Arsenic-Microbe-Mineral Interactions in Mining-Affected Environments
Abstract
:1. Introduction
2. Arsenic-Bearing Minerals in Mining-Affected Environments
| Mineral | Formula | Mineral Group |
|---|---|---|
| Native arsenic | As | Native metalloid |
| Arsenopyrite | Fe3+As−S2− | Sulfide |
| Arsenical pyrite | Fe2+(S−,As−)2 | Sulfide |
| Loellingite | Fe2+As−2 | Arsenide |
| Niccolite | Ni3+As3− | Arsenide |
| Realgar | As3+,1−S2− | Sulfide |
| Orpiment | As3+2S2−3 | Sulfide |
| Enargite | Cu3AsS2−4 | Sulfosalt |
| Tennanite | (Cu,Ag,Fe,Zn)12As4S2− | Sulfosalt |
| Cobaltite | CoAsS2− | Sulfosalt |
| Gersdorffite | NiAsS2− | Sulfosalt |
| Claudetite | As3+2O3 | Oxide |
| Manganarsite | Mn3As3+2O4(OH)4 | Oxyhydroxide |
| Arseniosiderite | Ca2Fe3+3O2(As5+O4)3·3H2O | Fe-arsenate |
| Kankite | Fe3+As5+O4·3.5H2O | Fe-arsenate |
| Parasymplesite | Fe3+3(As5+O4)2·8H2O | Fe-arsenate |
| Pharmacosiderite | K[Fe3+4(OH)4(As5+O4)3]·6.5H2O | Fe-arsenate |
| Scorodite | Fe3+As5+O4·2H2O | Fe-arsenate |
| Symplesite | Fe3+3(As5+O4)2·8H2O | Fe-arsenate |
| Tooeleite | Fe3+6(As3+O3)4(S2−O4)(OH)4·4H2O | Fe sulfoarsenites |
| Beudantite | PbFe3+3(As5+O4)(S2−O4)(OH)6 | Fe sulfoarsenates |
| Zýkaite | Fe3+4(As5+O4)3(S2−O4)(OH)·15H2O | Fe sulfoarsenates |
| Yukonite | Ca7Fe3+12(As5+O4)10(OH)20·15H2O | Ca-Fe-arsenates |
| Hörnesite | Mg3(As5+O4)2·8H2O | Ca-Mg-arsenates |
| Pharmacolite | Ca(HAs5+O4)·2H2O | Ca-Mg-arsenates |
| Annabergite | Ni3(As5+O4)2·8H2O | Other metal arsenates |
| Erythrite | Co3(As5+O4)2·8H2O | Other metal arsenates |
| Köttigite | Zn3(As5+O4)2·8H2O | Other metal arsenates |
| Mimetite | Pb5(As5+O4)3Cl | Other metal arsenates |
| As(V)-sorbed hydrous ferric oxide | Fe3+(OH)3 | Fe oxides/hydroxides |
| As(V)-sorbed akaganeite | β-Fe3+OOH | Fe oxides/hydroxides |
| As(V)-sorbed goethite | γ-Fe3+OOH | Fe oxides/hydroxides |
| As(V)-sorbed lepidocrocite | α-Fe3+OOH | Fe oxides/hydroxides |
| As(III)- & As(V)-sorbed maghemite | γ-Fe3+2O3 | Fe oxides/hydroxides |
| As(III)- & As(V)-sorbed hematite | α-Fe3+2O3 | Fe oxides/hydroxides |
| As(V)-sorbed jarosite, natrojarosite, hydronium jarosite | (K,Na,H3O)Fe3+3(SO4)2(OH)6 | Fe oxyhydroxysulfates |

3. Prokaryotes in Arsenic-Bearing, Mining-Affected Environments
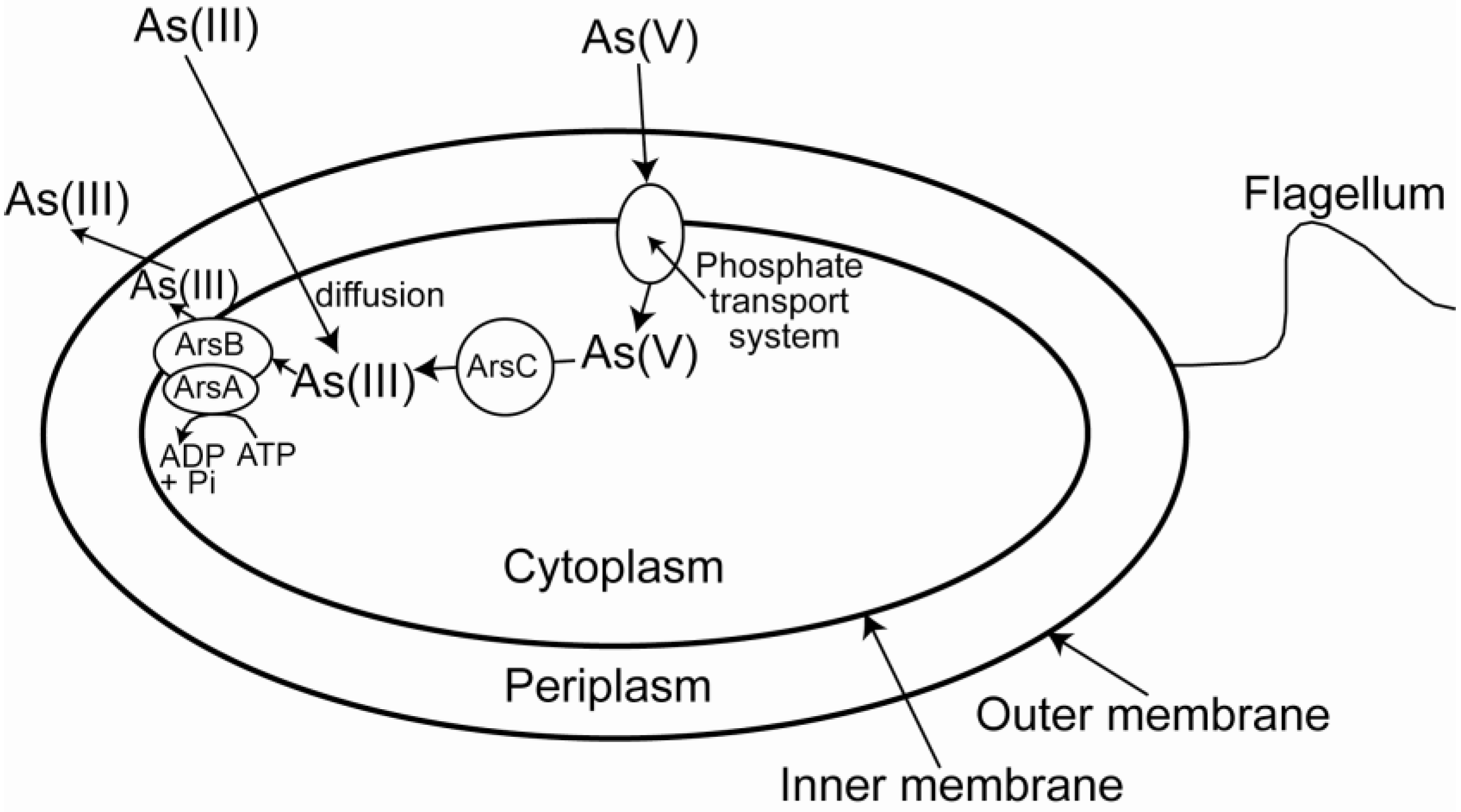
| Organism | Phylogenetic affiliation | Environment | Metabolism | Reference |
|---|---|---|---|---|
| Isolated bacteria | ||||
| BEN-4 | β | As-contaminated mine water | het | [33] |
| BEN-5 | α | As-contaminated mine water | fac chem | [33] |
| NT-4 | α | arsenopyrite from gold mine | het | [33] |
| NT-10 | β | arsenopyrite from gold mine | het | [33] |
| NT-14 | β | arsenopyrite from gold mine | fac chem. | [33] |
| NT-26 | α | arsenopyrite from gold mine | fac chem | [34] |
| GM1 | β | As-contamined biofilm inside gold mine | het | [11] |
| Leptothrix sp. str. S1.1 | β | mine drainage water | het | [35] |
| Ralstonia sp. str. 22 | β | soil from mine site | het | [36] |
| Sinorhizobium sp. str. M14 | α | gold mine waters | het | [37] |
| Thiomonas arsenivorans | β | gold mine | fac chem | [38] |
| Thiomonas sp. str. 4As | β | acid mine drainage | fac chem | [39] |
| Variovorax paradoxus | β | mine drainage water | het | [35] |
| Acinetobacter junni | γ | mining-affected sediment | het | [40] |
| Marinobacter sp. | γ | mining-affected sediment | het | [40] |

4. Arsenic-Microbe-Mineral Interactions in Mining-Affected Environments
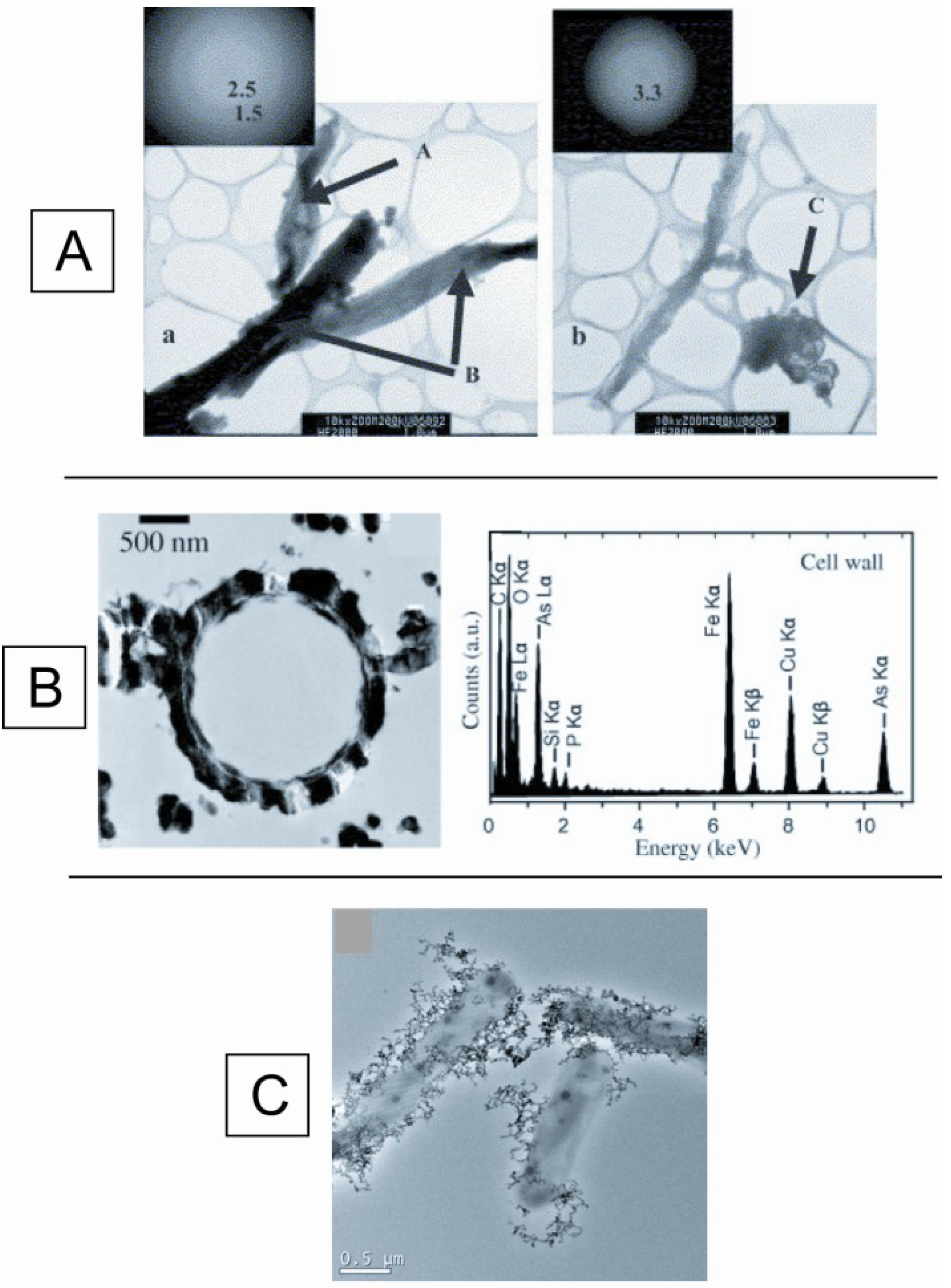
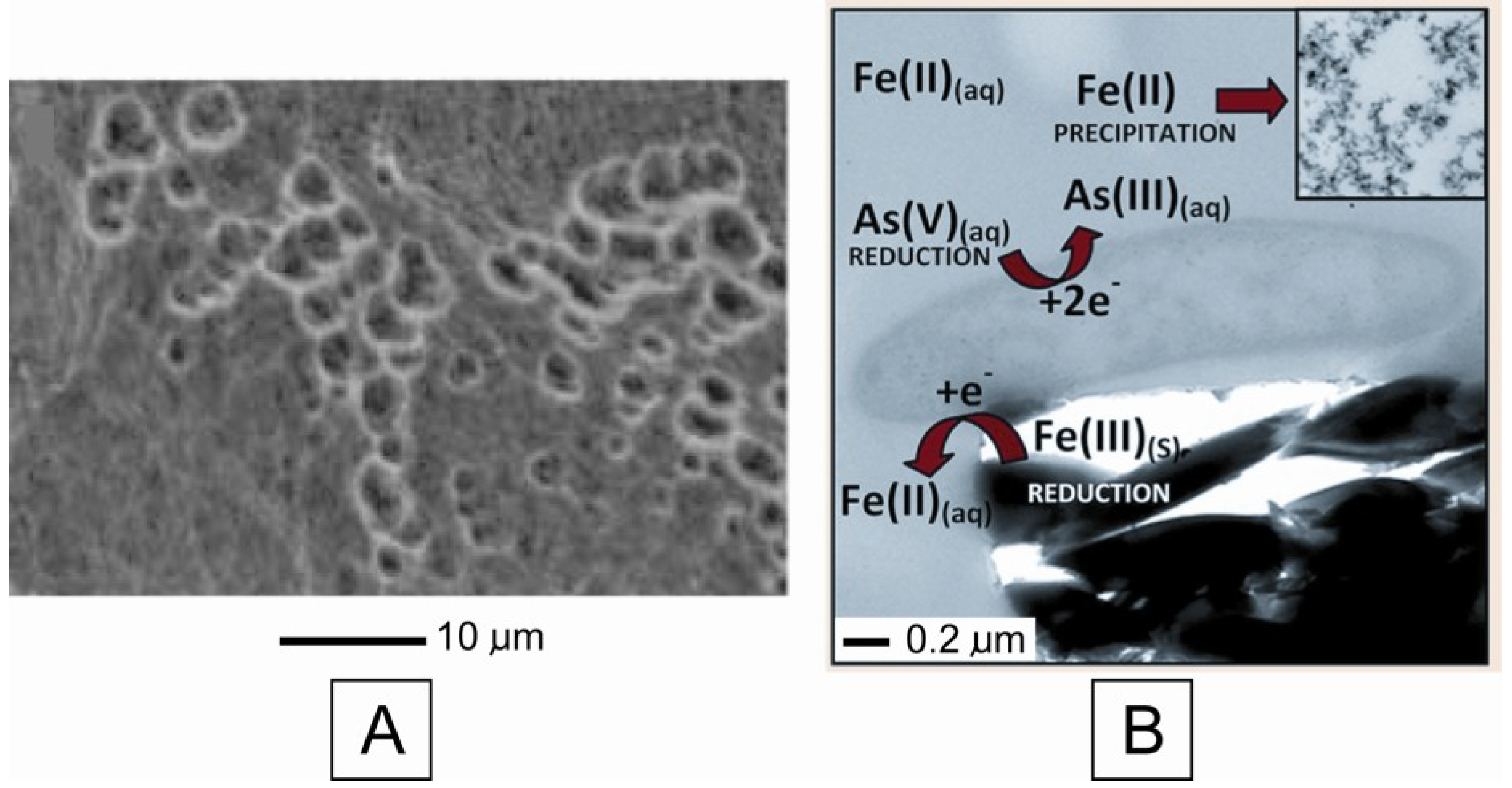
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Drahota, P.; Filippi, M. Secondary arsenic minerals in the environment: A review. Environ. Int. 2009, 35, 1243–1255. [Google Scholar] [CrossRef]
- Kossoff, D.; Hudson-Edwards, K.A.; Dubbin, W.E.; Alfredsson, M.; Geraki, T. Cycling of As, P, Pb and Sb during weathering of mine tailings: Implications for fluvial environments. Mineral. Mag. 2012, 76, 1209–1228. [Google Scholar] [CrossRef]
- Bowell, R.; Parshley, J. Arsenic Cycling in the Mining Environment. In U.S. EPA Workshop on Managing Arsenic Risks to the Environment: Characterization of Waste, Chemistry, and Treatment and Disposal Proceedings and Summary Report; U.S. Environmental Protection Agency: Washington, DC, USA, 2003; pp. 10–11. [Google Scholar]
- Foster, A.L.; Brown, G.E.; Tingle, T.N.; Parks, G.A. Quantitative arsenic speciation in mine tailings using X-ray absorption spectroscopy. Am. Mineral. 1998, 83, 553–568. [Google Scholar]
- Hudson-Edwards, K.A.; Jamieson, H.E.; Charnock, J.M.; Macklin, M.G. Arsenic speciation in waters and sediments of ephemeral floodplain pools, Ríos Agrio—Guadiamar, Aznalcóllar, Spain. Chem. Geol. 2005, 219, 175–192. [Google Scholar]
- Meunier, L.; Walker, S.R.; Wragg, J.; Parsons, M.B.; Koch, I.; Jamieson, H.E.; Reimer, K.J. Effects of soil composition and mineralogy on the bioaccessibility of arsenic from tailings and soil in gold mine districts of Nova Scotia. Environ. Sci. Technol. 2010, 44, 2667–2674. [Google Scholar]
- Lattanzi, P.; da Pelo, S.; Musu, E.; Atzei, D.; Elsener, B.; Fantauzzi, M.; Rossi, A. Enargite oxidation: A review. Earth Sci. Rev. 2008, 86, 62–88. [Google Scholar] [CrossRef]
- Bruckard, W.J.; Davey, K.J.; Jorgensen, F.R.A.; Wright, S.; Brew, D.R.M.; Haque, N.; Vance, E.R. Development and evaluation of an early removal process for the beneficiation of arsenic-bearing copper ores. Miner. Eng. 2010, 23, 1167–1173. [Google Scholar] [CrossRef]
- Kwong, Y.T.J.; Beauchemin, S.; Hossain, M.F.; Gould, W.D. Transformation and mobilization of arsenic in the historic Cobalt mining camp, Ontario, Canada. J. Geochem. Explor. 2007, 92, 133–150. [Google Scholar] [CrossRef]
- Senior, G.D.; Smith, L.K.; Silvester, E.; Bruckard, W.J. The flotation of gersdorffite in sulfide nickel systems—A single mineral study. Int. J. Miner. Process. 2009, 93, 165–171. [Google Scholar] [CrossRef]
- Osborne, T.H.; Jamieson, H.E.; Hudson-Edwards, K.A.; Nordstrom, D.K.; Walker, S.R.; Ward, S.A.; Santini, J.M. Microbial oxidation of arsenite in a subarctic environment: Diversity of arsenite oxidase genes and identification of a psychrotolerant arsenite oxidiser. BMC Microbiol. 2010, 10, 205. [Google Scholar] [CrossRef]
- Walker, S.R.; Jamieson, H.E.; Lanzirotti, A.; Andrade, C.F.; Hall, G.E.M. The speciation of arsenic in iron oxides in mine wastes from the Giant gold mine, NWT: Application of synchrotron micro-XRD and micro-XANES at the grain scale. Can. Mineral. 2005, 43, 1205–1224. [Google Scholar] [CrossRef]
- Fawcett, S.E.; Jamieson, H.E. The distinction between ore processing and post-depositional transformation on the speciation of arsenic and antimony in mine waste and sediment. Chem. Geol. 2011, 283, 109–118. [Google Scholar] [CrossRef]
- Walker, S.R.; Parsons, M.B.; Jamieson, H.E.; Lanzirotti, A. Arsenic mineralogy of near-surface tailings and soils: Influences on arsenic mobility and bioaccessibility in the Nova Scotia gold mining districts. Can. Mineral. 2009, 47, 533–556. [Google Scholar] [CrossRef]
- Morin, G.; Juillot, F.; Casiot, C.; Bruneel, O.; Personne, J.C.; Elbaz-Poulichet, F.; Leblanc, M.; Ildefonse, P.; Calas, G. Bacterial formation of tooeleite and mixed arsenic (III) or arsenic (V)-iron (III) gels in the Carnoules acid mine drainage, France. A XANES, XRD, and SEM study. Environ. Sci. Technol. 2003, 37, 1705–1712. [Google Scholar] [CrossRef]
- Corriveau, M.C.; Jamieson, H.E.; Parsons, M.B.; Hall, G.E.M. Mineralogical characterization of arsenic in gold mine tailings from three sites in Nova Scotia. Geochem. Explor. Environ. Anal. 2011, 11, 179–192. [Google Scholar] [CrossRef]
- Weisener, C.G.; Guthrie, J.W.; Smeaton, C.M.; Paktunc, D.; Fryer, B.J. The effects of Ca-Fe-As coatings on microbial leaching of metals in arsenic bearing mine waste. J. Geochem. Explor. 2011, 110, 23–30. [Google Scholar] [CrossRef]
- Ashley, P.M.; Lottermoser, B.G. Arsenic contamination at the Mole River Mine, northern New South Wales. Aust. J. Earth Sci. 1999, 46, 861–874. [Google Scholar] [CrossRef]
- Hochella, M.F., Jr.; Moore, J.N.; Golla, U.; Putnis, A. A TEM study of samples from acid mine drainage systems: Metal-mineral association with implications for transport. Geochim. Cosmochim. Acta 1999, 63, 3395–3406. [Google Scholar] [CrossRef]
- Roussell, C.; Néel, C.; Bril, H. Minerals controlling arsenic and lead solubility in an abandoned gold mine tailings. Sci. Total Environ. 2000, 263, 209–219. [Google Scholar] [CrossRef]
- Savage, K.S.; Tingle, T.N.; O’Day, P.A.; Waychunas, G.A.; Bird, D.K. Arsenic speciation in pyrite and secondary weathering phases, Mother Lode Gold District, Tuolumne County, California. Appl. Geochem. 2000, 15, 1219–1244. [Google Scholar] [CrossRef]
- Mao, M.; Lin, J.; Pan, Y. Hemimorphite as a natural sink for arsenic in zinc deposits and related mine tailings: Evidence from single-crystal EPR spectroscopy and hydrothermal synthesis. Geochim. Cosmochim. Acta 2010, 74, 2943–2956. [Google Scholar] [CrossRef]
- Majzlan, J.; Lalinska, B.; Chovan, M.; Bläß, U.; Brecht, B.; Göttlicher, J.; Steininger, R.; Hug, K.; Ziegler, S.; Gescher, J. A mineralogical, geochemical, and microbiological assessment of the antimony- and arsenic-rich neutral mine drainage tailings near Pezinok, Slovakia. Am. Mineral. 2011, 96, 1–13. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U., Jr. Arsenic removal from water/wastewater using adsorbents—A critical review. J. Hazard. Mater. 2007, 142, 1–53. [Google Scholar] [CrossRef]
- Hudson-Edwards, K.A.; Schell, C.; Macklin, M.G. Mineralogy and geochemistry of alluvium contaminated by metal mining in the Rio Tinto area, southwest Spain. Appl. Geochem. 1999, 14, 1015–1030. [Google Scholar] [CrossRef]
- Jamieson, H.E.; Robinson, C.; Alpers, C.N.; Nordstrom, D.K.; Poustovetov, A.; Lowers, H.A. The composition of coexisting jarosite-group minerals and water from the Richmond mine, Iron Mountain, California. Can. Mineral. 2005, 43, 1225–1242. [Google Scholar] [CrossRef]
- Egal, M.; Casiot, C.; Morin, G.; Parmentier, M.; Bruneel, O.; Lebrun, S.; Elbaz-Poulichet, F. Kinetic control on the formation of tooeleite, schwertmannite and jarosite by Acidithiobacillus ferrooxidans strains in an As(III)-rich acid mine water. Chem. Geol. 2009, 265, 432–441. [Google Scholar] [CrossRef]
- Rhine, E.D.; Garcia-Dominguez, E.; Phelps, C.D.; Young, L.Y. Environmental microbes can speciate and cycle arsenic. Environ. Sci. Technol. 2005, 39, 9569–9573. [Google Scholar] [CrossRef]
- Stolz, J.F. Overview of Microbial Arsenic Metabolism and Resistance. In The Metabolism of Arsenite; Arsenic in the Environment 5; Santini, J.M., Ward, S.A., Eds.; CRC Press: London, UK, 2012; pp. 55–60. [Google Scholar]
- Osborne, T.H.; Santini, J.M. Prokaryotic Aerobic Oxidation of Arsenite. In The Metabolism of Arsenite; Arsenic in the Environment 5; Santini, J.M., Ward, S.A., Eds.; CRC Press: London, UK, 2012; pp. 61–72. [Google Scholar]
- Stolz, J.F.; Basu, P.; Santini, J.M.; Oremland, R.S. Selenium and arsenic in microbial metabolism. Annu. Rev. Microbiol. 2006, 60, 107–130. [Google Scholar] [CrossRef]
- Tamaki, S.; Frankenberger, W.T., Jr. Environmental biochemistry of arsenic. Rev. Environ. Contam. Toxicol. 1992, 124, 79–110. [Google Scholar] [CrossRef]
- Santini, J.M.; Sly, L.I.; Wen, A.; Comrie, D.; De Wulf-Durand, P.; Macy, J.M. New arsenite-oxidizing bacteria isolated from Australian gold-mining environments—Phylogenetic relationships. Geomicrobiol. J. 2002, 19, 67–76. [Google Scholar] [CrossRef]
- Santini, J.M.; Sly, L.I.; Schnagl, R.D.; Macy, J.M. A new chemolithoautotrophic arsenite-oxidizing bacterium isolated from a gold mine: Phylogenetic, physiological, and preliminary biochemical studies. Appl. Environ. Microbiol. 2000, 66, 92–97. [Google Scholar]
- Battaglia-Brunet, F.; Itard, Y.; Garrido, F.; Delorme, F.; Crouzet, C.; Greffie, C.; Joulian, C. A simple biogeochemical process removing arsenic from mine drainage water. Geomicrobiol. J. 2006, 23, 201–211. [Google Scholar] [CrossRef]
- Lieutaud, A.; van Lis, R.; Duval, S.; Capowiez, L.; Muller, D.; Lebrun, R.; Lignon, S.; Fardue, M.L.; Lett, M.C.; Nitschke, W.; et al. Arsenite oxidase from Ralstonia sp. 22: Characterization of the enzyme and its interaction with soluble cytochromes. J. Biol. Chem. 2010, 285, 20433–20441. [Google Scholar] [CrossRef]
- Drewniak, L.; Matlakowska, R.; Sklodowsska, A. Arsenite and arsenate metabolism of Sinorhizobium sp. M14 living in the extreme environment of the Zloty Stok gold mine. Geomicrobiol. J. 2008, 25, 363–370. [Google Scholar] [CrossRef]
- Battaglia-Brunet, F.; Joulian, C.; Garrido, F.; Dictor, M.C.; Morin, D.; Coupland, K.; Johnson, D.B.; Hallberg, K.B.; Baranger, P. Oxidation of arsenite by Thiomnonas strains and characterization of Thiomonas arsenivorans sp. nov. Antonie Leeuwenhoek 2006, 89, 99–108. [Google Scholar] [CrossRef]
- Duquesne, K.; Lieautaud, A.; Retarouchniak, J.; Muller, D.; Lett, M.C.; Bonnefoy, V. Arsenite oxidation by a chemoautotrophic moderately acidophilic Thiomonas sp.: From the strain isolation to the gene study. Environ. Microbiol. 2008, 10, 228–237. [Google Scholar]
- Chang, J.S.; Lee, J.H.; Kim, I.S. Bacterial aox genotype from arsenic contaminated mine to adjacent coastal sediment: Evidences for potential biogeochemical arsenic oxidation. J. Hazard. Mater. 2011, 193, 233–242. [Google Scholar] [CrossRef]
- Macy, J.M.; Nunan, K.; Hagen, K.D.; Dixon, D.R.; Harbour, P.J.; Cahill, M.; Sly, L.I. Chrysiogenes arsenatis gen. nov., sp. nov., a new arsenate-respiring bacterium isolated from gold mine wastewater. Int. J. Syst. Bacteriol. 1996, 46, 1153–1157. [Google Scholar] [CrossRef]
- Macy, J.M.; Santini, J.M.; Pauling, B.V.; O’Neill, A.H.; Sly, L.I. Two new arsenate/sulfate-reducing bacteria: Mechanisms of arsenate reduction. Arch. Microbiol. 2000, 173, 49–57. [Google Scholar] [CrossRef]
- Santini, J.M.; Stolz, J.F.; Macy, J.M. Isolation of a new arsenate-respiring bacterium—Physiological and phylogenetic studies. Geomicrobiol. J. 2002, 19, 41–52. [Google Scholar] [CrossRef]
- Santini, J.M.; Streimann, I.C.A.; van den Hoven, R.N. Bacillus macyae sp. nov., an arsenate-respiring bacterium isolated from an Australian gold mine. Int. J. Syst. Evol. Microbiol. 2004, 54, 2241–2244. [Google Scholar] [CrossRef]
- Heath, M.D.; Schoepp-Cothenet, B.; Osborne, T.H.; Santini, J.M. Arsenite Oxidase. In The Metabolism of Arsenite; Arsenic in the Environment 5; Santini, J.M., Ward, S.A., Eds.; CRC Press: London, UK, 2012; pp. 81–97. [Google Scholar]
- Oremland, R.S.; Stolz, J.F.; Saltikov, C.W. Anaerobic Oxidation of Arsenite by Autotrophic Bacteria: The View from Mono Lake, California. In The Metabolism of Arsenite; Arsenic in the Environment 5; Santini, J.M., Ward, S.A., Eds.; CRC Press: London, UK, 2012; pp. 73–80. [Google Scholar]
- Jones, R.A.; Koval, S.F.; Nesbitt, H.W. Surface alteration of arsenopyrite (FeAsS) by Thiobacillus ferrooxidans. Geochim. Cosmochim. Acta 2003, 67, 955–965. [Google Scholar] [CrossRef]
- Smeaton, C.M.; Walshe, G.E.; Smith, A.M.L.; Hudson-Edwards, K.A.; Dubbin, W.E.; Wright, K.; Beale, A.M.; Fryer, B.J.; Weisener, C.G. Simultaneous release of Fe and As during the reductive dissolution of Pb-As jarosite by Shewanella putrefaciens CN32. Environ. Sci. Technol. 2012, 46, 12823–12831. [Google Scholar] [CrossRef]
- Edwards, K.J.; Hu, B.; Hamers, R.J.; Banfield, J.F. A new look at microbial leaching patterns on sulfide minerals. FEMS Microbiol. Ecol. 2001, 34, 197–206. [Google Scholar]
- Saltikov, C.W.; Cifuentes, A.; Venkateswaran, K.; Newman, D.K. The ars detoxification system is advantageous but not required for As(V) respiration by the genetically tractable Shewanella species strain ANA-3. Appl. Environ. Microbiol. 2003, 69, 2800–2809. [Google Scholar] [CrossRef]
- Ohnuki, T.; Sakamoto, F.; Kozai, N.; Ozaki, T.; Yoshida, T.; Narumi, I.; Wakai, E.; Sakai, T.; Francis, A.J. Mechanisms of arsenic immobilization in a biomat from mine discharge water. Chem. Geol. 2004, 212, 279–290. [Google Scholar] [CrossRef]
- Benzerara, K.; Morin, G.; Yoon, T.H.; Miot, J.; Tyliszczak, T.; Casiot, C.; Bruneel, O.; Farges, F.; Brown, G.E., Jr. Nanoscale study of As biomineralization in an acid mine drainage system. Geochim. Cosmochim. Acta 2008, 72, 3949–3963. [Google Scholar] [CrossRef]
- Duquesne, K.; Leburn, S.; Casiot, C.; Bruneel, O.; Personne, J.C.; Leblanc, M.; Elbaz-Poulichet, F.; Morin, G.; Bonnefoy, V. Immobilization of arsenite and ferric iron by Acidithiobacillus ferrooxidans and its relevance to acid mine drainage. Appl. Environ. Microbiol. 2003, 69, 6165–6173. [Google Scholar] [CrossRef]
- Chen, P.; Yan, L.; Wang, Q.; Li, H. Arsenic precipitation in the bioleaching of realgar using Acidithiobacillus ferrooxidans. J. Appl. Chem. 2013, 424253. [Google Scholar] [CrossRef]
- Barker, W.W.; Welch, S.A.; Chu, S.; Banfield, J.F. Experimental observations of the effects of bacteria on aluminosilicate weathering. Am. Mineral. 1998, 83, 1551–1563. [Google Scholar]
- Welch, S.A.; Barker, W.W.; Banfield, J.F. Microbial extracellular polysaccharides and plagioclase dissolution. Geochim. Cosmochim. Acta 1999, 63, 1405–1419. [Google Scholar] [CrossRef]
- Sutton, J.A.; Corrick, J.D. Bacteria in Mining and Metallurgy: Leaching Selected Ores and Minerals; Experiments with Thiobacillus ferrooxidans; Rept. Invest. RI 5839; Bureau of Mines, U.S. Department of the Interior: Washington, DC, 1964. [Google Scholar]
- Ehrlich, H.L. Bacterial action on orpiment. Econ. Geol. 1963, 58, 991–994. [Google Scholar] [CrossRef]
- Corkhill, C.L.; Wincott, P.L.; Lloyd, J.R.; Vaughan, D.J. The oxidative dissolution of arsenopyrite (FeAsS) and enargite (Cu3AsS4) by Leptospirillum ferrooxidans. Geochim. Cosmochim. Acta 2006, 70, 3593–3612. [Google Scholar] [CrossRef]
- Hallberg, K.B.; Johnson, D.B. Biodiversity of acidophilic prokaryotes. Adv. Appl. Microbiol. 2001, 49, 37–84. [Google Scholar] [CrossRef]
- Baker, B.J.; Banfield, J.F. Microbioal communities in acid mine drainage. FEMS Microbiol. Ecol. 2003, 44, 139–152. [Google Scholar] [CrossRef]
- González-Pastor, J.E.; Mirete, S. Novel metal resistance genes from microorganisms: A functional metagenomic approach. Methods Mol. Biol. 2010, 668, 273–285. [Google Scholar] [CrossRef]
- Yelton, A.P.; Comolli, L.R.; Justice, N.B.; Castelle, C.; Denef, V.J.; Thomas, B.C.; Banfield, J.F. Comparative genomics in acid mine drainage biofilm communities reveals metabolic and structural differentiation of co-occurring archaea. BMC Genomics 2013, 14, 485. [Google Scholar] [CrossRef]
- Drewniak, L.; Sklodowska, A. Arsenic-transforming microbes and their role in biomining processes. Environ. Sci. Pollut. Res. 2013. [Google Scholar] [CrossRef]
- Islam, A.B.M.R.; Maity, J.P.; Bundschuh, J.; Chen, C.-Y.; Bhowmik, B.K.; Tazaki, K. Arsenic mineral dissolution and possible mobilization in mineral-microbe-groundwater environment. J. Hazard. Mater. 2012. [Google Scholar] [CrossRef]
- Corkhill, C.L.; Vaughan, D.J. Arsenopyrite oxidation—A review. Appl. Geochem. 2009, 24, 2342–2361. [Google Scholar] [CrossRef]
- Henao, D.M.O.; Godoy, M.A.M. Jarosite pseudomorph formation from arsenopyrite oxidation using Acidithiobacillus ferrooxidans. Hydrometallurgy 2010, 104, 162–168. [Google Scholar] [CrossRef]
- Drewniak, L.; Matlakowska, R.; Rewerski, B.; Sklodowska, A. Arsenic release from gold mine rocks mediated by the activity of indigenous bacteria. Hydrometallurgy 2010, 104, 437–442. [Google Scholar] [CrossRef]
- Drahota, P.; Falteisek, L.; Redlich, A.; Rohovec, J.; Matoušek, T.; Čepička, I. Microbial effects on the release and attenuation of arsenic in the shallow subsurface of a natural geochemical anomaly. Environ. Pollut. 2013, 180, 84–91. [Google Scholar] [CrossRef]
- Cummings, D.E.; Caccavo, F., Jr.; Fendorf, S.; Resonzweigh, R.F. Arsenic mobilization by the dissimilatory Fe(III)-reducing bacterium Shewanella alga BrY. Environ. Sci. Technol. 1999, 33, 723–729. [Google Scholar] [CrossRef]
- Papassiopi, N.; Vaxevanidou, K.; Paspaliaris, I. Investigating the use of iron reducing bacteria for the removal of arsenic from contaminated soils. Water Air Soil Pollut. 2003, 3, 81–90. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hudson-Edwards, K.A.; Santini, J.M. Arsenic-Microbe-Mineral Interactions in Mining-Affected Environments. Minerals 2013, 3, 337-351. https://doi.org/10.3390/min3040337
Hudson-Edwards KA, Santini JM. Arsenic-Microbe-Mineral Interactions in Mining-Affected Environments. Minerals. 2013; 3(4):337-351. https://doi.org/10.3390/min3040337
Chicago/Turabian StyleHudson-Edwards, Karen A., and Joanne M. Santini. 2013. "Arsenic-Microbe-Mineral Interactions in Mining-Affected Environments" Minerals 3, no. 4: 337-351. https://doi.org/10.3390/min3040337




