Montbrayite from the Svetlinsk Gold–Telluride Deposit (South Urals, Russia): Composition Variability and Decomposition
Abstract
:1. Introduction
2. Geological Setting
3. Materials and Methods
4. Results
4.1. Mineral Assemblages and Physical Properties
4.2. Chemical Composition
4.3. EBSD Study
4.4. Raman Spectroscopy
5. Discussion
5.1. Chemical Variability of Montbrayite
5.2. Formation Conditions of Montbrayite
5.3. Decomposition of Montbrayite
6. Conclusions
- Two substitution mechanisms for antimony were proposed for montbrayite from the Svetlinsk gold–telluride deposit: Sb → Au (2.5–5.6 wt% Sb) and Sb → Te (7–8 wt% Sb).
- The slope of the reflectance spectra decreases, and the curve in the blue–green region of the spectrum disappears with increasing Sb content in montbrayite.
- The average positions of the peak with high intensity are ~64 cm−1 and ~90 cm−1 for montbrayite with Sb → Te and Sb → Au, respectively. Variations of the peak position are the result of Sb substitution of Au in a disordered fashion.
- The upper temperature range of montbrayite crystallization is 410–440 °C.
- A possible scenario of montbrayite decomposition is as follows: local temperature increase → montbrayite melting and decomposition → positive volume change → reaction-induced fracturing → fluid inflow through cracks → leaching of calaverite, dissolution of gold, precipitation of new phases.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| No. | Wt% | Formula Calculated on the Basis of 61 Atoms | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ag | Au | Pb | Sb | Bi | Te | Total | Ag | Au | Pb | Sb | Bi | Te | |
| Bi-rich montbrayite | |||||||||||||
| 1 | 0.55 | 44.32 | 1.61 | 0.90 | 2.81 | 49.80 | 99.99 | 0.48 | 21.15 | 0.73 | 0.70 | 1.26 | 36.68 |
| 2 | 0.60 | 47.70 | 1.30 | 0.30 | 2.90 | 47.00 | 99.80 | 0.53 | 23.13 | 0.60 | 0.24 | 1.33 | 35.18 |
| 3 | – | 46.10 | 1.10 | 1.10 | 3.80 | 47.10 | 99.20 | 0 | 22.46 | 0.51 | 0.87 | 1.74 | 35.42 |
| 4 | – | 46.00 | 1.10 | 1.00 | 4.00 | 47.40 | 99.50 | 0 | 22.34 | 0.51 | 0.79 | 1.83 | 35.53 |
| 5 | – | 45.09 | 0.02 | 1.79 | 3.68 | 49.15 | 99.91 | 0 | 21.60 | 0 | 0.68 | 1.31 | 37.41 |
| 6 | – | 46.74 | 0.79 | 3.59 | 47.00 | 98.12 | 0 | 23.10 | 0.37 | 0 | 1.67 | 35.86 | |
| 7 | 0.07 | 47.38 | 1.30 | 4.46 | 47.48 | 100.69 | 0.06 | 22.85 | 0.60 | 0 | 2.03 | 35.35 | |
| 8 | – | 49.64 | – | – | 3.58 | 46.32 | 99.54 | 0 | 24.32 | 0 | 0 | 1.65 | 35.03 |
| 9 | – | 49.34 | – | – | 3.43 | 46.96 | 99.73 | 0 | 24.07 | 0 | 0 | 1.58 | 35.36 |
| 10 | – | 46.10 | 1.10 | 1.10 | 3.80 | 47.10 | 99.20 | 0 | 22.46 | 0.51 | 0.87 | 1.74 | 35.42 |
| Sb-rich montbrayite | |||||||||||||
| 11 | – | 48.73 | – | 5.63 | 1.95 | 42.97 | 99.28 | 0 | 23.59 | 0 | 4.41 | 0.89 | 32.11 |
| 12 | 0.92 | 44.86 | – | 3.73 | 1.75 | 49.17 | 100.43 | 0.79 | 21.03 | 0 | 2.83 | 0.77 | 35.58 |
| 13 | – | 50.76 | – | 5.03 | 1.79 | 46.55 | 104.13 | 0 | 23.38 | 0 | 3.75 | 0.78 | 33.10 |
| 14 | – | 49.71 | – | 4.94 | 1.90 | 46.20 | 102.75 | 0 | 23.18 | 0 | 3.73 | 0.84 | 33.26 |
| 15 | – | 50.60 | – | – | – | 49.40 | 100.00 | 0 | 24.33 | 0 | 0 | 0 | 36.67 |
| 16 | – | 47.70 | – | 4.90 | – | 45.20 | 97.80 | 0 | 23.20 | 0 | 3.86 | 0 | 33.94 |
| 17 | 0.10 | 47.90 | – | 5.00 | – | 44.70 | 97.70 | 0.09 | 23.34 | 0 | 3.94 | 0 | 33.63 |
| 18 | – | 48.30 | – | 4.60 | – | 46.40 | 99.30 | 0 | 23.13 | 0 | 3.56 | 0 | 34.30 |
| 19 | – | 48.60 | 0.40 | 6.40 | – | 46.20 | 101.60 | 0 | 22.69 | 0.18 | 4.83 | 0 | 33.30 |
| 20 | – | 49.60 | – | 6.00 | – | 45.10 | 100.70 | 0 | 23.47 | 0 | 4.59 | 0 | 32.94 |
| 21 | – | 48.00 | 0.30 | 6.10 | – | 47.20 | 101.60 | 0 | 22.35 | 0.13 | 4.59 | 0 | 33.92 |
| 22 | – | 47.40 | 0.10 | 6.80 | – | 46.40 | 100.70 | 0 | 22.22 | 0.05 | 5.16 | 0 | 33.58 |
| 23 | – | 47.90 | – | 6.40 | 0.10 | 47.10 | 101.50 | 0 | 22.30 | 0 | 4.82 | 0.04 | 33.84 |
| 24 | – | 47.10 | – | 7.40 | 0.10 | 46.00 | 100.60 | 0 | 22.07 | 0 | 5.61 | 0.04 | 33.27 |
| 25 | – | 47.10 | 0.20 | 7.50 | – | 45.40 | 100.20 | 0 | 22.19 | 0.09 | 5.72 | 0 | 33.01 |
| 26 | 0.10 | 46.90 | 0.10 | 7.10 | – | 46.90 | 101.10 | 0.08 | 21.83 | 0.04 | 5.35 | 0 | 33.70 |
| 27 | – | 46.71 | – | 8.36 | 43.19 | 98.26 | 0 | 22.27 | 0 | 6.45 | 0 | 31.79 | |
| 28 | – | 46.06 | – | 6.36 | 46.94 | 99.36 | 0 | 21.81 | 0 | 4.87 | 0 | 34.31 | |
| Pb-rich montbrayite | |||||||||||||
| 29 | 3.13 | 46.75 | 4.41 | 1.41 | 45.00 | 100.70 | 2.72 | 22.21 | 1.99 | 1.08 | 0 | 33.00 | |
| 30 | 3.32 | 44.95 | 4.45 | 1.69 | 44.75 | 99.16 | 2.91 | 21.58 | 2.03 | 1.31 | 0 | 33.16 | |
| Synthetic montbrayite | |||||||||||||
| 31 | 0.40 | 47.40 | 1.00 | 1.10 | 3.20 | 46.70 | 99.80 | 0.35 | 22.95 | 0.46 | 0.86 | 1.46 | 34.91 |
| 32 | 20.13 | 1.53 | 39.35 | ||||||||||
| 33 | 48.24 | 7.38 | 43.78 | 99.40 | 0 | 23.03 | 0 | 5.70 | 0 | 32.27 | |||
| 34 | 48.07 | 7.82 | 43.21 | 99.10 | 0 | 23.01 | 0 | 6.06 | 0 | 31.93 | |||
| 35 | 49.06 | 6.67 | 43.66 | 99.39 | 0 | 23.52 | 0 | 5.17 | 0 | 32.31 | |||
| Raman Shift (cm−1) | Corresponding Raman Modes | References | ||||||
|---|---|---|---|---|---|---|---|---|
| A* | B | C | D | E | F | G | ||
| 38 | 41 | 38 | 40 | 43 | Mode at 42 cm−1 of AuTe2 | [29] | ||
| 62 | 62 | 60 | 60 | 62 | Mode at 57 cm−1 of AuTe2 | [29] | ||
| Modes at 58 and 61 cm−1 of AuAgTe4 | [42] | |||||||
| Sb-Te vibration (63 cm−1) of Sb2Te3 | [43] | |||||||
| 71 | 65 | 66 | 71 | 67 | 68 | 71 | Mode at 69 cm−1 of Sb2Te3 | [44] |
| Mode at 71 cm−1 of AuSbTe | [45] | |||||||
| 88 | 89 | 90 | 87 | 90 | 89 | 92 | Modes at 88 and 92 cm−1 of AuTe2 | [29] |
| Modes at 88 and 95 cm−1 of AuAgTe4 | [42] | |||||||
| Mode at 88 cm−1 | [46] | |||||||
| Mode at 90 cm−1 of Te | [47] | |||||||
| 99 | 103 | 104 | Mode at 101 cm−1 of AuTe2 | [29] | ||||
| Mode at 103 cm−1 of Bi2Te3 | [44] | |||||||
| Mode at 105 cm−1 of Cu-doped Sb2Te3 | [48] | |||||||
| Mode at 102 cm−1 of AuAgTe4 | [42] | |||||||
| Mode at 98 cm−1 of Se-rich AuTe2 | [49] | |||||||
| Mode at 98 cm−1 of Bi2Te3 | [50] | |||||||
| 116 | 112 | 118 | 112 | 120 | 114 | 120 | Mode at 119 cm−1 of AuTe2 | [29] |
| Te-Te vibration (116 cm−1) of Sb2Te3 | [43] | |||||||
| Modes at 117 and 121 cm−1 of AuSb2 | [51] | |||||||
| Mode at 120 cm−1 of Bi2Te3 and mode at 112 cm−1 of Sb2Te3 | [44] | |||||||
| Mode at 120 cm−1 of Cu-doped Sb2Te3 | [48] | |||||||
| Mode at 117 cm−1 | [46] | |||||||
| Mode at 112 cm−1 of Sb2Te3 | [52] | |||||||
| Mode at 119 cm−1 | [53] | |||||||
| Mode at 120 cm−1 | [47] | |||||||
| Mode at 116 cm−1 of Bi2Te3 | [50] | |||||||
| Modes 114 and 121 cm−1 of AuAgTe4 | [42] | |||||||
| 135 | Te-Te vibration (137 cm−1) of Sb2Te3 | [43] | ||||||
| Mode at 134 cm−1 of Bi2Te3 | [44] | |||||||
| Mode at 135 cm−1 of Cu-doped Sb2Te3 | [48] | |||||||
| Mode at 137 cm−1 | [46] | |||||||
| Mode at 136 cm−1 of Bi2Te3 | [50] | |||||||
| Modes at 132 and 134 cm−1 of AuAgTe4 | [42] | |||||||
| 149 | 142 | 146 | 148 | Mode at 143 cm−1 of AuTe2 | [29] | |||
| Mode at 147 cm−1 of AuSbTe | [45] | |||||||
| 155 | 155 | 153 | 157 | Modes at 152 and 162 cm−1of AuTe2 | [29] | |||
| Sb-Te vibration (162 cm−1) of Sb2Te3 | [43] | |||||||
| Modes at 155 and 162 cm−1 of AuSb2 | [51] | |||||||
| Mode at 158 cm−1 of AuAgTe4 | [42] | |||||||
| Mode at 160 cm−1 of Cu-doped Sb2Te3 | [48] | |||||||
| Mode at 159 cm−1 of AuSbTe | [45] | |||||||
| 177 | 171 | Mode at 172 cm−1 of AuTe2 | [29] | |||||
| Mode at 176 cm−1 of Au2.73Te6.23Se3.84 | [49] | |||||||
| Mode at 174 to 178 cm−1 of synthetic Au3X10 (X = Te, Se, S) | [49] | |||||||
| 184 | 190 | 190 | 185 | Mode at 183 cm−1 of PbTe | [53] | |||
| 193 | 198 | 197 | Mode at 206 cm−1 of synthetic AuX (X = Te,Se,S) | [49] | ||||
| 218 | 222 | 222 | 217 | 216 | Mode at 210 and 238 cm−1 of synthetic AuX (X = Te, Se, S) | [49] | ||
| 240 | 254 | 257 | 262 | 255 | 245 | Sb-O vibration (251 cm−1) | [43] | |
| 279 | Mode at 276 to 284 cm−1 of synthetic Au3X10 (X = Te, Se, S) | [49] | ||||||
| 303 | 301 | 302 | 303 | 302 | 302 | 294 | Mode at 302 cm−1 of PbTe | [53] |
Appendix B
References
- Peacock, M.A.; Thompson, R.M. Montbrayite, a new gold telluride. Am. Miner. 1946, 31, 515–526. [Google Scholar]
- Markham, N.L. Synthetic and natural phases in the system Au-Ag-Te. Econ. Geol. 1960, 55, 1148–1178. [Google Scholar]
- Cabri, L.J. Phase relations in the Au-Ag-Te system and their mineralogical significance. Econ. Geol. 1965, 60, 1569–1606. [Google Scholar] [CrossRef]
- Howie, F.H.; Veale, C.R. Low temperature synthesis of gold telluride. J. Inorg. Nucl. Chem. 1966, 28, 1149–1154. [Google Scholar] [CrossRef]
- Rucklidge, J. Frohbergite, montbrayite, and a new Pb-Bi telluride. Can. Miner. 1969, 9, 709–716. [Google Scholar]
- Bachechi, F. Synthesis and stability of montbrayite, Au2Te3. Am. Miner. 1972, 57, 146–154. [Google Scholar]
- Gather, B.; Blachnik, R. Das ternäre System Gold-Antimon-Tellur. Int. J. Mater. Res. 1976, 67, 395–399. [Google Scholar] [CrossRef]
- Blachnik, R.; Gather, B. Notizen: Einige neue ternäre Goldchalkogenide. Z. Naturforschung B 1976, 31, 526–527. [Google Scholar] [CrossRef]
- Nakamura, Y.; Ikeda, K. Isothermal phase relations in the Au–Sb–Te system at 350 °C. Neues Jahrb. Mineral. Monatshefte 2002, H6, 276–288. [Google Scholar] [CrossRef]
- Shackleton, J.M.; Spry, P.G. Antimony-rich montbrayite ((Au,Sb)2Te3) from the Golden Mile, Western Australia, and its compositional implications. Neues Jahrb. Miner. Monatshefte 2003, 3, 113–125. [Google Scholar] [CrossRef]
- Bachechi, F. Crystal structure of montbrayite. Nat. Phys. Sci. 1971, 231, 67–68. [Google Scholar] [CrossRef]
- Edenharter, A.; Bente, K.; Steins, M. Strukturuntersuchungen an Montbrayit, Au2Te3. Z. Krist. Suppl. 1991, 3, 63–64. (In German) [Google Scholar]
- Bindi, L.; Paar, W.H.; Lepore, G.O. Montbrayite, (Au,Ag,Sb,Pb,Bi)23(Te,Sb,Pb,Bi)38, from the Robb-Montbray mine, Montbray, Québec: Crystal structure and revision of the chemical formula. Can. Miner. 2018, 56, 129–142. [Google Scholar] [CrossRef]
- Travis, G.A. A Study of the Gold and Telluride Mineralization at Kalgoorlie, Western Australia. Master’s Thesis, Imperial College, London, UK, 1966. [Google Scholar]
- Golding, Y.L. Mineralogy, Geochemistry and Origin of the Kalgoorlie Gold Deposits, Western Australia. Ph.D. Thesis, University of Melbourne, Melbourne, Australia, 1978. [Google Scholar]
- Mueller, A.G.; Muhling, J.R. Early pyrite and late telluride mineralization in vanadium-rich gold ore from the Oroya Shoot, Paringa South mine, Golden Mile, Kalgoorlie: 3. Ore mineralogy, Pb-Te (Au-Ag) melt inclusions, and stable isotope constraints on fluid sources. Miner. Depos. 2020, 55, 733–766. [Google Scholar] [CrossRef]
- Nysten, P.; Annersten, H. The gold mineralization at Enasen, Central Sweden. Geol. Foereningen I Stockh. Foerhandlingar 1984, 106, 245–256. [Google Scholar] [CrossRef]
- Criddle, A.J.; Stanley, C.J.; Paar, W.H. The optical properties of montbrayite, Au2Te3, from Robb Montbray, Quebec, compared with those of the other gold tellurides. Can. Miner. 1991, 29, 223–229. [Google Scholar]
- Spiridonov, E.M. Mineralogy of the metamorphosed Kokchar plutogenic gold-quartz deposit (Southern Urals): 1. Gold-telluride ores (new minerals Bi-Pb-Te-S; kochkarite, ruklidgeite, aleksite, gold, montbrayite, tellurides and sulphides of Bi). Proc. Russ. Mineral. Soc. 1995, 124, 24–39. [Google Scholar]
- Spiridonov, E.M. New data on mineralogy of deposits of plutonogenic gold-quartz formation in the northern Central Kazakhstan. Part I. New Data Miner. 2014, 49, 57–73. [Google Scholar]
- Genkin, A.D.; Safonov, Y.G.; Tsepin, A.I.; Scherbachev, D.K. Montbrayite from Voronezhsky Massif. Proc. Russ. Mineral. Soc. 1999, 128, 93–94. [Google Scholar]
- Palyanova, G.A. Gold and silver minerals in sulfide ore. Geol. Ore Dep. 2020, 62, 383–406. [Google Scholar] [CrossRef]
- Sazonov, V.N.; Popov, V.A.; Grigoryev, N.A.; Murzin, V.V.; Metsner, E.I. Crust-Mantle Mineralisation in the Salic Blocks of Eugeosyncline; UrO USSR AN: Sverdlovsk, Russia, 1989; 113p. (In Russian) [Google Scholar]
- Vikent’eva, O.V.; Bortnikov, N.S. The large Svetlinsk Au-Te deposit, South Urals: Telluride mineralization for genetic reconstructions. In Proceedings of the 13th Biennial SGA Meeting, Nancy, France, 24–27 August 2015; pp. 851–854. [Google Scholar]
- Bortnikov, N.S.; Murzin, V.V.; Sazonov, V.N.; Prokof’ev, V.Y.; Stolyarov, M.I. The Svetlinsk gold-telluride deposit, Urals, Russia: Mineral paragenesis, fluid inclusions and stable isotope studies. In Proceedings of the Fifth Biennial SGA Meeting and the Tenth Quadrennial JAGOD Symposium, London, UK, 22–25 August 1999; Volume 1, pp. 21–24. [Google Scholar]
- Vikent’eva, O.; Prokofiev, V.; Borovikov, A.; Kryazhev, S.; Groznova, E.; Pritchin, M.; Vikentyev, I.; Bortnikov, N. Contrasting Fluids in the Svetlinsk Gold-Telluride Hydrothermal System, South Urals. Minerals 2020, 10, 37. [Google Scholar] [CrossRef]
- Shackleton, J.M.; Spry, P.G.; Bateman, R. Telluride mineralogy of the Golden Mile deposit, Kalgoorlie, Western Australia. Can. Miner. 2003, 41, 1503–1524. [Google Scholar] [CrossRef]
- Mueller, A.G.; Hagemann, S.G.; Brugger, J.; Xing, Y.; Roberts, M.P. Early Fimiston and late Oroya Au–Te ore, Paringa South mine, Golden Mile, Kalgoorlie: 4. Mineralogical and thermodynamic constraints on gold deposition by magmatic fluids at 420–300 °C and 300 MPa. Miner. Depos. 2020, 55, 767–796. [Google Scholar] [CrossRef]
- van Loosdrecht, P.H.M.; van Bentum, P.J.M.; Balzuweit, K. Raman study of incommensurately modulated calaverite (AuTe2). Ferroelectrics 1992, 125, 517–522. [Google Scholar] [CrossRef]
- Gather, B.; Blachnik, R. Das System Gold–Wismut–Tellur. Z. Met. 1974, 65, 653–656, (In German with English Abstract). [Google Scholar] [CrossRef]
- Winkler, E.W.; Bright, N.F.H. Experiments in the Au–Bi–Te system. Solid State Communications 1964. 2, 293–295.
- Legendre, B.; Souleau, C. Etude du systeme ternaire Au-Pb-Te. Bull. Chim. Fr. 1972, 2, 473–479. (In French) [Google Scholar]
- Kitakaze, A.; Nakamura, M.; Komatsu, R. Phase relation of some sulfide systems-(6): Especially Pb-Sb-S ternary system. Mem. Fac. Eng. Yamaguchi Univ. 2020, 71, 7–16. [Google Scholar]
- Polekhovsky, Y.S.; Petrov, S.V.; Kalinin, A.A.; Koval, A.V. New data on mineralogy of the Rompas gold-uranium occurrence, Finland. In Proceedings of the Fersman Scientific Session; Kola Science Center RAS: Apatity, Russia, 2018; Volume 15, pp. 482–486. (In Russian) [Google Scholar]
- Putnis, A. Mineral replacement reactions: From macroscopic observations to microscopic mechanisms. Miner. Mag. 2002, 66, 689–708. [Google Scholar] [CrossRef]
- Ciobanu, C.L.; Birch, W.D.; Cook, N.J.; Pring, A.; Grundler, P.V. Petrogenetic significance of Au–Bi–Te–S associations: The example of Maldon, Central Victorian gold province, Australia. Lithos 2010, 116, 1–17. [Google Scholar] [CrossRef]
- Gamyanin, G.N.; Nekrasov, I.Y.; Samusikov, V.P. Maldonite from gold occurences of Eastern Yakutia. Miner. J. 1986, 8, 65–71. [Google Scholar]
- Zhao, J.; Brugger, J.; Grundler, P.V.; Xia, F.; Chen, G.; Pring, A. Mechanism and kinetics of a mineral transformation under hydrothermal conditions: Calaverite to metallic gold. Am. Miner. 2009, 94, 1541–1555. [Google Scholar] [CrossRef]
- Xu, W.; Zhao, J.; Brugger, J.; Chen, G.; Pring, A. Mechanism of mineral transformations in krennerite, Au3AgTe8, under hydrothermal conditions. Am. Miner. 2013, 98, 2086–2095. [Google Scholar] [CrossRef]
- Zhao, J.; Brugger, J.; Xia, F.; Ngothai, Y.; Chen, G.; Pring, A. Dissolution-reprecipitation vs. solid-state diffusion: Mechanism of mineral transformations in sylvanite, (AuAg)2Te4, under hydrothermal conditions. Am. Miner. 2013, 98, 19–32. [Google Scholar] [CrossRef]
- Vikent’eva, O.V.; Bortnikov, N.S. Evidences of mineral melting in the ores of the Svetlinsk gold deposit, South Urals, Russia. Dokl. Earth Sci. 2023, 513, 2. [Google Scholar]
- Amiel, Y.; Kafle, G.P.; Komleva, E.V.; Greenberg, E.; Ponosov, Y.S.; Chariton, S.; Lavina, B.; Zhang, D.; Palevski, A.; Ushakov, A.V.; et al. Silvanite AuAgTe4: A rare case of gold superconducting material. J. Mater. Chem. C 2023, 11, 10016. [Google Scholar] [CrossRef]
- Zybała, R.; Mars, K.; Mikuła, A.; Bogusławski, J.; Soboń, G.; Sotor, J.; Schmidt, M.; Kaszyca, K.; Chmielewski, M.; Ciupiński, L.; et al. Synthesis and characterization of antimony telluride for thermoelectric and optoelectronic applications. Arch. Metall. Mater. 2017, 62, 1067–1070. [Google Scholar] [CrossRef]
- Richter, W.; Köhler, H.; Becker, C.R. A Raman and far-infrared investigation of phonons in the rhombohedral V2-VI3 compounds Bi2Te3, Bi2Se3, Sb2Te3 and Bi2(Te1−xSex)3 (0 < x < l), (Bi1−ySby)2Te3 (0 < y < 1). Phys. Stat. Sol. 1977, 6, 619–628. [Google Scholar]
- Vikent’eva, O.V.; Shilovskikh, V.V.; Shcherbakov, V.D.; Vikentyev, I.V.; Bortnikov, N.S. A Rare Au-Sb telluride pampaloite from the Svetlinsk gold-telluride deposit, South Urals, Russia. Minerals 2022, 12, 1274. [Google Scholar] [CrossRef]
- Rodríguez-Fernández, C.; Manzano, C.V.; Romero, A.H.; Martín, J.; Martín-González, M.; Morais de Lima Jr, M.; Cantarero, A. The fingerprint of Te-rich and stoichiometric Bi2Te3 nanowires by Raman spectroscopy. Nanotechnology 2016, 27, 075706. [Google Scholar] [CrossRef]
- Volodin, V.A.; Sinyukov, M.P.; Shcheglov, D.V.; Latyshev, A.V.; Fedosenko, E.V. Raman scattering in PbTe and PbSnTe films: In situ phase transformations. Semiconductors 2014, 48, 173–177. [Google Scholar] [CrossRef]
- Shi, D.; Wang, R.; Wang, G.; Li, C.; Shen, X.; Nie, Q. Enhanced thermoelectric properties in Cu-doped Sb2Te3 films. Vacuum 2017, 145, 347–350. [Google Scholar] [CrossRef]
- Palyanova, G.; Beliaeva, T.; Kokh, K.; Seriotkin, Y.; Moroz, T.; Tolstykh, N. Characterization of synthetic and natural gold chalcogenides by electron microprobe analysis, X-ray powder diffraction, and Raman spectroscopic methods. J. Raman Spectrosc. 2022, 53, 1012–1022. [Google Scholar] [CrossRef]
- Russo, V.; Bailini, A.; Zamboni, M.; Passoni, M.; Conti, C.; Casari, C.S.; Li Bassi, A.; Bottani, C.E. Raman spectroscopy of Bi-Te thin films. J. Raman Spectrosc. 2008, 39, 205–210. [Google Scholar] [CrossRef]
- Freund, G.A.; Kirby, R.D.; Sellmyer, D.J. Raman scattering from pyrite structure AuSb2. Solid State Commun. 1977, 22, 5–7. [Google Scholar] [CrossRef]
- Goncalves, L.M.; Alpuim, P.; Rolo, A.G.; Correia, J.H. Thermal co-evaporation of Sb2Te3 thin-films optimized for thermoelectric applications. Thin Solid Film. 2011, 519, 4152–4157. [Google Scholar] [CrossRef]
- Zhang, B.; Cai, C.; Zhu, H.; Wu, F.; Ye, Z.; Chen, Y.; Li, R.; Kong, W.; Wu, H. Phonon blocking by two dimensional electron gas in polar CdTe/PbTe heterojunctions. Appl. Phys. Lett. 2014, 104, 161601. [Google Scholar] [CrossRef]
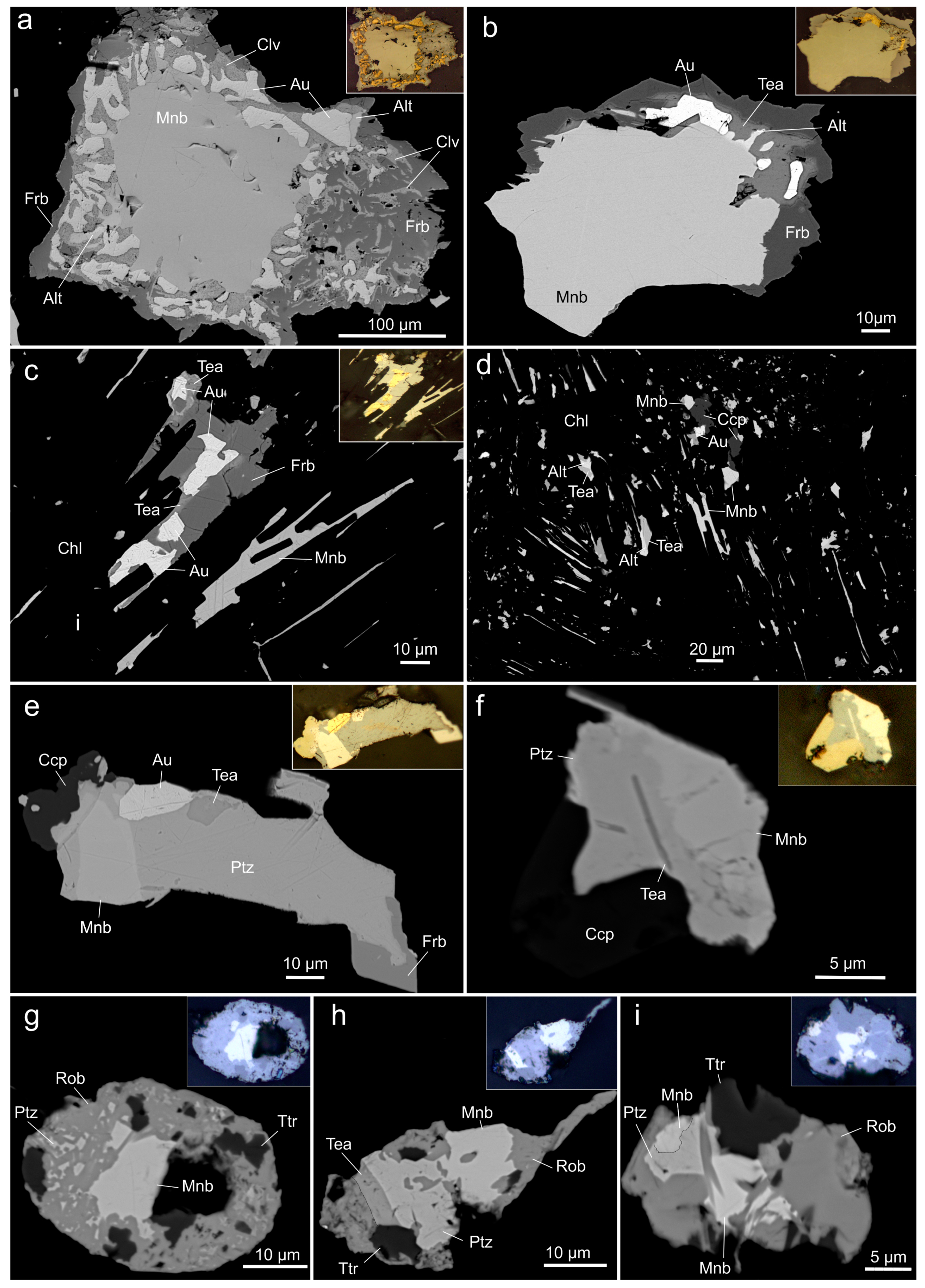
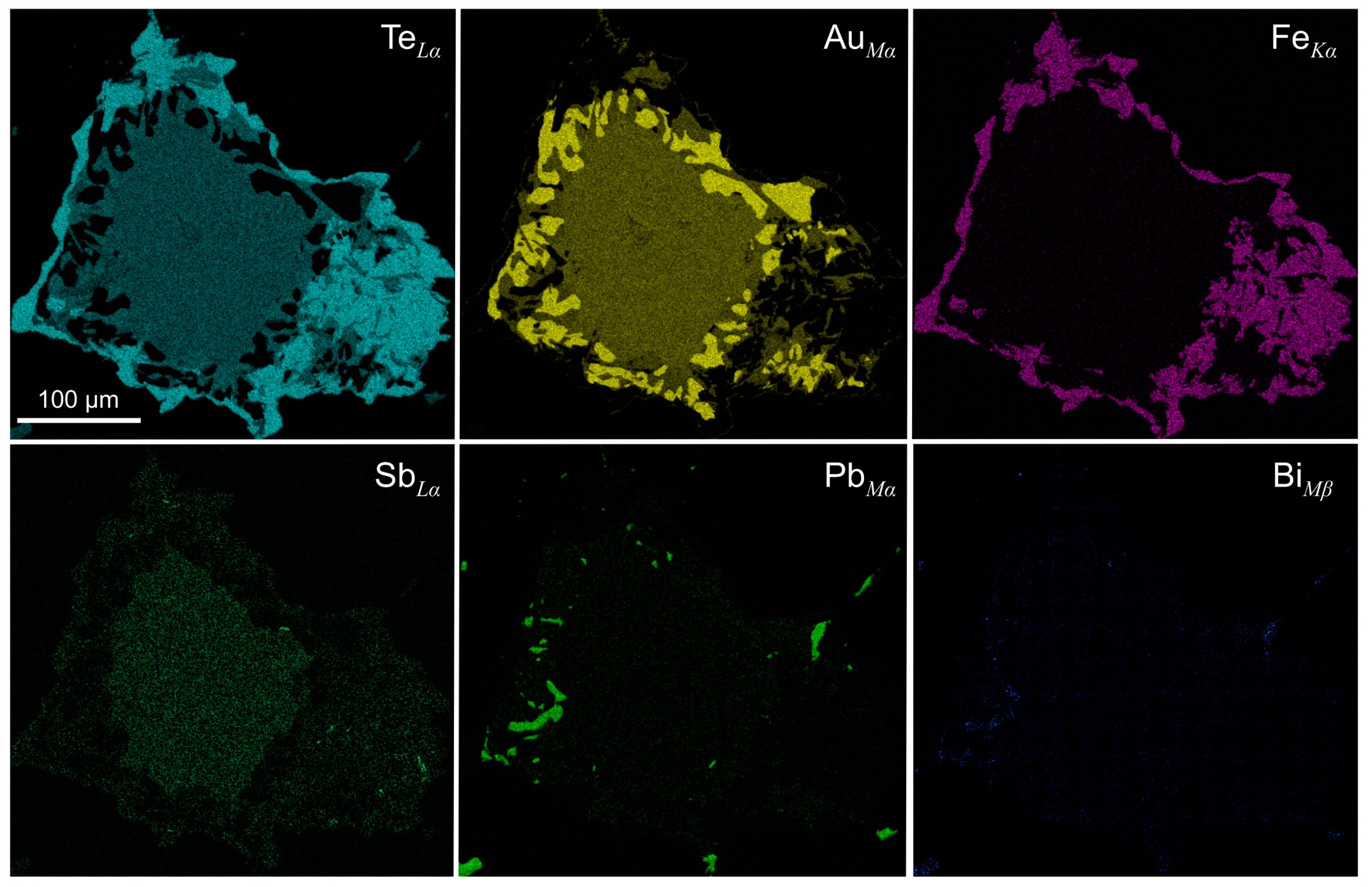


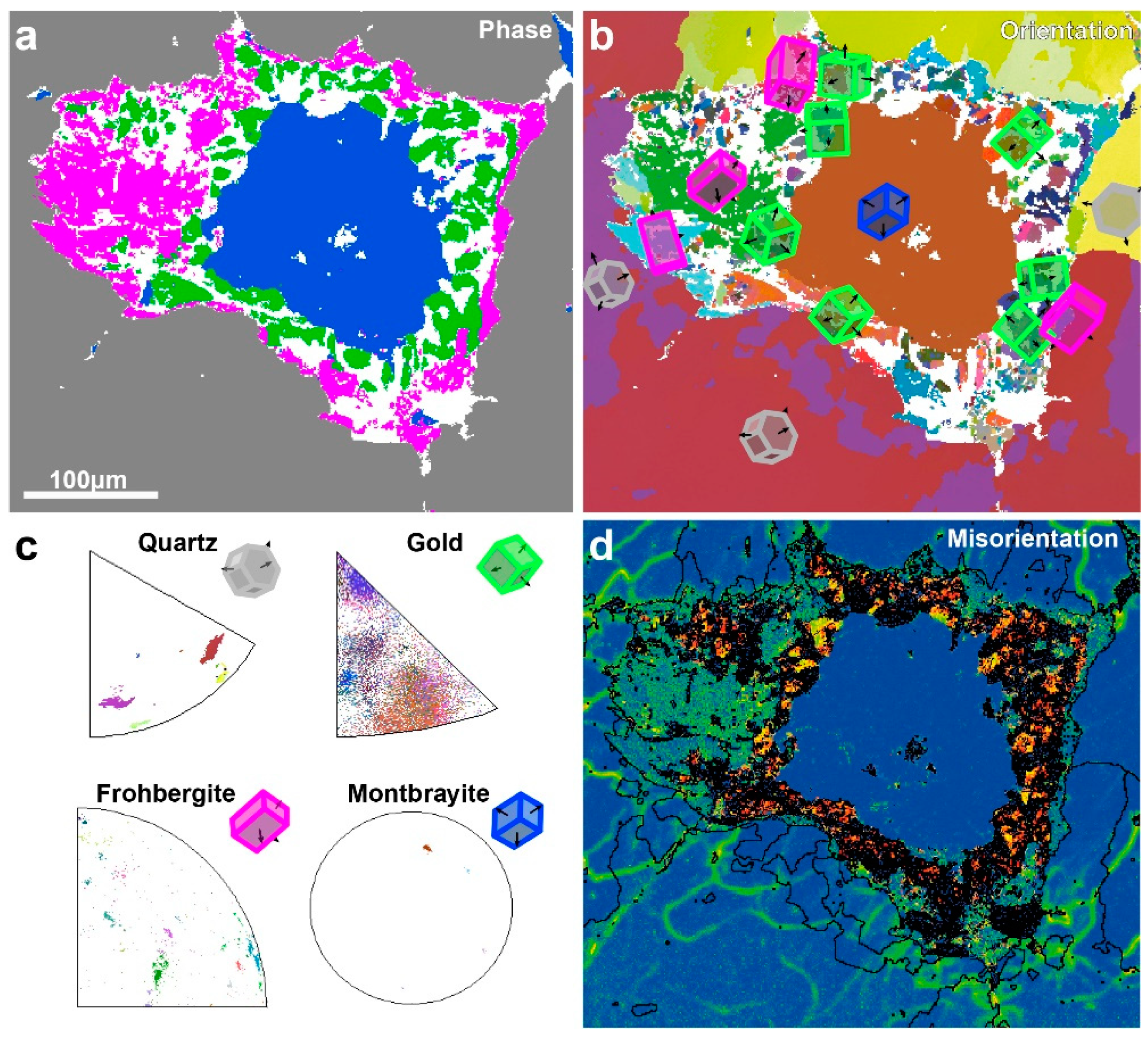
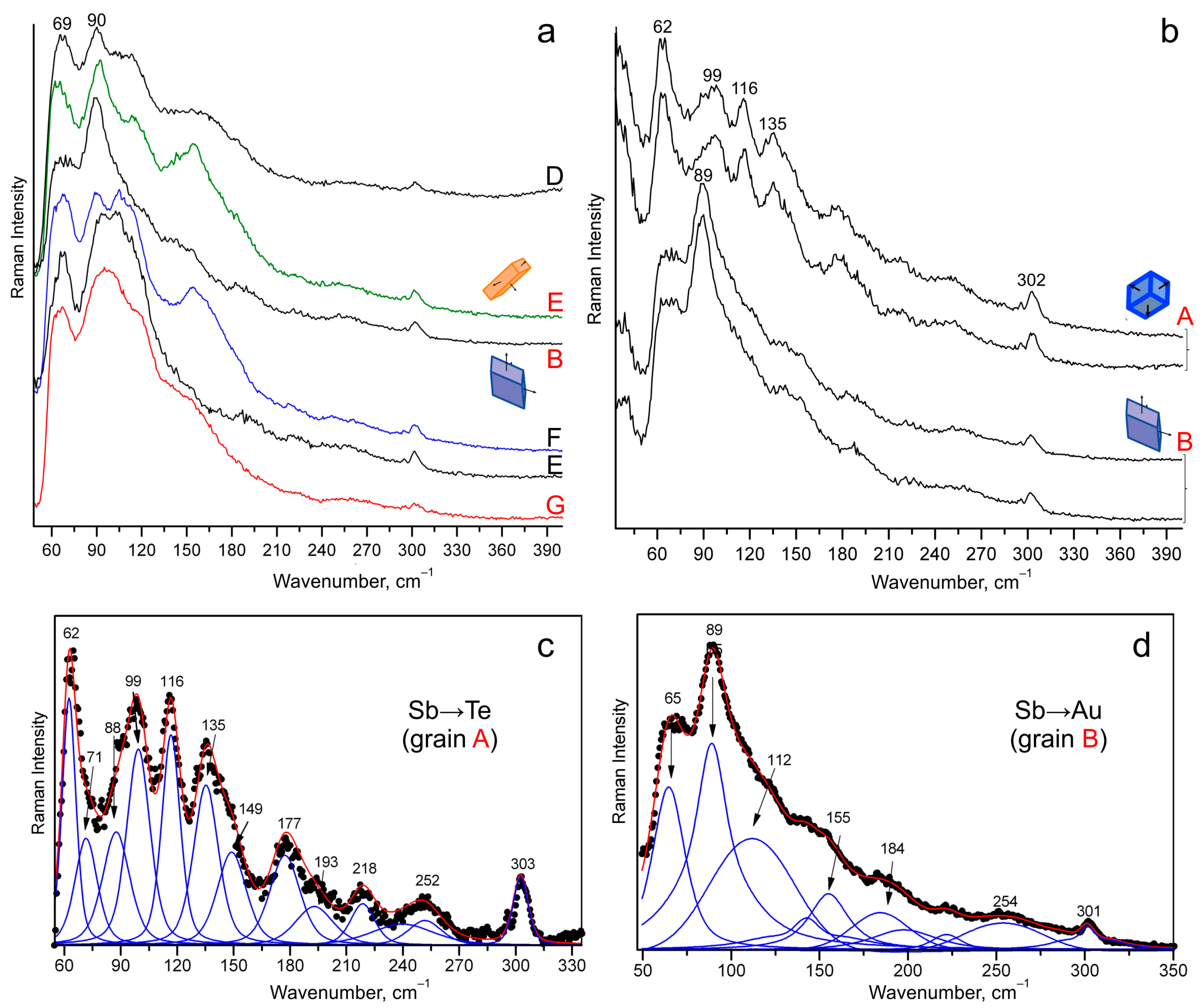
| Mnb | Deposit/Location | Mineral Assemblages | References |
|---|---|---|---|
| Bi-rich | Robb-Montbray Mine/Quebec, Canada | Mnb-Au-Tbi-Alt-Ptz-Mlt | [1] |
| Mnb-Clv | [5] | ||
| Mnb-Tbi | [18] | ||
| Mnb-Tbi-Frb-Ptz-Alt-Mlt-Ccp-Au | [13] | ||
| Kochkar/S. Urals, Russia | Mnb-Au-Koc | [19] | |
| Sb-rich | Golden Mile/Kalgoorlie, Western Australia | Mnb-Mlt, Mnb-Syl, Mnb-Clv, Mnb-Alt-Ptz | [14] |
| Mnb-Alt-Ptz, Mnb-Au, Mnb-Ptz, Mnb-Clv-Au, Mnb-Au-Mtg, Mnb-Au-Tea | [15] | ||
| Mnb-Alt, Mnb-Alt-Ptz, Mnb-Au-Ptz | [27] | ||
| Mnb-Alt-Ptz, Mnb-Au-Ptz | [10] | ||
| Mnb-Au-Mlt, Mnb-Au-Tea, Mnb-Alt-Tea-Clr-Mlt-Clv-Ptz | [16] | ||
| Enasen/Sweden | Mnb-Au-Tea, Mnb-Frb | [17] | |
| Svetlinsk/S. Urals, Russia | Mnb-Frb-Au-Clv-Alt, Mnb-Frb-Au-Tea-Alt, Mnb-Frb-Au-Tea-Chl-Ccp, Mnb-Frb-Au-Tea-Ptz-Ccp, Mnb-Tea-Ptz-Ccp, Mnb-Tea-Ptz-Rob-Ttr | This study | |
| Pb-rich | Zhana-Tyube, South Aksu, Zholymbet/Kazakhstan | Mnb-Mlt-Frb-Au in the pyrrhotite ores | [20] |
| No. | wt.% | Formula Calculated on the Basis of 61 Atoms | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ag | Au | Cu | Pb | Sb | Bi | Te | Total | Ag | Au | Cu | Pb | Sb | Bi | Te | |
| 1 | — | 48.66 | — | 0.26 | 7.22 | — | 43.35 | 99.49 | 0 | 23.28 | 0 | 0.12 | 5.59 | 0 | 32.01 |
| 2 | — | 48.47 | — | 0.23 | 7.15 | — | 43.39 | 99.24 | 0 | 23.24 | 0 | 0.10 | 5.54 | 0 | 32.12 |
| 3 | — | 47.88 | — | 0.22 | 7.15 | — | 43.50 | 98.75 | 0 | 23.03 | 0 | 0.10 | 5.57 | 0 | 32.30 |
| 4 * | — | 48.19 | 0.61 | 8.10 | — | 44.92 | 101.82 | 0 | 22.40 | 0 | 0.27 | 6.09 | 0 | 32.24 | |
| 5 * | — | 47.86 | — | 7.79 | — | 44.14 | 99.79 | 0 | 22.70 | 0 | 0 | 5.98 | 0 | 32.32 | |
| 6 * | — | 48.62 | 0.34 | 7.88 | — | 44.71 | 101.55 | 0 | 22.69 | 0 | 0.15 | 5.95 | 0 | 32.21 | |
| 7 | 0.93 | 41.16 | 0.38 | — | 4.95 | — | 52.95 | 100.37 | 0.77 | 18.77 | 0.54 | 0 | 3.65 | 0 | 37.27 |
| 8 | 0.97 | 40.99 | 0.47 | — | 5.02 | — | 53.29 | 100.74 | 0.81 | 18.58 | 0.65 | 0 | 3.68 | 0 | 37.28 |
| 9 | 0.96 | 41.22 | 0.39 | — | 5.08 | — | 53.11 | 100.76 | 0.79 | 18.71 | 0.55 | 0 | 3.73 | 0 | 37.21 |
| 10 | 2.36 | 39.19 | 0.46 | — | 3.79 | 0.11 | 55.42 | 101.33 | 1.92 | 17.49 | 0.63 | 0 | 2.73 | 0.05 | 38.17 |
| 11 | 1.54 | 37.69 | 0.96 | — | 5.15 | — | 55.06 | 100.40 | 1.26 | 16.80 | 1.33 | 0 | 3.71 | 0 | 37.90 |
| 12 | 1.36 | 39.34 | 1.16 | — | 4.07 | 0.33 | 53.47 | 99.73 | 1.13 | 17.80 | 1.63 | 0 | 2.98 | 0.14 | 37.34 |
| 13 | 1.19 | 38.90 | 1.42 | — | 4.25 | 0.32 | 54.89 | 100.97 | 0.96 | 17.27 | 1.96 | 0 | 3.05 | 0.14 | 37.62 |
| 14 | 2.22 | 39.83 | 0.47 | — | 3.78 | 0.33 | 52.93 | 99.56 | 1.85 | 18.20 | 0.67 | 0 | 2.80 | 0.14 | 37.34 |
| 15 | 1.60 | 39.58 | 0.30 | — | 4.38 | — | 52.58 | 98.44 | 1.35 | 18.34 | 0.42 | 0 | 3.28 | 0 | 37.61 |
| 16 | 4.56 | 37.18 | 0.16 | 0.09 | 2.52 | — | 56.53 | 101.04 | 3.69 | 16.50 | 0.22 | 0.04 | 1.81 | 0 | 38.74 |
| 17 | 4.42 | 37.18 | 0.11 | — | 2.49 | — | 56.53 | 100.73 | 3.60 | 16.57 | 0.15 | 0 | 1.79 | 0 | 38.89 |
| 18 | 2.48 | 38.81 | 0.26 | 2.04 | 5.57 | — | 49.35 | 98.50 | 2.10 | 18.03 | 0.37 | 0.90 | 4.19 | 0 | 35.40 |
| 19* | 4.03 | 36.98 | 3.86 | 55.13 | 100.00 | 3.31 | 16.63 | 2.81 | 38.26 | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peak | FWHM | Peak | FWHM | Peak | FWHM | Peak | FWHM | Peak | FWHM | Peak | FWHM | Peak | FWHM |
| 38 | 5 | 41 | 9 | 38 | 29 | 40 | 13 | 43 | 10 | ||||
| 62 | 9 | 65 | 22 | 66 | 24 | 62 | 12 | 60 | 8 | 60 | 8 | 62 | 11 |
| 71 | 14 | 71 | 13 | 67 | 15 | 68 | 15 | 71 | 10 | ||||
| 88 | 17 | 89 | 25 | 90 | 17 | 87 | 27 | 90 | 17 | 89 | 25 | 92 | 38 |
| 99 | 15 | 103 | 25 | 104 | 13 | ||||||||
| 116 | 12 | 112 | 54 | 118 | 29 | 112 | 31 | 116 120 | 30 25 | 114 | 27 | 120 | 28 |
| 135 | 16 | ||||||||||||
| 149 | 18 | 142 | 27 | 146 | 34 | 148 | 70 | ||||||
| 155 | 27 | 155 | 60 | 153 | 45 | 157 | 60 | ||||||
| 177 | 19 | 171 | 18 | ||||||||||
| 184 | 38 | 190 | 39 | 190 | 22 | 185 | 31 | ||||||
| 193 | 23 | 198 | 43 | 197 | 170 | ||||||||
| 218 | 15 | 222 | 20 | 222 | 34 | 217 | 98 | 216 | 33 | ||||
| 240 | 40 | 254 | 50 | 257 | 53 | 262 | 30 | 255 | 52 | 245 | 68 | ||
| 279 | 21 | ||||||||||||
| 303 | 10 | 301 | 15 | 302 | 8 | 303 | 14 | 302 | 13 | 302 | 14 | 294 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vikent’eva, O.V.; Shilovskikh, V.V.; Shcherbakov, V.D.; Moroz, T.N.; Vikentyev, I.V.; Bortnikov, N.S. Montbrayite from the Svetlinsk Gold–Telluride Deposit (South Urals, Russia): Composition Variability and Decomposition. Minerals 2023, 13, 1225. https://doi.org/10.3390/min13091225
Vikent’eva OV, Shilovskikh VV, Shcherbakov VD, Moroz TN, Vikentyev IV, Bortnikov NS. Montbrayite from the Svetlinsk Gold–Telluride Deposit (South Urals, Russia): Composition Variability and Decomposition. Minerals. 2023; 13(9):1225. https://doi.org/10.3390/min13091225
Chicago/Turabian StyleVikent’eva, Olga V., Vladimir V. Shilovskikh, Vasily D. Shcherbakov, Tatyana N. Moroz, Ilya V. Vikentyev, and Nikolay S. Bortnikov. 2023. "Montbrayite from the Svetlinsk Gold–Telluride Deposit (South Urals, Russia): Composition Variability and Decomposition" Minerals 13, no. 9: 1225. https://doi.org/10.3390/min13091225





