Kinetic and Isotherm Studies for Cu2+ and Cs+ Uptake with Mono- and Bimetallic FeO(OH)-MnOx-Clinoptilolite
Abstract
:1. Introduction
2. Experimental Section
2.1. Adsorption Materials Examined
2.2. Batch Adsorption Experiments
2.3. Analytical Methods
3. Results and Discussion
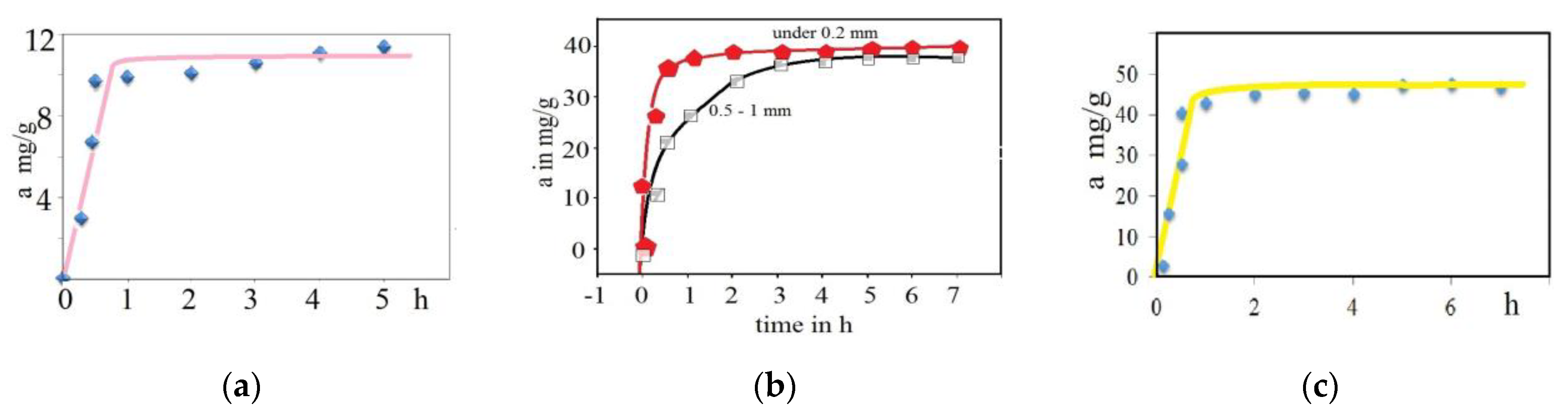
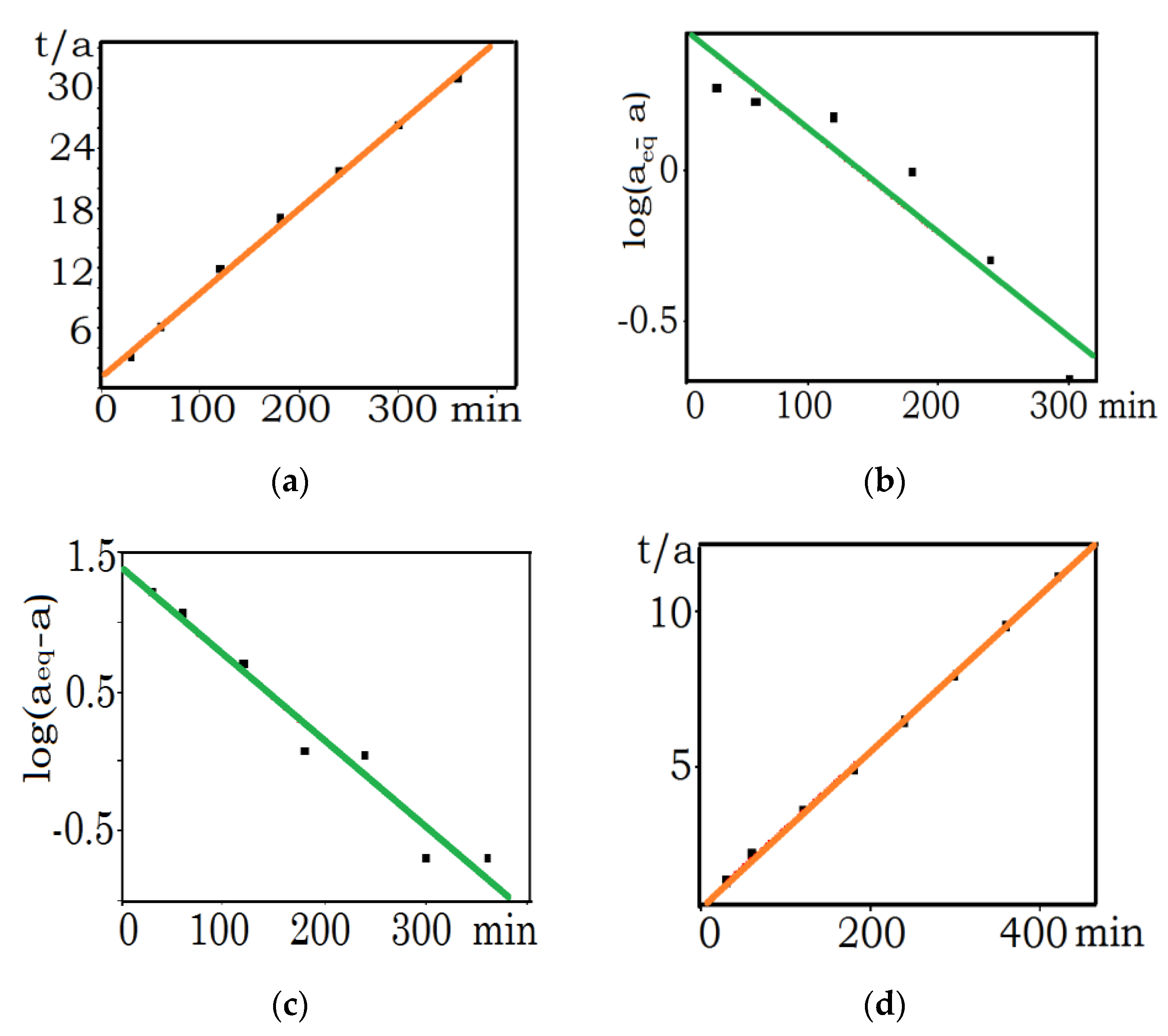
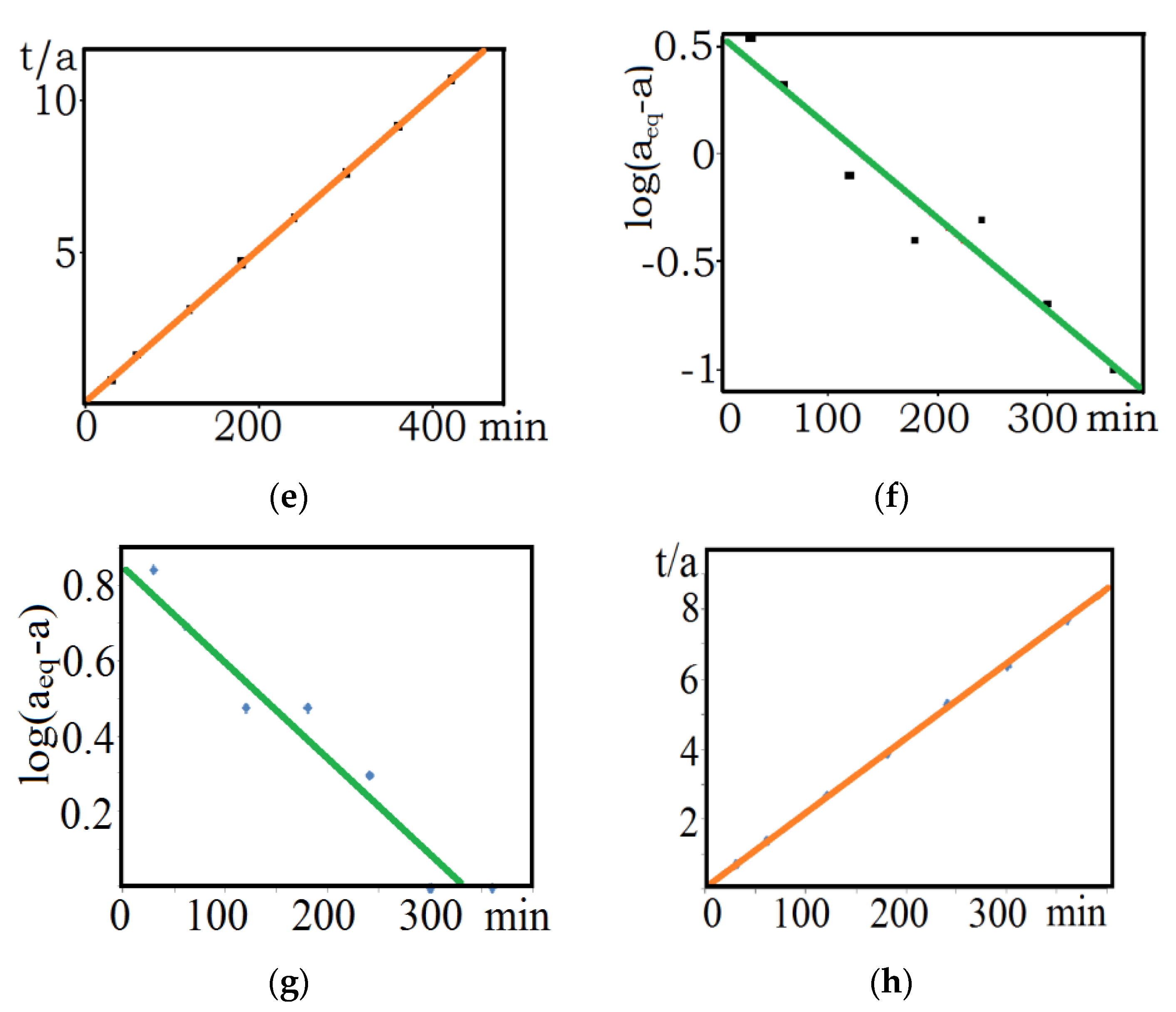
3.1. Kinetic Studies
3.2. Isotherm Studies
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rowshanfarzada, P.; Sabetb, M.; Reza Jaliliana, A.; Kamalidehghan, M. An overview of copper radionuclides and production of 61Cu by proton irradiation of nat. Zn at a medical cyclotron. Appl. Radiat. Isot. 2006, 64, 1563–1573. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.D.; Larry, E. (Eds.) “Copper”. Merck Manual Home Health Handbook; Merck Sharp & Dohme Corp., a Subsidiary of Merck & Co., Inc.: Gujarat, India, 2008; Archived from the Original on 7 March 2016. [Google Scholar]
- Copper, Chemical Element—Overview, Discovery and Naming, Physical Properties, Chemical Properties, Occurrence in Nature, Isotopes. Available online: http://www.chemistryexplained.com/elements/C-K/Copper.html (accessed on 16 October 2023).
- Haynes, W.M. (Ed.) CRC Handbook of Chemistry and Physics, 95th ed.; CRC Press/Taylor and Francis: Boca Raton, FL, USA, 2015. [Google Scholar]
- Coursey, J.S.; Schwab, D.J.; Tsai, J.J.; Dragoset, R.A. Atomic Weights and Isotopic Compositions; Version 4.1; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2015.
- George, L.; Trigg Immergut, E.H. Encyclopedia of Applied Physics, Vol. 4: Combustion to Dimagnetism; VCH Publishers: Hoboken, NJ, USA, 1992; pp. 267–272. [Google Scholar]
- Delacroix, D.; Guerre, J.P.; Leblanc, P.; Hickman, C. Radionuclide and Radiation Protection Handbook; Nuclear Technology Publishing: Ashford, UK, 2002. [Google Scholar]
- Voronina, A.V.; Kulyaeva, I.O.; Gupta, D.K. Determination of parameters of selective 137 Cs sorption onto natural and ferrocyanide– modified glauconite and clinoptilolite. Radiochem 2018, 60, 35–41. [Google Scholar] [CrossRef]
- Voronina, A.V.; Yu, N.A.; Semenishchev, V.S.; Gupta, D.K. Decontamination of seawater from 137Cs and 90Sr radionuclides using inorganic. Sorbents J. Environ. Radioact. 2020, 217, 106–210. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, M.; Du, X.; Feng, S.; Miyamoto, N.; Kano, N. Removal of Cesium from Radioactive Waste Liquids Using Geomaterials. Appl. Sci. 2021, 11, 8407. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, G.; Ma, C.; Guo, Y.; Duo, J.; Li, M.; Deng, T. Cesium removal from wastewater: High-efficient and reusable adsorbent K1.93Ti0.22Sn3S6.43. Chemosphere 2022, 305, 135406. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Kim, Y.; Roh, Y. Effective cesium removal from Cs-containing water using chemically activated opaline mudstone mainly composed of opal-cristobalite/tridymite (opal-CT). Sci. Rep. 2021, 11, 15362. [Google Scholar] [CrossRef] [PubMed]
- Awual, R. Ring size dependent crown ether based mesoporous adsorbent for high cesium adsorption from wastewater. Chem. Eng. J. 2016, 303, 539–546. [Google Scholar] [CrossRef]
- Lv, K.; Xiong, L.-P.; Luo, Y.-M. Ion exchange properties of cesium ion sieve based on zirconium molybdopyrophosphate. Colloids Surf. A Physicochem. Eng. Asp. 2013, 433, 37–46. [Google Scholar] [CrossRef]
- Ofomaja, A.E.; Pholosi, A.; Naidoo, E.B. Application of raw and modified pine biomass material for cesium removal from aqueous solution. Ecol. Eng. 2015, 82, 258–266. [Google Scholar] [CrossRef]
- Hasan, M.N.; Shenashen, M.A.; Hasan, M.M.; Znad, H.; Awual, M.R. Assessing of cesium removal from wastewater using functionalized wood cellulosic adsorbent. Chemosphere 2021, 270, 128668. [Google Scholar] [CrossRef]
- Parab, H.; Sudersanan, M. Engineering a lignocellulosic biosorbent–Coir pith for removal of cesium from aqueous solutions: Equilibrium and kinetic studies. Water Res. 2010, 44, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhuang, S.; Liu, Y. Metal hexacyanoferrates-based adsorbents for cesium removal. Coord. Chem. Rev. 2018, 374, 430–438. [Google Scholar] [CrossRef]
- Jiménez-Castañeda, M.E.; Medina, D.I. Use of Surfactant-Modified Zeolites and Clays for the Removal of Heavy Metals from Water. Water 2017, 9, 235. [Google Scholar] [CrossRef]
- Ab Hamid, N.H.; bin Mohd Tahir, M.I.; Chowdhury, A.; Nordin, A.H.; Alshaikh, A.A.; Suid, M.A.; Nazaruddin, N.I.; Nozaizeli, N.D.; Sharma, S.; Rushdan, A.I. The Current State-of-Art of Copper Removal from Wastewater: A Review. Water 2022, 14, 3086. [Google Scholar] [CrossRef]
- Aydına, H.; Buluta, Y.; Yerlikaya, C. Removal of copper (II) from aqueous solution by adsorption onto low-cost adsorbents. J. Environ. Manag. 2008, 87, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Stojakovic, D.; Milenkovic, J.; Daneu, N.; Rajic, N. A Study of the Removal of Copper Ions from aqueous solution using Clinoptilolite from Serbia. Clays Clay Miner. 2011, 59, 277–285. [Google Scholar] [CrossRef]
- Ezati, F.; Sepehr, E.; Ahmadi, F. The efficiency of nano TiO2 and γ Al2O3 in copper removal from aqueous solution by characterization and adsorption study. Sci. Rep. 2021, 11, 18831. [Google Scholar] [CrossRef]
- Dasgupta, S.; Das, M.; Klunk, M.A.; Xavier, S.J.; Caetano, N.R.; Wander, P.R. Copper and chromium removal from synthetic textile Wastewater using clay minerals and zeolite through the effect of pH. J. Iran. Chem. Soc. 2021, 18, 3377–3386. [Google Scholar] [CrossRef]
- Irannajad, M.; Kamran Haghighi, H.; Soleimanipour, M. Adsorption of Zn2+, Cd2+ and Cu2+ on Zeolites coated by Manganese and Iron Oxides. Physicochem. Probl. Min. Process 2016, 52, 894–908. [Google Scholar]
- Bai, Y.; Rong, F.; Wang, H.; Zhou, Y.; Xie, X.; Teng, J. Removal of Copper from Aqueous Solutions by Adsorption on Elemental Selenium nanoparticles. J. Chem. Eng. Data 2011, 56, 2563–2568. [Google Scholar] [CrossRef]
- de O. Primo, J.; Bittencourt, C.; Acosta, S.; Sierra-Castillo, A.; Colomer, J.F.; Jaerger, S.; Teixeira, V.C.; Anaissi, F.J. Synthesis of Zinc Oxide Nanoparticles by Ecofriendly Routes: Adsorbent for Copper Removal from Wastewater. Front. Chem. 2020, 57, 1790. [Google Scholar]
- Pavan Kumar, G.V.; Malla, K.A.; Yerra, B.; Srinivasa Rao, K. Removal of Cu(II) using three low-cost adsorbents and prediction of adsorption using artificial neural networks. Appl. Water Sci. 2019, 9, 44. [Google Scholar] [CrossRef]
- Gong, J.-L.; Wang, X.-Y.; Zeng, G.-M.; Chen, L.; Deng, J.-H.; Zhang, X.-R.; Niu, Q.-Y. Copper (II) removal by pectin–iron oxide magnetic nanocomposite adsorbent. Chem. Eng. J. 2012, 185–186, 100–107. [Google Scholar] [CrossRef]
- Pan, S.-C.; Lin, C.-C.; Tseng, D.-H. Reusing sewage sludge ash as adsorbent for copper removal from wastewater. Res. Conserv. Recycl. 2003, 39, 79–90. [Google Scholar] [CrossRef]
- Chmielewská, E.; Majzlan, J.; Bujdoš, M. Clinoptilolite with surface-enhanced functionality for radionuclide and inorganic pollutants removal. J. Radioanal. Nucl. Chem. 2022, 331, 3495–3504. [Google Scholar] [CrossRef]
- Taffarel, S.R.; Rubio, J. Removal of Mn2+ from aqueous solution by manganese oxide coated zeolite. Miner. Eng. 2010, 23, 1131–1138. [Google Scholar] [CrossRef]
- Chmielewská, E.; Tylus, W.; Bujdoš, M. Study of mono- and bimetallic Fe and Mn oxides supported clinoptilolite for improved Pb(II) removal. Molecules 2021, 26, 4143. [Google Scholar] [CrossRef] [PubMed]
- Chmielewská, E.; Tylus, W. A potential refinement of water endangered with Zn-65 onto modified zeolite. J. Radioanal. Nucl. Chem. 2021, 327, 31–37. [Google Scholar] [CrossRef]
- Chmielewská, E. Environmental Zeolites and Aqueous Media: Examples of Practical Solutions; Betham Science Publishers: Sharjah, United Arab Emirates, 2014. [Google Scholar]
- İnan, S.; Kusumkar, V.V.; Galamboš, M.; Viglašová, E.; Rosskopfová, O.; Daňo, M. Isotherm, Kinetic, and Selectivity Studies for the Removal of 133Ba and 137Cs from Aqueous Solution Using Turkish Perlite. Materials 2022, 15, 7816. [Google Scholar] [CrossRef]
- Baskan, M.B.; Pala, A. Removal of arsenic from drinking water using modified natural zeolite. Desalination 2011, 281, 396–403. [Google Scholar] [CrossRef]
- Morris, D.F.C. Ionic radii and enthalpies of hydration of ions. In Structure and Bonding; Jørgensen, C.K., Neilands, J.B., Nyholm, R.S., Reinen, D., Williams, R.J.P., Eds.; Springer: Berlin/Heidelberg, Germany, 1969; Volume 6. [Google Scholar]

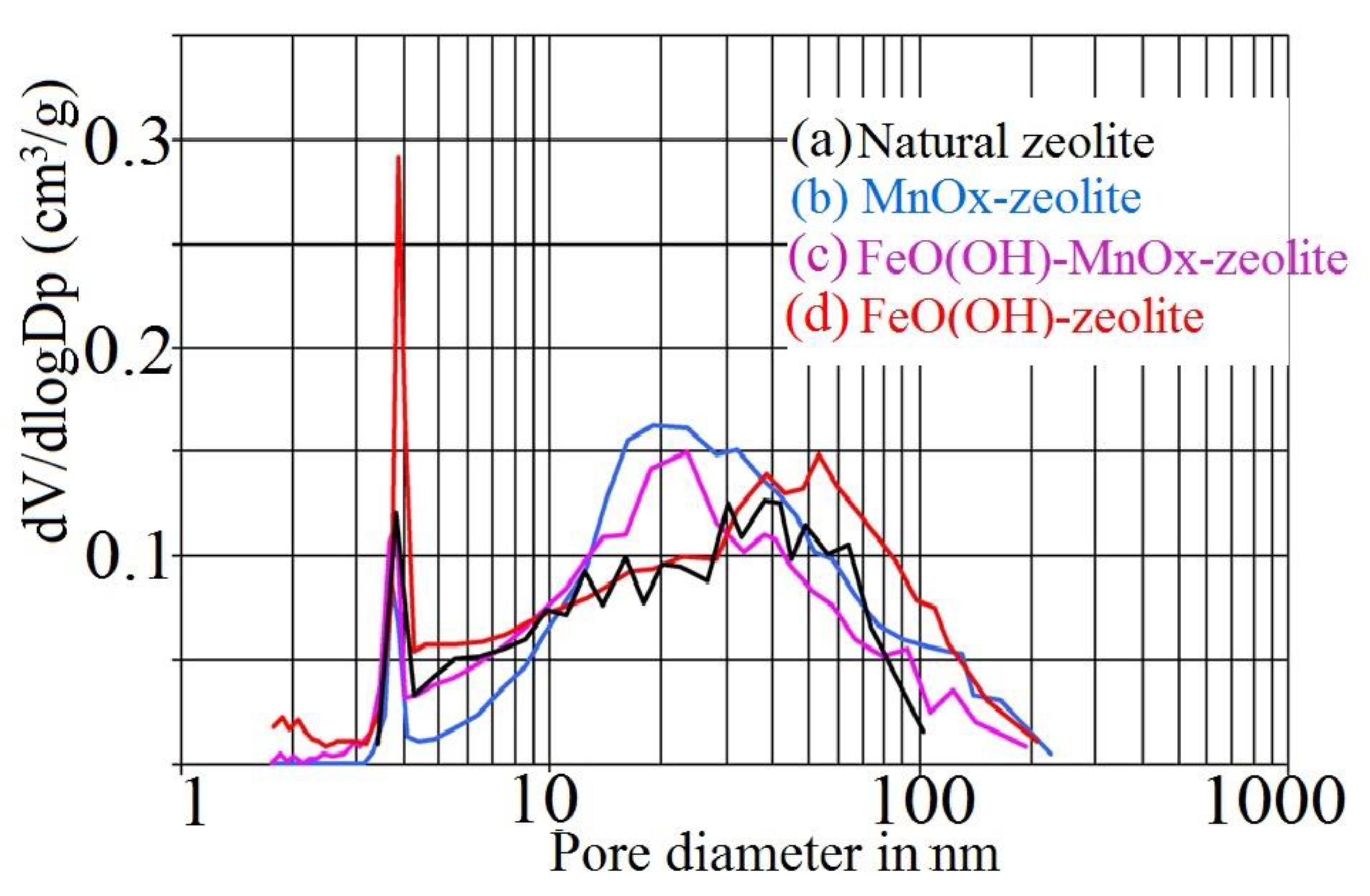
| Sample | Grain Size | SBET (m2/g) | St (m2/g) | Vmicro (cm3/g) |
|---|---|---|---|---|
| Slovak natural zeolite of clinoptilolite type | 0.2–0.6 mm | 31.7 | 21.4 | 0.004 |
| Slovak natural clinoptilolite | ≤20 µm | 59.2 | - | |
| FeO(OH)–MnOx-clinoptilolite of Slovak origin | ≤100 µm | 31.4 | 28.8 | 0.001 |
| MnOx–Slovak clinoptilolite | ≤100 µm | 27.5 | 22.9 | 0.002 |
| FeO(OH)-clinoptilolite of Slovak origin | ≤100 µm | 52.2 | 38.2 | 0.007 |
| aeq | aeq mg/g (cal.) | K1 (1/min) | R2 | K2 (g/mg·min) | aeq mg/g (cal.) | R2 | Ki (g/mg·min−1/2) | Kfd (mg/g·min−1) | |
|---|---|---|---|---|---|---|---|---|---|
| Sample Type | mg/g (exp.) | Pseudo | First | Order | Pseudo | Second | Order | Intraparticle Diffusion | Liquid Film Diffusion |
| MnOx-zeolite grain size less than 0.2 mm/Cu(II) | 39.4 | 3.6 | 0.00186 | 0.93396 | 0.00709 | 39.7 | 0.99998 | 0.23125 | 0.00984 |
| MnOx-zeolite 0.5–1.0 mm/Cu(II) | 37.8 | 24.7 | 0.00271 | 0.95596 | 0.00090 | 40.6 | 0.99903 | 1.21873 | 0.0123 |
| Natural zeolite less than 0.2 mm/Cu(II) | 11.6 | 3.1 | 0.00151 | 0.88450 | 0.00609 | 11.9 | 0.99726 | 0.14631 | 0.00763 |
| Natural zeolite less than 0.2 mm/Cs-137 | 48.0 | 7.1 | 0.00564 | 0.83985 | 0.00239 | 48.3 | 0.99903 | 0.42684 | 0.00592 |
|
Adsorbent/ Adsorbate | Langmuir Isotherm | Freundlich Isotherm | BET Isotherm | Redlich–Peterson Isotherm | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| amax | amax | amax | n | KF | R2 | KBET | R2 | g | A | B | R2 | |
| mg/g | mg/g | mg/g | L/mg | L/mg | L/g | (L/mg)g | ||||||
| MnOx-zeolite/Cu(II) | 60.97 | 0.0351 | 0.9995 | 5.7372 | 18.8191 | 0.9312 | −13.2438 | 0.8621 | 0.4240 | 2.2782 | 0.7814 | 0.7164 |
| FeO(OH)-MnOx-zeolite/Cu(II) | 35.34 | 0.0048 | 0.9836 | 5.5710 | 7.4611 | 0.9708 | −6.2651 | 0.8271 | 0.6170 | 63.0545 | 0.4746 | 0.9218 |
| FeO(OH)-zeolite/Cu(II) | 17.89 | 0.0048 | 0.9725 | 4.2230 | 2.6669 | 0.9127 | −1.7427 | 0.7220 | 0.7811 | 2.8011 | 0.3246 | 0.9841 |
| Natural zeolite/Cu(II) | 17.24 | 0.0104 | 0.9896 | 4.8733 | 3.6392 | 0.9297 | −1.8110 | 0.5848 | 0.8474 | 2.7475 | 0.1899 | 0.9951 |
| Natural zeolite/Cs-137 | 119.33 | 0.0159 | 0.9965 | 1.9981 | 4.2459 | 0.9577 | 3.7671 | 0.2034 | 0.1899 | 1.8087 | 1.6084 | 0.6077 |
| Adsorbent | Max. Adsorption Capacity in mg/g to Cu(II) | Reference in This Study |
|---|---|---|
| Coconut seed powder (CCP) | 4.3 | [21,28] |
| Sesame seed cake powder (SSCP) | 4.2 | [28] |
| Ground nut seed cake powder (GNCSP) | 4.8 | [15,28] |
| Shells of lentil (LS) | 9.6 | [15,21] |
| Wheat (WS) | 17.4 | [17,21] |
| Rice (RS) | 2.9 | [21] |
| Iron oxide-clinoptilolite, Iran | 8.8 | [24,25] |
| Manganese oxide-clinoptilolite, Iran | 6.9 | [25,32] |
| Natural zeolite (clinoptilolite), Zlatokop, Serbia | 16.8 | [22] |
| CTAB—surfactant-coated kaolin | 38.5 | [19,20] |
| Natural kaolin | 19.2 | [21,28] |
| Sewage sludge ash (SSA) | 4.1 | [30] |
| Pectin-coated iron oxide magnetic nanocomposite adsorbent | 49.0 | [29] |
| ZnO2 nanoparticles green synthesised with Aloe vera | 20.4 | [27] |
| Elemental selenium nanoparticles | 890 | [26] |
| TiO2 nanosorbent | 9.3 | [23] |
| Adsorbent | Max. Adsorption Capacity in mg/g to Cs(I) | Reference in This Study |
|---|---|---|
| Prussian Blue encapsulated alginate/calcium beads | 142.8 | [18] |
| Prussian Blue analogue on chitosan/carbon nanotubes | 219.8 | [8,9,10] |
| Polyacrylonitrile–K-Ni-Cyanoferrates | 157.7 | [18] |
| Zirconium molybdopyrophosphate | 183.4 | [14] |
| Dibenzo-30-crown-10-ether immobilised mesoporous adsorbent | 107.2 | [13] |
| Fly ash-based geomaterials | 89.3 | [10,13] |
| K1.93Ti0.22Sn3S6.43 adsorbent | 450.1 | [11] |
| Opaline mudstone | 32.1 | [12] |
| Slovak natural clinoptilolite | 99.8 | [31] |
| Na-exchanged Slovak clinoptilolite | 249.6 | [31] |
| Russian natural clinoptilolite | 135 | [8,9] |
| Russian ferrocyanide-modified clinoptilolite | 136 | [8,9] |
| Ferrocyanide-modified silica gel | 53.2 | [13] |
| Ammonium-pillared montmorillonite/CoFe2O4-Ca-alginate | 86.5 | [19] |
| Lignocellulosic coir pith with nickel hexacyanoferrate | 93.3 | [15,16,17] |
| Mangrove charcoal-modified adsorbent | 133.5 | [15,16] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chmielewská, E.; Bujdoš, M.; Hupian, M.; Galamboš, M. Kinetic and Isotherm Studies for Cu2+ and Cs+ Uptake with Mono- and Bimetallic FeO(OH)-MnOx-Clinoptilolite. Minerals 2023, 13, 1536. https://doi.org/10.3390/min13121536
Chmielewská E, Bujdoš M, Hupian M, Galamboš M. Kinetic and Isotherm Studies for Cu2+ and Cs+ Uptake with Mono- and Bimetallic FeO(OH)-MnOx-Clinoptilolite. Minerals. 2023; 13(12):1536. https://doi.org/10.3390/min13121536
Chicago/Turabian StyleChmielewská, Eva, Marek Bujdoš, Marek Hupian, and Michal Galamboš. 2023. "Kinetic and Isotherm Studies for Cu2+ and Cs+ Uptake with Mono- and Bimetallic FeO(OH)-MnOx-Clinoptilolite" Minerals 13, no. 12: 1536. https://doi.org/10.3390/min13121536









