1. Introduction
The term kaolin has a different meaning to geologists, mineralogists, and ceramists. To geologists, is a rock comprised primarily of one of the kaolin groups of clay minerals. To mineralogists, the term refers to the group for the kaolin minerals; namely kaolinite, halloysite, dickite, and nacrite. To ceramists, the term is synonymous with “china clay” meaning that it is a white ceramic raw material. Industrially, the term refers to a clay consisting of substantially pure kaolinite, or related clay minerals. They are of natural origin, or can be beneficiated to reach white or nearly white color. Its beneficiation by known methods is desirable to make it suitable for use in a white product such as paper, rubbers, paints, etc. [
1,
2].
Clays possess organic matter, generally lignite, as well as other colloidal minerals that confer them its characteristic colors. The red clays have ferric oxide, in minerals such as hematite, magnetite, and amorphous hydrated oxides, among others. Iron is the main contaminant in minerals of clay and kaolin. The removal of the iron from kaolin is of particular importance in the paper industry, among others, where purity requirements are high. In these minerals, iron may or may not form part the kaolinite crystalline lattice. When iron is inside the lattice, low concentrations do not affect coloration [
3]. The iron oxyhydroxides are often deposited along with the kaolinite, contaminating and making much of the kaolin unusable for commercial applications due to the leaking of whiteness. On the other hand, other secondary minerals accompanying the clay and kaolin such as hematite, maghemite, and pyrite, iron can be removed by magnetic separation [
4,
5]. According to Mexican Geological Service studies, the kaolin reserves in the state of Hidalgo exceed 25 million tons; those of the neighboring states of Puebla and Veracruz are unquantified. Conduct leaching studies for bleaching kaolin in this region opens the way to applied research with the aim that in the medium term can be an impetus for the economic development of these localities currently considered to be in high poverty.
Different inorganic and organic acids or complexing reagents have been used in the dissolution of iron from these kinds of kaolin minerals. Most of these are focused on the mechanism of dissolution on hematite and magnetite, testing different acids and experimental conditions [
6,
7]. Different researchers have used oxalic acid for the dissolution of iron in the kaolin [
6,
7,
8,
9,
10]. Early works, phosphoric acid was reported as leaching iron in kaolin clay [
11]. Similarly, the influence of phosphoric acid in iron removal from quartz sand bleaching was also studied [
12]. Also, statistical analysis was conducted to determine the most influential factors in the dissolution with organics acids used as a leaching reagent to remove iron from a kaolin mineral [
13,
14]. Recently, the general concepts and fundamentals of the biological dissolution of iron from kaolin clays had been studied by Hosseini et al. [
15]. Among the organic and inorganic acids [
6,
7,
8,
9,
10,
11,
12,
13,
16], the biological beneficiation, [
15,
17,
18] a combination of both [
19,
20,
21] and the anionic surfactants, the first are the most common reagents to remove iron oxides and oxyhydroxides contained in kaolin clay [
22]. However, goethite species have been removed with anionic surfactants, increasing the whiteness index [
23]. The advantages of using phosphoric acid as the leaching agent if compared with other acids are: the whiteness index that is achieved is higher, they have rapid dilution in water, and simple operation as well as ease of adding directly to the pulp. Bioleaching is an environmentally friendly method and it is also an alternative; however, bacteria are usually quite sensitive to changes in the environment in which they develop. It is crucial to consider some factors such as atmosphere, temperature, and pH which leads to a more complex process.
Considerable attention has been to the studies of mechanisms of dissolution, which employ synthetic minerals such as hematite, goethite, and magnetite and ignoring the complex interactions that can take place in iron dissolution from industrial minerals such as kaolin. The contribution of kinetics data for industrial-use minerals like kaolin clays it is of high importance as they are clays with complex interactions, variable iron content, and in other words very different each other due to the different geological origin they have. The kinetic data for the iron extraction from kaolin clay using phosphoric acid are very important for industrial applications such as the supply of raw material for the industry of paper and the ceramic. The aim of this work was to examine the iron leaching kinetics from kaolin clay using phosphoric acid solutions.
2. Experimental
For performing the experiments, Kaolin from the city of Agua Blanca Iturbide, Hidalgo State, Mexico, was used. This mineral was firstly grinded and classified by particle size. For the experimental procedure, particles of average size of 35 μm were used. To characterize the mineral samples (Mineralogy and composition) X-ray diffraction (XRD) and Atomic Absorption Spectroscopy (AAS) were used.
The leaching experiments were carried out in a round bottom reactor (500 mL) with mechanical stirring and constant temperature. The temperature was regulated with a thermo-regulator, which is part of the equipment. In the leaching tests at 94 °C, the reactor, equipped with a thermometer and a reflux condenser, was heated with a thermostatically controlled heating mantle. All leaching tests were made at atmospheric pressure for 120 min.
Two different experiments were carried out. Firstly, the effect of the concentration (0.1, 0.5, 1.0, and 3.0 M) of the phosphoric acid at a fixed temperature of 367.15 K (94 °C) was evaluated, adding the kaolin to the solution. Secondly, the acid concentration was fixed at 3.0 M varying the temperature in between 273 to 353 K. For both experiments, the pH was kept constant at 1.0 adding small amounts of saturated NH
4OH or H
2SO
4 solutions. Early works confirmed that optimum dissolution rate is at a pH value of 1, approximately [
1]. The progress of extraction reaction was carried out by taking samples from solution throughout the experiments, and testing for Fe content by atomic absorption spectrometry. Variations in the mass balance due to the sampling take and the addition of reagent were taken into account for the calculations.
3. Results and Discussion
The results found through mineralogical and chemical analysis of the kaolin are shown in
Table 1,
Figure 1 and
Figure 2. The chemical composition is shown in
Table 1. The values of SiO
2, Al
2O
3 and low contents of Fe
3O
4, indicates that this material has an ideal composition for the leaching kinetic study.
Figure 1 shows the X-ray diffraction spectrum; for (a) natural kaolin mineral and (b) leached kaolin; upon a comparison of both diffractograms, it is possible to appreciate the reduction of the compound of iron oxyhydroxide (FH). A major peak corresponding to kaolinite can be appreciated, with minor signals of silica in the forms of quartz and tridymite.
For this work, the phosphoric acid has been used as leaching reagent due to their theoretical advantage for this use. Regarding the iron leaching, hydrogen ions play an important role in the dissolution of iron oxides with inorganic acids. The H
3PO
4 in solution provides H
+, that reacts with iron oxide, and the possible reaction according Zhang et al. [
12], might be:
These hydrogen ions are adsorbed on the activated surface of the iron oxyhydroxide species. The hydrogen ions are adsorbed on sites of the solid surface, thus creating a surface of active centers on which the main reaction of dissolution takes place. As the hydrogen ion concentration in the solution increases, the amount of adsorbed hydrogen ions also increases, according to the adsorption theory. An increase in the number of active centers results in a corresponding increase in the dissolution rate [
3].
The H3PO4 not only provides more H+ ions (pKa1 = 2.12), which will react with iron oxide; but also provides PO43− ions, which are produced while the H+ is being ionized, and also have a larger complexing ability to iron ions. All these features lead to high leaching percentages of the H3PO4.
The possible reaction according to Zhang et al. [
12], might be:
These multiple synergisms of the H
3PO
4, lead to the best leaching results [
2].
4. Kinetic Study
The results obtained during kinetics study of iron extraction, are related with the effect of phosphoric acid concentration and temperature in the range 298 to 367 K and were treated with the shrinking core model, for heterogeneous reactions both for diffusive and chemical control.
Extraction of iron from kaolin clay can be explained with the shrinking core model [
4], in which:
If diffusion controls the reaction rate through an inert product layer, the integrated rate equation is as follows:
The above equation represents the shrinking core model for diffusive control. Additionally, if the reaction is controlled by a chemical reaction, Equation (4) is transformed to Equation (5) [
24].
where
X is the fraction that has reacted,
t is the reaction time and
Kd and
Kc are the rate constants, which are calculated from Equations (4) and (5), respectively.
5. Effect of the Acid Concentration
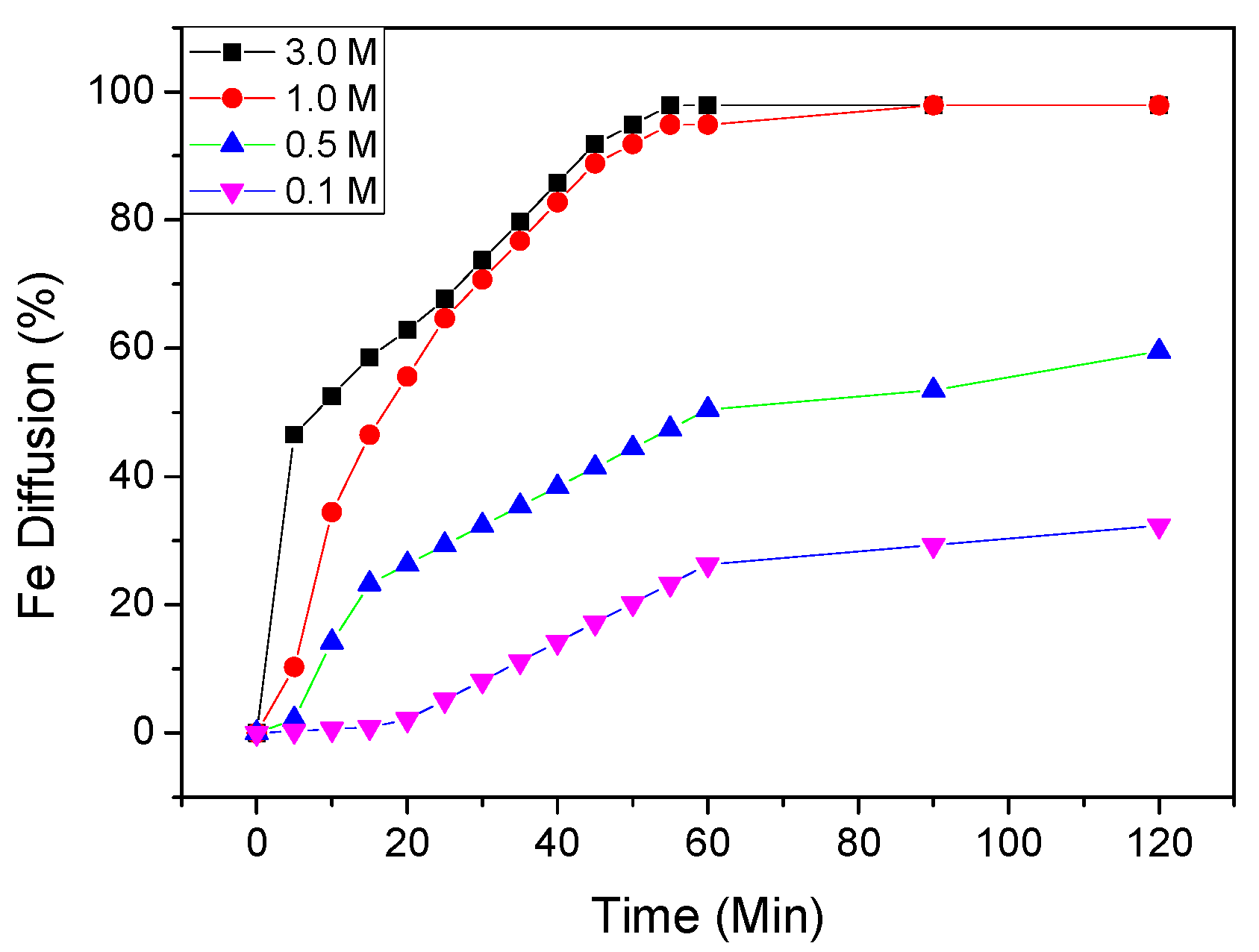
In order to observe the effect of phosphoric acid concentration in the range of 0.10, 0.50, 1.0 and 3.0 M, experiments were performed in the temperatures ranging of 298 to 367 K. The results are shown in
Figure 2. It shows that increasing phosphoric acid concentration increases the iron dissolution rate in the kaolin clay, especially in lower concentrations.
It was also observed that the leaching rate of iron at 1.0 M is higher than at 0.10 M; the iron extractions were of 98.65% and 19.81%, respectively.
The results found in this study are in good accordance with another study [
2], where the high efficiency of iron removal was reported; but unlike our study, this work was carried out in quartz sands, using phosphoric acid.
The apparent rate constants
Kd, and
Kc obtained from Equations (4) and (5), and the regression coefficients, were calculated from the graph at each concentration. The graph of Equation (4) (
Figure 3a) yield straight lines with a global regression coefficient
R2 equal to 0.99 for phosphoric acid concentrations of 3.0 M. The
Figure 3b displays the graph of the Equation (5), it exhibits straight lines with a regression coefficient
R2 higher than 0.98 showing a slight deviation from linearity. These values of regression coefficient show that iron dissolution from kaolin clay, is controlled by diffusion through an inert product layer.
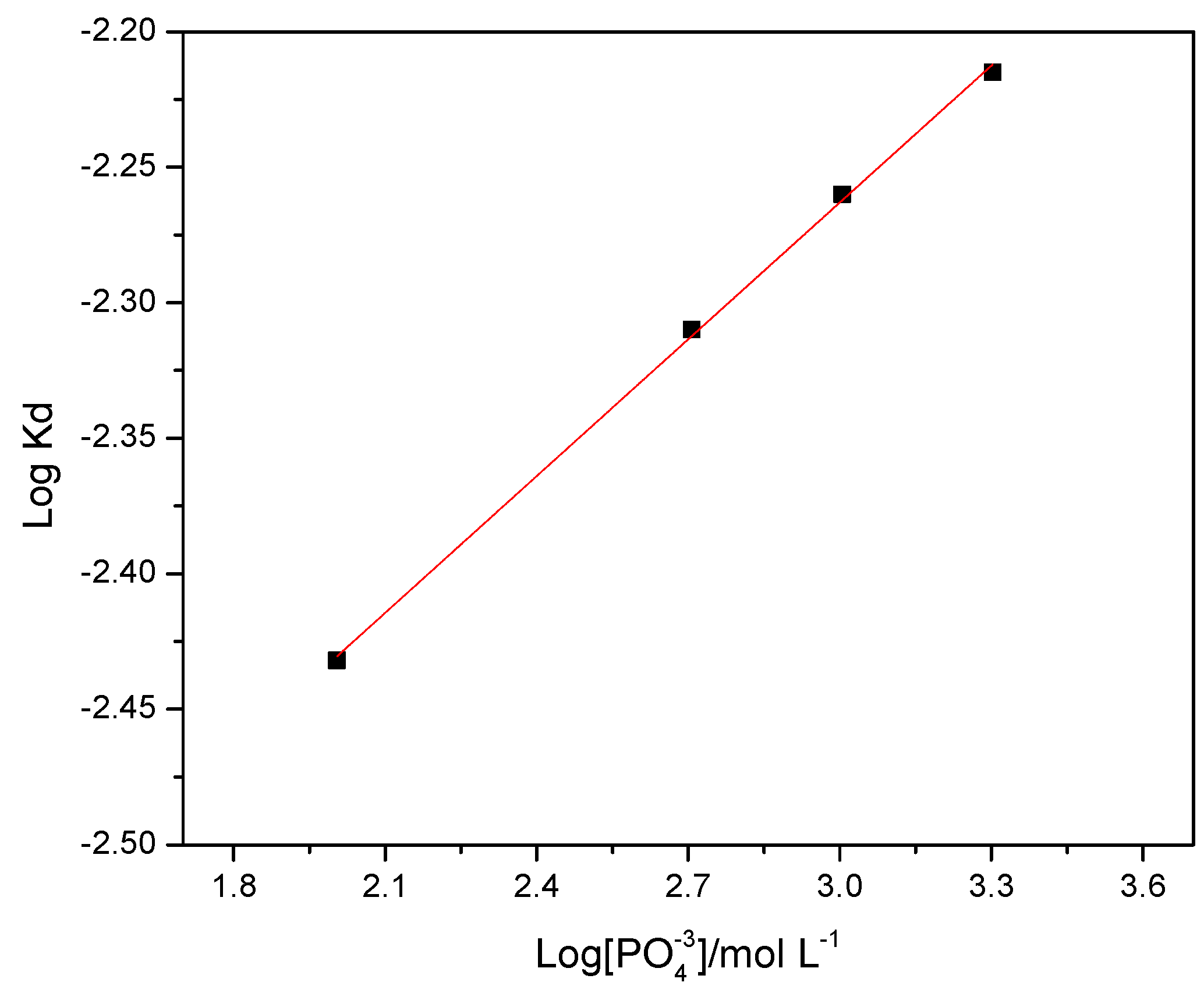
The obtained slopes for the graph were calculated according to the model 1 − (2
X/3) − (1 −
X)
2/3, from which a graph of log(
Kd) vs. log[PO
43−] was obtained as can be seen further in
Figure 4. A straight line from this graph gave a slope from where was calculated a reaction order of
n = 0.168, according to the Equation (4) and that is shown in
Figure 3.
6. Effect of Temperature
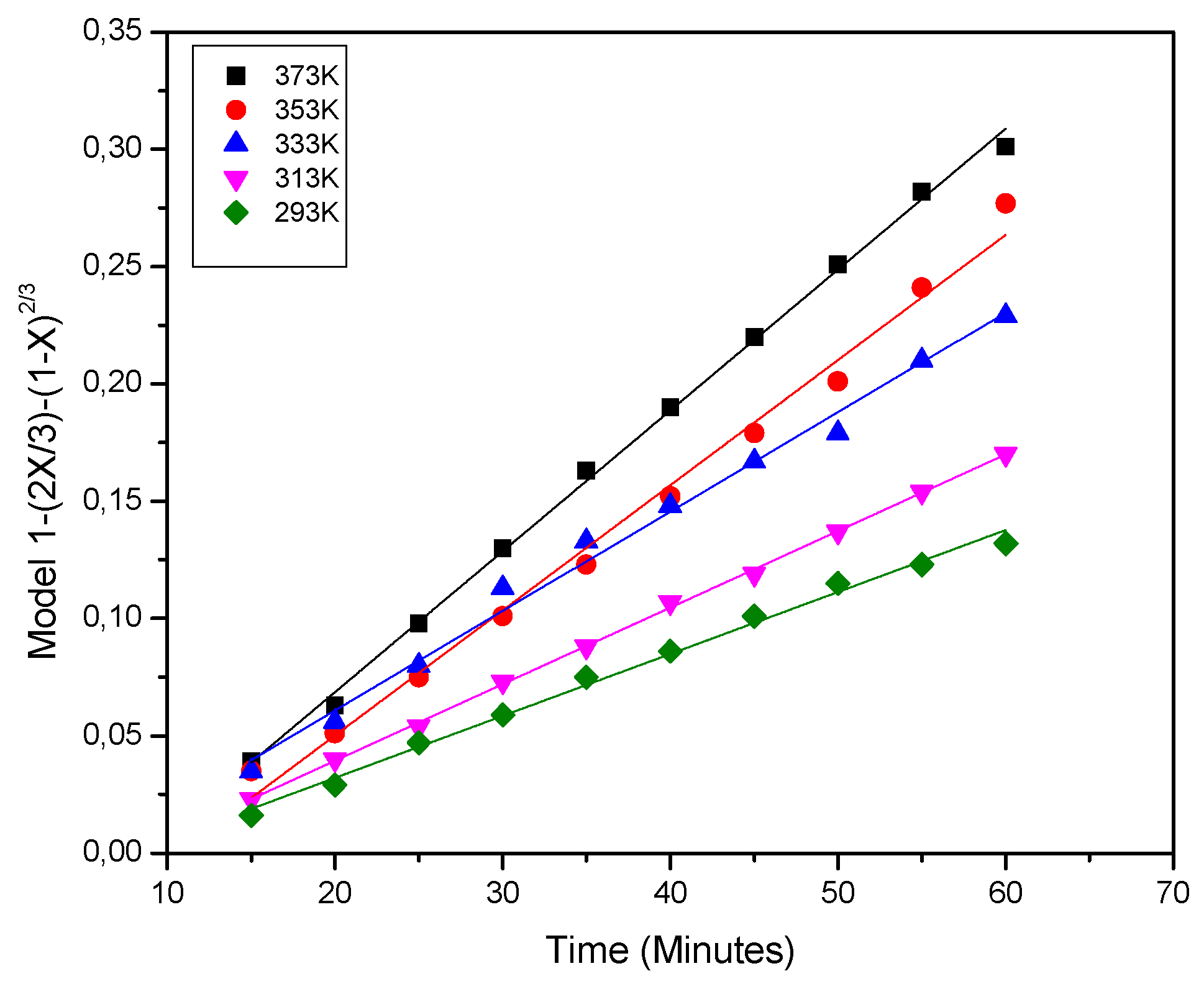
With the aim of determining the effect of temperature in the kinetics of the iron extraction, several experiments in an acid solution 0.3 M were performed, varying the temperature in the range from 298 to 373 K. Typical rate curves with a strong temperature dependence were found. It denoted a very slow rate in the temperature range of 273 to 353 K exhibiting a very strong growth rate above 353 K. This suggests that it is reasonable to use temperatures above 353 K. That means that iron dissolution using phosphoric acid could be thermally activated to improve its efficiency. The results in
Table 2 show that iron dissolution from kaolin clay in the temperature range of 298 to 373 K, fit well with the diffusion through an inert product layer model shown in Equation (4).
The apparent rate constants,
Kd and
Kc obtained from Equations (4) and (5) and their corresponding regression coefficients were calculated from the graph at each temperature tested. These values were treated with Equation 4, and the resulting data is shown in
Figure 4. The analysis of these graph revealed straight lines for 298, 313, 333, 353 and 373 K with regression coefficients (
R2) of around 0.99 in average. It is important to mention that the results obtained from the graph of the temperature effect were also analyzed by chemical control model (Equation (5)) and the data did not fit well with this model. Therefore, it can be dismissed that the controlling stage being the chemical reaction [
25].
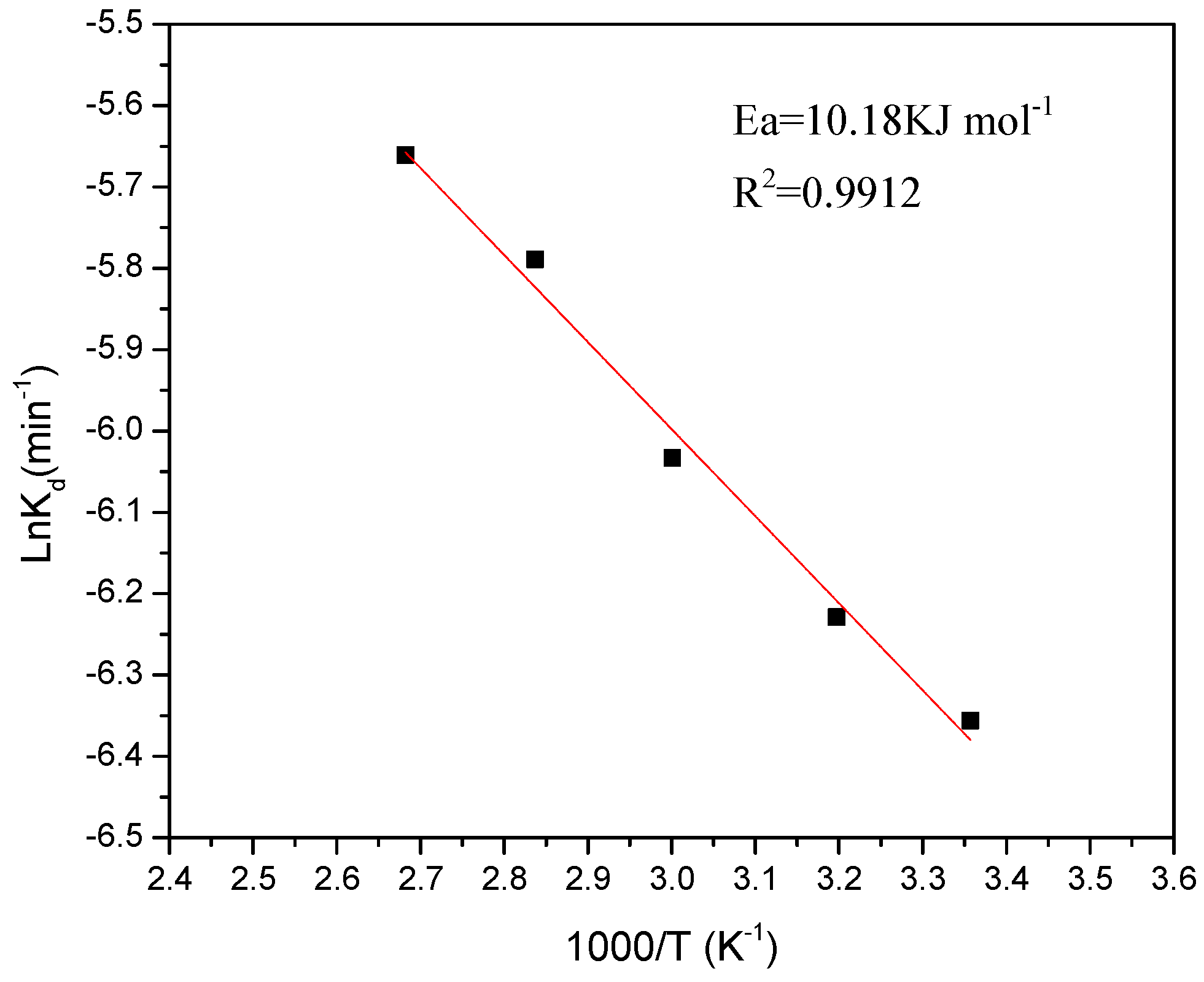
The apparent rate constants,
Kd, for iron dissolution were calculated from the slopes in the graph of
Figure 5. On the other hand, a graph was made, showing the variation of ln
Kd vs. 1000/
T where
T is the temperature in the range of 298 to 373 K and from which yields the straight line shown in the
Figure 6. The regression coefficient
R2 was 0.99. The apparent activation energy calculated from the Arrhenius plot was, 10.18 kJ·mol
−1. This result confirmed that iron dissolution from the kaolin clay is controlled by diffusion through an inert product layer. In fact, this low energy is considered within the stated range for the controlling step of diffusion through a layer of products (below 20 kJ·mol
−1) [
6].
7. Conclusions
In this work, the kinetics of iron dissolution from kaolin clay in phosphoric acid solution was studied. The results showed that the temperature, concentration of phosphoric acid, and particle size, were of importance for the determination of the reaction kinetics. Importantly, the effect of temperature on iron dissolution is more significant at 94 °C. Also, the iron dissolution increased with phosphoric acid concentration and temperature. Leaching data showed that the iron dissolution from kaolin clay occurs by diffusion through the product layer, showing an order of reaction n = 0.168. Finally, the activation energy of the process was found to be 10.18 kJ·mol−1.