min6030076
Liu J, Li Q, Sand W, Zhang R. Influence of Sulfobacillus thermosulfidooxidans on Initial Attachment and Pyrite Leaching by Thermoacidophilic Archaeon Acidianus sp. DSM 29099. Minerals. 2016; 6(3):76. https://doi.org/10.3390/min6030076
Chicago/Turabian StyleLiu, Jing, Qian Li, Wolfgang Sand, and Ruiyong Zhang. 2016. "Influence of Sulfobacillus thermosulfidooxidans on Initial Attachment and Pyrite Leaching by Thermoacidophilic Archaeon Acidianus sp. DSM 29099" Minerals 6, no. 3: 76. https://doi.org/10.3390/min6030076