Demonstration Plant Equipment Design and Scale-Up from Pilot Plant of a Leaching and Solvent Extraction Process
Abstract
:1. Introduction
| Fly Ash | Date | Limestone (%) | Coal/Pet-Coke Dose | Ge Content (mg/kg) |
|---|---|---|---|---|
| FA#2 | 11/09/1999 | 4.1 | 50:50 | 347 |
| FA#7 | 24/10/2000 | 2.6 | 50:50 | 244 |
| FA#10 | --/11/2002 | 2.2 | 50:50 | 356 |
| FA#13 | 20/10/2005 | 2.5 | 50:50 | 319 |
| FA#15 | 03/06/2008 | 2.7 | 50:50 | 235 |
| FA#16 | 30/06/2008 | 2.8 | 50:50 | 268 |
| FA#17 | 02/07/2008 | 2.8 | 50:50 | 228 |
| FA#18 | 19/09/2008 | 2.4 | 50:50 | 174 |
2. Process and Pilot Plant
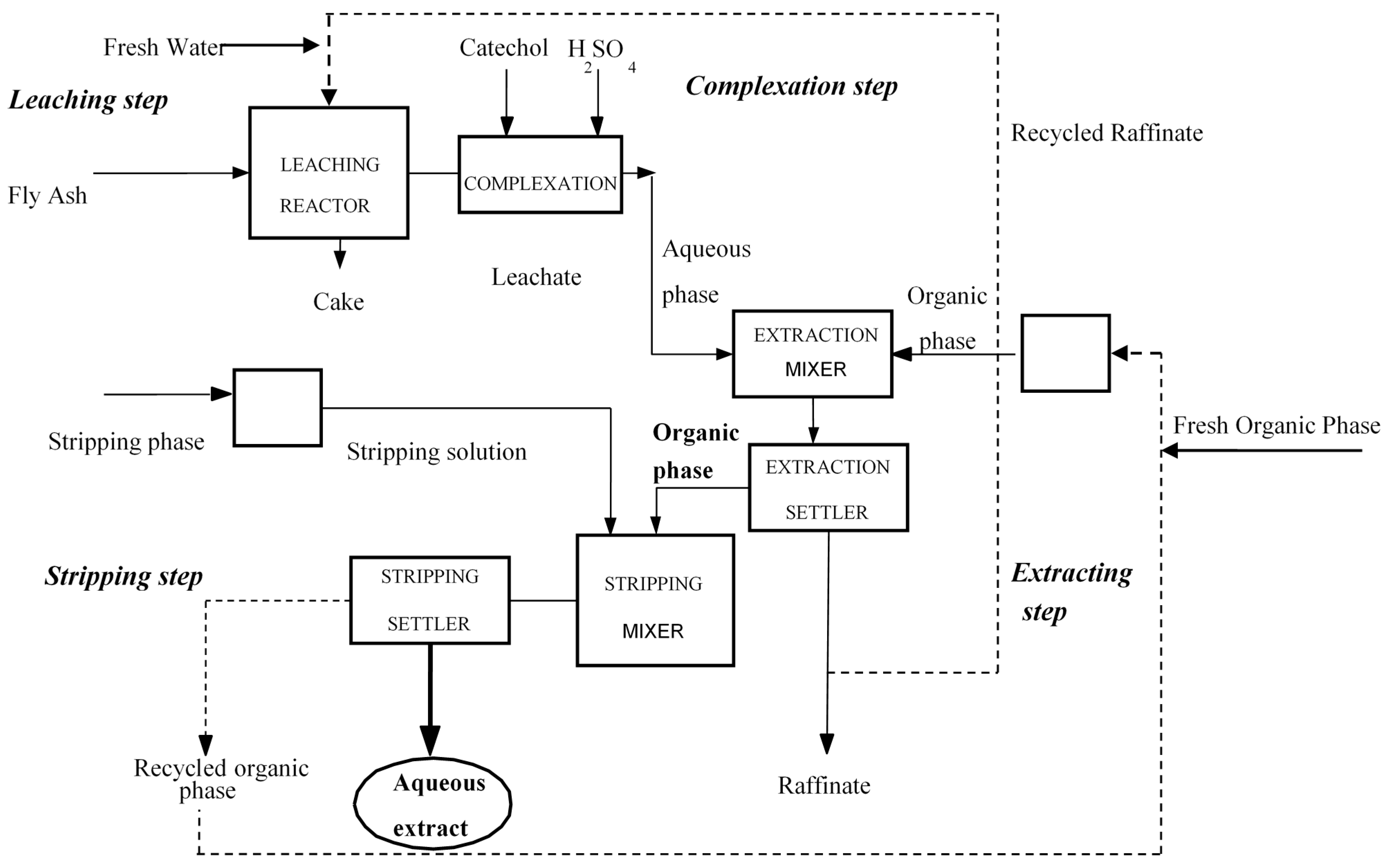
| Leaching Solution | Leaching Time (h) | Ge in Leachate (mg/L) |
|---|---|---|
| 65% raffinate 35% water | 1.5 | 30 |
| 2 | 45 | |
| 6 | 40 | |
| 75% raffinate 25% water | 1.5 | 32 |
| 2 | 53 | |
| 6 | 46 | |
| 85% raffinate 15% water | 1.5 | 43 |
| 2 | 52 | |
| 6 | 49 |
3. Preliminary Mass Balance
| Main Compounds | Flow | Units | Concentration (mg/L) | Flow | Units | Ge Total (g) | |
|---|---|---|---|---|---|---|---|
| Fly ash | FA | 5.0 | kg/h | 350 | 200 | kg/h | 70.0 |
| Leaching solution (W/FA = 5) | Fresh water (FW) | 6.3 | L/h | 0 | 250 | L/h | 0.0 |
| R recycled (RR) | 18.8 | L/h | 4 | 750 | L/h | 3.0 | |
| Total | 25.0 | L/h | - | 1000 | L/h | 3.0 | |
| Leachate/Aqueous phase | AP | 22.4 | L/h | 53 | 896 | L/h | 46.8 |
| Cake | C (FA) | 5.0 | kg/h | - | 200 | kg/h | 20.7 |
| C (leachate) | 2.6 | L/h | 53 | 104 | L/h | 5.5 | |
| Total (Wet FA) | 7.5 | - | - | 300 | - | 26.2 | |
| Organic phase (AP/OP = 5) | Fresh OP (FOP) | 0.4 | L/h | 0 | 17.9 | L/h | 0.0 |
| ROP (recycled) | 4.0 | L/h | 20 | 161.3 | L/h | 3.2 | |
| Total | 4.5 | L/h | - | 179.2 | L/h | 3.2 | |
| Organic extract | OE | 4.3 | L/h | 259 | 170.2 | L/h | 44.0 |
| Losses | 0.2 | L/h | 259 | 9.0 | L/h | 2.4 | |
| Total | 4.5 | L/h | - | 180 | L/h | - | |
| Raffinate | Residual raffinate | 3.7 | L/h | 4 | 146 | L/h | 0.6 |
| For recycling (RR) | 18.8 | L/h | 4 | 750 | L/h | 3.0 | |
| Total Raffinate | 22.4 | L/h | 4 | 896 | L/h | 3.6 | |
| Stripping phase (OE/SP = 5) | SP | 0.9 | L/h | 0 | 34.0 | L/h | 0.0 |
| Aqueous extract | AE | 0.9 | L/h | 1243.2 | 34.0 | L/h | 42.3 |
| Residual organic phase | ROP | 4.0 | L/h | 20 | 161.3 | L/h | 3.2 |
| Losses | 0.2 | L/h | 20 | 9.0 | L/h | 0.2 | |
4. Demonstration Equipment Design
4.1. Leaching Equipment

4.2. Filter
4.3. Extraction Equipment
4.3.1. Number of Stages
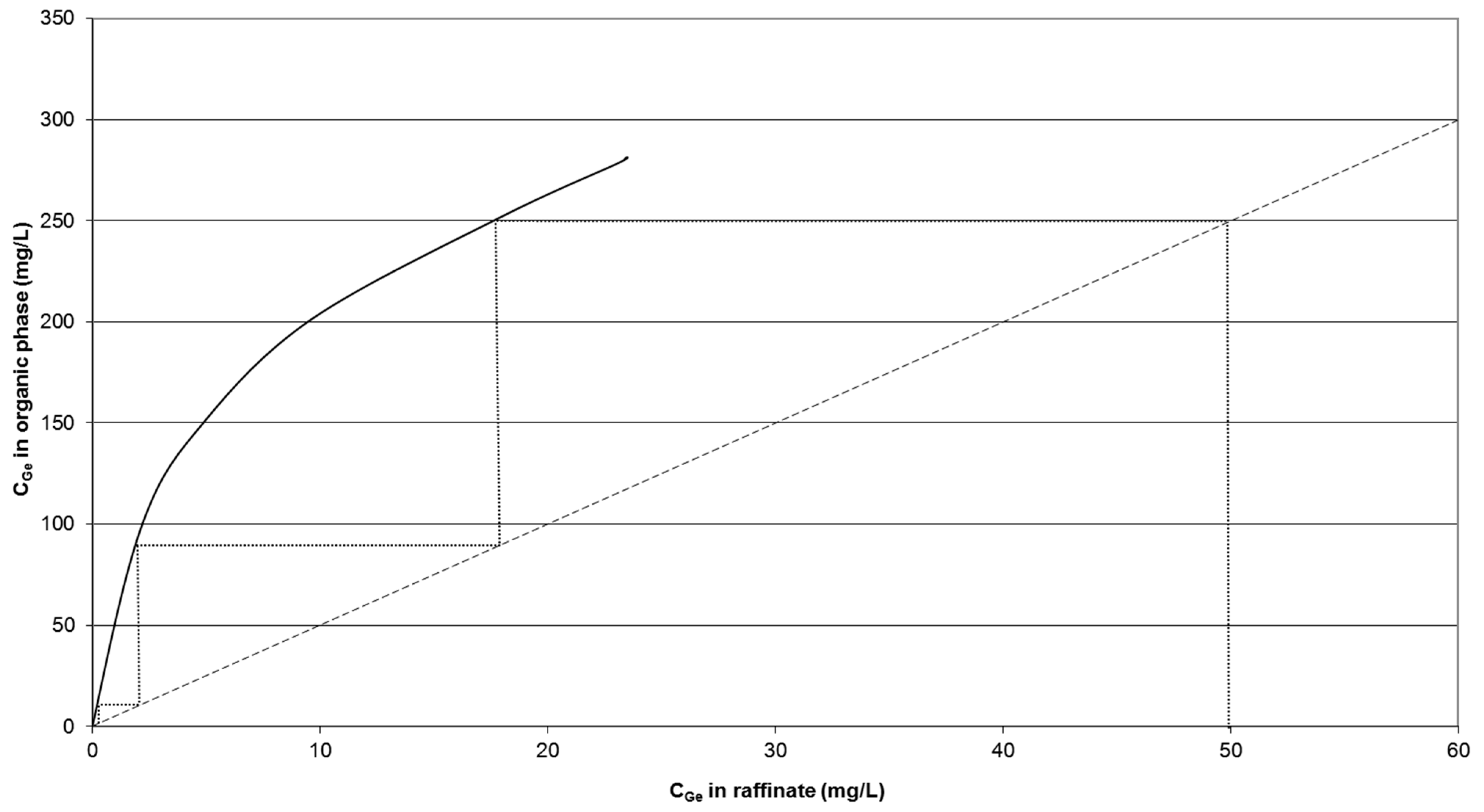
4.3.2. Mixer Geometry
4.3.3. Settler Geometry
| Settler Design Parameters | Options | ||||||
|---|---|---|---|---|---|---|---|
| Q/A (m3/m2·h) | 3.6 | 4.0 | 4.4 | 4.8 | 5.2 | 5.6 | 6.0 |
| A (m2) | 0.27 | 0.25 | 0.22 | 0.21 | 0.19 | 0.18 | 0.16 |
| W (m) | 0.18 | 0.16 | 0.15 | 0.14 | 0.13 | 0.12 | 0.11 |
4.4. Stripping Equipment
4.4.1. Number of Stages

4.4.2. Mixer Geometry
4.4.3. Settler Geometry
5. Demonstration Plant Scheme
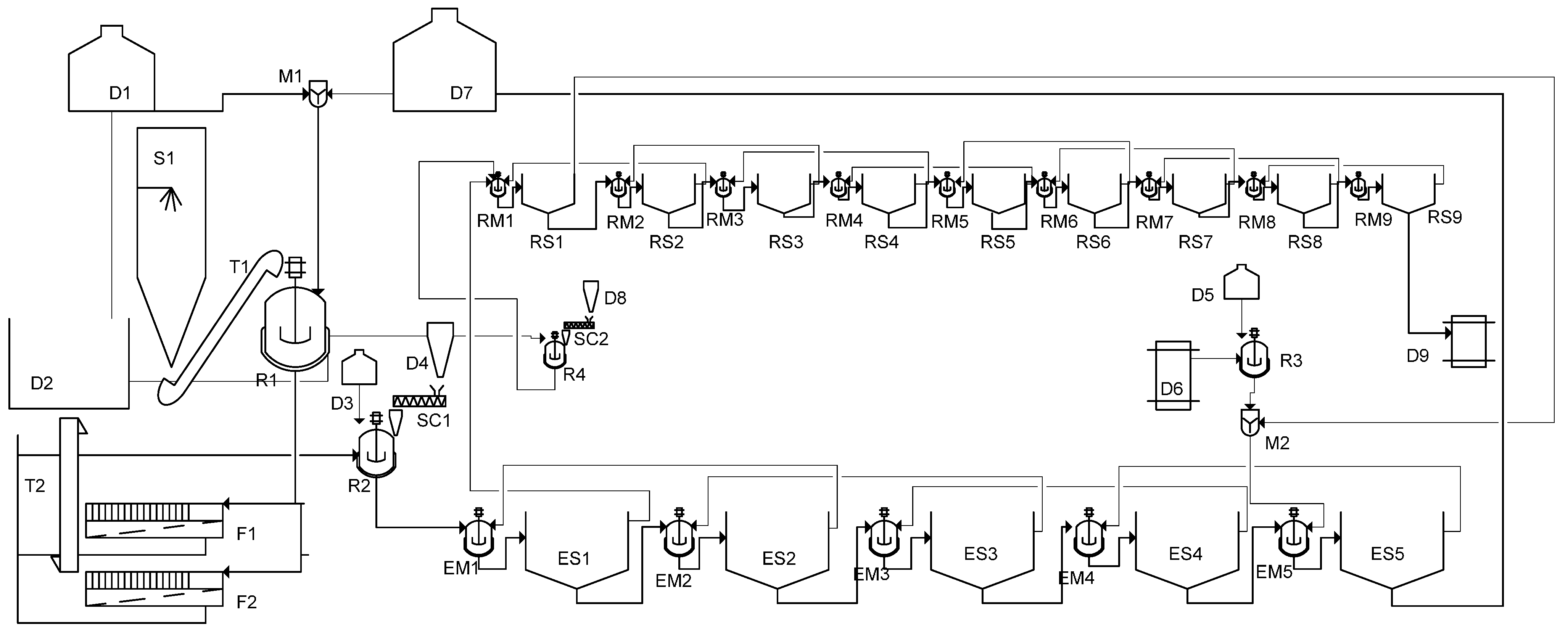
6. Cost Estimation
| Equipment | Cost (€) |
|---|---|
| Extraction equipment | - |
| Mixers | 75,000 |
| Settlers | 25,000 |
| Stripping equipment | - |
| Mixers | 70,000 |
| Settlers | 30,000 |
| Auxiliary reactors | 50,000 |
| Pumps | 30,000 |
| Liquid store | 70,000 |
| Total | 360,000 |
7. Conclusions
Author Contributions
Conflicts of Interest
References and Notes
- Adams, J.H.; Thomas, D. Germanium and germanium compounds. In Ullman’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH: New York, NY, USA, 2000. [Google Scholar]
- MetalPrices. Available online: http://www.metalprices.com (accessed on 26 May 2015).
- Querol, X.; Fernández-Turiel, J.L.; López-Soler, A. Trace elements in coal and their behaviour during coal combustion in a large power station. Fuel 1995, 74, 331–343. [Google Scholar] [CrossRef]
- Font, O.; Querol, X.; Huggins, F.; Chimenos, J.M.; Fernández, A.I.; Burgos, S.; García Peña, F. Speciation of major and selected trace elements in IGCC fly ash. Fuel 2005, 84, 1364–1371. [Google Scholar] [CrossRef]
- Font, O.; Querol, X.; Lopez-Soler, A.; Chimenos, J.M.; Fernandez, A.I.; Burgos, S.; Garcia Pena, F. Ge extraction from gasification fly ash. Fuel 2005, 84, 1384–1392. [Google Scholar] [CrossRef]
- Font, O.; Querol, X.; Plana, F.; Coca, P.; Burgos, S.; Pena, F.G. Condensing species from flue gas in Puertollano gasification power plant, Spain. Fuel 2006, 85, 2229–2242. [Google Scholar] [CrossRef]
- Font, O.; Querol, X.; Juan, R.; Casado, R.; Ruiz, C.R.; López-Soler, A.; Coca, P.; García Peña, F. Recovery of gallium and vanadium from gasification fly ash. J. Hazard. Mater. 2007, 139, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Font, O. Trace Elements in Integrated Gasification Combiner Fly Ash: Extraction of Potential Valuable Elements. Ph.D. Thesis, Universitat Politècnica de Catalunya, Barcelona, Spain, 2007. [Google Scholar]
- Jandova, J.; Vu, H. Recovery of Germanium-Bearing Fly Ash. In Metallurgy, Refractories and Environment; Technical University of Košice: Košice, Slovakia, 2001; pp. 107–112. [Google Scholar]
- Matis, K.A.; Mavros, P. Recovery of Metals by Ion Flotation from Dilute Aqueous Solutions. Sep. Purif. Rev. 1991, 20, 1–48. [Google Scholar] [CrossRef]
- Hernández-Expósito, A.; Chimenos, J.M.; Fernández, A.I.; Font, O.; Querol, X.; Coca, P.; García Peña, F. Ion flotation of germanium from fly ash aqueous leachates. Chem. Eng. J. 2006, 118, 69–75. [Google Scholar] [CrossRef]
- Marco-Lozar, J.P.; Cazorla-Amorós, D.; Linares-Solano, A. A new strategy for germanium adsorption on activated carbon by complex formation. Carbon 2007, 45, 2519–2528. [Google Scholar] [CrossRef]
- Arroyo, F.; Fernández-Pereira, C.; Querol, X.; Font, O.; Coca, P.; Chimenos, J.M.; Fernández, A.I. Method for the Recovery of Germanium Present in Coal Ash. Patent WO/2008/003808, 10 January 2008. [Google Scholar]
- Arroyo, F.; Fernandez-Pereira, C. Hydrometallurgical recovery of germanium from coal gasification fly ash, solvent extraction method. Ind. Eng. Chem. Res. 2008, 47, 3186–3191. [Google Scholar] [CrossRef]
- Menendez, F.J.S.; Menendez, F.M.S.; de La Cuadra Herrera, A.; Tamargo, F.A.; Valcárcel, M.R.; Fernandez, V.A. Process for the Recovery of Germanium from Solutions that Contain It. US Patent 4,886,648, 29 June 1989. [Google Scholar]
- Harada, A.; Tarutani, T.; Yoshimura, K. Spectrophotometric determination of germanium in rocks after selective adsorption on Sephadex gel. Anal. Chim. Acta 1998, 209, 333–336. [Google Scholar] [CrossRef]
- Arroyo Torralvo, F.; Fernández-Pereira, C. Recovery of germanium form fly ash leachate by ion-exchange extraction. Miner. Eng. 2011, 24, 35–41. [Google Scholar] [CrossRef]
- Moskalyk, R.R. Review of germanium processing worldwide. Miner. Eng. 2004, 17, 393–402. [Google Scholar] [CrossRef]
- Arroyo Torralvo, F.; Font, O.; Pereira Fernandez, C.; Querol, X.; Juan, R. Germanium recovery from gasification fly ash: Evaluation of end-products. J. Hazard. Mater. 2009, 167, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Arroyo Torralvo, F.; Fernandez-Pereira, C.; Coca, P. Precipitación of germanium from coal fly ash leachates. Coal Combust. Gasif. Prod. 2010, 2, 28–34. [Google Scholar]
- Arroyo Torralvo, F. Recuperación del Germanio Presente en la Ceniza Volante Producida en Una Planta GICC que Emplea Carbones de la Cuenca de Encasur en Puertollano. Ph.D. Thesis, University of Seville, Seville, Spain, 2008. [Google Scholar]
- Arroyo, F.; Fernández-Pereira, C.; Olivares, J.; Coca, P. Hydrometallurgical Recovery of Germanium from Coal Gasification Fly Ash: Pilot Plant Scale Evaluation. Ind. Eng. Chem. Res. 2009, 48, 3573–3579. [Google Scholar] [CrossRef]
- Palluzi, R.P. Pilot plants. In Ullman’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH: New York, NY, USA, 2005. [Google Scholar]
- Crittenden, J.C.; Trussell, R.; Hand, D.W.; Howe, K.J.; Tchobanoglous, G. Principles of Reactor Analysis and Mixing. In MWH’s Water Treatment: Principles and Design, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA.
- Perry, R.H.; Green, D.W. Perry’s Chemical Engineers’ Handbook, 7th ed.; McGraw-Hill: New York, NY, USA, 1997. [Google Scholar]
- Thakur, R.K.; Vial, C.; Djelveh, G.; Labbafi, M. Mixing of complex fluids with flat-bladed impellers: Effect of impeller geometry and highly shear-thinning behavior. Chem. Eng. Process. 2004, 43, 1211–1222. [Google Scholar] [CrossRef]
- Wei, J.; Anderson, J.L.; Bischoff, K.B. Advances in Chemical Engineering; Academic Press: Waltham, MA, USA, 1991; Volume 17. [Google Scholar]
- Al-Khafaji, A.A.A.R. Prediction of Filter Life by Measurement of Cake Resistance. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 1967. [Google Scholar]
- Sincero, A.P.; Sincero, G.A. Environmental Engineering. A Design Approach; Prentice Hall: Upper Saddle River, NJ, USA, 1996. [Google Scholar]
- Stevens, G.W.; The, C.L.; Malcolm, H.B. Extraction, Liquid-Liquid. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley and Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Cox, M.; Musikas, C. Solvent Extraction Principles and Practice; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Plant Design and Economics for Chemical Engineers. Max S. Peters, University of Colorado, Klaus Timmerhaus, University of Colorado, Boulder, Ronald E. West, University of Colorado, Boulder. Available online: http://highered.mheducation.com/sites/0072392665/student_view0/index.html (accessed on 22 May 2015).
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arroyo, F.; Fernández-Pereira, C.; Bermejo, P. Demonstration Plant Equipment Design and Scale-Up from Pilot Plant of a Leaching and Solvent Extraction Process. Minerals 2015, 5, 298-313. https://doi.org/10.3390/min5020298
Arroyo F, Fernández-Pereira C, Bermejo P. Demonstration Plant Equipment Design and Scale-Up from Pilot Plant of a Leaching and Solvent Extraction Process. Minerals. 2015; 5(2):298-313. https://doi.org/10.3390/min5020298
Chicago/Turabian StyleArroyo, Fátima, Constantino Fernández-Pereira, and Pilar Bermejo. 2015. "Demonstration Plant Equipment Design and Scale-Up from Pilot Plant of a Leaching and Solvent Extraction Process" Minerals 5, no. 2: 298-313. https://doi.org/10.3390/min5020298





