Phosphate plays a significant economic role in developing countries because of the increasing demand on phosphate rock for fertilizer production and its importance in animal feed stocks, as well as food-grade phosphates and other industrial uses. The high demand for phosphate is typically fulfilled through mining and processing [
1], an industry which, globally, produced 224 million tons in 2013 and is expected to reach 260 million tons in 2017 [
2]. About 75% of the world’s phosphate rocks are of sedimentary origin and 75%–80% of those contain carbonate gangue [
3]. To meet the needs of the agricultural sector to produce phosphate and chemical fertilizers, several methods have been proposed for mining based on the characteristics and depth of phosphate ore [
4]. Similarly, to concentrate low-grade phosphate ore to a marketable grade (~30% P
2O
5) [
5] several pre-processing and processing methods are defined [
1,
6,
7]. These are based on the ore type, associated gangue minerals and the amount of impurities, as well as factors such as the degree of liberation of apatite minerals, the cost of the beneficiation method [
1,
6]. The methods employed include gravity separation [
6], magnetic separation [
8,
9,
10], electrostatic separation [
11,
12,
13,
14], size reduction, screening [
15], attrition, scrubbing, classification [
7], heavy media separation [
16], calcination [
17], acidic leaching [
18,
19,
20], direct flotation [
21,
22,
23,
24], reverse flotation [
25,
26] or the use of multiple methods. Sedimentary rocks have various chemical and mineralogical compositions in the gangue phase [
16] and, therefore, based on the major associated minerals, the Lar phosphate deposit of southwest Iran is classified as a calcareous ore of sedimentary origin. In terms of processing, conventional techniques such as flotation and physical separation are difficult to remove the carbonate minerals from such ores (because of the similarity in physical properties of carbonates and phosphates) [
6] and Calcination is another solution for upgrading these difficult-to-treat types of ores. Beneficiation by calcination is one of the better known processes which has been proposed in the past to treat carbonate-bearing sedimentary phosphate ores [
27]. Calcination can be an effective mineral concentration method, and is used in the processing and production of more than 10% of global phosphate sales [
28]. Calcination involves the thermal decomposition of carbonates and burning intended organic materials but has several disadvantages [
11,
29], such as high energy costs [
5], low reactivity of final products and the high initial capital cost of calcination plants [
6].
The thermal dissociation of carbonates is an endothermic reaction (Equation 1) with a significant energy requirement.
Furthermore, calcination produces low reactivity of the resulting phosphate [
4] as well as a lower ratio of CaO/P
2O
5 compared with raw francolite [
30]. It should be noted that despite the disadvantages, the calcined phosphate product is used as raw material in the production of phosphoric acid and chemical fertilizers [
31].
Using gravity methods after calcination is an effective process to increase the concentrate grade and leads to a more economical design. There are numerous advantages to gravity separation: low startup cost, low energy consumption during the crushing process, high efficiency, lack of environmental impacts and high selectivity compared with other methods, such as flotation [
32]. The gravity separation method is not applicable to all mineral compounds and requires the determination of a concentration criterion (CC) based on the relationship between the specific gravity (SG) of heavy and light minerals and the fluid (water or air) in which they are found [
33], as follows:
Mineralogical studies have shown that calcite, quartz, apatite (collophane) and glauconite with specific gravities of 2.7, 2.65, 3.2 and 2.4 g/cm
3, respectively, are the primary minerals of the phosphate ore. Consequently, the CC, based on the equation of Taggart (1951) [
33] is 1.29, a value which indicates that gravity separation will not be commercially viable and cannot be used as the only pre-concentration method. Because carbonate is very finely dispersed in phosphate, gravity separation is also ineffective [
16] and the similarity in specific gravities of dolomite, calcite and apatite also render the technique ineffective as the only mineral concentration process. Dependence of the foregoing phenomena it can be stated that this method should be applied together with calcination as an effective process to concentrate the low-grade phosphate ore. In this study the degree of liberation was considered the fundamental selection criterion for screening using the shaking table and, hence, parameters such as deck slope, feed water flow rate and dressing water flow rate were optimized to increase the efficiency of process.
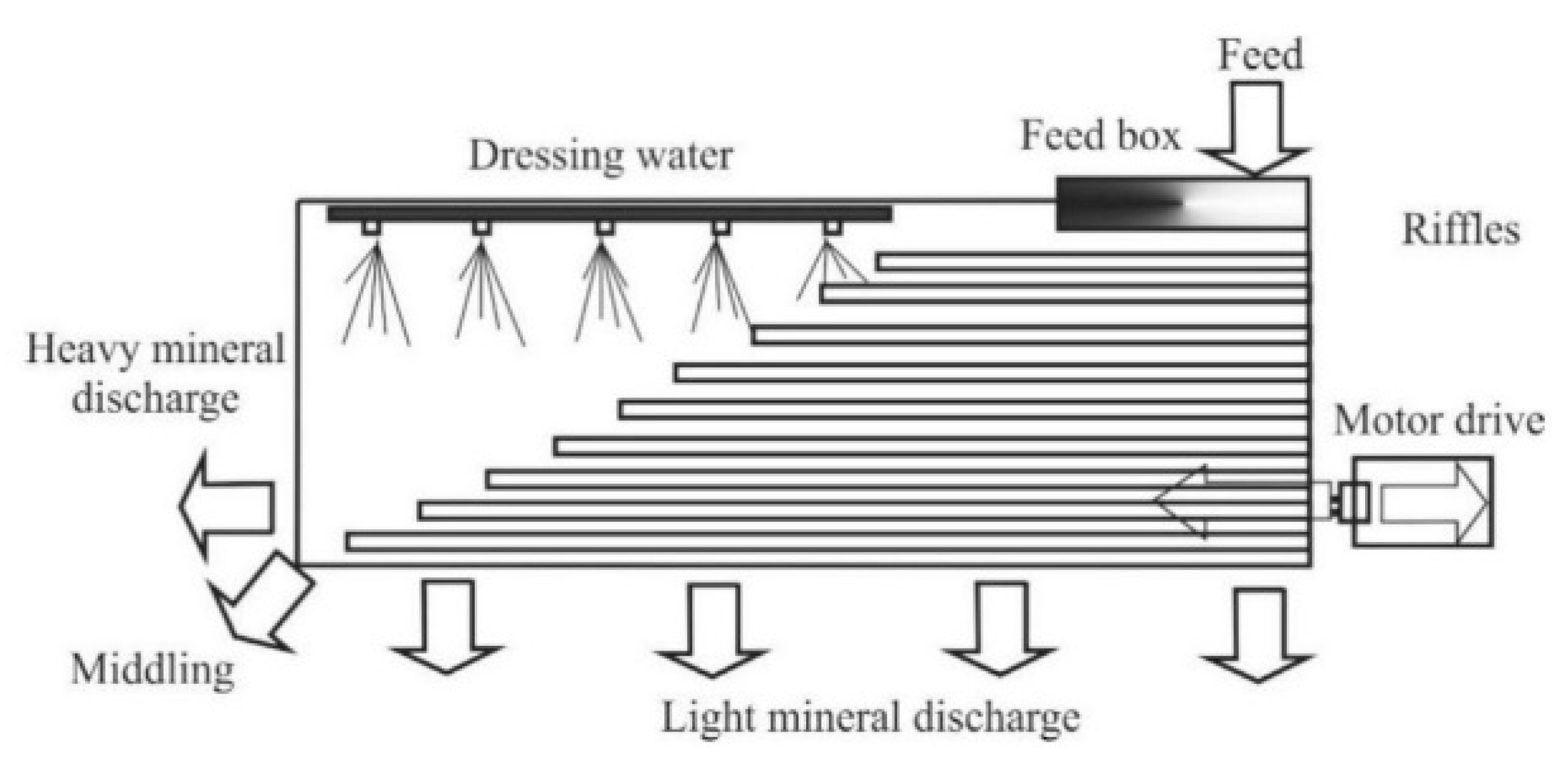
Of the gravitational methods, the use of shaking tables (such as the Wilfley table) is considered an efficient method for ore concentration. In this method, a solid-liquid separation process is based on cross flow of light and heavy particles on an inclined, riffled table as the particles simultaneously spread out. As the table shakes the differential motion, riffled deck and cross flowing water causes particle separation (
Figure 1), with the riffles helping to transmit the shaking motion to the particles as well as preventing direct washing of particles off the table. The vibration is asymmetrical, being slow in the forward direction and quick in the reverse direction. The particle feed enters at the corner of the table at a concentration of 25% solids, along with dressing water introduced from the upper edge to aid separation and displacement across the table. Finally, the particles move diagonally across the deck in accordance with their specific gravity where they can be variously collected.
Figure 1.
Schematic illustration of the arrangement of a riffled shaking table; note the feed input at the top right corner of the table.