Effect of Particle Size and Grinding Time on Gold Dissolution in Cyanide Solution
Abstract
:1. Introduction
2. Objective and Experimental Procedure
2.1. Objective of the Test Work
2.2. Ore Characteristics
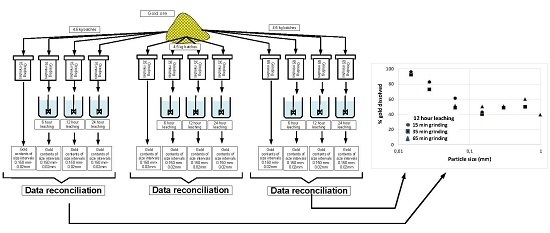
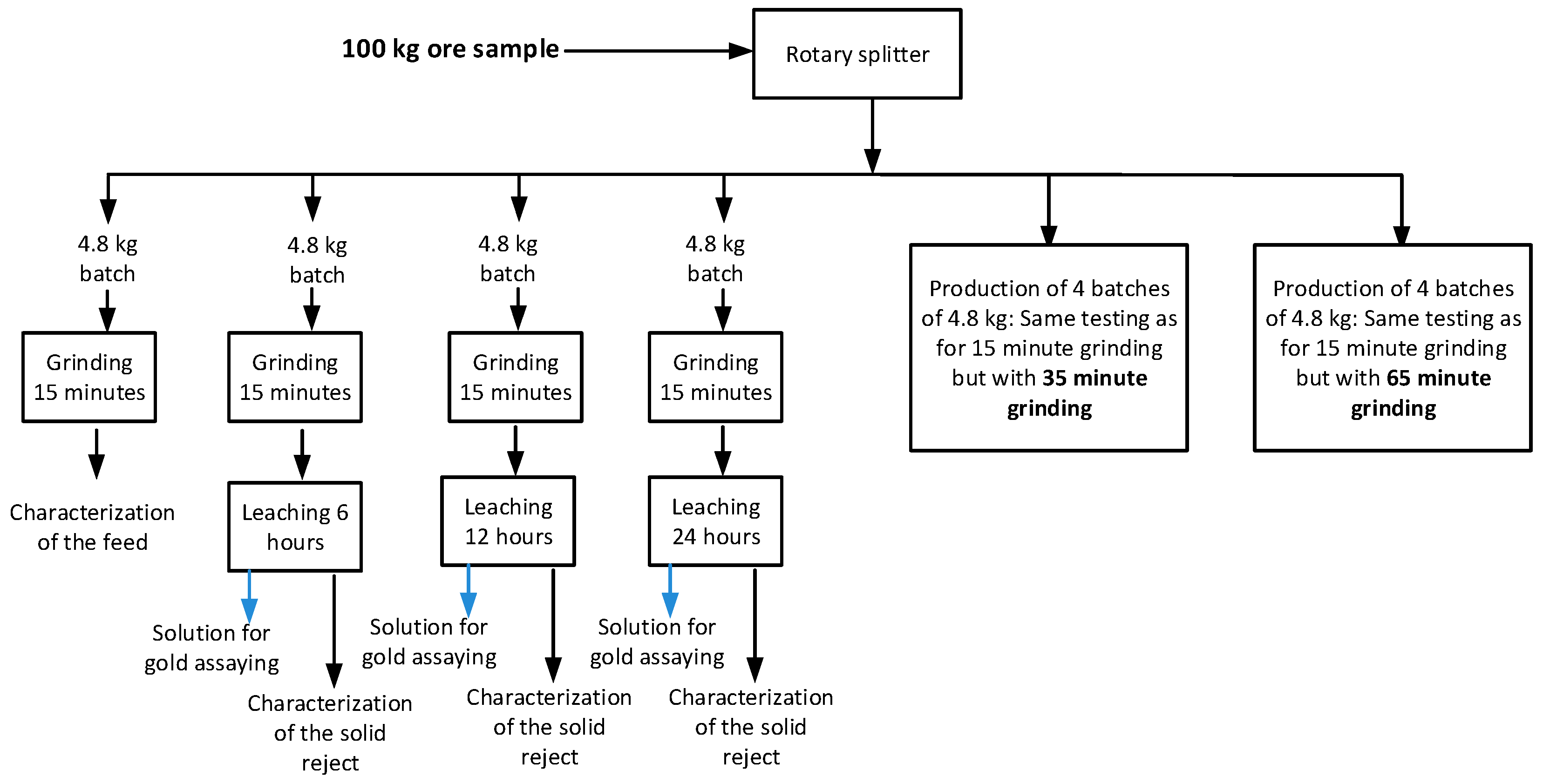
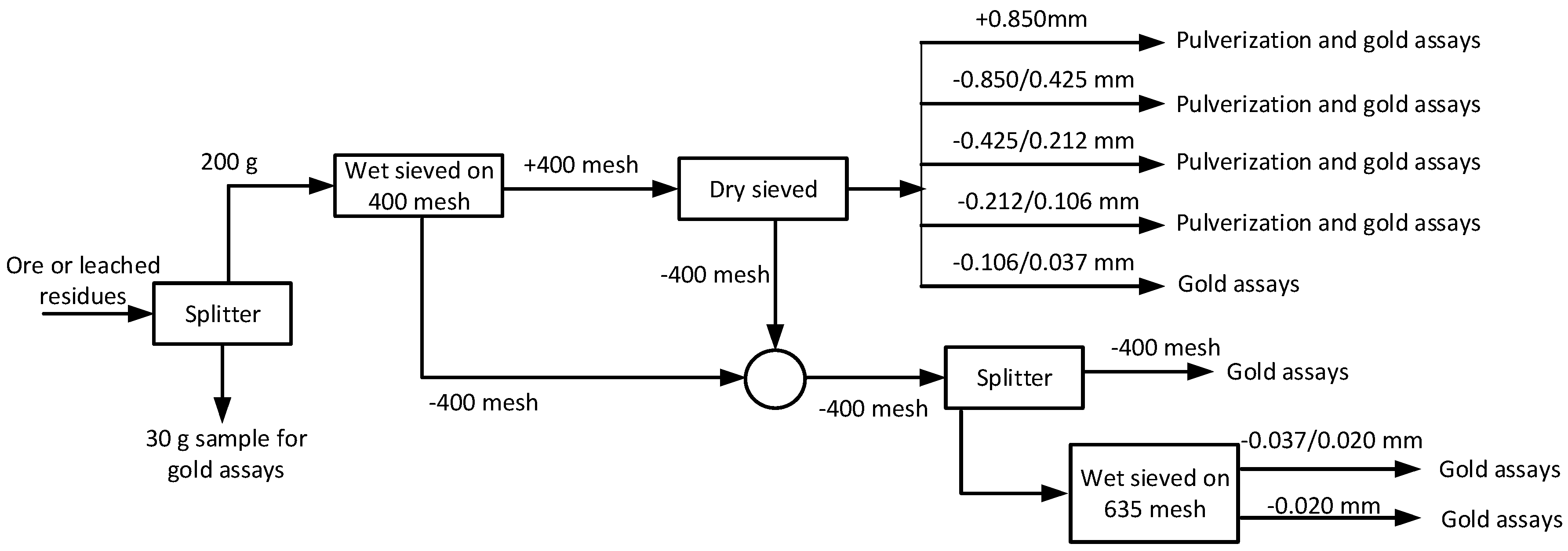
2.3. Experimental Procedure
3. Analysis of the Results
3.1. Measurements
3.2. Global Gold Dissolution
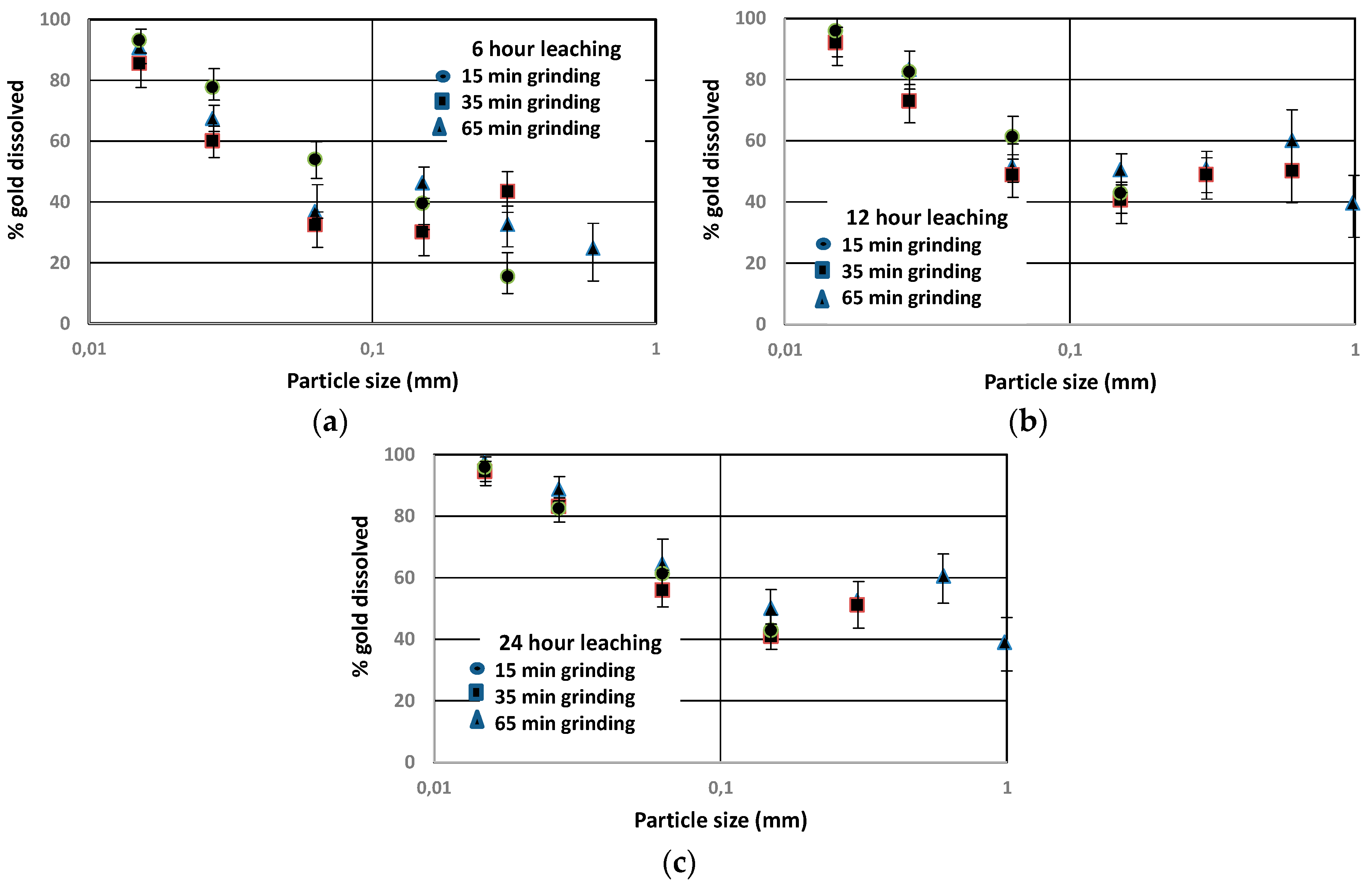
3.3. Gold Dissolution as a Function of Particle Size
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Marsden, J.O.; House, C.I. The Chemistry of Gold Extraction; Society for Mining, Metallurgy, and Exploration (SME): Englewood, CO, USA, 2006. [Google Scholar]
- Deschênes, G.; Fleming, C.; Zhou, J.; Hodouin, D.; Amankwah, R.; Ghali, E.; Choi, Y. Highlights of the Past Five Decades of Gold Ore Processing in Canada. In Proceedings of the 50th World Gold Conference, Montreal, QC, Canada, 2–5 October 2011; Deschênes, G., Ed.; Canadian Institute of Mining, Metallurgy and Petroleum (CIM): Montreal, QC, Canada, 2011; pp. 20–45. [Google Scholar]
- Free, M.L. Modelling of heterogeneous material performance. Can. Metall. Q. 2008, 47, 277–284. [Google Scholar] [CrossRef]
- Bouffard, S.C.; Dixon, D.G. Evaluation of kinetic and diffusion phenonmena in cyanide leaching of crushed and run-of-mine gold ores. Hydrometallurgy 2007, 86, 63–71. [Google Scholar] [CrossRef]
- De Andrade Lima, L.R.P.; Hodouin, D. A lumped kinetic model for gold ore cyanidation. Hydrometallurgy 2005, 3–4, 121–137. [Google Scholar] [CrossRef]
- Bellec, S.; Hodouin, D.; Bazin, C.; Khalesi, M.R.; Duchesne, C. Hydrocyclone classification modeling for gold ore grinding circuit simulation. In Proceedings of the 50th World Gold Conference, Montreal, QC, Canada, 2–5 October 2011; Deschênes, G., Ed.; Canadian Institute of Mining, Metallurgy and Petroleum (CIM): Montreal, QC, Canada, 2011; pp. 150–162. [Google Scholar]
- Deschênes, G.; Fulton, M.; Smith, C. A new tool to improve lead nitrate control in cyanidation. In Proceedings of the 39th Annual Meeting of the Canadian Mineral Processors, Ottawa, ON, Canada, 23–25 January 2007; pp. 231–248.
- Gy, P. Sampling of Particulate Materials: Theory and Practice; Elsevier: Amsterdam, The Netherlands, 1979. [Google Scholar]
- Bazin, C.; Hodouin, D.; Blondin, M.R. Estimation of the variance of the fundamental error associated to the sampling of low grade ores. Int. J. Miner. Process. 2013, 124, 117–123. [Google Scholar] [CrossRef]
- Egan, J.; Bazin, C.; Hodouin, D. Effect of ore particle size on gold dissolution in a cyanide solution. In Proceedings of the SME Annual Meeting, Denver, CO, USA, 24–27 February 2013.
- Hodouin, D.; Flament, F.; Bazin, C. Reliability of material balance calculations—A sensitivity approach. Miner. Eng. 1989, 2, 157–170. [Google Scholar] [CrossRef]
- Gagnon, C.; Landry, G.; Ourriban, M.; Pelletier, P.; Bouajila, A. Implementation of Cyanidation by Size Class at Sleeping Giant Mill. In Proceedings of the 41st Annual Meeting of the Canadian Mineral Processors, Ottawa, ON, Canada, 22 January 2009.
| Elements | Minerals | ||
|---|---|---|---|
| Au | 10.4 g/t | Quartz, albite, phogopite | 58.5% |
| Ag | 10.5 g/t | Calcite, chlinochlore | 31.1% |
| Si | 26.0% | Pyrite | 3.2% |
| Fe | 8.0% | Pyrrhotite | 0.8% |
| Al | 6.0% | Chalcopyrite | 0.4% |
| Ca | 4.4% | Sphalerite | 0.1% |
| Grinding Time (min) | D80 (mm) | % <0.037 mm (−400 mesh) | % <0.106 mm (−150 mesh) | % <0.212 mm (−65 mesh) |
|---|---|---|---|---|
| 15 | 0.850 | 21 | 26 | 40 |
| 35 | 0.318 | 25 | 33 | 51 |
| 65 | 0.106 | 35 | 49 | 76 |
| Material | Note | Retained % | Non Leached Au(g/t) | 6 h Leach Au(g/t) | 12 h Leach Au(g/t) | 24 h Leach Au(g/t) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Meas. | Std | Meas. | Std | Meas. | Std | Meas. | Std | Meas. | Std | ||
| Calculated Feed | a | - | - | 12.38 | - | 12.05 | 1.60 | 12.64 | 1.70 | 11.43 | 1.60 |
| Solution | - | - | - | 0.00 | - | 3.66 | 0.35 | 5.25 | 0.52 | 5.42 | 0.55 |
| Measured Head (solids) | - | - | - | 12.38 | 1.65 | 8.23 | 0.85 | 7.16 | 0.81 | 5.77 | 0.65 |
| Calculated Head (solids) | b | - | - | 13.26 | - | 8.67 | - | 5.99 | - | 6.25 | - |
| +0.850 mm | - | 1.0 | 0.2 | 0.41 | 0.14 | 0.47 | 0.16 | 0.55 | 0.19 | 1.29 | 0.45 |
| 0.850/0.425 mm | - | 8.5 | 1.2 | 11.54 | 2.31 | 14.52 | 2.90 | 5.67 | 1.13 | 14.49 | 2.90 |
| 0.425/0.212 mm | - | 21.5 | 0.6 | 11.64 | 1.75 | 8.78 | 1.32 | 5.80 | 0.87 | 5.67 | 0.85 |
| 0.212/0.106 mm | - | 18.1 | 0.5 | 18.75 | 1.88 | 13.16 | 1.32 | 11.02 | 1.10 | 11.31 | 1.13 |
| 0.106/0.037 mm | - | 18.2 | 0.5 | 21.05 | 1.68 | 14.27 | 1.14 | 10.72 | 0.86 | 9.30 | 0.74 |
| 0.037/0.020 mm | - | 8.6 | 0.4 | 14.90 | 0.75 | 5.92 | 0.30 | 4.04 | 0.20 | 2.58 | 0.13 |
| −0.020 mm | - | 24.1 | 1.2 | 4.10 | 0.16 | 0.58 | 0.02 | 0.32 | 0.01 | 0.22 | 0.01 |
| Measured −0.037 mm | - | - | - | 6.65 | 0.33 | 2.00 | 0.10 | 1.10 | 0.06 | 0.71 | 0.04 |
| Calculated −0.037 mm | c | - | - | 7.37 | - | 2.02 | - | 1.25 | - | 0.77 | - |
| Material | Ore Retained | Gold Content (g/t) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 h | 6 h | 12 h | 24 h | |||||||
| Obs. | Rec. | Obs. | Rec. | Obs. | Rec. | Obs. | Rec. | Obs. | Rec. | |
| Feed Sample | - | - | - | 12.63 | - | 11.74 | - | 11.40 | - | 11.74 |
| Solution | - | - | 0.00 | 0.00 | 3.66 | 3.64 | 5.25 | 5.21 | 5.42 | 5.37 |
| Residues | - | - | 12.38 | 12.63 | 8.23 | 7.94 | 7.16 | 5.96 | 5.77 | 6.13 |
| +0.850 mm | 1.0 | 0.9 | 0.41 | 0.41 | 0.47 | 0.47 | 0.55 | 0.55 | 1.29 | 1.29 |
| 0.850/0.425 mm | 8.5 | 7.6 | 11.54 | 11.36 | 14.52 | 14.29 | 5.67 | 5.66 | 14.49 | 14.13 |
| 0.425/0.212 mm | 21.5 | 21.3 | 11.64 | 11.31 | 8.78 | 6.39 | 5.80 | 5.77 | 5.67 | 5.52 |
| 0.212/0.106 mm | 18.1 | 17.9 | 18.75 | 18.49 | 13.16 | 12.91 | 11.02 | 10.97 | 11.31 | 10.92 |
| 0.106/0.037 mm | 18.2 | 17.9 | 21.05 | 20.95 | 14.27 | 14.15 | 10.72 | 10.71 | 9.30 | 9.22 |
| 0.037/0.020 mm | 8.6 | 8.2 | 14.90 | 14.89 | 5.92 | 5.94 | 4.04 | 4.01 | 2.58 | 2.50 |
| −0.020 mm | 24.1 | 26.2 | 4.10 | 4.09 | 0.58 | 0.59 | 0.32 | 0.32 | 0.22 | 0.22 |
| −0.037 mm | - | - | 6.65 | 6.66 | 2.00 | 1.86 | 1.10 | 1.19 | 0.71 | 0.76 |
| Grinding Time (min) | Feed | 6 h Leaching | 12 h Leaching | 24 h Leaching | |||
|---|---|---|---|---|---|---|---|
| % Dissolved | Std | % Dissolved | Std | % Dissolved | Std | ||
| 15 | 0.0 | 26.3 | 2.7 | 47.5 | 5.3 | 50.8 | 5.5 |
| 35 | 0.0 | 32.4 | 3.4 | 47.7 | 5.1 | 47.8 | 4.8 |
| 65 | 0.0 | 45.8 | 5.1 | 46.8 | 5.2 | 64.5 | 5.1 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egan, J.; Bazin, C.; Hodouin, D. Effect of Particle Size and Grinding Time on Gold Dissolution in Cyanide Solution. Minerals 2016, 6, 68. https://doi.org/10.3390/min6030068
Egan J, Bazin C, Hodouin D. Effect of Particle Size and Grinding Time on Gold Dissolution in Cyanide Solution. Minerals. 2016; 6(3):68. https://doi.org/10.3390/min6030068
Chicago/Turabian StyleEgan, Jessica, Claude Bazin, and Daniel Hodouin. 2016. "Effect of Particle Size and Grinding Time on Gold Dissolution in Cyanide Solution" Minerals 6, no. 3: 68. https://doi.org/10.3390/min6030068