Lithium Extraction from Lithium-Bearing Clay Minerals by Calcination-Leaching Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experiments
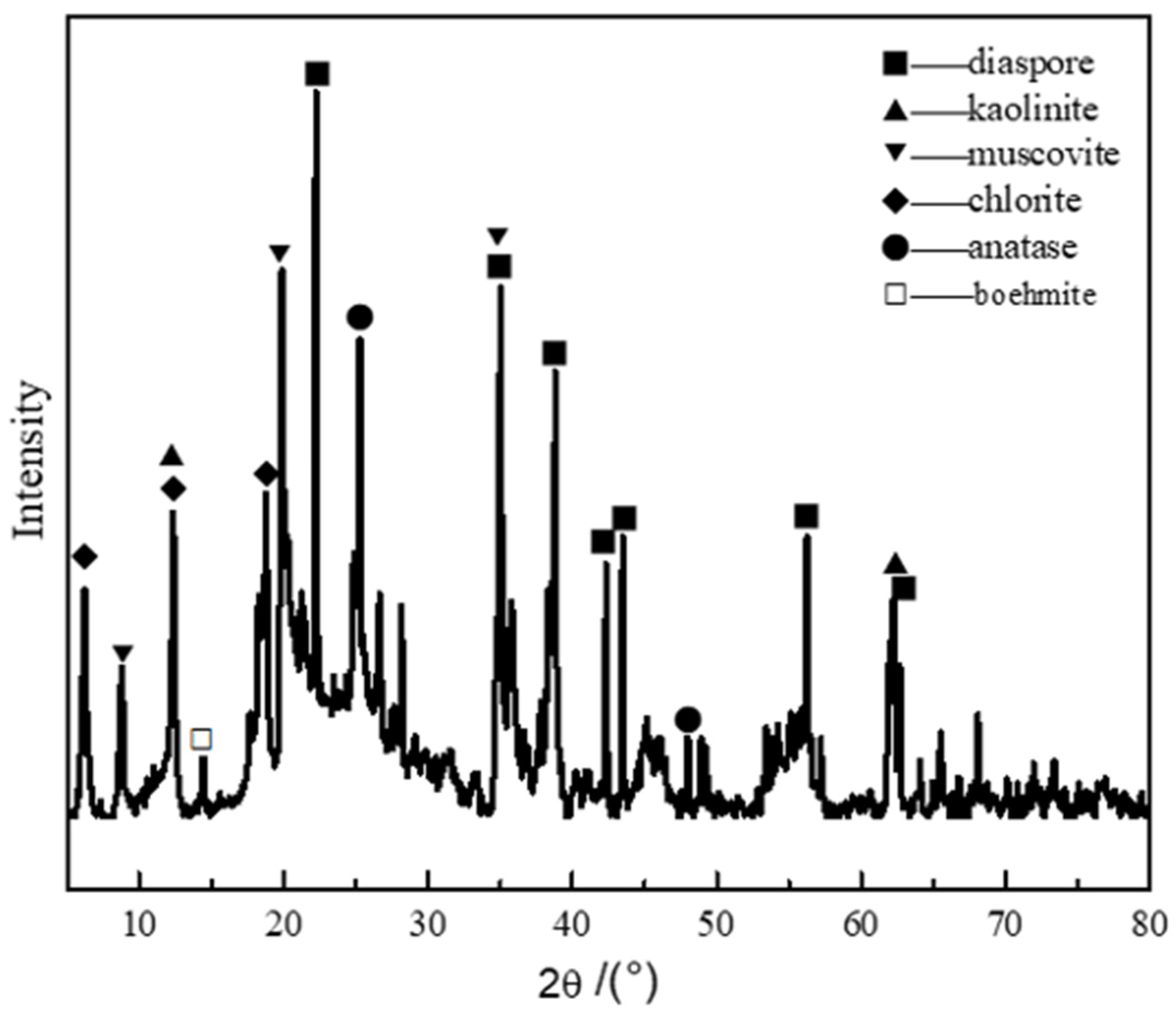
2.3. Characterization
3. Results and Discussion
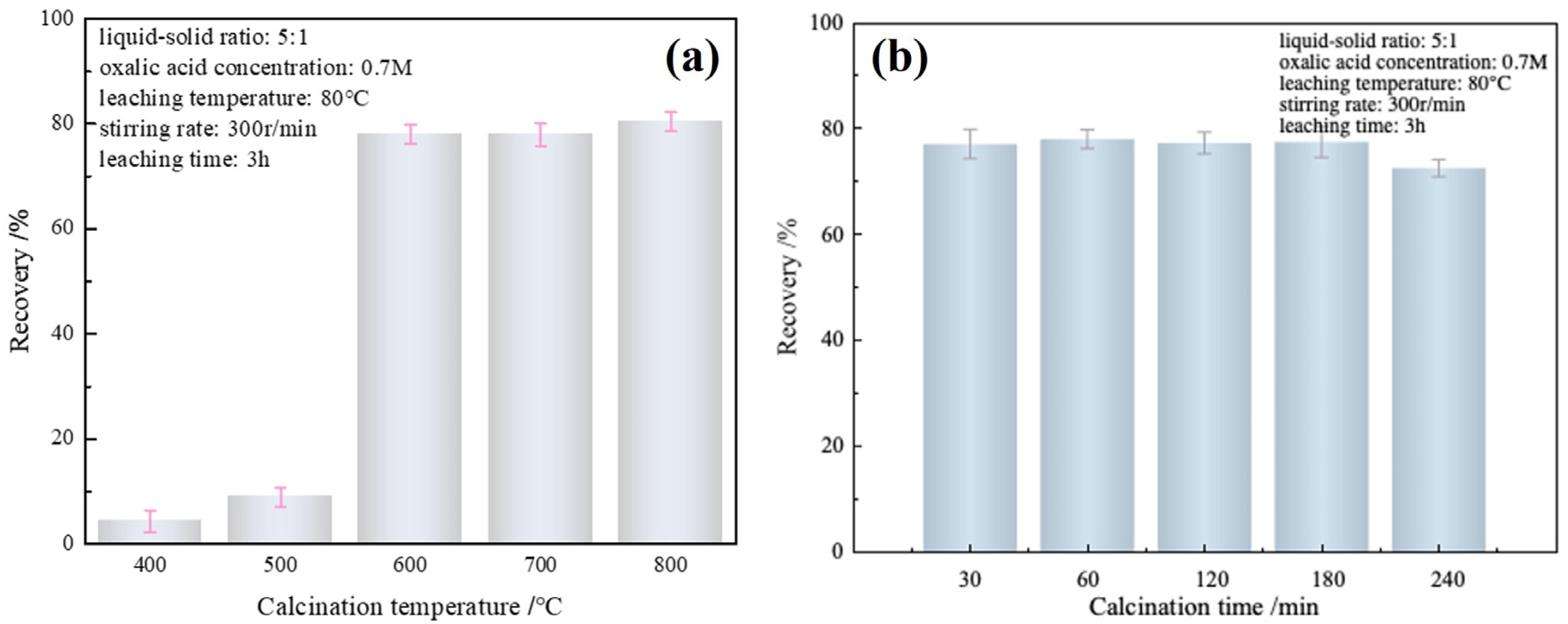
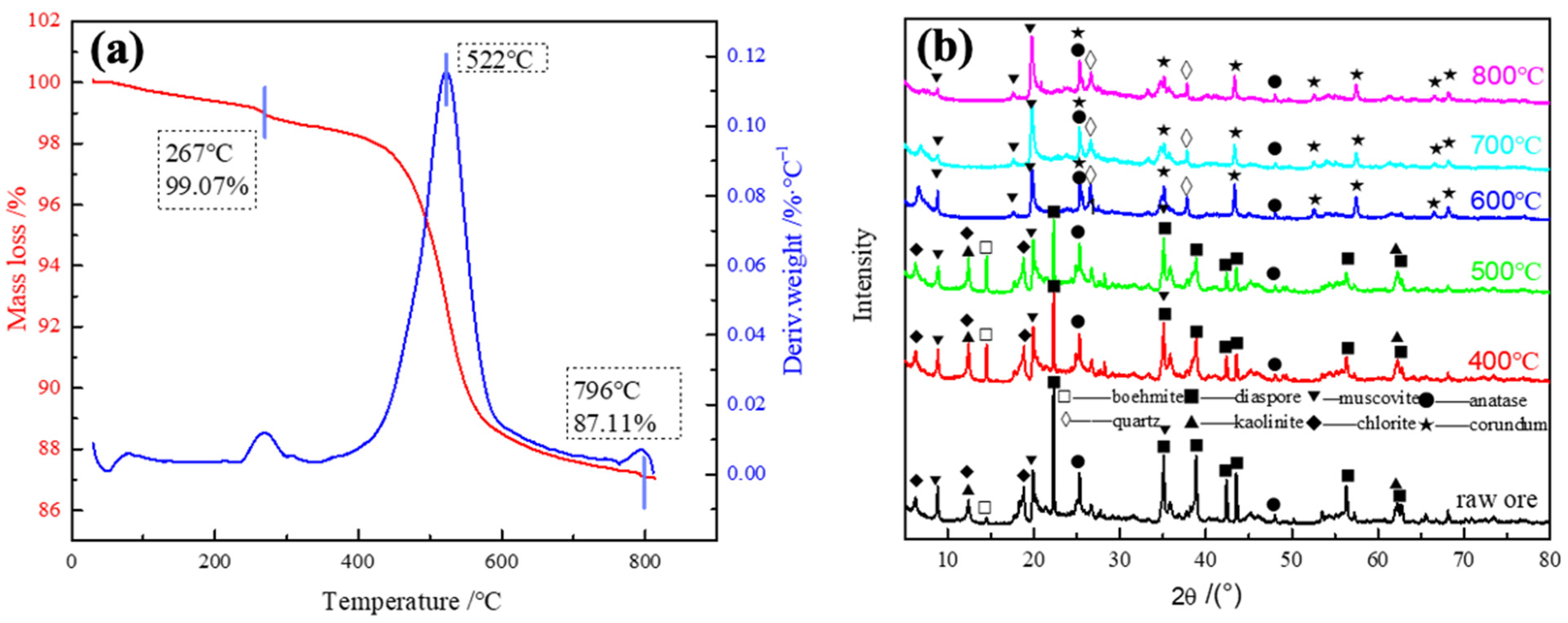
3.1. Calcination Process
3.2. Leaching Process
3.2.1. Effect of Stirring Rate
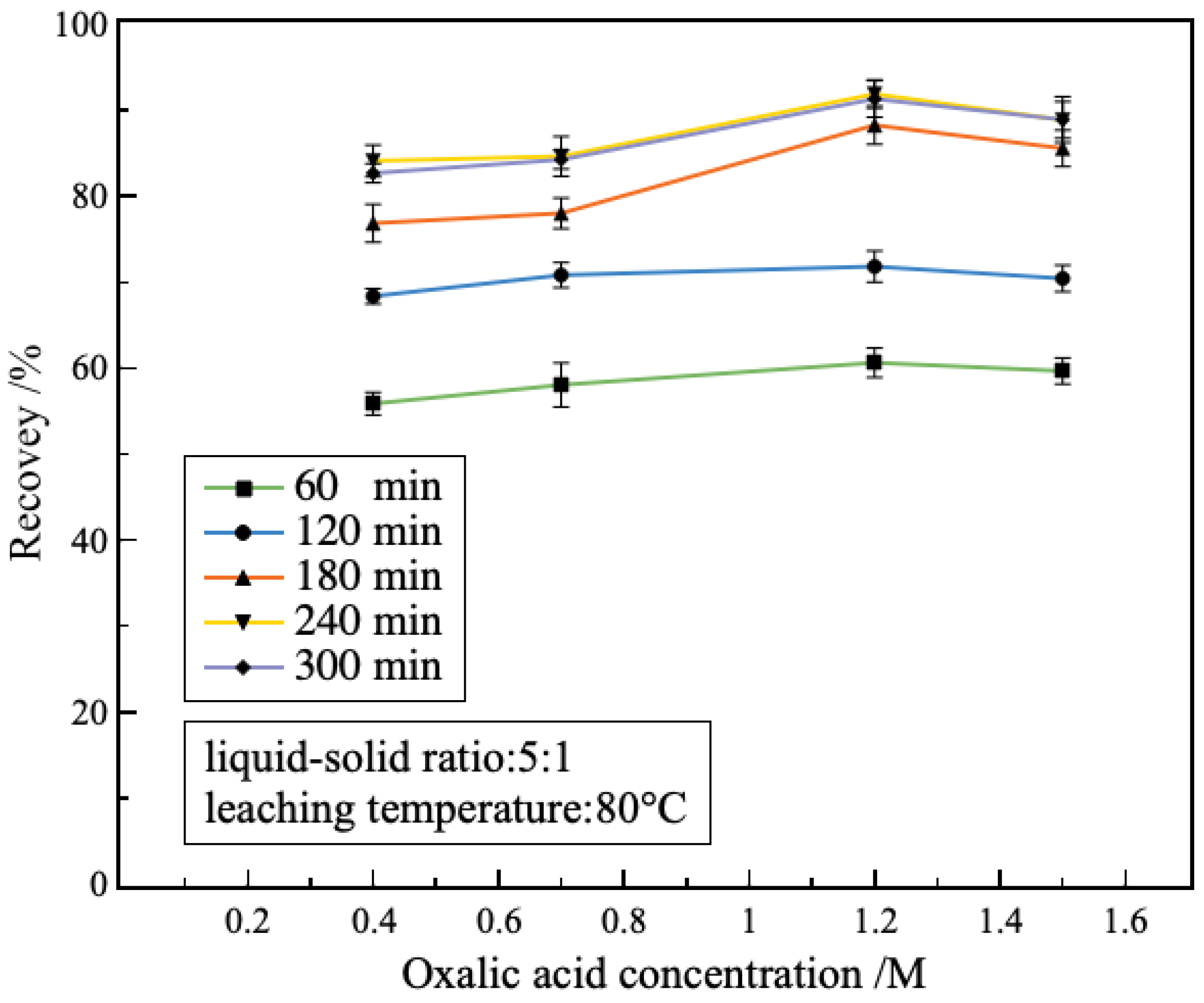
3.2.2. Effect of Oxalic Acid Concentrations
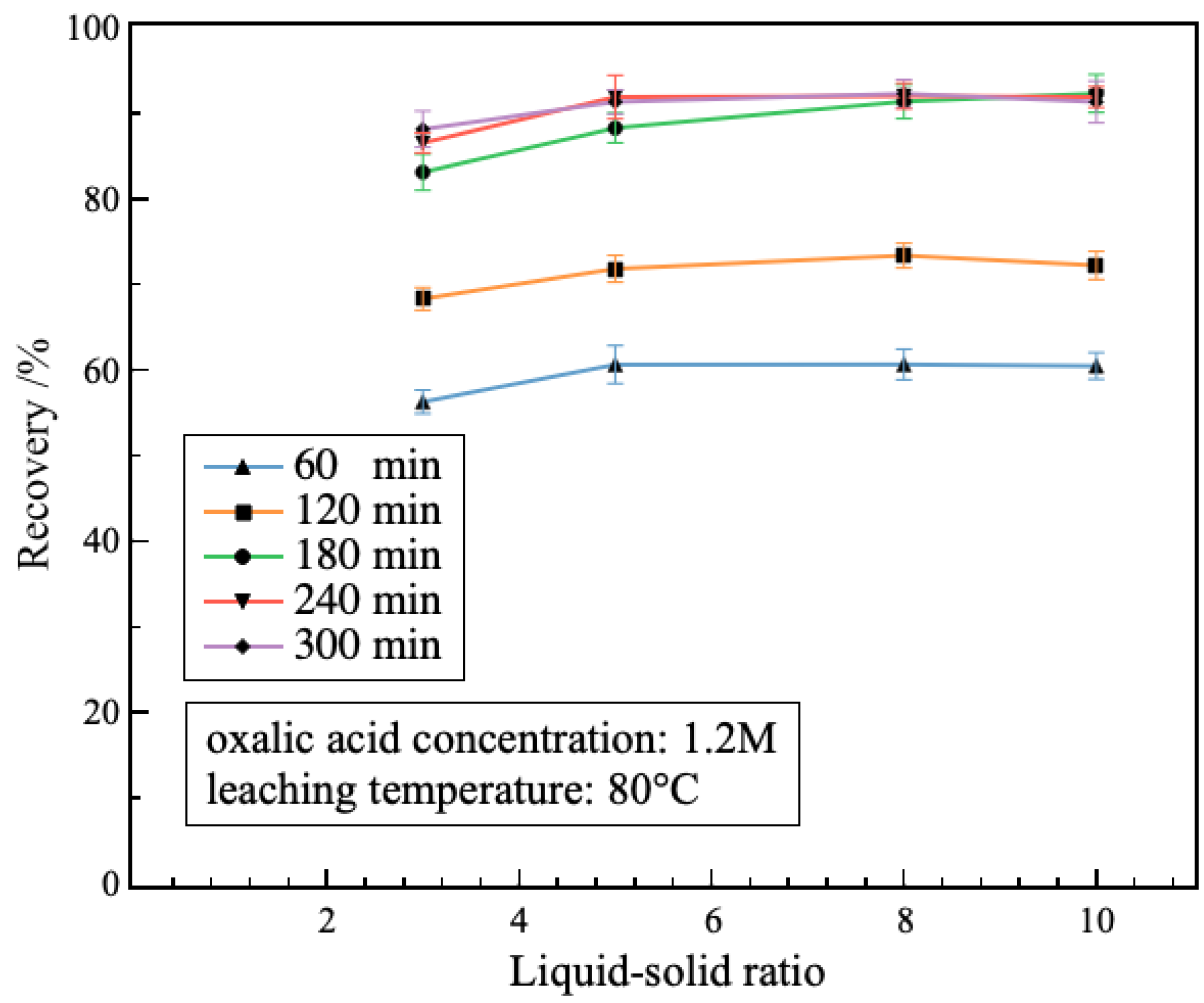
3.2.3. Effect of Liquid–Solid Ratio
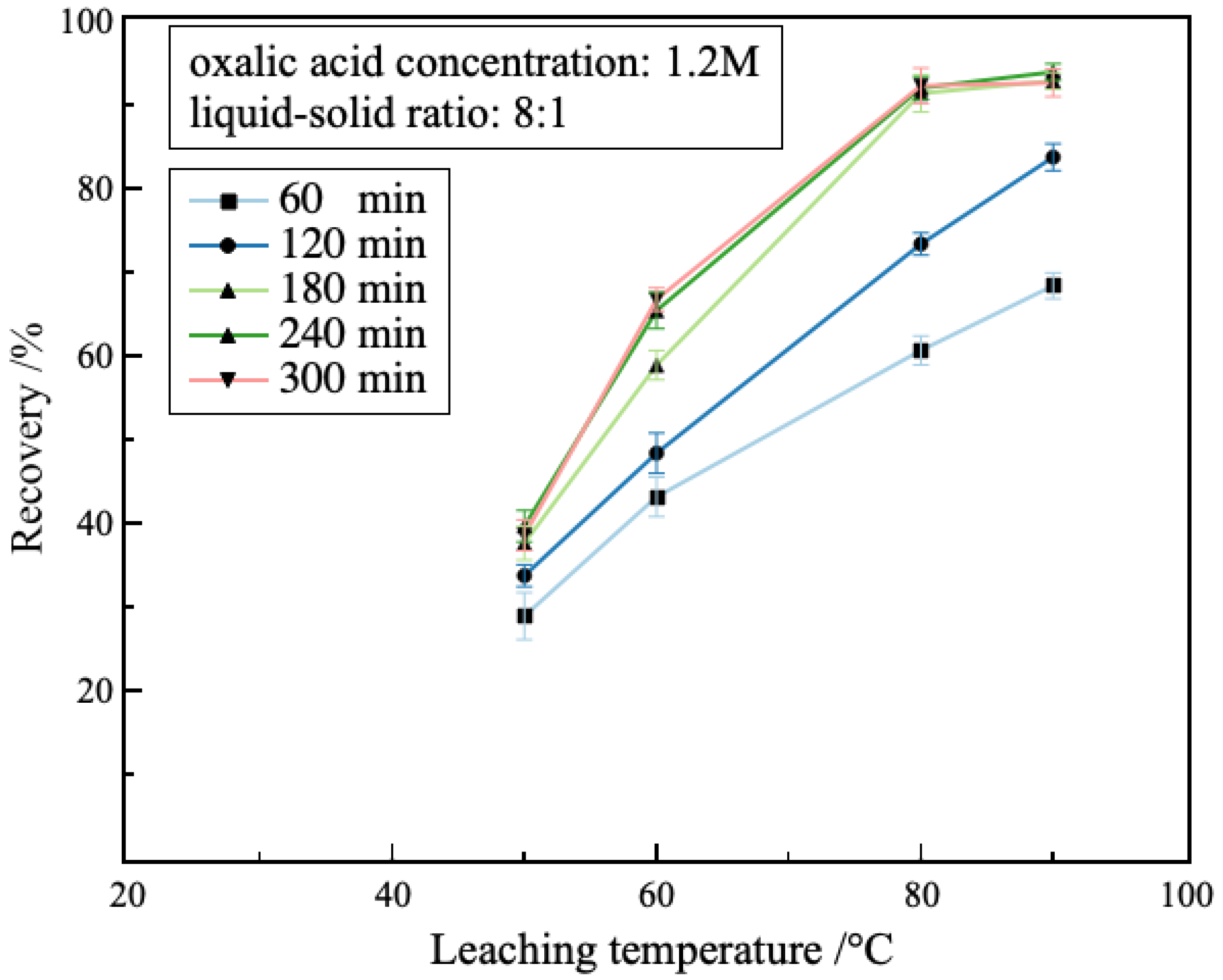
3.2.4. Effect of Leaching Temperature
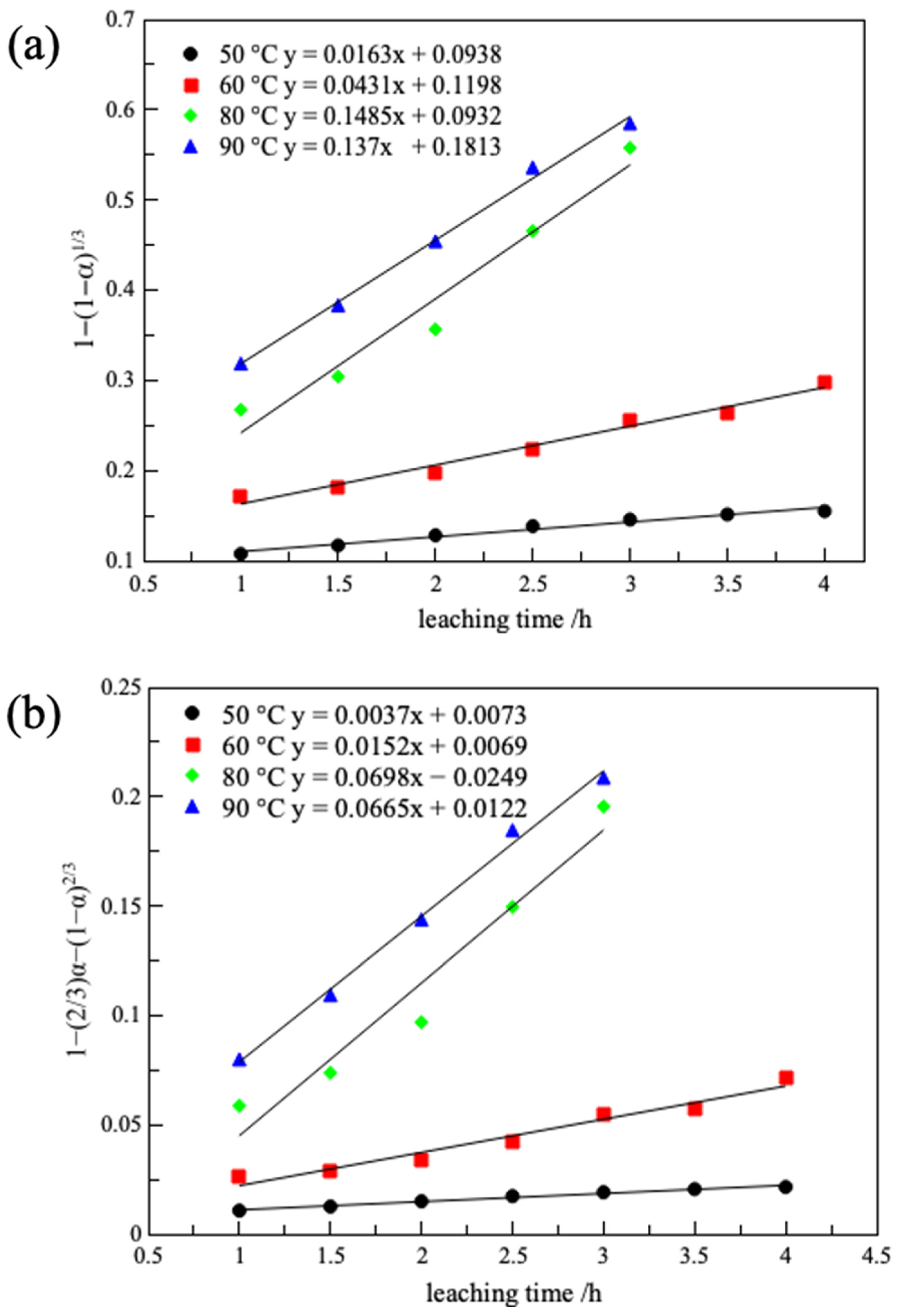
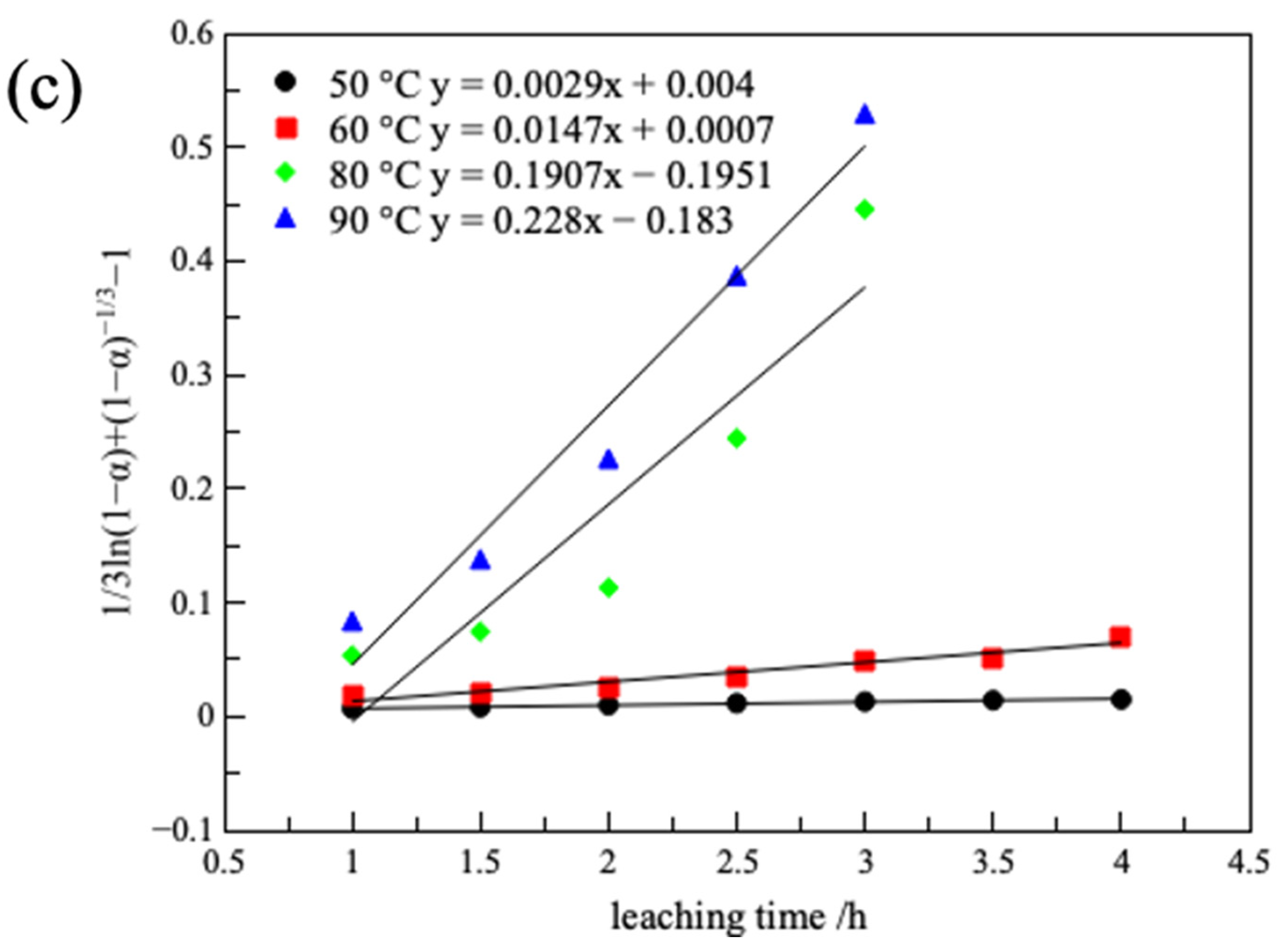
3.2.5. Leaching Kinetics
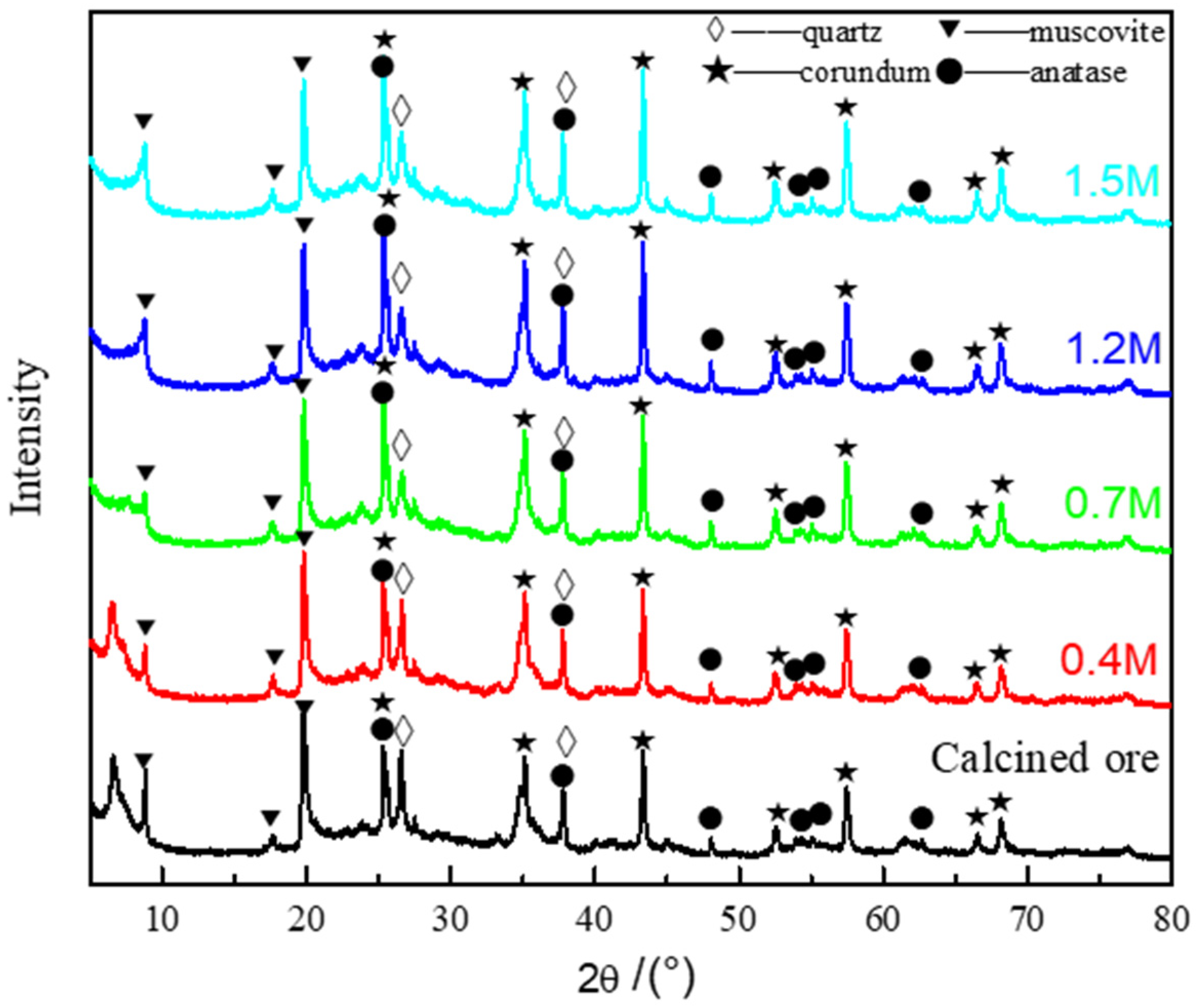
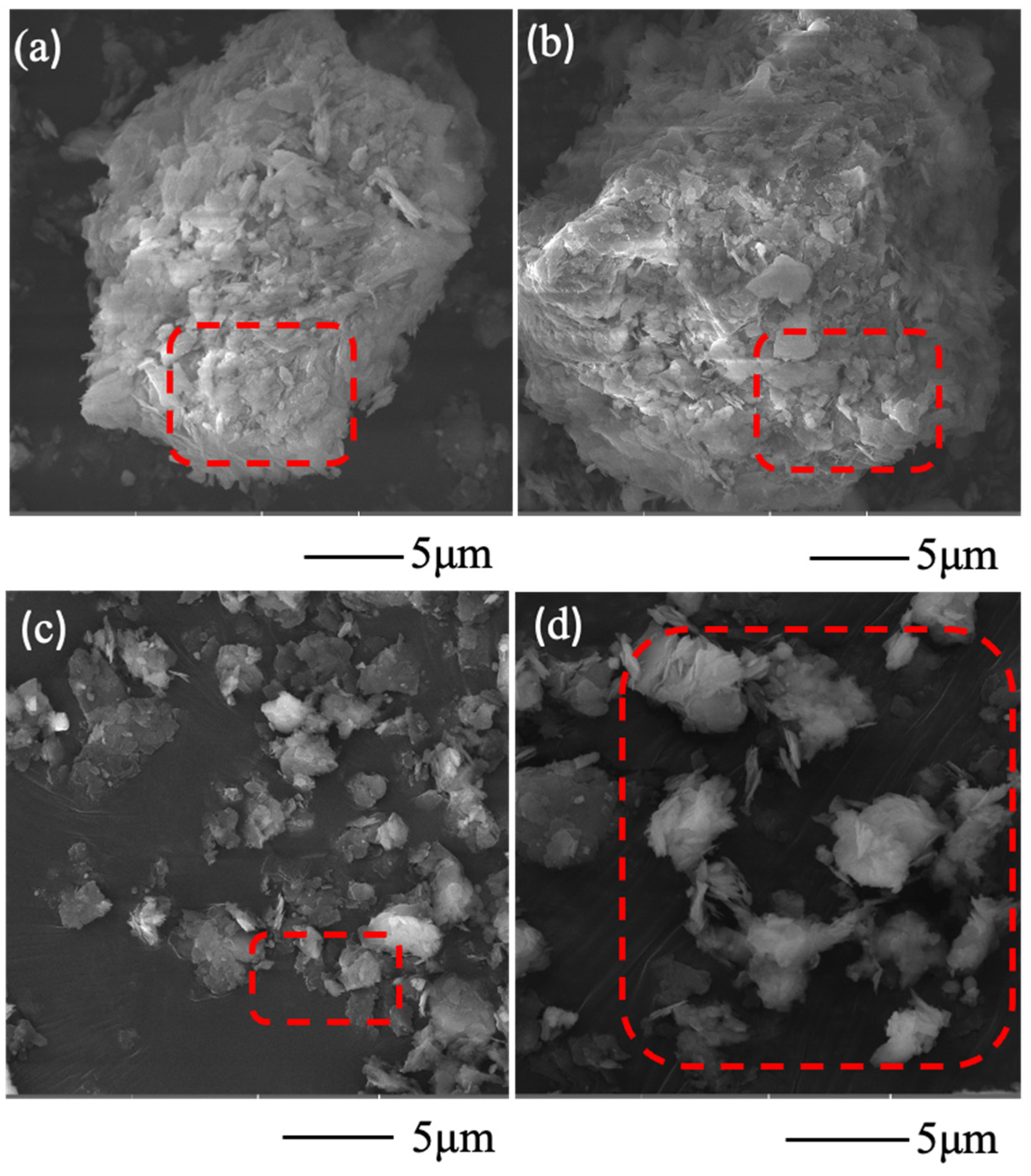
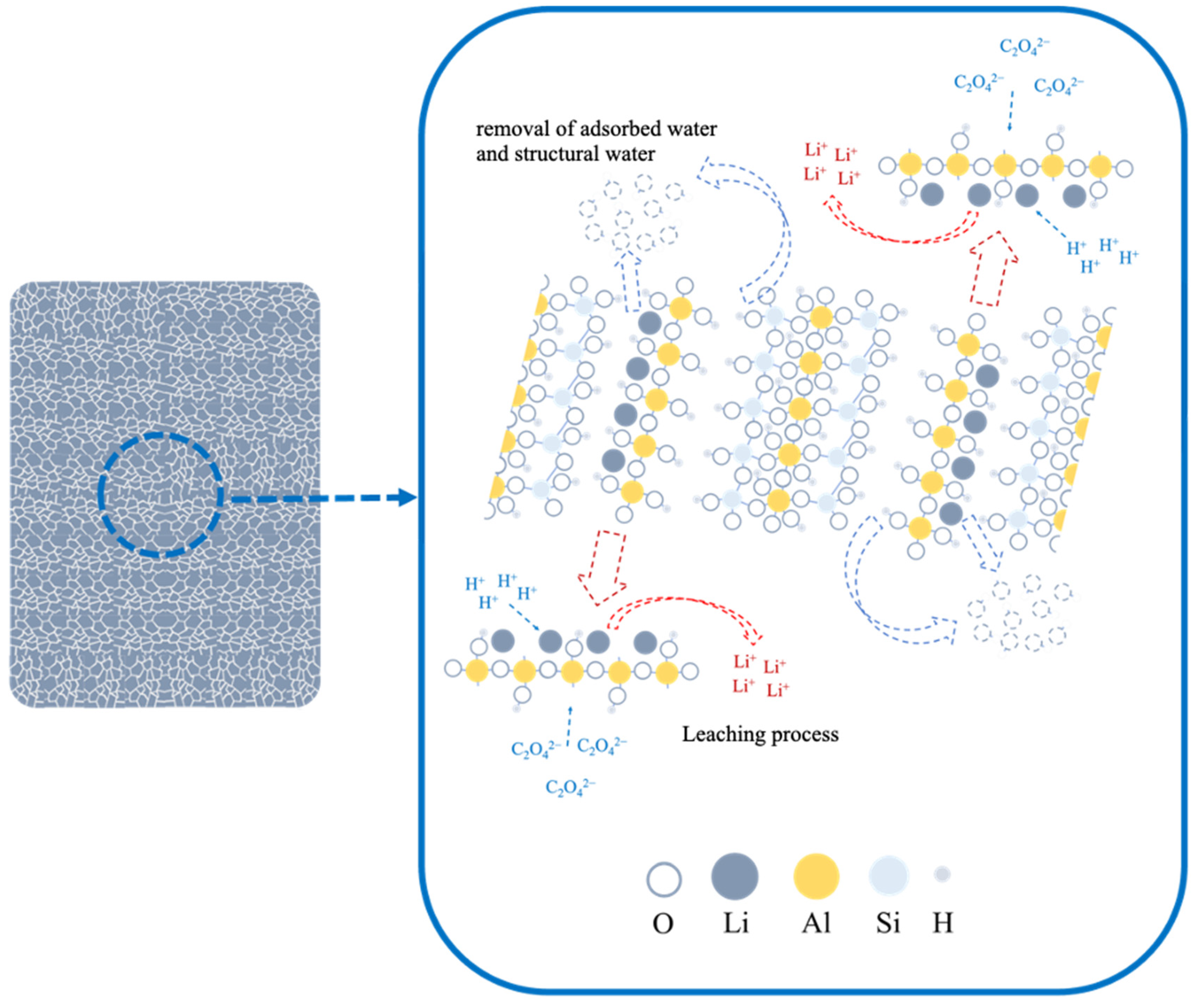
3.3. Lithium Extraction Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Xu, R.; Sun, W.; Wang, L.; Tang, H. Li extraction from model brine via electrocoagulation: Processing, kinetics, and mechanism. Sep. Purif. Technol. 2020, 250, 117234. [Google Scholar] [CrossRef]
- Tarascon, J.M. Is lithium the new gold? Nat. Chem. 2010, 2, 510. [Google Scholar] [CrossRef]
- Peiró, L.T.; Méndez, G.V.; Ayres, R.U. Lithium: Sources, Production, Uses, and Recovery Outlook. Jom 2013, 65, 986–996. [Google Scholar] [CrossRef]
- Seredin, V.V.; Tomson, I.N. The West Primorye noble-rare metal zone: A new Cenozoic metallogenic taxon in the Russian Far East. Dokl. Earth Sci. 2008, 421, 745–750. [Google Scholar] [CrossRef]
- Crocker, L.; Lien, R.H. Lithium and Its Recovery from Low-Grade Nevada Clays; Bureau of Mines, Department of Interior: Washington, DC, USA, 1987; pp. 8–9. [Google Scholar]
- Amer, A.M. The hydrometallurgical extraction of lithium from egyptian montmorillonite-type clay. Jom 2008, 60, 55–57. [Google Scholar] [CrossRef]
- May, J.T.; Witkowsky, D.S.; Seidel, D.C. Extracting Lithium from Clays by Roast-Leach Treatment; Bureau of Mines: Washington, DC, USA, 1980. [Google Scholar]
- Gu, H.; Guo, T.; Wen, H.; Luo, C.; Cui, Y.; Du, S.; Wang, N. Leaching efficiency of sulfuric acid on selective lithium leachability from bauxitic claystone. Miner. Eng. 2020, 145, 106076. [Google Scholar] [CrossRef]
- Zhang, W.; Noble, A.; Yang, X.; Honaker, R. Lithium leaching recovery and mechanisms from density fractions of an Illinois Basin bituminous coal. Fuel 2020, 268, 117319. [Google Scholar] [CrossRef]
- Li, L.; Dunn, J.B.; Zhang, X.X.; Gaines, L.; Chen, R.J.; Wu, F.; Amine, K. Recovery of metals from spent lithium-ion batteries with organic acids as leaching reagents and environmental assessment. J. Power Sources 2013, 233, 180–189. [Google Scholar] [CrossRef]
- Li, S.Y.; Zhao, W.; Zhou, Z.F.; Cui, X.L.; Shang, Z.C.; Liu, H.N.; Zhang, D.Q. Studies on Electrochemical Performances of Novel Electrolytes for Wide-Temperature-Range Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2014, 6, 4920–4926. [Google Scholar] [CrossRef]
- Irassar, E.F.; Bonavetti, V.L.; Castellano, C.C.; Trezza, M.A.; Rahhal, V.F.; Cordoba, G.; Lemma, R. Calcined illite-chlorite shale as supplementary cementing material: Thermal treatment, grinding, color and pozzolanic activity. Appl. Clay Sci. 2019, 179, 105143. [Google Scholar] [CrossRef]
- Caritat, P.D.; Hutcheon, I.; Walshe, J. Chlorite Geothermometry: A Review. Clays Clay Miner. 1993, 41, 219–239. [Google Scholar] [CrossRef]
- Abdeldayem, O.M.; Al Noman, M.A.; Dupont, C.; Ferras, D.; Ndiaye, L.G.; Kennedy, M. Hydrothermal carbonization of Typha australis: Influence of stirring rate. Environ. Res. 2023, 236, 116777. [Google Scholar] [CrossRef]
- Li, W.; Guo, H.; Huang, Q.; Han, P.; Hou, Y.; Zou, W. Effect of stirring rate on microstructure and properties of microporous mullite ceramics. J. Mater. Process. Technol. 2018, 261, 159–163. [Google Scholar] [CrossRef]
- Liu, Q.; Tu, T.; Guo, H.; Cheng, H.; Wang, X. High-efficiency simultaneous extraction of rare earth elements and iron from NdFeB waste by oxalic acid leaching. J. Rare Earths 2021, 39, 323–330. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Y.; Sun, N.; Khoso, S.A.; Wang, L.; Sun, W. A novel precipitant for separating lithium from magnesium in high Mg/Li ratio brine. Hydrometallurgy 2019, 187, 125–133. [Google Scholar] [CrossRef]
- Zhu, K.; He, Y.; Feng, D.; Jiang, W.; Zhang, K. Leaching behavior of copper tailings solidified/stabilized using hydantoin epoxy resin and red clay. J. Environ. Manag. 2023, 345, 118876. [Google Scholar] [CrossRef]
- Zhou, F.; Zhang, Y.; Liu, Q.; Huang, S.; Wu, X.; Wang, Z.; Zhang, L.; Chi, R. Modified tailings of weathered crust elution-deposited rare earth ores as adsorbents for recovery of rare earth ions from solutions: Kinetics and thermodynamics studies. Miner. Eng. 2023, 191, 107937. [Google Scholar] [CrossRef]
- Smith, I.W.M. (Ed.) Chapter 3—Molecular collision dynamics. In Kinetics and Dynamics of Elementary Gas Reactions; Butterworth-Heinemann: Oxford, UK, 1980; pp. 59–109. [Google Scholar]
- Cheng, H.; Yang, J.; Liu, Q.; He, J.; Frost, R.L. Thermogravimetric analysis–mass spectrometry (TG–MS) of selected Chinese kaolinites. Thermochim. Acta 2010, 507–508, 106–114. [Google Scholar] [CrossRef]
- Zhan, L.; Xia, F.; Ye, Q.; Xiang, X.; Xie, B. Novel recycle technology for recovering rare metals (Ga, In) from waste light-emitting diodes. J. Hazard. Mater. 2015, 299, 388–394. [Google Scholar] [CrossRef]
- Bailey, S.W. (Ed.) Chapter 10. Chlorites: Structures and crystal chemistry. In Hydrous Phyllosilicates (Exclusive of Micas); De Gruyter: Berlin, Germany, 1988; pp. 347–404. [Google Scholar]
- Subramanian, N.; Lammers, L.N. Thermodynamics of ion exchange coupled with swelling reactions in hydrated clay minerals. J. Colloid. Interface Sci. 2022, 608, 692–701. [Google Scholar] [CrossRef]
- Yang, X.; An, C.; Feng, Q.; Boufadel, M.; Ji, W. Aggregation of microplastics and clay particles in the nearshore environment: Characteristics, influencing factors, and implications. Water Res. 2022, 224, 119077. [Google Scholar] [CrossRef] [PubMed]
- Martial, N.G.; Yao, S.; Hamer, U.; Zhang, Y.; Zhang, B. Positive and negative priming effects induced by freshly added mineral-associated oxalic acid in a Mollisol. Rhizosphere 2023, 26, 100708. [Google Scholar] [CrossRef]

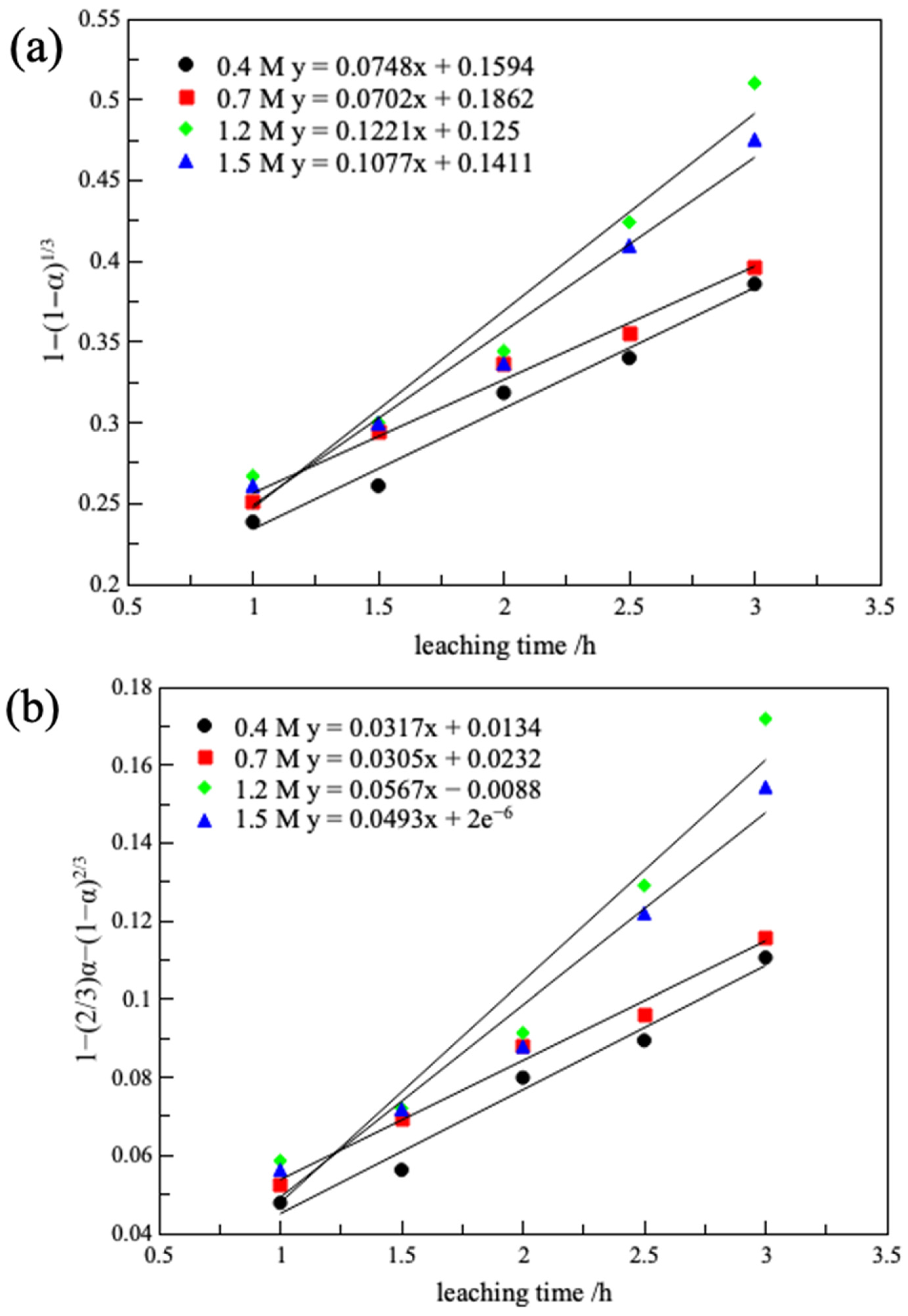
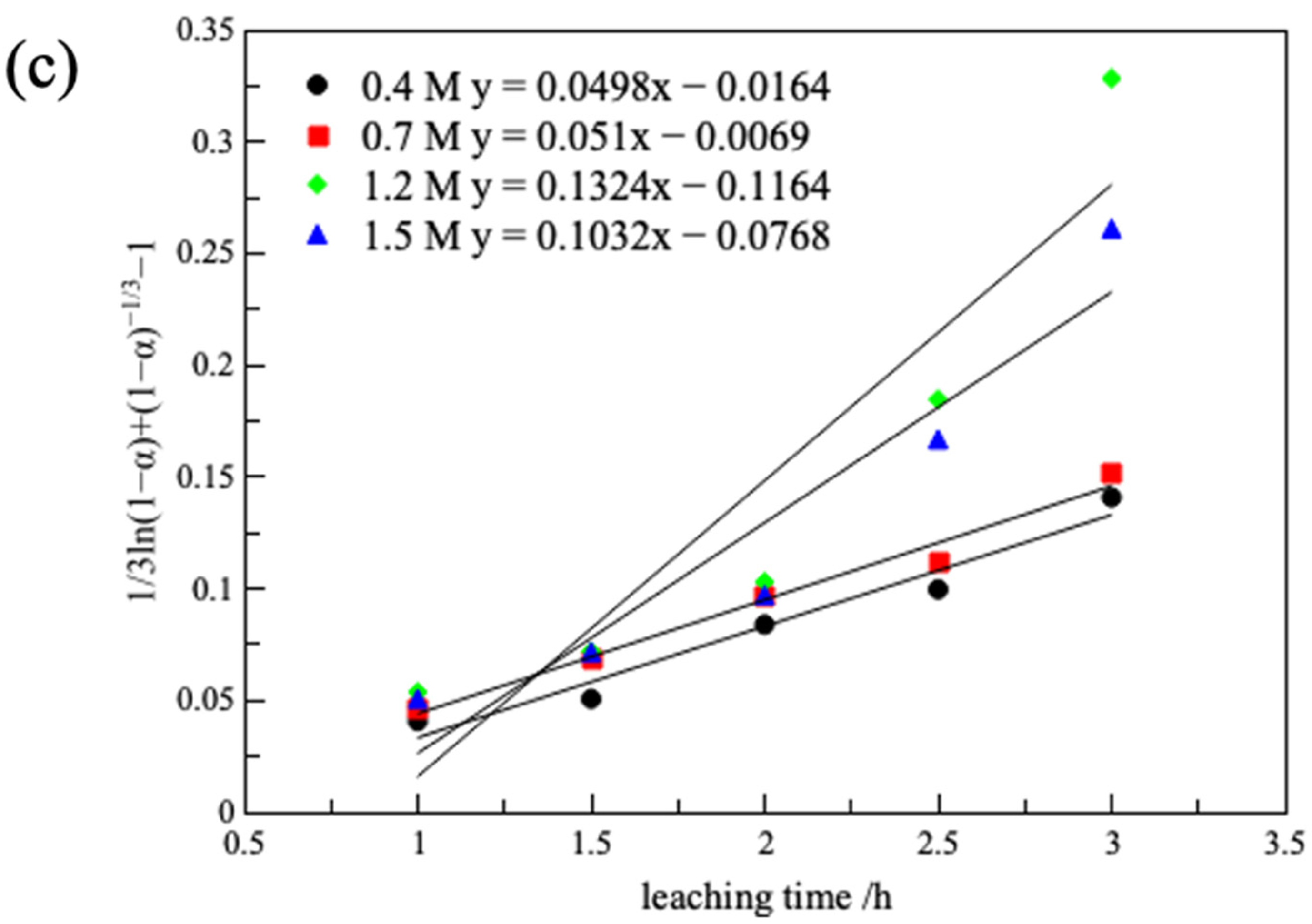
| Oxalic Acid Concentration/(M) | Chemical Reaction Control | Internal Diffusion Control | Mixed Reaction Control | |||
|---|---|---|---|---|---|---|
| R2 | R2 | R2 | ||||
| 0.4 | 0.0748 | 0.987 | 0.0317 | 0.979 | 0.0498 | 0.962 |
| 0.7 | 0.0702 | 0.985 | 0.0305 | 0.988 | 0.051 | 0.981 |
| 1.2 | 0.1221 | 0.961 | 0.0567 | 0.948 | 0.1324 | 0.867 |
| 1.5 | 0.1077 | 0.977 | 0.0493 | 0.966 | 0.1032 | 0.908 |
| Temperature/(K) | Chemical Reaction Control | Internal Diffusion Control | Mixed Reaction Control | |||
|---|---|---|---|---|---|---|
| R2 | R2 | R2 | ||||
| 323 | 0.0163 | 0.973 | 0.0037 | 0.983 | 0.0029 | 0.987 |
| 333 | 0.0431 | 0.977 | 0.0152 | 0.966 | 0.0147 | 0.949 |
| 353 | 0.1485 | 0.960 | 0.0698 | 0.948 | 0.1907 | 0.857 |
| 363 | 0.137 | 0.995 | 0.0665 | 0.994 | 0.228 | 0.963 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Xu, R.; Sun, W.; Wang, L.; Zhang, Y. Lithium Extraction from Lithium-Bearing Clay Minerals by Calcination-Leaching Method. Minerals 2024, 14, 248. https://doi.org/10.3390/min14030248
Liu J, Xu R, Sun W, Wang L, Zhang Y. Lithium Extraction from Lithium-Bearing Clay Minerals by Calcination-Leaching Method. Minerals. 2024; 14(3):248. https://doi.org/10.3390/min14030248
Chicago/Turabian StyleLiu, Jie, Rui Xu, Wei Sun, Li Wang, and Ye Zhang. 2024. "Lithium Extraction from Lithium-Bearing Clay Minerals by Calcination-Leaching Method" Minerals 14, no. 3: 248. https://doi.org/10.3390/min14030248







