Primary Composition of Kimberlite Melt
Abstract
:1. Introduction
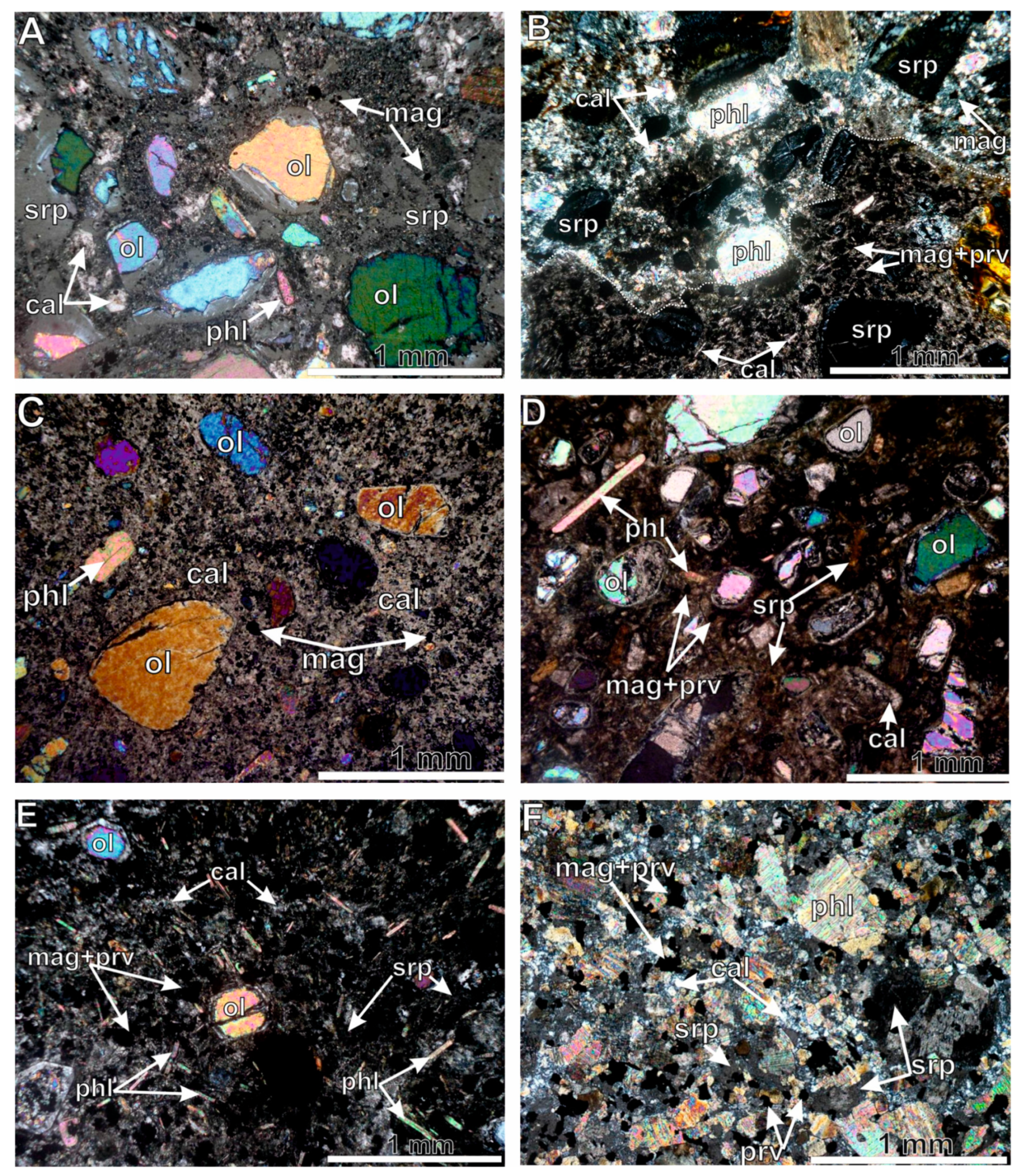
2. General Information about the Obnazhennaya Pipe and Velikan Dyke, and a Petrographic Description
3. Analytical Methods
4. Results
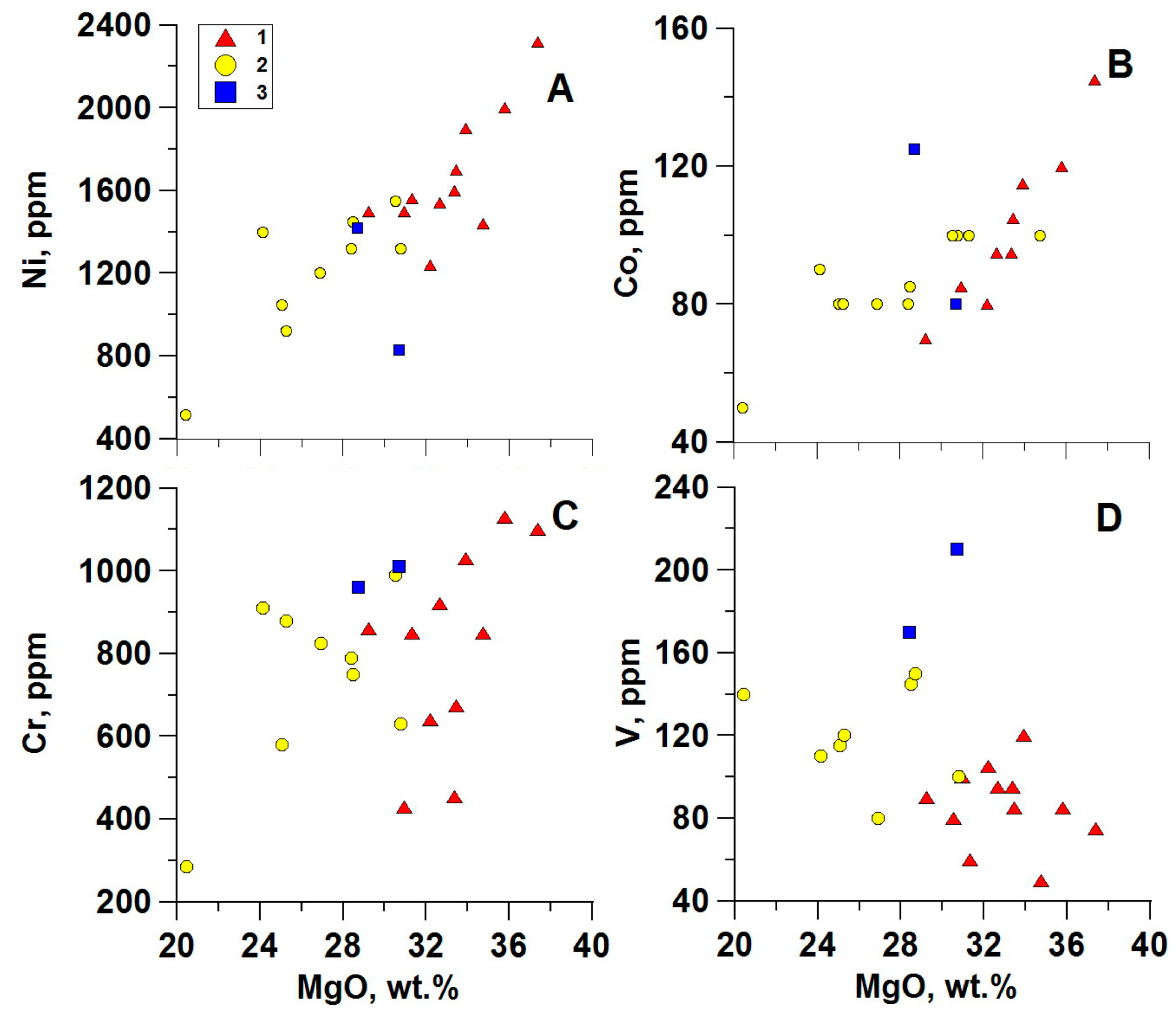
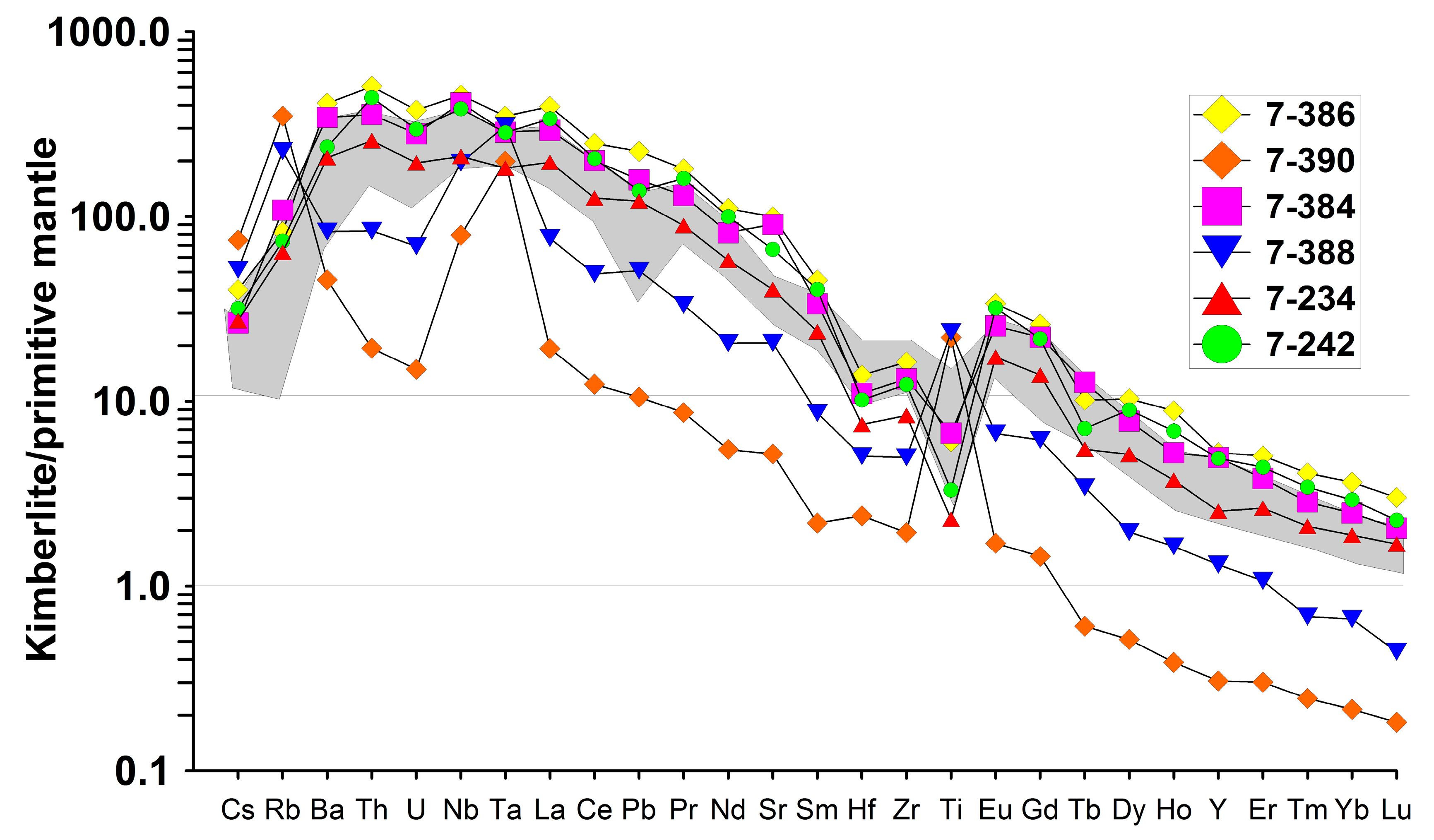
4.1. Major and Rare Elements Chemistry
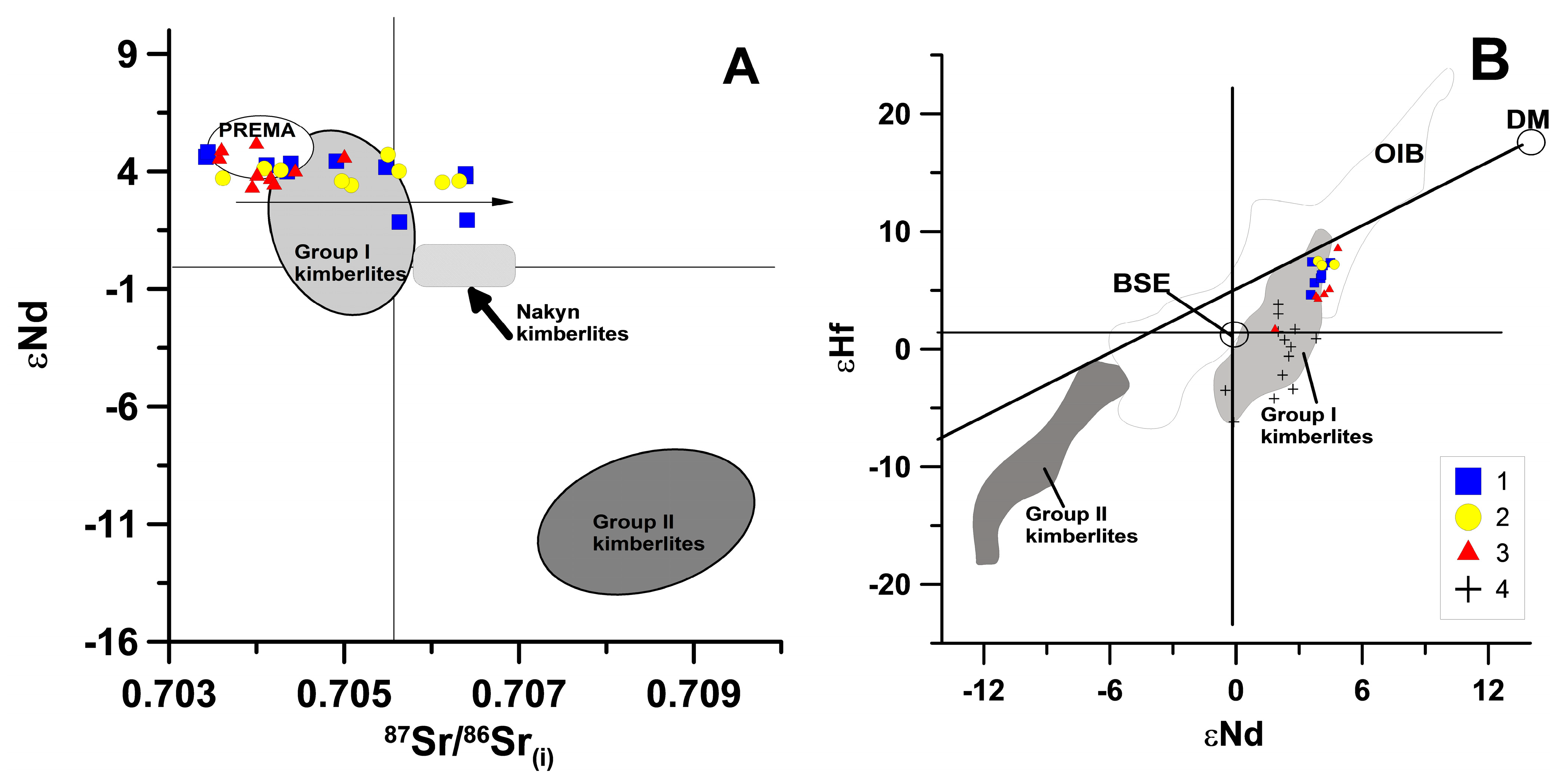
4.2. Sr-Nd-Hf Systematics
4.3. Mineral Composition
5. Discussion
5.1. Primary Kimberlitic Melt (PKM)
5.2. Evolution of Kimberlites in Multi-Phase Pipes
5.3. How Was Pyroclastic Kimberlite Formed?
5.4. The Origin of Olivine in Kimberlites
5.5. Mantle Sources of Kimberlites
6. Conclusions
- The contrasting compositions of two closely spaced kimberlite bodies, the Obnazhennaya pipe and the Velikan dyke, have been studied. The Obnazhennaya pipe is filled with volcanoclastic and pyroclastic types of high-Mg kimberlite; the latter contains rare fragments of coherent previous intrusion phases (autoliths, according to [25]). The pyroclastic kimberlite is highly saturated with olivine, pyroxene, garnet macrocrysts and mantle xenoliths. The Velikan dyke consists of high-Fe, high-Ti kimberlite that does not contain any mantle clastic material (neither Ol xenocrysts nor mantle xenoliths).
- In the multiphase Obnazhennaya pipe, three types of coherent kimberlite were found only in the pyroclastic kimberlite, indicating a later intrusion of the latter. Since a similar sequence of intrusion of different types of kimberlites was observed in most of the pipes we studied [4] (for example, in the pipes of Udachnaya-Western, Udachnaya-Eastern, Uybileynaya, Sytykanskaya, Aykhal, Komsomol’skaya and others), we believe that this regularity is general for kimberlite volcanism.
- The spatial proximity of the Obnazhennaya pipe and the Velikan dyke, the similarity of the Sr-Nd-Hf isotopic and trace element taxonomy (in terms of incoherent elements) for the corresponding kimberlites and, finally, the coincidence of their formation ages was the basis for concluding that they had a single magmatic asthenospheric source.
- The formation of compositionally contrasting kimberlites filling the Obnazhennaya pipe and the Velikan dyke was most likely due to the process of differentiation of the asthenospheric melt into two parts with different densities, viscosities and, consequently, disintegration capabilities. Their hypothetical composition is essentially carbonate and carbonate–silicate, probably characterized by different H2O contents. A melt of essentially carbonate composition, which had high integrability, formed a high-Mg petrochemical type of kimberlite; a carbonate–silicate melt formed Mg-Fe and Fe-Ti petrochemical types of kimberlite.
- The melt that formed the coherent kimberlite dyke of Velikan is, according to the authors, primary, as it does not contain xenogenic material from the lithospheric mantle and therefore has not been subjected to the process of its assimilation.
- A comparison of the composition of olivine from pyroclastic and coherent kimberlites, as well as from mantle xenoliths of the Obnazhennaya pipe showed that olivine from pyroclastic kimberlite is completely xenogenic; olivine from coherent types of kimberlite from the Obnazhennaya pipe is of both xenogenic and phenocryst origin; and olivine from coherent kimberlites of the Velikan dyke is completely crystallized from the melt.
- The geochemical homogeneity of the asthenospheric source under the YaKP, which persisted for a long time (410–160 Ma), is confirmed by the high level of similarity of the incompatible trace element patterns and the Sr-Nd-Hf isotopic systematics of the kimberlites for most of the kimberlites from different fields with different ages.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Kharkiv, A.D.; Zuenko, V.V.; Zinchuk, N.N.; Ukhanov, V.A.; Bogatykh, M.M. Petrochemistry of Kimberlites; Nedra: Moscow, Russia, 1991; 304p. (In Russian) [Google Scholar]
- Kharkiv, A.D.; Zinchuk, N.N.; Kryuchkov, A.I. Indigenous Deposits of Diamonds in the World; Nedra: Moscow, Russia, 1998; 555p. (In Russian) [Google Scholar]
- Ilupin, I.P.; Kaminsky, F.V.; Francesson, E.V. Geochemistry of Kimberlites; Nedra: Moscow, Russia, 1978; 352p. (In Russian) [Google Scholar]
- Kostrovitsky, S.I.; Spetsius, Z.V.; Yakovlev, D.A.; Fon-Der-Flaas, G.S.; Suvorova, L.F.; Bogush, I.N. Atlas of Primary Diamond Deposits of the Yakutian Kimberlite Province; NIGP ALROSA (PJSC): Mirny, Russia, 2015; 480p. (In Russian) [Google Scholar]
- Kostrovitsky, S.I.; Morikiyo, T.; Serov, I.V.; Yakovlev, D.A.; Amirzhanov, A.A. Isotope-geochemical systematics of kimberlites and related rocks from the Siberian Platform. Russ. Geol. Geophys. 2007, 48, 272–290. [Google Scholar] [CrossRef]
- Kornilova, V.P.; Nikolaev, L.I. Petrography and chemistry of kimberlite and comagmatic rocks of the Kuoika field. In Kimberlite and Basic Magmatism of the Olenek Uplift; Yakutian Branch of the Academy of Sciences of the USSR: Yakutsk, Russia, 1980; pp. 92–111. [Google Scholar]
- Milashev, V.A. Parent inclusions in the Obnazhennaya kimberlite pipe (Olenek River Basin). Papes USSR-Union Mineral. Soc. 1960, 89, 284–298. (In Russian) [Google Scholar]
- Sobolev, N.V. Mantle Xenoliths in Kimberlites; Nauka: Novosibirsk, Russia, 1974; 264p. (In Russian) [Google Scholar]
- Ukhanov, A.V.; Ryabchikov, I.D.; Kharkiv, A.D. Lithospheric Mantle of the Yakutian Kimberlite Province; Nauka: Moscow, Russia, 1988; 286p. (In Russian) [Google Scholar]
- Solov’eva, L.V.; Vladimirov, B.M.; Dneprovskaya, L.V.; Maslovskaya, M.N.; Brandt, S.B. Kimberlites and Kimberlitic Rocks: Mantle Material Beneath Precambrian Cratons; Nauka: Novosibirsk, Russia, 1994; 256p. (In Russian) [Google Scholar]
- Brooker, R.A.; Sparks RS, J.; Kavanagh, J.; Field, M. The volatile content of hypabyssal kimberlite magmas: Some constraints from experiments on natural rock compositions. Bull. Volcanol. 2011, 73, 959–981. [Google Scholar] [CrossRef]
- Mitchell, R.H. Petrology of hypabyssal kimberlites: Relevance to primary magma compositions. J. Volcanol. Geotherm. Res. 2008, 174, 1–8. [Google Scholar] [CrossRef]
- Russell, J.K.; Porritt, L.A.; Lavallee, Y.; Dingwell, D.B. Kimberlite ascent by assimilation—Fuelled buoyancy. Nature 2012, 481, 352–356. [Google Scholar] [CrossRef]
- Sparks, R.S.J.; Baker, L.; Brown, R.J.; Field, M.; Schumacher, J.; Stripp, G.; Walters, A. Dynamical constraints on kimberlite volcanism. J. Volcanol. Geotherm. Res. 2006, 155, 18–48. [Google Scholar] [CrossRef]
- Sparks, R.S.J.; Brooker, R.A.; Field, M.; Kavanagh, J.; Schumacher, J.; Walters, A.; White, J. The nature of erupting kimberlite melts. Lithos 2009, 112, 429–438. [Google Scholar] [CrossRef]
- Wilson, L.; Head, J.W. An integrated model of kimberlite ascent and eruption. Nature 2007, 447, 53–57. [Google Scholar] [CrossRef]
- Pearson, D.G.; Woodhead, J.; Janney, P.E. Kimberlites as Geochemical Probes of Earth’s Mantle. Elements 2019, 15, 387–392. [Google Scholar] [CrossRef]
- Soltys, A.; Giuliani, A.; Phillips, D. A new approach to reconstructing the composition and evolution of kimberlite melts: A case study of the archetypal Bultfontein kimberlite (Kimberley, South Africa). Lithos 2018, 304–307, 1–15. [Google Scholar] [CrossRef]
- Mitchell, R.H.; Giuliani, A.; O’Brien, H. What is a kimberlite? Petrology and Mineralogy of hypabyssal kimberlites. Elements 2019, 15, 381–386. [Google Scholar] [CrossRef]
- Giuliani, A.; Pearson, D.G. Kimberlites: From deep earth to diamond mines. Elements 2019, 15, 377–380. [Google Scholar] [CrossRef]
- Giuliani, A.; Pearson, D.G.; Soltys, A.; Dalton, H.; Phillips, D.; Foley, S.F.; Lim, E.; Goemann, K.; Griffin, W.L.; Mitchell, R.H. Kimberlite genesis from a common carbonate-rich primary melt modified by lithospheric mantle assimilation. Sci. Adv. 2020, 6, eaaz0424. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.H. Kimberlites: Mineralogy, Geochemistry, and Petrology; Plenum Press: New York, NY, USA, 1986; 442p. [Google Scholar]
- Kopylova, M.G.; Hayman, P. Petrology and textural classification of the Jericho kimberlite, northern Slave Province, Nunavut, Canada. Can. J. Earth Sci. 2008, 45, 701–723. [Google Scholar] [CrossRef]
- Cas, R.A.F.; Porritt, L.A.; Pittari, A.; Hayman, P.C. A new approach to kimberlite facies terminology using a revised general approach to the nomenclature of all volcanic rocks and deposits: Descriptive to genetic. J. Volcanol. Geotherm. Res. 2008, 174, 226–240. [Google Scholar] [CrossRef]
- Smith, S.B.H.; Nowicki, T.E.; Russell, J.K.; Webb, K.J.; Mitchell, R.H.; Hetman, C.M.; Harder, M.; Skinner, E.M.W.; Robey, J.V. Kimberlites terminology and classification. In Proceedings of the 10th International Kimberlite Conference, Bangalore, India, 5–11 February 2012; pp. 1–18. [Google Scholar] [CrossRef]
- Arndt, N.T.; Guitreau, M.; Boullier, A.M.; le Roex, A.; Tommasi, A.; Cordier, P.; Sobolev, A. Olivine, and the origin kimberlite. J. Petrol. 2010, 51, 573–602. [Google Scholar] [CrossRef]
- Brett, R.S.; Russel, J.K.; Moss, S. Origin of Olivine in kimberlite: Phenocryst or imposter? Lithos 2009, 112, 201–212. [Google Scholar] [CrossRef]
- Kamenetsky, V.S.; Maas, R.; Kamenetsky, M.B.; Paton, C.; Phillips, D.; Golovin, A.V.; Gornova, M. Chlorine from the mantle: Magmatic halides in the Udachnaya-East kimberlite, Siberia. Earth Planet. Sci. Lett. 2009, 285, 96–104. [Google Scholar] [CrossRef]
- Lorenz, V.; Kurszlaukis, S. Root zone processes in the phreatomagmatic pipe emplacement model and consequences for the evolution of maar–diatreme volcanoes. J. Volcanol. Geotherm. Res. 2007, 159, 4–32. [Google Scholar] [CrossRef]
- McCallum, M.E. An emplacement model to explain contrasting mineral assemblages in adjacent pipes. J. Geol. 1976, 84, 673–684. [Google Scholar] [CrossRef]
- Dawson, J.B. Kimberlites and their Xenoliths; Springer: Berlin, Germany, 1980; 252p. [Google Scholar]
- Kurszlaukis, S.; Lorenz, V. Formation of “tuffisitic kimberlites” by phreatomagmatic processes. J. Volcanol. Geotherm. Res. 2008, 174, 68–80. [Google Scholar] [CrossRef]
- Leonov, B.N.; Prokopchuk, B.I.; Orlov, Y.u.L. Diamonds in the Lena Province; Nauka: Moscow, Russia, 1966; 279p. (In Russian) [Google Scholar]
- Brakhfogel, F.F. Kimberlite Magmatism in the Northeastern Siberian Craton: Geological Aspects; YaF SO AN SSSR: Yakutsk, Russia, 1984; 128p. (In Russian) [Google Scholar]
- Malkov, B.A.; Gustomesov, V.A. Jurassic fauna in kimberlites from the Olenek uplift and the age of kimberlite paroxysm in the northeastern Siberian craton. Dokl. Akad. Nauk USSR 1976, 229, 435–438. (In Russian) [Google Scholar]
- Kostrovitsky, S.I.; Admakin, L.A. A find of xenolithic wood remnants in the Obnazhennaya kimberlite pipe. Russ. Geol. Geophys. 1991, 32, 82–84. [Google Scholar]
- Sun, J.; Liu, C.-Z.; Tappe, S.; Kostrovitsky, S.I.; Wu Fu-Yuan Yakovlev, D.; Yang, Y.-H.; Yang, J.-H. Repeated kimberlite magmatism beneath Yakutia and its relationship to Siberian flood paroxysm: Insights from in situ U-Pb and Sr-Nd perovskite isotope analysis. Earth Planet. Sci. Lett. 2014, 404, 283–295. [Google Scholar] [CrossRef]
- Davis, G.L.; Sobolev, N.V.; Kharkiv, A.D. New data on the age of kimberlites of Yakutia obtained by the uranium-lead method on zircons. Dokl. Akad. Nauk USSR 1980, 254, 175–179. (In Russian) [Google Scholar]
- Griffin, W.L.; Ryan, C.G.; Kaminsky, F.V.; O’Reilly, S.Y.; Natapov, L.M.; Win, T.T.; Kinny, D.; Ilupin, I. The Siberian lithosphere traverse: Mantle terranes and the assembly of the Siberian Craton. Tectonophysics 1999, 310, 1–35. [Google Scholar] [CrossRef]
- Sun, J.; Tappe, S.; Kostrovitsky, S.I.; Liu, C.-Z.; Skuzovatov, S.Y.; Wu, F.-Y. Mantle sources of kimberlites through time: A U-Pb and Lu-Hf isotope study of zircon megacrysts from the Siberian diamond fields. Chem. Geol. 2018, 479, 228–240. [Google Scholar] [CrossRef]
- Clement, C.R. Comparative Geological Study of Some Major Kimberlite Pipes in Northern Cape and Orange Free State. Ph.D. Thesis, University of Cape Town, Cape Town, South Africa, 1982. [Google Scholar]
- Clement, C.R.; Skinner, E.M.W. A textural-genetic classification of kimberlites. Trans. Geol. Soc. S. Afr. 1985, 88, 403–409. [Google Scholar]
- Mitchell, R.H. Kimberlites, Orangeites, and Related Rocks; Plenum Press: New York, NY, USA, 1995; 410p. [Google Scholar]
- Le Roex, A.P.; Bell, D.R.; Davis, P. Petrogenesis of group I kimberlites from Kimberley, South Africa: Evidence from bulk-rock geochemistry. J. Petrol. 2003, 44, 2261–2286. [Google Scholar] [CrossRef]
- Mitchell, R.H. Kimberlites, Orangeites, Lamproites, Melilitites, and Minettes: A Petrographic Atlas; Almaz Press: Thunder Bay, ON, Canada, 1997; 243p. [Google Scholar]
- Kjarsgaard, B.A. Kimberlite pipe models: Significance for exploration. In Ore Deposits and Exploration Technology, Proceedings of the Exploration 07: Fifth Decennial International Conference on Mineral Exploration, Toronto, ON, Canada 9–12 September 2007; Milkereit, B., Ed.; GEOSCAN: Totonto, ON, Canada, 2007; pp. 667–677. [Google Scholar]
- Bogatikov, O.A.; Kononova, V.A.; Golubeva, Y.; Zinchuk, N.N.; Ilupin, I.P.; Rotman, A.Y.; Levsky, L.K.; Ovchinnikova, G.V.; Kondrashov, I.A. Petrogeochemical and isotopic variations in the composition of kimberlites of Yakutia and their causes. Geochemistry 2004, 9, 915–939. [Google Scholar]
- Afonin, V.P.; Finkelshtein, A.L.; Borkhodoev, V.J.; Gunicheva, T.N. X-Ray-Fluorescence analysis of rocks by the fundamental parameter method. X-Ray Spectrom 1992, 21, 69–75. (In Russian) [Google Scholar] [CrossRef]
- Yang, Y.-H.; Zhang, H.-F.; Chu, Z.-Y.; Xie, L.-W.; Wu, F.-Y. Combined chemical separation of Lu, Hf, Rb, Sr, Sm and Nd from a single rock digest and precise and accurate isotope determinations of Lu–Hf, Rb–Sr and Sm–Nd isotope systems using Multi-Collector ICP-MS and TIMS. Intern. J. Mass Spectrom. 2010, 290, 120–126. [Google Scholar] [CrossRef]
- Münker, C.; Weyer, S.; Scherer, E.; Mezger, K. Separation of high field strength elements (Nb, Ta, Zr, Hf) and Lu from rock samples for MC-ICPMS measurements. Geochem. Geophys. Geosyst. 2001, 2, 2001GC000183. [Google Scholar] [CrossRef]
- Smith, C.B. Pb, Sr and Nd isotopic evidence for sources of African Cretaceous kimberlites. Nature 1983, 304, 51–54. [Google Scholar] [CrossRef]
- Tainton, K.M.; McKenzie, D. The generation of kimberlites, lamproites, and their source rocks. J. Petrol. 1994, 35, 787–817. [Google Scholar] [CrossRef]
- Zindler, A.; Hart, S. Chemical geodynamics. Ann. Rev. Earth Planet. Sci. 1986, 14, 493–571. [Google Scholar] [CrossRef]
- Nowell, G.M.; Pearson, D.G.; Bell, D.R.; Carlson, R.W.; Smith, C.B.; Kempton, P.D.; Noble, S.R. Hf isotope systematics of kimberlites and their megacrysts: New constraints on their source regions. J. Petrol. 2004, 45, 1583–1612. [Google Scholar] [CrossRef]
- Kalashnikova, T.V. Geochemical Characteristics and Petrogenesis of Mantle Xenoliths from the Obnazhnaya Kimberlite Pipe Yakutsk Kimberlite Province. Ph.D. Thesis, Institute of Geochemistry SB RAS, Irkutsk, Russia, 2017; 254p. [Google Scholar]
- Sobolev, N.V.; Pokhilenko, N.P.; Lavrentiev, Y.u.G.; Usova, L.V. Peculiarities of composition of chromespinelides from diamonds and kimberlites of Yakutia. Russ. Geol. Geophys. 1975, 11, 7–24. (In Russian) [Google Scholar]
- Nimis, P.; Taylor, W.R. Single Clinopyroxene thermobarometery for garnet peridotites. Part 1, Calibration and testing of a Cr-in-Cpx barometer and an enstatite-in-cpx thermometer. Contrib. Mineral. Petrol. 2000, 139, 541–554. [Google Scholar] [CrossRef]
- Nimis, P.; Grutter, G. Internally consistent geothermometers for garnet peridotites and pyroxenite. Contrib. Mineral. Petrol. 2010, 159, 411–427. [Google Scholar] [CrossRef]
- Boyd, F.R.; Pokhilenko, N.P.; Pearson, D.G.; Mertzman, S.A.; Sobolev, N.V.; Finger, L.W. Composition of the Siberian cratonic mantle: Evidence from Udachnaya peridotite xenoliths. Contrib. Mineral. Petrol. 1997, 128, 228–246. [Google Scholar] [CrossRef]
- Pokhilenko, N.; Sobolev, N.; Kuligin, S.; Shimizu, N. Peculiarities of distribution of pyroxenite paragenesis garnets in Yakutian kimberlites and some aspects of the evolution of the Siberian craton lithospheric mantle. In Proceedings of the 7th International Kimberlite Conference, Cape Town, South Africa, 11–17 April 1998; pp. 689–698. [Google Scholar]
- Oleinikov, O.B.; Suknev, V.S. Ankelite from kimberlites of Kuoyka intrusive field (Yakutia). In Proceedings of the Russian Mineralogical Assembly 1999; Volume 5. (In Russian).
- Borodin, L.S.; Lapin, A.V.; Pyatenko, I.K. Petrology and Geochemistry of Dykes of Alkaline-Ultrabasic Rocks and Kimberlites; Nedra: Moscow, Russia, 1976; 244p. (In Russian) [Google Scholar]
- Becker, M.; Le Roex, A. Geochemistry of South African on–and off craton, Group I and Group II Kimberlites: Petrogenesis and Source Region Evolution. J. Petrol. 2006, 47, 673–703. [Google Scholar] [CrossRef]
- Kjarsgaard, B.A.; Pearson, D.G.; Tappe, S.; Nowell, G.M.; Dowall, D.P. Geochemistry of hypabyssal kimberlites from Las de Gras, Canada: Comparison to a global database and applications to the parent magma problem. Lithos 2009, 112, 236–248. [Google Scholar] [CrossRef]
- Kopylova, M.G.; Matveev, S.; Raudsepp, M. Searching for parental kimberlite melt. Geochim. Cosmochim. Acta 2007, 71, 3616–3629. [Google Scholar] [CrossRef]
- Price, S.E.; Russel, J.K.; Kopylova, M.G. Primitive Magma From the Jericho Pipe, N.W.T., Canada: Constraints on Primary Kimberlite Melt Chemistry. J. Petrol. 2000, 41, 789–808. [Google Scholar] [CrossRef]
- Shee, S.R. The Petrogenesis of the Wesselton Mine kimberlites, Samples. Ph.D. Thesis, University of Cape Town, Kimberley, South Africa, 1986. [Google Scholar]
- Kamenetsky, V.S.; Kamenetsky, M.B.; Sobolev, A.V.; Golovin, A.V.; Demouchy, S.; Faure, K.; Sharygin, V.V.; Kuzmin, D.V. Olivine in the Udachnaya-East kimberlite (Yakutia, Russia): Types, compositions and origins. J. Petrol. 2008, 49, 823–839. [Google Scholar] [CrossRef]
- Kamenetsky, V.S.; Golovin, A.V.; Maas, R.; Giuliani, A.; Kamenetsky, M.B.; Weiss, Y. Towards a new model for kimberlite petrogenesis: Evidence from unaltered kimberlites and mantle minerals. Earth-Sci. Rev. 2014, 139, 145–167. [Google Scholar] [CrossRef]
- Jones, R.A. Sr and Nd isotopic and rare earth element evidence for the genesis of megacrysts in kimberlites of southern Africa. In Mantle Xenoliths; Nixon, H., Ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 1987; pp. 711–724. [Google Scholar]
- Kostrovitsky, S.I.; Solovieva, L.V.; Alymova, N.V.; Yakovlev, D.A.; Suvorova, L.F.; Sandimirova, G.P.; Travin, A.V.; Yudin, D.S. Kimberlites and their megacryst mineral assemblage: Isotope geochemical data. Petrology 2013, 21, 143–162. [Google Scholar] [CrossRef]
- Nielsen, T.F.D.; Sand, K.K. The Majuagaa kimberlite dyke, Maniitsoq, region, West Greenland: Constraints on a Mg-rich silicocarbonatitic melt composition from groundmass mineralogy and bulk compositions. Can. Mineral. 2008, 46, 1043–1061. [Google Scholar] [CrossRef]
- Kostrovitsky, S.I.; Yakovlev, D.A. Kimberlites of the Yakutian Kimberlite Province: Composition, Genesis; SB RAN. A.P. Vinogradov Institute of Geochemistry, Institute of the Earth’s Crust: Novosibirsk, Russia, 2022; 468p. (In Russian) [Google Scholar]
- Vladimirov, B.M.; Kostrovitsky, S.I.; Solovieva, L.V.; Botkunov, A.I.; Fiveiskaya, L.V.; Egorov, K.N. Classification of Kimberlites and Structure of Kimberlite Pipes; Nauka: Moscow, Russia, 1981; 131p. (In Russian) [Google Scholar]
- Kurszlaukis, S.; Makhotkin, I.; Rotman, A.Y.; Kolesnikov, G.V.; Makovchuk, I.V. Syn–and post-eruptive pyroc processes in the Yubileinaya kimberlite pipe, Yakutia, Russia, and implications for the emplacement of South African-style kimberlite pipes. Lithos 2009, 112, 579–591. [Google Scholar] [CrossRef]
- Kostrovitsky, S.I. Geochemistry of Kimberlitic Minerals; Nauka: Novosibirsk, Russia, 1986; 263p. (In Russian) [Google Scholar]
- Pell, J.; Russell, J.K.; Zhang, S. Kimberlite emplacement temperatures from conodont geothermometry. Earth Planet. Sci. Lett. 2015, 411, 131–141. [Google Scholar] [CrossRef]
- Woolsey, T.S.; McPhllum, M.E.; Schumm, S.A. Modeling of diatreme empalcement by fluidization. Phys. Chem. Earth 1975, 9, 29–42. [Google Scholar] [CrossRef]
- Gernon, T.M.; Gilbertson, M.A.; Sparks, R.S.J.; Field, M. The role of gas-fluidisation in the formation of massive volcaniclastic kimberlite. Lithos 2009, 112, 439–451. [Google Scholar] [CrossRef]
- Scott Smith, B.H.; Nowicki, T.E.; Russell, J.K.; Webb, K.J.; Mitchell, R.H.; Hetman, C.M.; Robey, J.V. A Glossary of Kimberlite and Related Terms; Scott-Smith Petrology Inc.: North Vancouver, BC, Canada, 2018; Part 1—144p; Part 2—59p; Part 3—56p. [Google Scholar]
- Kovalsky, V.V.; Nikishov, K.N.; Egorov, O.S. Kimberlite and Carbonatite Formations of the Eastern and Southeastern Slopes of Anabar Anteclise; Nauka: Moscow, Russia, 1969; 288p. (In Russian) [Google Scholar]
- Milashev, V.A.; Krutoyarsky, M.A.; Rabkin, M.I.; Erlich, E.N. Kimberlitic Rocks and Picritic Porphyry in the Northeastern Siberian Craton; Gosgeoltekhizdat: Moscow, Russia, 1963; 216p. (In Russian) [Google Scholar]
- Marshintsev, V.K. Vertical Heterogeneity of Kimberlite Bodies of Yakutia; Science: Novosibirsk, Russia, 1986; 240p. (In Russian) [Google Scholar]
- Hawthorne, J.B. Model of kimberlite pipe. Phys. Chem. Earth. 1975, 9, 1–15. [Google Scholar] [CrossRef]
- Straaten, B.I.; Kopylova, M.G.; Russell, J.K.; Scott Smith, B.H. A rare occurrence of a crater-filling clastogenic extrusive coherent kimberlite, Victor Northwest (Ontario, Canada). Bull. Volcanol. 2011, 73, 1047–1062. [Google Scholar] [CrossRef]
- Kurszlaukis, S.; Fulop, A. Factors controlling the internal facies architecture of maar-diatreme volcanoes. Bull. Volcanol. 2013, 75, 761. [Google Scholar] [CrossRef]
- Dawson, J.B. Advances in kimberlite geology. Earth Sci. Rev. 1971, 7, 187–214. [Google Scholar] [CrossRef]
- Dawson, J.B.; Hawthorne, J.B. Magmatic sedimentation and carbonatic differentiation in kimberlite sills at Benfontein, South Africa. J. Geol. Soc. Lond. 1973, 129, 61–85. [Google Scholar] [CrossRef]
- White, J.I.; Sparks, R.S.I.; Bailey, K.; Barnet, W.R.; Field, M.; Windsor, L. Kimberlite sills and dykes associated with Wesselton kimberlite pipe, Kimberley, South Africa. S. Afr. J. Geol. 2012, 115, 1–32. [Google Scholar] [CrossRef]
- Mitchell, R.H. Paragenesis and oxygen isotope studies of serpentine in kimberlite. In Proceedings of the 10th International Kimberlite Conference, Bangalore, India, 5–11 February 2012; pp. 1–12. [Google Scholar] [CrossRef]
- Ogilvie-Harris, R.C.; Field, M.; Sparks RS, J.; Walter, M.J. Perovskite from the Dutoitspan kimberlite, Kimberley, South Africa: Implications for magmatic processes. Mineral. Mag. 2009, 73, 915–928. [Google Scholar] [CrossRef]
- Pokhilenko, N.P. Polymict breccia xenoliths: Evidence for the complex character of kimberlite formation. Lithos 2009, 112, 934–941. [Google Scholar] [CrossRef]
- Castillo-Oliver, M.; Galí, S.; Melgarejo, J.C.; Griffin, W.L.; Belousova, E.; Pearson, N.J.; Watangua, M.; O’Reilly, S.Y. Trace-element geochemistry and U–Pb dating of perovskite in kimberlites of the Lunda Norte province (NE Angola): Petrogenetic and tectonic implications. Chem. Geol. 2016, 426, 118–134. [Google Scholar] [CrossRef]
- Pilbeam, L.N.; Nielsen, T.F.D.; Waight, T.E. Digestion fractional crystallization (DFC): An important process in the genesis of kimberlites. Evidence from Olivine in the Majuagaa kimberlite, Southern West Greenland. J. Petrol. 2013, 54, 1399–1425. [Google Scholar] [CrossRef]
- Bussweiler, Y.; Foley, S.F.; Prevelic, D.; Jacob, D.E. The Olivine Macrocryst Problem:New Insights from Minor and Trace Element Compositions of Olivine from Las de Gras Kimberlites, Canada. Lithos 2015, 220–223, 238–252. [Google Scholar] [CrossRef]
- Sobolev, N.V.; Sobolev, A.V.; Tomilenko, A.A.; Kovyazin, S.V.; Butanova, V.G.; Kuz’min, D.V. Paragenesis and complex zoning of Olivine macrocrysts from unaltered kimberlite of the Udachnaya-East pipe, Yakutia: Relationship with the kimberlite formation conditions and evolution. Russ. Geol. Geophys. 2015, 56, 260–279. [Google Scholar] [CrossRef]
- Kostrovitsky, S.I.; Spetsius, Z.V.; Alymova, N.V.; Suvorova, L.F. Clinopyroxene-olivine-ilmenite megacryst association from kimberlites of Udachnaya pipe. Dokl. Earth Sci. 2004, 396, 93–97. [Google Scholar]
- Kostrovitsky, S.I.; Yakovlev, D.A.; Soltys, A.; Ivanov, A.S.; Matsyuk, S.S.; Robles-Cruz, S.E. A genetic relationship between magnesian ilmenite and kimberlites of the Yakutian diamond fields. Ore Geol. Rev. 2020, 120, 103419. [Google Scholar] [CrossRef]
- Mercier, J.C.C. Single-pyroxene termobarometry. Tectonophysics 1980, 70, 1–37. [Google Scholar] [CrossRef]
- Tappe, S.; Kjarsgaard, B.A.; Kurszlukis, S.; Nowell, G.M.; Phillips, D. Petrology and Nd-Hf isotope geochemistry of Neoproterozoic Amon kimberlite sills, Baffin Island (Canada): Evidence for deep mantle magmatic activity linked to supercontinent cycles. J. Petrol. 2014, 55, 2003–2042. [Google Scholar] [CrossRef]
- Agashev, A.M.; Watanabe, T.; Bydaev, D.A.; Pokhilenko, N.P.; Fomin, A.S.; Maehara, K.; Maeda, J. Geochemistry of kimberlites from the Nakyn field, Siberia: Evidence for unique source composition. Geology 2001, 29, 267–270. [Google Scholar] [CrossRef]
- Torsvik, T.H.; Burke, K.; Steinberger, B.; Webb, S.J.; Ashwal, L.D. Diamonds sampled by plumes from the core-mantle boundary. Nature 2010, 466, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Pernet-Fisher, J.F.; Howarth, G.H.; Pearson, D.G.; Woodland, S.; Barry, P.H.; Pokhilenko, N.P.; Pokhilenko, L.N.; Agashev, A.M.; Taylor, L.A. Plume impingement on the Siberian SCLM: Evidence from Re-Os isotope systematics. Lithos 2015, 218–219, 141–154. [Google Scholar] [CrossRef]
- Tappe, S.; Brand, N.B.; Stracke, A.; van Acken, D.; Liu, C.-Z.; Strauss, H.; Wu, F.-Y.; Luguet, A.; Mitchell, R.H. Plates or plumes in the origin of kimberlites: U/Pb perovskite and Sr–Nd–Hf–Os–C–O isotope constraints from the Superior craton (Canada). Chem. Geol. 2017, 455, 57–83. [Google Scholar] [CrossRef]
- Jaques, A.I.; Lewis, J.D.; Smith, C.B. The kimberlites and lamproites of Western Australia. Geol. Surv. West. Aust. Bull. 1986, 132, 268. [Google Scholar]





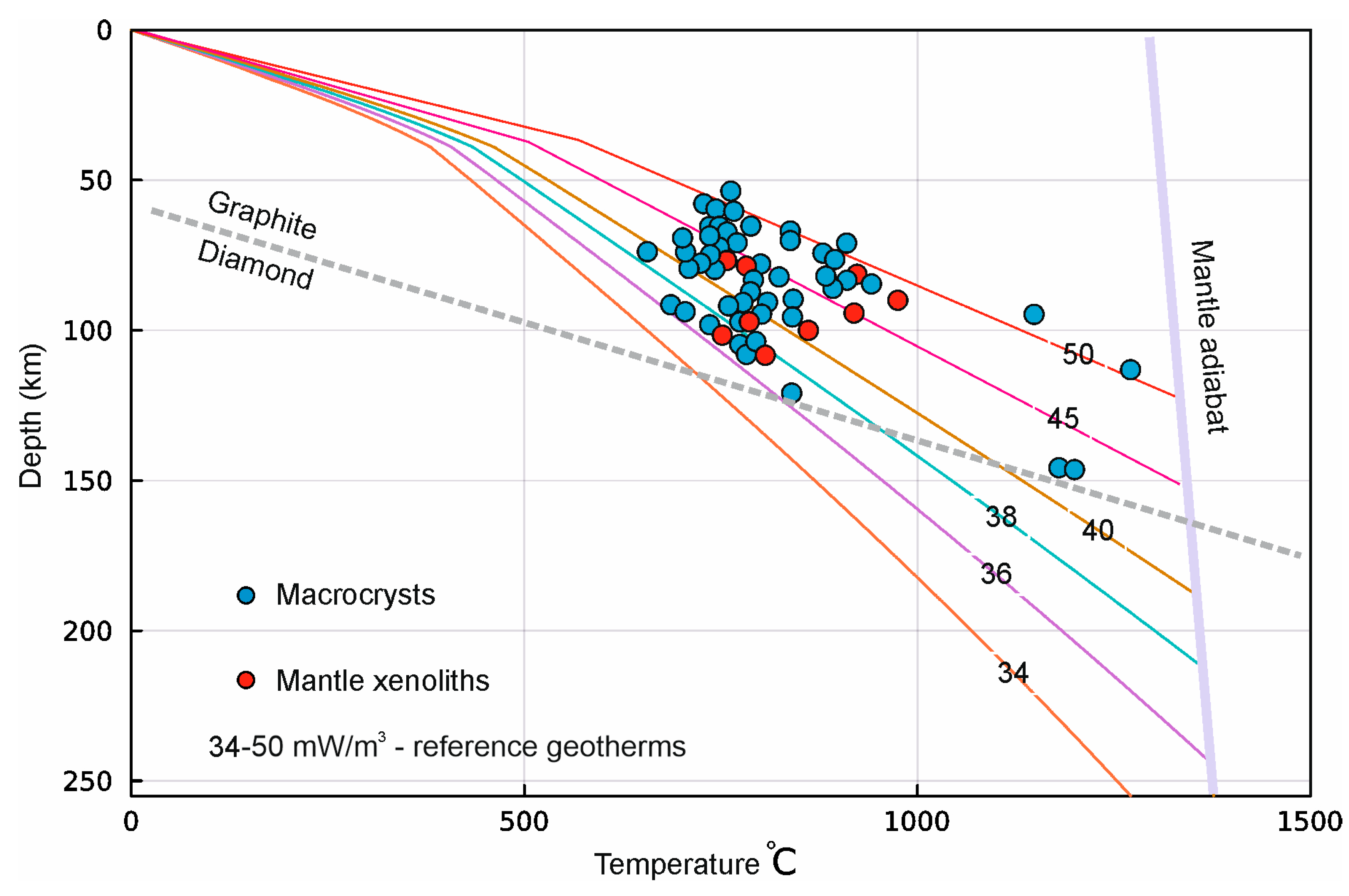
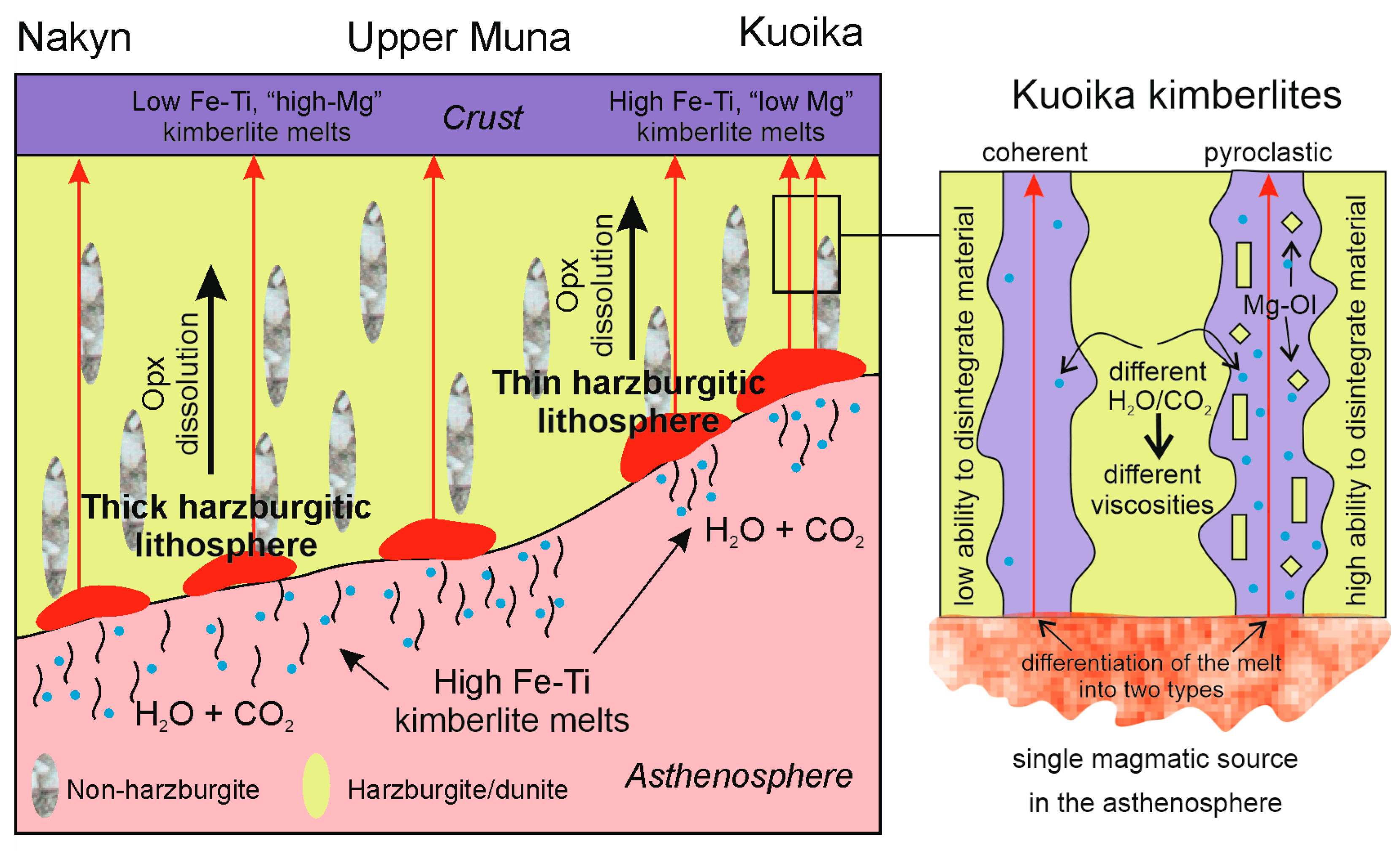
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 7-234 | 7-237 | 7-387 | 7-384 | 7-386 | 7-388 | 7-191 | 7-192 | 7-196 | 3 (7) | |
| SiO2 | 33.25 | 31.16 | 32.01 | 24.56 | 20.67 | 32.34 | 22.11 | 19.87 | 16.54 | 22.76 |
| TiO2 | 0.57 | 0.60 | 0.47 | 1.35 | 1.25 | 4.75 | 4.20 | 3.75 | 3.94 | 3.28 |
| Al2O3 | 2.87 | 1.82 | 2.69 | 2.65 | 2.92 | 3.72 | 3.25 | 2.89 | 3.10 | 4.39 |
| Fe2O3 | 5.95 | 7.06 | 4.00 | 5.25 | 6.58 | 6.25 | 5.53 | 4.65 | 7.12 | 7.64 |
| FeO | 2.40 | 1.26 | 3.60 | 3.43 | 1.43 | 4.1 | 5.73 | 5.85 | 3.95 | 3.74 |
| MnO | 0.13 | 0.14 | 0.15 | 0.26 | 0.20 | 0.12 | 0.20 | 0.19 | 0.16 | 0.19 |
| MgO | 32.67 | 32.22 | 30.78 | 25.25 | 20.43 | 28.71 | 22.14 | 19.09 | 13.30 | 22.23 |
| CaO | 6.98 | 8.45 | 8.92 | 16.52 | 20.42 | 4.56 | 16.32 | 20.84 | 23.40 | 15.90 |
| Na2O | 0.11 | 0.08 | 0.15 | 0.15 | 0.19 | 0.15 | 0.13 | 0.09 | 0.23 | 0.28 |
| K2O | 0.69 | 0.73 | 1.00 | 1.35 | 1.14 | 2.28 | 1.12 | 0.48 | 0.16 | 0.86 |
| P2O5 | 0.49 | 0.79 | 0.64 | 0.83 | 1.12 | 0.17 | 0.92 | 1.65 | 1.10 | 0.89 |
| H2O | 7.84 | 8.23 | 8.46 | 4.61 | 5.13 | 8.62 | 6.59 | 6.30 | 5.34 | 5.44 |
| CO2 | 5.48 | 6.64 | 7.01 | 12.98 | 16.04 | 3.58 | 10.00 | 13.07 | 20.64 | 11.8 |
| Total | 99.43 | 99.18 | 99.98 | 99.19 | 97.52 | 99.35 | 98.24 | 98.72 | 99.33 | 99.4 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|
| No. | 7-234 | 7-242 | 7-384 | 7-386 | 7-388 | 7-390 | 7-191 |
| Sc | 11.6 | 12.9 | n.d. | 17 | n.d. | 4.93 | 22 |
| V | 75 | 74 | 111 | 143 | 183 | 181 | 397 |
| Cr | 1644 | 1073 | 858 | 400 | 1057 | 1975 | 548 |
| Co | 82 | 68 | 69.4 | 46 | 110 | 60 | 82 |
| Ni | 1371 | 1027 | 833 | 407 | 1415 | 592 | 416 |
| Cu | 23 | 34 | 72.7 | 53 | 219 | 84 | 124 |
| Zn | 51 | 48 | 76.8 | 55 | 110 | 46 | 96 |
| Rb | 41 | 47 | 69 | 52 | 110 | 222 | 91 |
| Sr | 845 | 1395 | 1903 | 2094 | 435 | 109 | 1313 |
| Y | 11.5 | 22 | 22.6 | 24 | 5.9 | 1.39 | 29 |
| Zr | 94 | 138 | 148 | 183 | 55.6 | 22 | 297 |
| Nb | 151 | 273 | 294 | 324 | 139 | 56 | 1092 |
| Cs | 0.63 | 0.73 | 0.61 | 0.92 | 1.18 | 1.71 | 1.48 |
| Ba | 1455 | 1660 | 2405 | 2867 | 578 | 317 | 6123 |
| La | 139 | 239 | 207 | 279 | 54 | 13.7 | 301 |
| Ce | 231 | 377 | 367 | 455 | 89 | 23 | 524 |
| Pr | 23 | 41 | 33 | 46 | 8.4 | 2.2 | 51 |
| Nd | 79 | 136 | 112 | 151 | 28.2 | 7.5 | 176 |
| Sm | 10.6 | 18 | 14.9 | 20 | 3.8 | 0.97 | 24 |
| Eu | 2.67 | 4.92 | 3.9 | 5.2 | 1.03 | 0.26 | 5.4 |
| Gd | 7.5 | 11.8 | 12.1 | 14.2 | 3.36 | 0.79 | 17 |
| Tb | 0.54 | 0.7 | 1.26 | 1 | 0.34 | 0.06 | 1.33 |
| Dy | 3.47 | 6.1 | 5.26 | 7 | 1.32 | 0.35 | 7.8 |
| Ho | 0.53 | 0.95 | 0.8 | 1.08 | 0.2 | 0.06 | 1.13 |
| Er | 1.15 | 1.92 | 1.68 | 2.22 | 0.47 | 0.13 | 2.34 |
| Tm | 0.14 | 0.23 | 0.19 | 0.28 | 0.05 | 0.02 | 0.28 |
| Yb | 0.83 | 1.29 | 1.09 | 1.61 | 0.29 | 0.09 | 1.69 |
| Lu | 0.11 | 0.15 | 0.14 | 0.2 | 0.03 | 0.01 | 0.22 |
| Hf | 2.31 | 3.14 | 3.41 | 4.27 | 1.56 | 0.74 | 8.7 |
| Ta | 6.8 | 10.5 | 10.6 | 12.9 | 11.4 | 7.4 | 5.7 |
| Pb | 8.6 | 9.8 | 11.2 | 16 | 3.61 | 0.75 | 13.7 |
| Th | 22 | 37 | 29.9 | 42 | 7.01 | 1.63 | 70 |
| U | 4.1 | 6.2 | 5.88 | 7.9 | 1.45 | 0.31 | 8.7 |
| Sample | Pipe | Field | Rb | Sr | 87Rb/86Sr | 87Sr/86Sr | 2σ | (87Sr/86Sr) | Age |
| 00-289 | Inter | Mirniy | 19 | 689 | 0.0805 | 0.703856 | 0.000013 | 0.70344 | 360 |
| 03/33-1 | Udachnaya | Daldyn | 53 | 1510 | 0.1011 | 0.705995 | 0.000011 | 0.70548 | 360 |
| 03/91 | Udachnaya | Daldyn | 38 | 912 | 0.1199 | 0.705524 | 0.000012 | 0.70491 | 360 |
| 03-101 | Udachnaya | Daldyn | 59 | 1128 | 0.1525 | 0.707170 | 0.000012 | 0.70639 | 360 |
| 03-142 | Udachnaya | Daldyn | 70 | 1601 | 0.1263 | 0.707036 | 0.000012 | 0.70639 | 360 |
| 03-180 | Udachnaya | Daldyn | 91 | 1254 | 0.2096 | 0.706706 | 0.000014 | 0.70563 | 360 |
| 05-75 | Udachnaya | Daldyn | 31 | 1191 | 0.0749 | 0.705535 | 0.000013 | 0.70515 | 360 |
| 7-191 | Velikan | Kuoika | 85 | 1203 | 0.2037 | 0.704625 | 0.000015 | 0.70416 | 160 |
| 7-483 | Zenit | Kuoika | 15 | 926 | 0.0478 | 0.704116 | 0.000014 | 0.70401 | 160 |
| 7-487 | Jila 87/2 | Kuoika | 3 | 640 | 0.0130 | 0.705031 | 0.000013 | 0.70500 | 160 |
| 7-234 | Obnazhennaya | Kuoika | 39 | 828 | 0.1358 | 0.703924 | 0.000015 | 0.70362 | 160 |
| 7-237 | Obnazhennaya | Kuoika | 31 | 1114 | 0.08119 | 0.704361 | 0.000014 | 0.70418 | 160 |
| 7-242 | Obnazhennaya | Kuoika | 48 | 1512 | 0.0922 | 0.705836 | 0.000011 | 0.70563 | 160 |
| 7-280 | Obnazhennaya | Kuoika | 34 | 866 | 0.1140 | 0.706382 | 0.000015 | 0.70612 | 160 |
| 7-390 | Obnazhennaya | Kuoika | 216 | 106 | 5.9198 | 0.718436 | 0.000011 | 0.70497 | 160 |
| 7-390 | Obnazhennaya | Kuoika | 220 | 104 | 6.1251 | 0.718206 | 0.000012 | 0.70427 | 160 |
| 7-392 | Obnazhennaya | Kuoika | 30 | 920 | 0.0944 | 0.704303 | 0.000013 | 0.70409 | 160 |
| Sample | Pipe | Sm | Nd | 147Sm/144Nd | 143Nd/144Nd | 2σ | eNd(t) | 2σ | Age |
| 00-289 | Inter | 11.8 | 83.6 | 0.085221 | 0.512623 | 0.000015 | 4.84 | 0.29 | 360 |
| 03/33-1 | Udachnaya | 11.0 | 85.4 | 0.078185 | 0.512573 | 0.000009 | 4.18 | 0.18 | 360 |
| 03/91 | Udachnaya | 6.9 | 50.3 | 0.083196 | 0.512598 | 0.000012 | 4.44 | 0.23 | 360 |
| 03-101 | Udachnaya | 8.0 | 62.4 | 0.077734 | 0.512552 | 0.000011 | 3.80 | 0.21 | 360 |
| 03-142 | Udachnaya | 10.2 | 78.2 | 0.078820 | 0.512559 | 0.000011 | 3.89 | 0.21 | 360 |
| 03-180 | Udachnaya | 5.1 | 36.9 | 0.082929 | 0.512464 | 0.000013 | 1.85 | 0.25 | 360 |
| 7-191 | Velikan | 18.0 | 126.2 | 0.086474 | 0.512715 | 0.000011 | 3.76 | 0.21 | 160 |
| 7-483 | Zenit | 24.8 | 179.3 | 0.083792 | 0.512719 | 0.000011 | 3.89 | 0.21 | 160 |
| 7-487 | Jila 87/2 | 7.9 | 52.4 | 0.091687 | 0.512767 | 0.000015 | 4.67 | 0.29 | 160 |
| 7-234 | Obnazhennaya | 9.5 | 70.0 | 0.081715 | 0.512708 | 0.000015 | 3.72 | 0.29 | 160 |
| 7-237 | Obnazhennaya | 14.1 | 105.9 | 0.080360 | 0.512746 | 0.000013 | 4.48 | 0.25 | 160 |
| 7-242 | Obnazhennaya | 18.2 | 134.4 | 0.081806 | 0.512724 | 0.000012 | 4.02 | 0.23 | 160 |
| 7-280 | Obnazhennaya | 6.4 | 47.7 | 0.081011 | 0.512698 | 0.000012 | 3.54 | 0.23 | 160 |
| 7-390 | Obnazhennaya | 0.9 | 6.6 | 0.081050 | 0.512701 | 0.000014 | 3.59 | 0.27 | 160 |
| 7-390 | Obnazhennaya | 0.9 | 6.6 | 0.081001 | 0.512725 | 0.000014 | 4.06 | 0.27 | 160 |
| 7-392 | Obnazhennaya | 10.5 | 77.8 | 0.081684 | 0.512729 | 0.000012 | 4.13 | 0.23 | 160 |
| Sample | Pipe | Lu | Hf | 176Lu/177Hf | 176Hf/177Hf | 2σ | εHf(t) | 2σ | Age |
| 00-289 | Inter | 0.0785 | 3.810 | 0.0029 | 0.282810 | 0.000015 | 8.57 | 0.52 | 360 |
| 03/33-1 | Udachnaya | 0.0600 | 2.859 | 0.0030 | 0.282700 | 0.000015 | 4.65 | 0.53 | 360 |
| 03/91 | Udachnaya | 0.0537 | 2.471 | 0.0031 | 0.282712 | 0.000013 | 5.08 | 0.45 | 360 |
| 03-101 | Udachnaya | 0.0529 | 3.158 | 0.0024 | 0.282691 | 0.000012 | 4.48 | 0.43 | 360 |
| 03-142 | Udachnaya | 0.0698 | 3.468 | 0.0029 | 0.282688 | 0.000018 | 4.26 | 0.63 | 360 |
| 03-180 | Udachnaya | 0.0462 | 2.203 | 0.0030 | 0.282616 | 0.000019 | 1.69 | 0.69 | 360 |
| 05-75 | Udachnaya | 0.0807 | 3.418 | 0.0034 | 0.282686 | 0.000010 | 4.08 | 0.37 | 360 |
| 7-191 | Velikan | 0.1677 | 5.214 | 0.0046 | 0.282898 | 0.000012 | 7.48 | 0.44 | 160 |
| 7-483 | Zenit | 0.1529 | 8.036 | 0.0027 | 0.282884 | 0.000009 | 7.20 | 0.33 | 160 |
| 7-487 | Jila 87/2 | 0.0666 | 4.403 | 0.0022 | 0.282881 | 0.000013 | 7.13 | 0.45 | 160 |
| 7-234 | Obnazhennaya | 0.0863 | 2.039 | 0.0060 | 0.282850 | 0.000014 | 5.64 | 0.48 | 160 |
| 7-237 | Obnazhennaya | 0.0985 | 2.756 | 0.0051 | 0.282895 | 0.000023 | 7.34 | 0.83 | 160 |
| 7-242 | Obnazhennaya | 0.1463 | 3.101 | 0.0067 | 0.282863 | 0.000014 | 6.03 | 0.51 | 160 |
| 7-242R | Obnazhennaya | 0.1463 | 3.092 | 0.0067 | 0.282851 | 0.000016 | 5.59 | 0.57 | 160 |
| 7-280 | Obnazhennaya | 0.0640 | 1.647 | 0.0055 | 0.282820 | 0.000032 | 4.63 | 1.13 | 160 |
| 7-390 | Obnazhennaya | 0.0104 | 0.661 | 0.0022 | 0.282889 | 0.000016 | 7.41 | 0.56 | 160 |
| 7-390 | Obnazhennaya | 0.0104 | 0.680 | 0.0022 | 0.282857 | 0.000012 | 6.29 | 0.44 | 160 |
| 7-392 | Obnazhennaya | 0.0844 | 2.369 | 0.0051 | 0.282889 | 0.000019 | 7.11 | 0.67 | 160 |
| 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|
| 237c (9) | 7-237r (8) | 7-387c (24) | 7-387r (12) | 386c (6) | 7-386r (9) | |
| SiO2 | 39.2–42.0 40.73 | 39.1–41.1 40.3 | 39.8–42.2 41.1 | 39.5–41.0 40.4 | 40.3–41.6 40.9 | 40.3–41.4 40.7 |
| TiO2 | 0–0.01 0.01 | 0.01–0.04 0.02 | 0–0.02 0.01 | 0–0.05 0.02 | 0–0.04 0.02 | 0–0.04 0.03 |
| Al2O3 | 0–0.1 0.05 | 0–0.11 0.03 | 0–0.09 0.02 | 0–0.06 0.01 | 0–0.1 0.03 | 0–0.03 0.01 |
| Cr2O3 | 0–0.02 0.01 | 0.04–0.08 0.06 | 0–0.04 0.01 | 0.01–0.11 0.06 | 0–0.07 0.03 | 0.03–0.16 0.1 |
| FeO | 7.04–8.0 7.51 | 11.8–12.9 12.3 | 6.5–10 7.5 | 11.9–13.6 12.4 | 7.7–10.3 9.0 | 11.2–12.0 11.5 |
| MnO | 0.08–0.11 0.09 | 0.15–0.25 0.19 | 0.05–0.13 0.1 | 0.11–0.23 0.18 | 0.08–0.12 0.1 | 0.14–0.18 0.16 |
| MgO | 45.4–51.2 49.05 | 45.3–47.5 46.4 | 48.6–51.7 50.4 | 45.6–47.2 46.5 | 47.9–51.0 49.5 | 46.5–48.4 47.7 |
| CaO | 0–0.38 0.05 | 0.04–0.12 0.08 | 0–0.19 0.02 | 0.04–0.14 0.08 | 0–0.08 0.04 | 0.05–0.15 0.09 |
| NiO | 0.35–0.42 0.38 | 0.08–0.27 0.18 | 0.34–0.42 0.38 | 0.09–0.32 0.21 | 0.32–0.42 0.36 | 0.24–0.39 0.32 |
| Lherzolite (21) | Olivine Websterites (13) | |
|---|---|---|
| SiO2 | 39.8–42.1 (40.9) | 40.5–42.1 (41.0) |
| TiO2 | 0–0.11 (0.02) | 0–0.06 (0.01) |
| Al2O3 | 0–0.04 (0.01) | 0–0.04 (0.01) |
| Cr2O3 | 0–0.04 (0.01) | 0–0.04 (0.01) |
| FeO | 7.25–8.64 (8.0) | 6.95–8.41 (7.8) |
| MnO | 0.06–0.11 (0.10) | 0.07–0.1 (0.09) |
| MgO | 48.8–52.2 (50.4) | 49.1–51.6 (50.7) |
| CaO | 0–0.03 (0.01) | 0–0.02 (0.01) |
| NiO | 0.35–0.49 (0.4) | 0.37–0.44 (0.41) |
| Total | 98.8–101.7 (99.9) | 99.4–100.9 (100.2) |
| Mg# | 91.0–92.6 (91.8) | 91.4–92.9 (92.1) |
| SiO2 | FeO | MnO | MgO | CaO | NiO | Total | |
|---|---|---|---|---|---|---|---|
| 1 | 40.33 | 12.21 | 0.12 | 45.64 | 0.06 | 0.35 | 98.83 |
| 2 | 39.91 | 12.80 | 0.13 | 45.26 | 0.00 | 0.26 | 98.27 |
| 3 | 39.67 | 13.02 | 0.14 | 46.41 | 0.06 | 0.28 | 99.65 |
| 4 | 40.33 | 12.74 | 0.15 | 46.00 | 0.07 | 0.28 | 99.67 |
| 5 | 39.92 | 12.12 | 0.13 | 46.72 | 0.06 | 0.32 | 99.34 |
| 6 | 40.50 | 12.04 | 0.12 | 46.60 | 0.07 | 0.29 | 99.68 |
| 7 | 40.72 | 11.33 | 0.13 | 46.52 | 0.08 | 0.34 | 99.28 |
| 8 | 39.67 | 12.31 | 0.13 | 46.81 | 0.07 | 0.26 | 99.38 |
| 9 | 40.57 | 12.16 | 0.13 | 46.21 | 0.07 | 0.25 | 99.53 |
| 10 | 40.58 | 11.41 | 0.11 | 46.63 | 0.00 | 0.39 | 99.34 |
| 11 | 40.21 | 11.27 | 0.10 | 46.95 | 0.07 | 0.39 | 99.12 |
| Middle (11) | 40.22 | 12.13 | 0.13 | 46.34 | 0.05 | 0.31 | 99.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostrovitsky, S.; Dymshits, A.; Yakovlev, D.; Sun, J.; Kalashnikova, T.; Ashchepkov, I.; Belozerova, O. Primary Composition of Kimberlite Melt. Minerals 2023, 13, 1404. https://doi.org/10.3390/min13111404
Kostrovitsky S, Dymshits A, Yakovlev D, Sun J, Kalashnikova T, Ashchepkov I, Belozerova O. Primary Composition of Kimberlite Melt. Minerals. 2023; 13(11):1404. https://doi.org/10.3390/min13111404
Chicago/Turabian StyleKostrovitsky, Sergey, Anna Dymshits, Dmitry Yakovlev, Jing Sun, Tatiana Kalashnikova, Igor Ashchepkov, and Olga Belozerova. 2023. "Primary Composition of Kimberlite Melt" Minerals 13, no. 11: 1404. https://doi.org/10.3390/min13111404






