Effect of Gangue Minerals on Pulp Rheology and Flotation Behavior of Smithsonite
Abstract
:1. Introduction
2. Materials and Methods
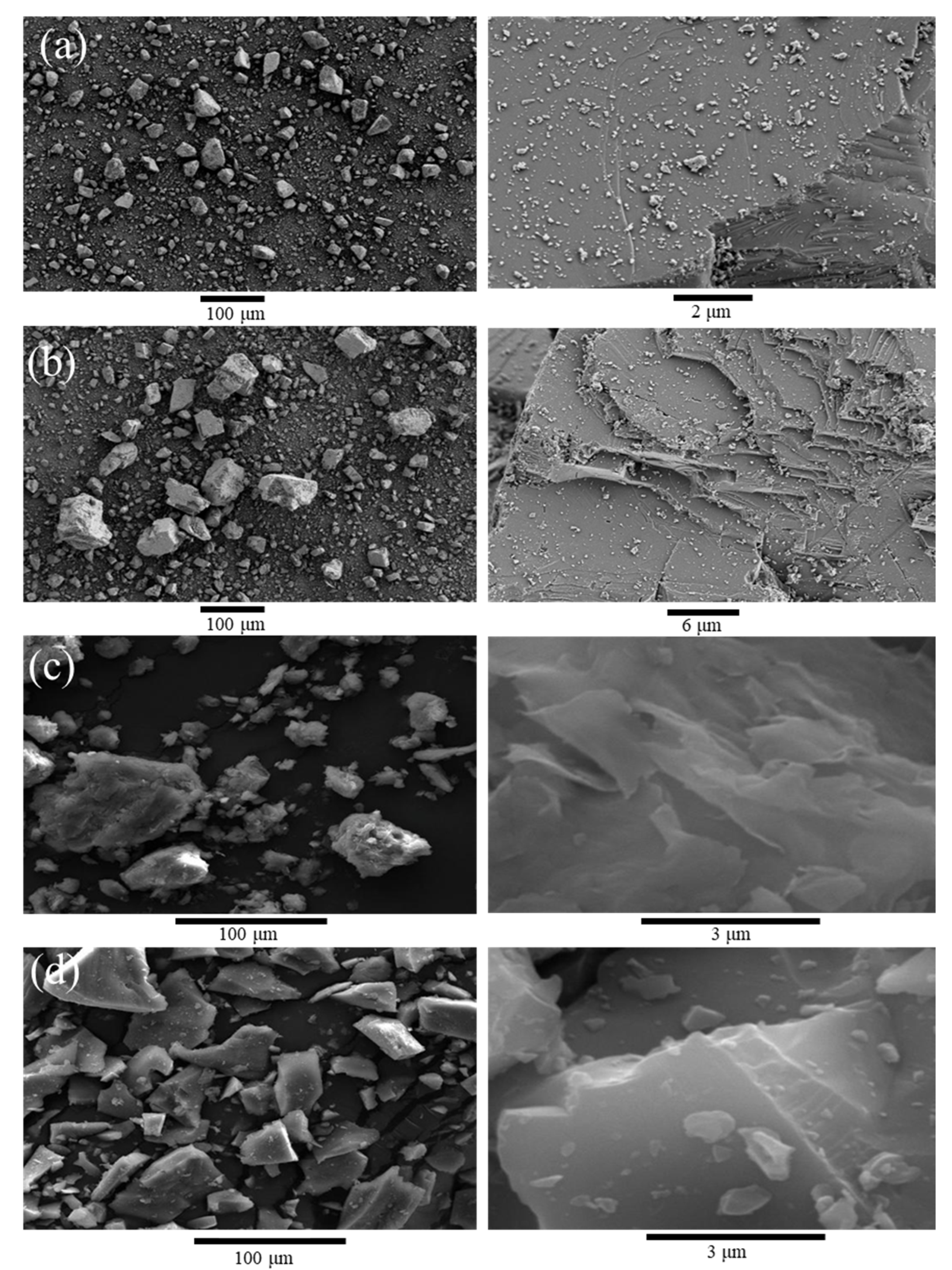
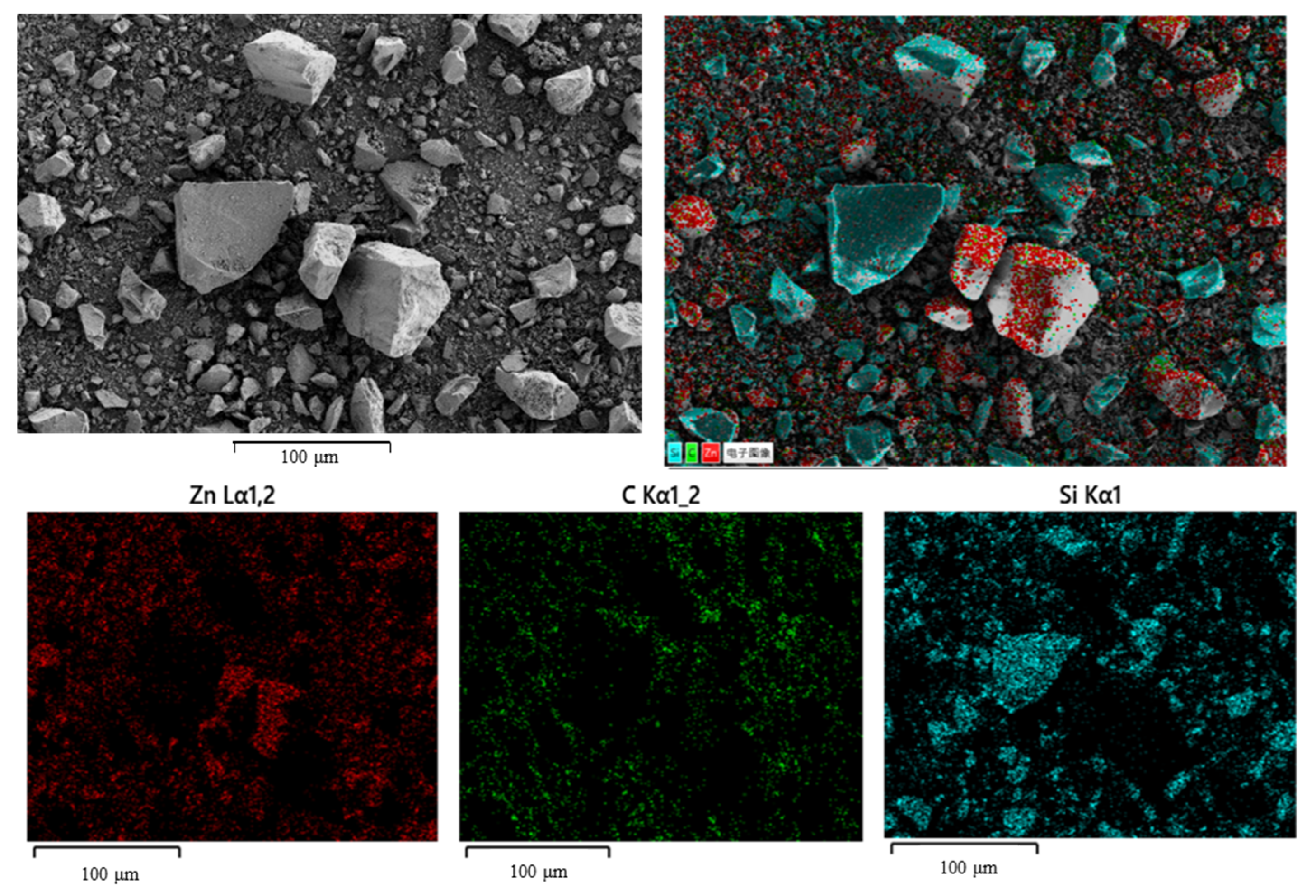
2.1. Materials
2.2. Pulp Rheology Measurement
2.3. Flotation Test
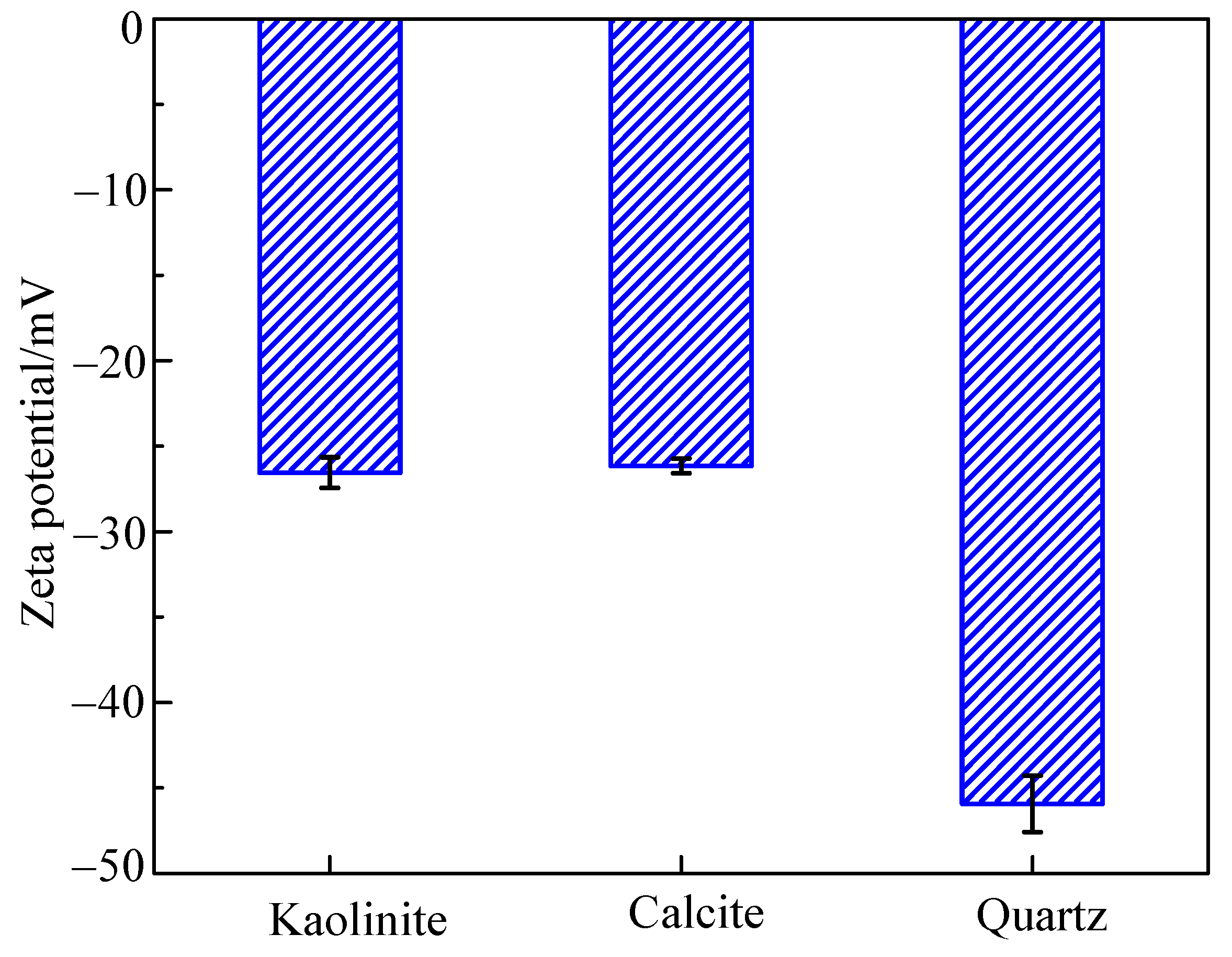
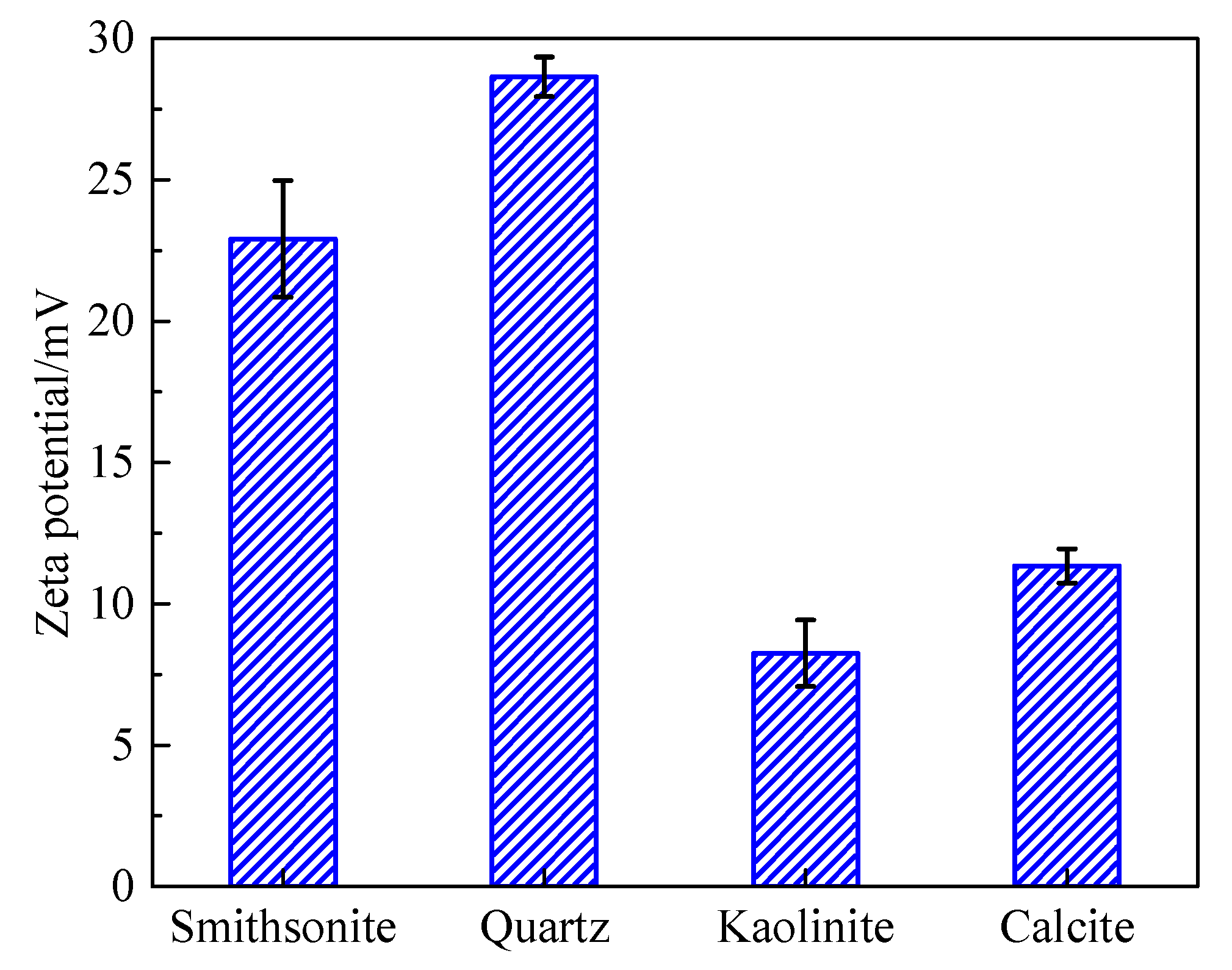
2.4. Zeta Potential Measurements
3. Results and Discussions
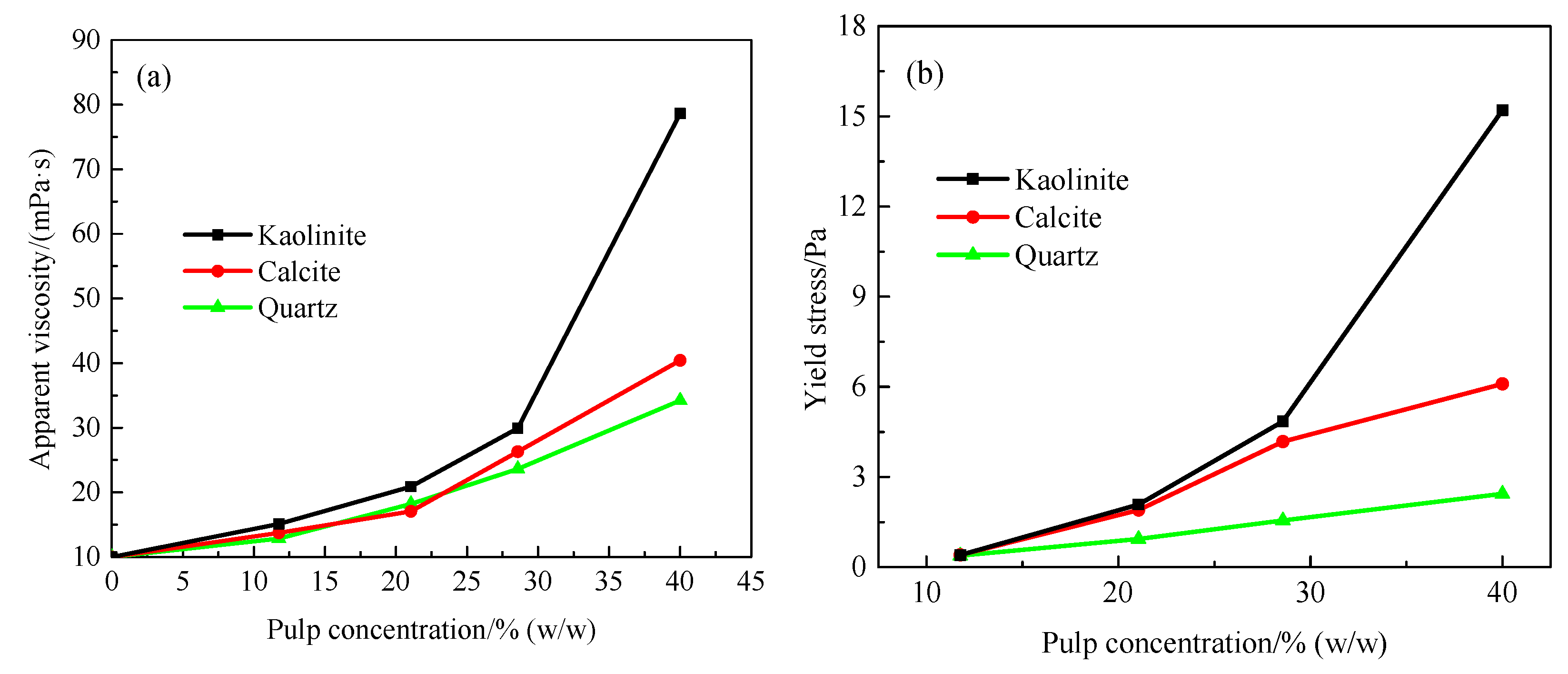
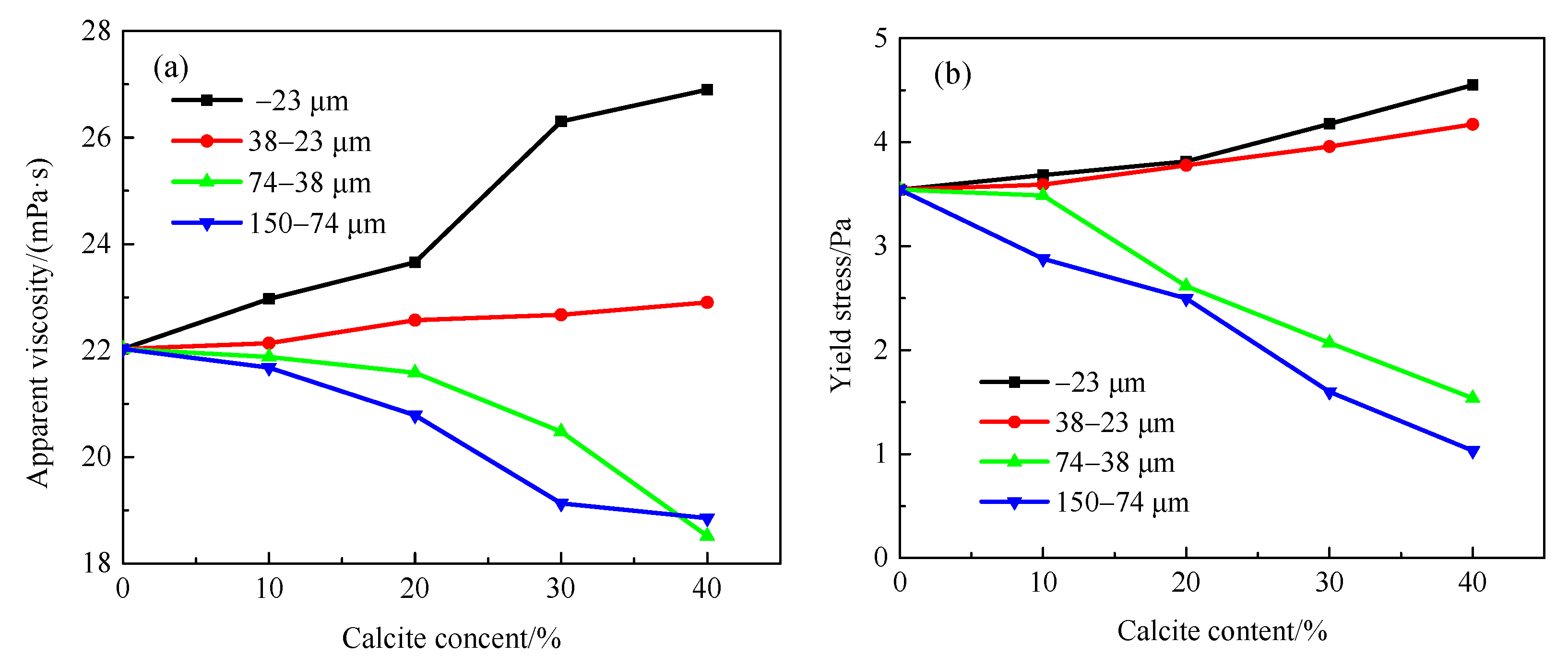
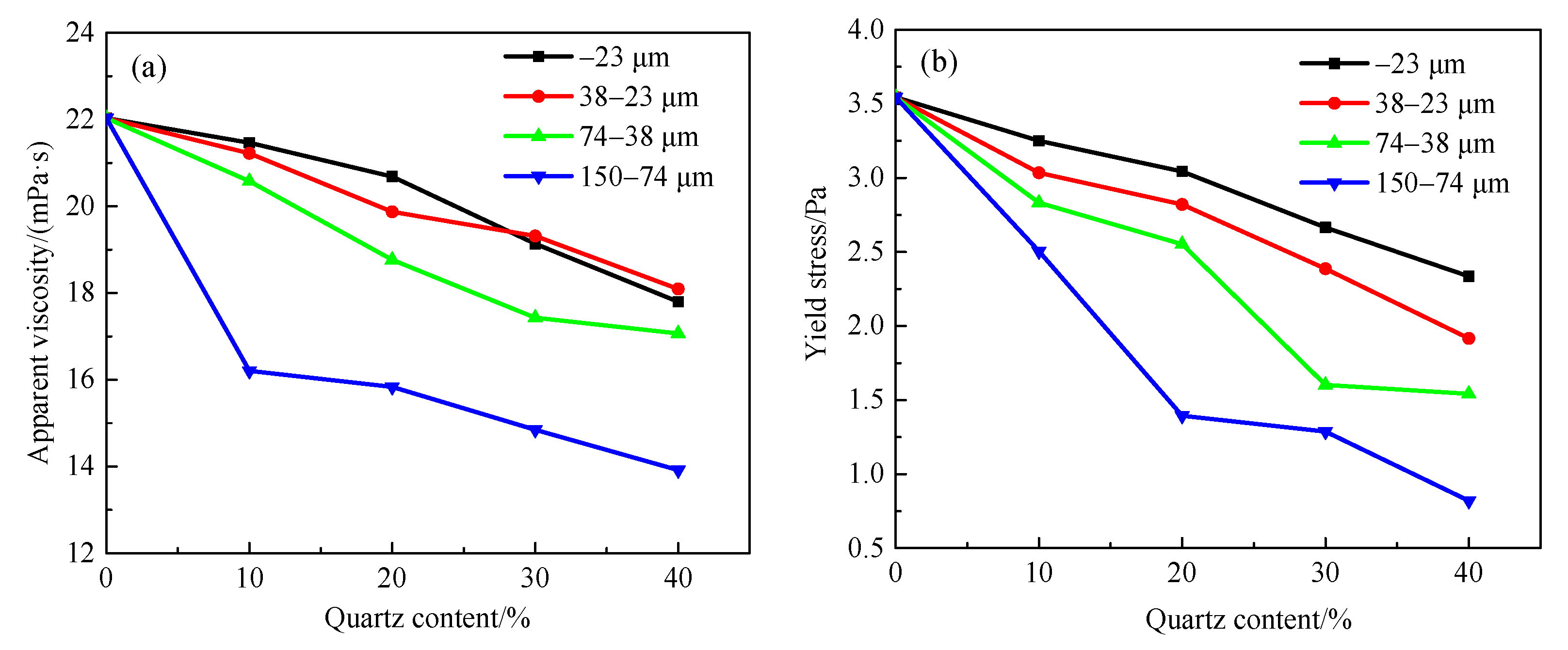
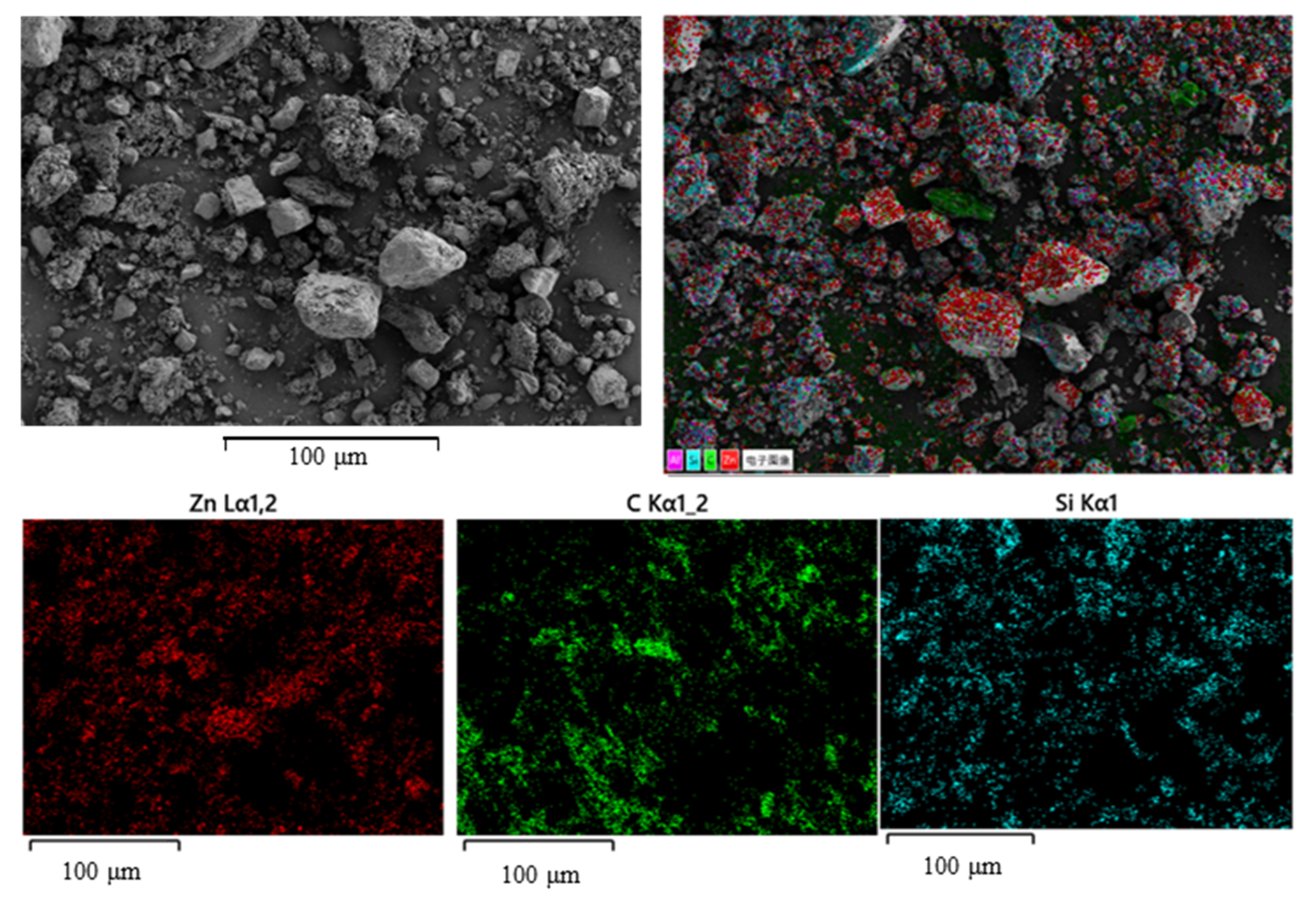
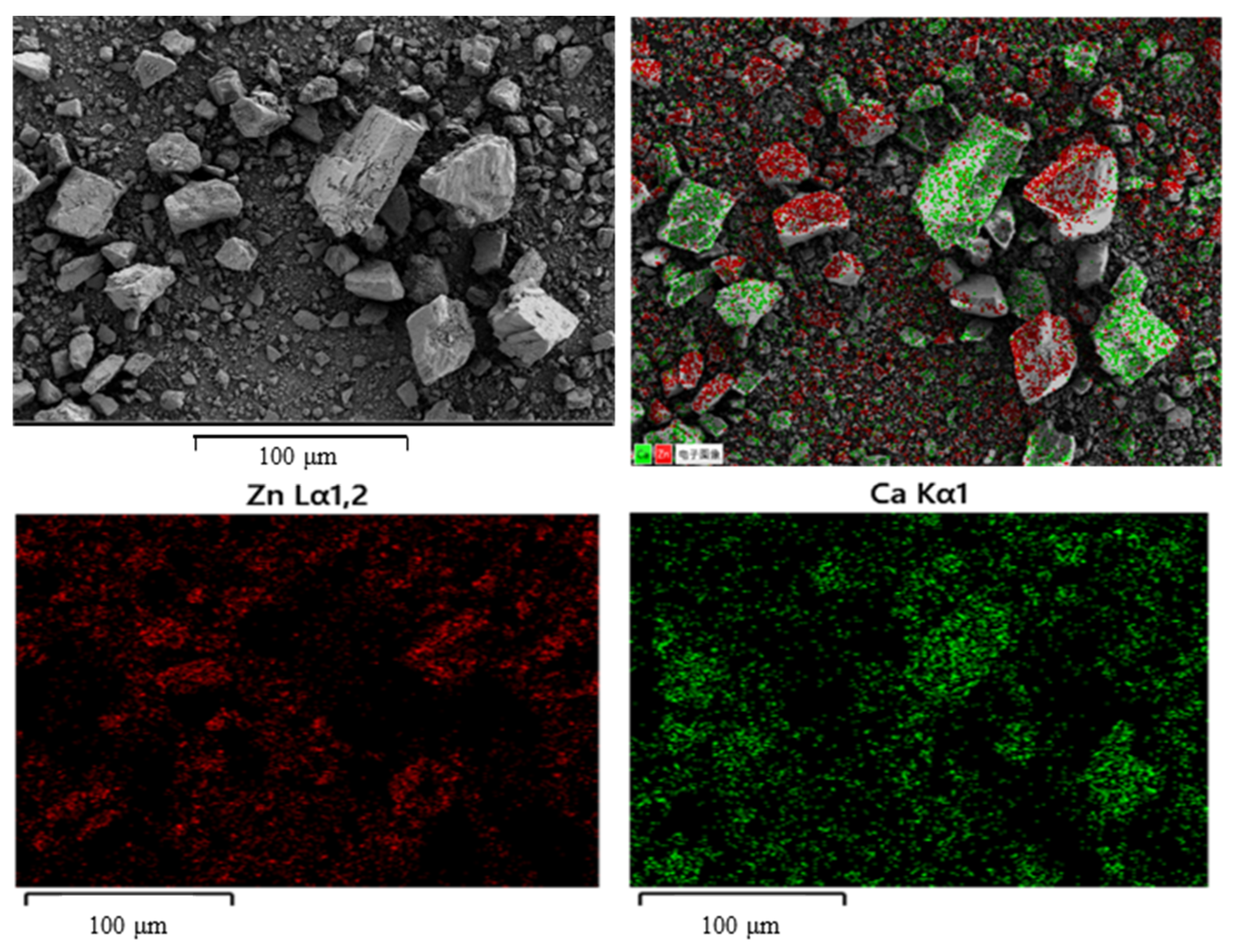
3.1. Effect of Gangue Mineral Content on Apparent Viscosity and Yield Stress of Smithsonite Pulp
3.2. Effect of Concentration on Pulp Apparent Viscosity and Yield Stress of Smithsonite
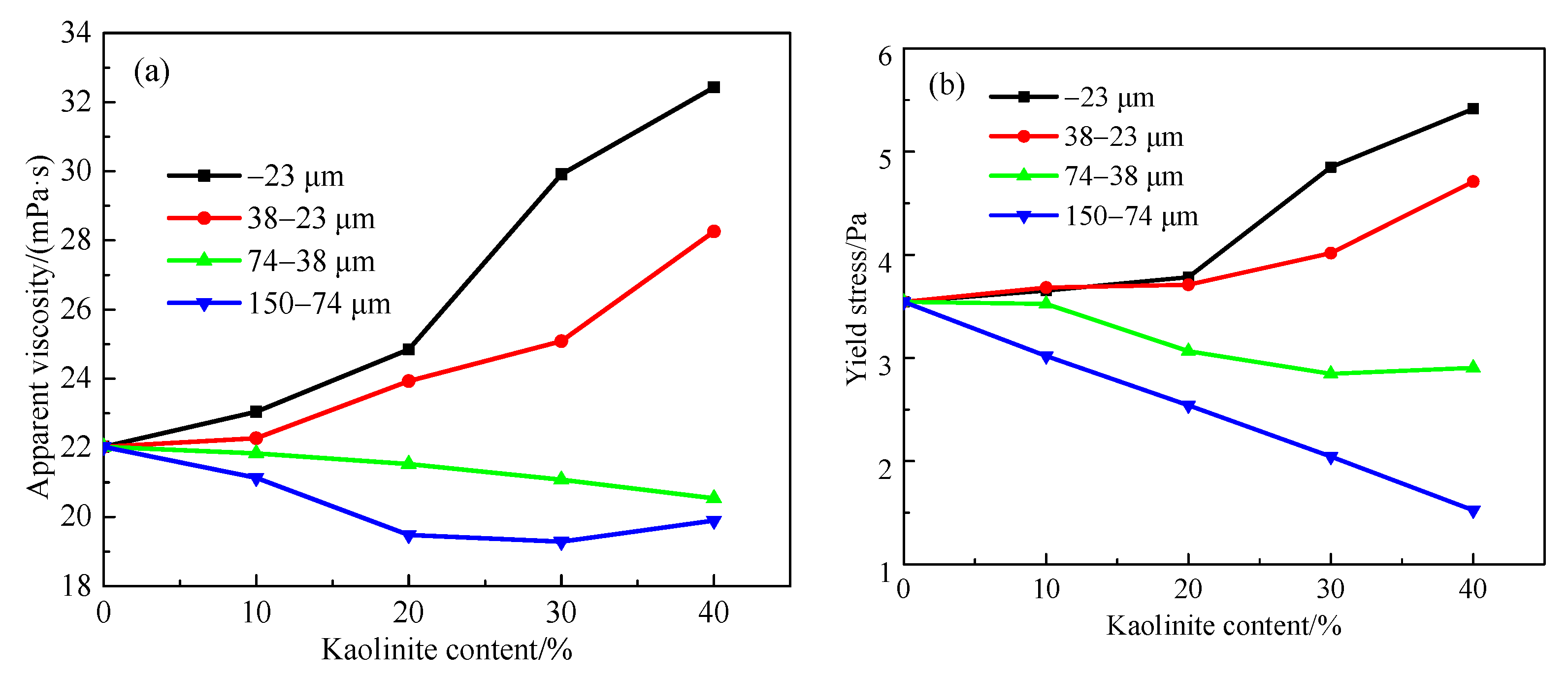
3.3. Effect of Particle Sizes on Apparent Viscosity and Yield Stress of Smithsonite Slurry
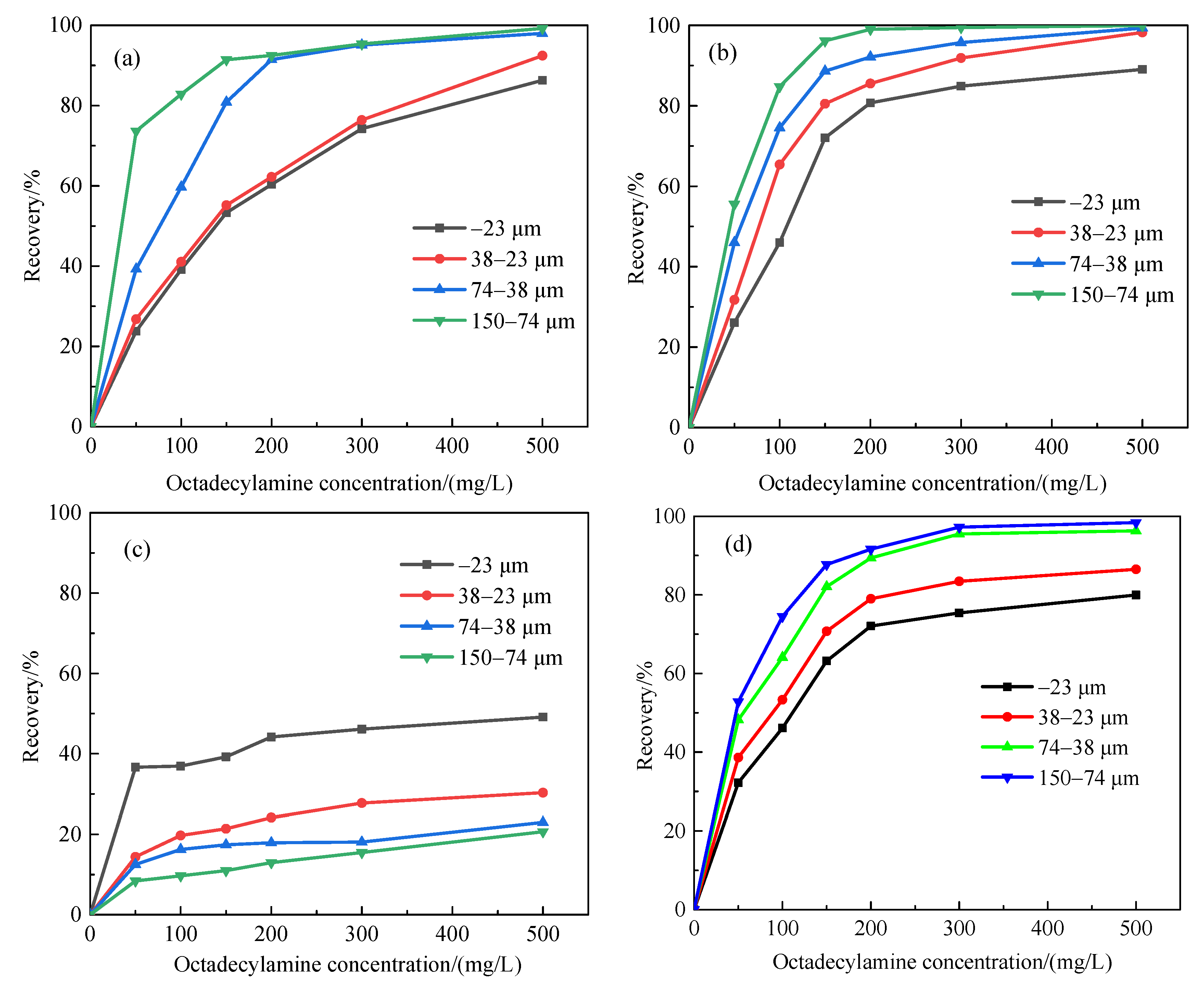
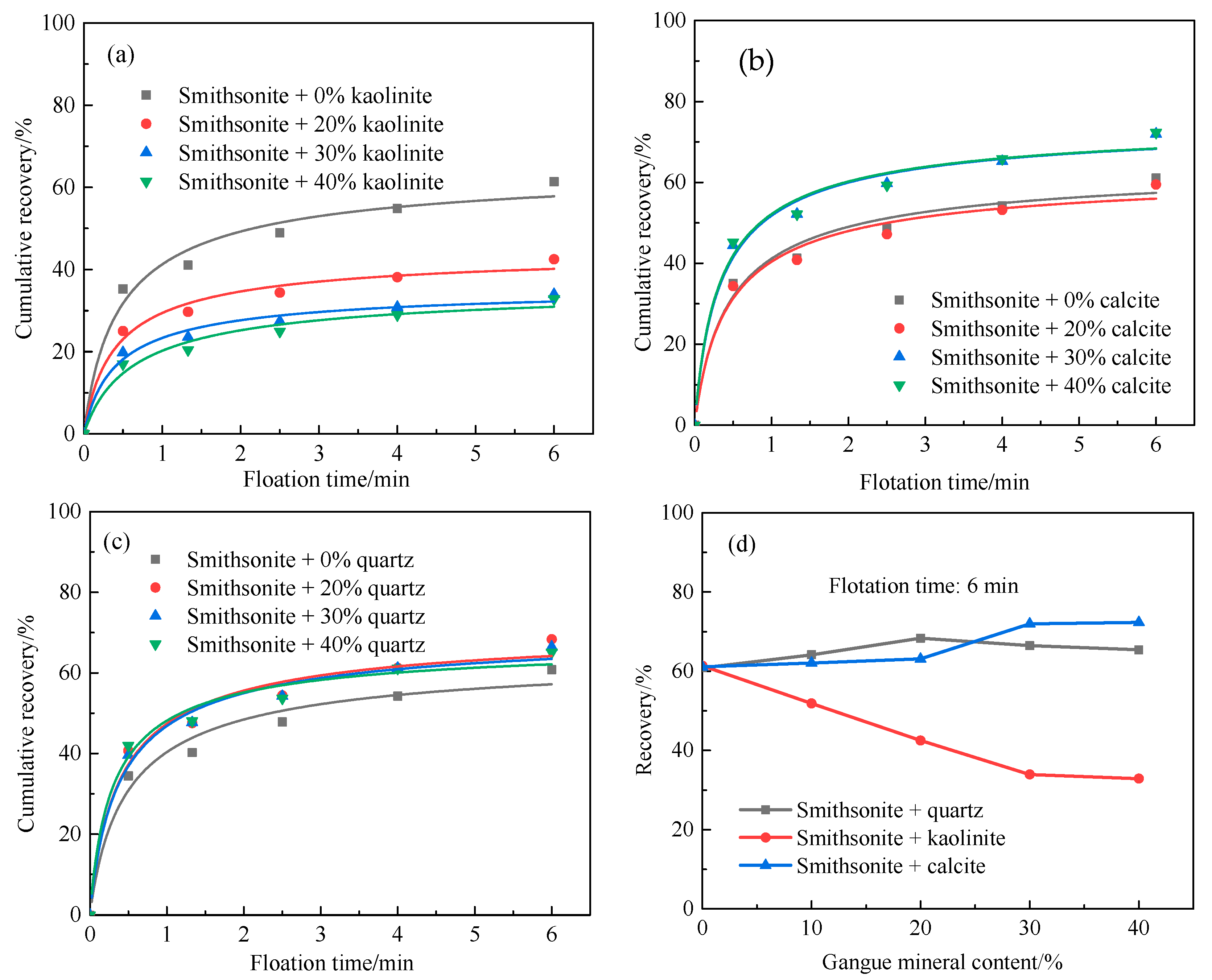
3.4. Basic Floatability of Minerals
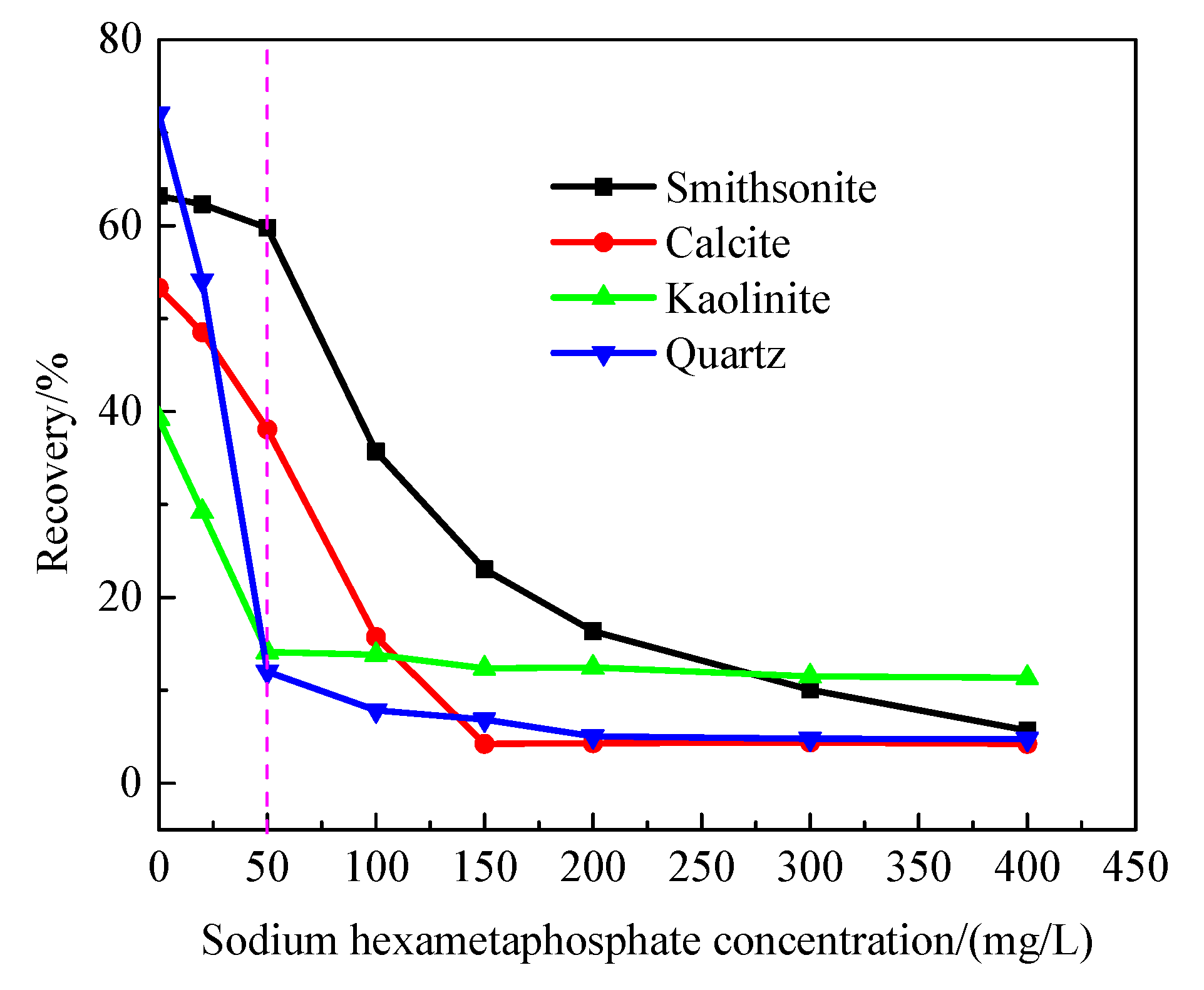
3.5. Flotation Behavior of Gangue Minerals under the Action of Sodium Hexametaphosphate as a Depressant
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feng, D.X.; Lin, L.B.; Xie, H.X.; Tong, X. Study on separation of low-grade zinc oxide ore with sulfurization-amination flotation. Physicochem. Probl. Miner. Process. 2019, 55, 1082–1090. [Google Scholar]
- Mehdilo, A.; Irannajad, M.; Zarei, H. Smithsonite flotation from zinc oxide ore using alkyl amine acetate collectors. Sep. Sci. Technol. 2014, 49, 445–457. [Google Scholar] [CrossRef]
- He, D.S.; Chen, Y.; Xiang, P.; Yu, Z.J.; Potgieter, J.H. Study on the pre-treatment of oxidized zinc ore prior to flotation. Int. J. Miner. Metall. Mater. 2018, 25, 117–122. [Google Scholar] [CrossRef]
- Duarte, G.M.P.; Lima, R.M.F. Quartz and hematite activation by Zn, Ca and Mg ions in the cationic flotation route for oxidized zinc ore. Miner. Process. Extr. Metall. Rev. 2022, 43, 720–727. [Google Scholar] [CrossRef]
- Ejtemaei, M.; Gharabaghi, M.; Irannajad, M. A review of zinc oxide mineral beneficiation using flotation method. Adv. Colloid Interface Sci. 2014, 206, 68–78. [Google Scholar] [CrossRef]
- Ferreira, P.H.T.; Lima, R.M.F. Concentration of oxidized Brazilian zinc ore by flotation: Comparative study between anionic and cationic routes. Sep. Sci. Technol. 2022, 57, 2625–2634. [Google Scholar] [CrossRef]
- Hosseini, H.; Taji, M. Flotation behavior of iranian oxidized zinc ore using different types of collectors (cationic, anionic and mixed (cationic/anionic)). Int. J. Min. Eng. Miner. Process. 2015, 4, 18–27. [Google Scholar]
- Irannajad, M.; Ejtemaei, M.; Gharabaghi, M. The effect of reagents on selective flotation of smithsonite–calcite–quartz. Miner. Eng. 2009, 22, 766–771. [Google Scholar] [CrossRef]
- Kashani, A.; Rashchi, F. Separation of oxidized zinc minerals from tailings: Influence of flotation reagents. Miner. Eng. 2008, 21, 967–972. [Google Scholar] [CrossRef]
- Zhao, W.J.; Liu, D.W.; Zhang, X.L.; Zeng, S.Q.; Xu, G.Y.; Feng, Q.C. Impact of pre-desliming on flotation of low-grade zinc oxide ore from Lanping. Adv. Mater. Res. 2013, 634–638, 3385–3389. [Google Scholar] [CrossRef]
- Boger, D.V. Rheology and the Minerals Industry. Miner. Process. Extr. Metall. Rev. 2000, 20, 1–25. [Google Scholar] [CrossRef]
- Farrokhpay, S. The importance of rheology in mineral flotation: A review. Miner. Eng. 2012, 36–38, 272–278. [Google Scholar] [CrossRef]
- Wang, L.; Li, C. A brief review of pulp and froth rheology in mineral flotation. J. Chem. 2020, 2020, 3894542. [Google Scholar] [CrossRef]
- Richmond, W.R.; Jones, R.L.; Fawell, P.D. The relationship between particle aggregation and rheology in mixed silica–titania suspensions. Chem. Eng. J. 1998, 71, 67–75. [Google Scholar] [CrossRef]
- Farrokhpay, S.; Morris, G.E.; Fornasiero, D.; Self, P. Influence of polymer functional group architecture on titania pigment dispersion. Colloids Surf. A Physicochem. Eng. Asp. 2005, 253, 183–191. [Google Scholar] [CrossRef]
- Sajjad, M.; Otsuki, A. Correlation between flotation and rheology of fine particle suspensions. Metals 2022, 12, 270. [Google Scholar] [CrossRef]
- Chen, X.; Peng, Y.J.M. Managing clay minerals in froth flotation—A critical review. Miner. Process. Extr. Metall. Rev. 2018, 39, 289–307. [Google Scholar] [CrossRef] [Green Version]
- Basnayaka, L.; Subasinghe, N.; Albijanic, B. Influence of clays on the slurry rheology and flotation of a pyritic gold ore. Appl. Clay Sci. 2017, 136, 230–238. [Google Scholar] [CrossRef]
- Cruz, N.; Peng, Y.; Wightman, E.; Xu, N. The interaction of pH modifiers with kaolinite in copper-gold flotation. Miner. Eng. 2015, 84, 27–33. [Google Scholar] [CrossRef]
- Cruz, N.; Peng, Y.J.; Wightman, E.; Xu, N. The interaction of clay minerals with gypsum and its effects on copper–gold flotation. Miner. Eng. 2015, 77, 121–130. [Google Scholar] [CrossRef]
- Zhang, M.; Peng, Y.J. Effect of clay minerals on pulp rheology and the flflotation of copper and gold minerals. Miner. Eng. 2015, 70, 8–13. [Google Scholar] [CrossRef]
- Chen, W.; Chen, F.F.; Bu, X.Z.; Zhang, G.F.; Zhang, C.H.; Song, Y.H. A significant improvement of fine scheelite flotation through rheological control of flotation pulp by using garnet. Miner. Eng. 2019, 138, 257–266. [Google Scholar] [CrossRef]
- Liu, D.Z.; Zhang, G.F.; Gao, Y.W. New perceptions into the detrimental influences of serpentine on Cu-Ni sulfide flotation through rheology studies and improved the separation by applying garnet. Miner. Eng. 2021, 171, 107110. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Q.; Mao, S.; Qin, S.H. Effects of fine minerals on pulp rheology and the flotation of diaspore and pyrite mixed ores. Minerals 2020, 10, 60. [Google Scholar] [CrossRef] [Green Version]
- Farrokhpay, S.; Morris, G.E.; Fornasiero, D.; Self, P. Stabilisation of titania pigment particles with anionic polymeric dispersants. Powder Technol. 2010, 202, 143–150. [Google Scholar] [CrossRef]
- He, M.Z.; Wang, Y.M.; Forssberg, E. Slurry rheology in wet ultrafine grinding of industrial minerals: A review. Powder Technol. 2004, 147, 94–112. [Google Scholar] [CrossRef]
- Becker, M.; Yorath, G.; Ndlovu, B.; Harris, M.; Deglon, D.; Franzidis, J.P. A rheological investigation of the behaviour of two Southern African platinum ores. Miner. Eng. 2013, 49, 92–97. [Google Scholar] [CrossRef]
- Cruz, N.; Peng, Y.J.; Farrokhpay, S.; Bradshaw, D. Interactions of clay minerals in copper-gold flotation: Part 1—Rheological properties of clay mineral suspensions in the presence of flotation reagents. Miner. Eng. 2013, 50–51, 30–37. [Google Scholar] [CrossRef]
- Das, G.K.; Kelly, N.; Muir, D.M. Rheological behaviour of lateritic smectite ore slurries. Miner. Eng. 2011, 24, 594–602. [Google Scholar] [CrossRef]
- Cruz, N.; Peng, Y.J.; Wightman, E. Interactions of clay minerals in copper–gold flotation: Part 2—Influence of some calcium bearing gangue minerals on the rheological behaviour. Int. J. Miner. Process. 2015, 141, 51–60. [Google Scholar] [CrossRef]
- Yalçın, T.; Alemdar, A.; Ece, Ö.I. The viscosity and zeta potential of bentonite dispersions in presence of anionic surfactants. Mater. Lett. 2002, 57, 420–424. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shang, Y.; Sun, C. Effect of Gangue Minerals on Pulp Rheology and Flotation Behavior of Smithsonite. Minerals 2023, 13, 66. https://doi.org/10.3390/min13010066
Shang Y, Sun C. Effect of Gangue Minerals on Pulp Rheology and Flotation Behavior of Smithsonite. Minerals. 2023; 13(1):66. https://doi.org/10.3390/min13010066
Chicago/Turabian StyleShang, Yanbo, and Chuanyao Sun. 2023. "Effect of Gangue Minerals on Pulp Rheology and Flotation Behavior of Smithsonite" Minerals 13, no. 1: 66. https://doi.org/10.3390/min13010066



