Recycling of Coal Fly Ash in Building Materials: A Review
Abstract
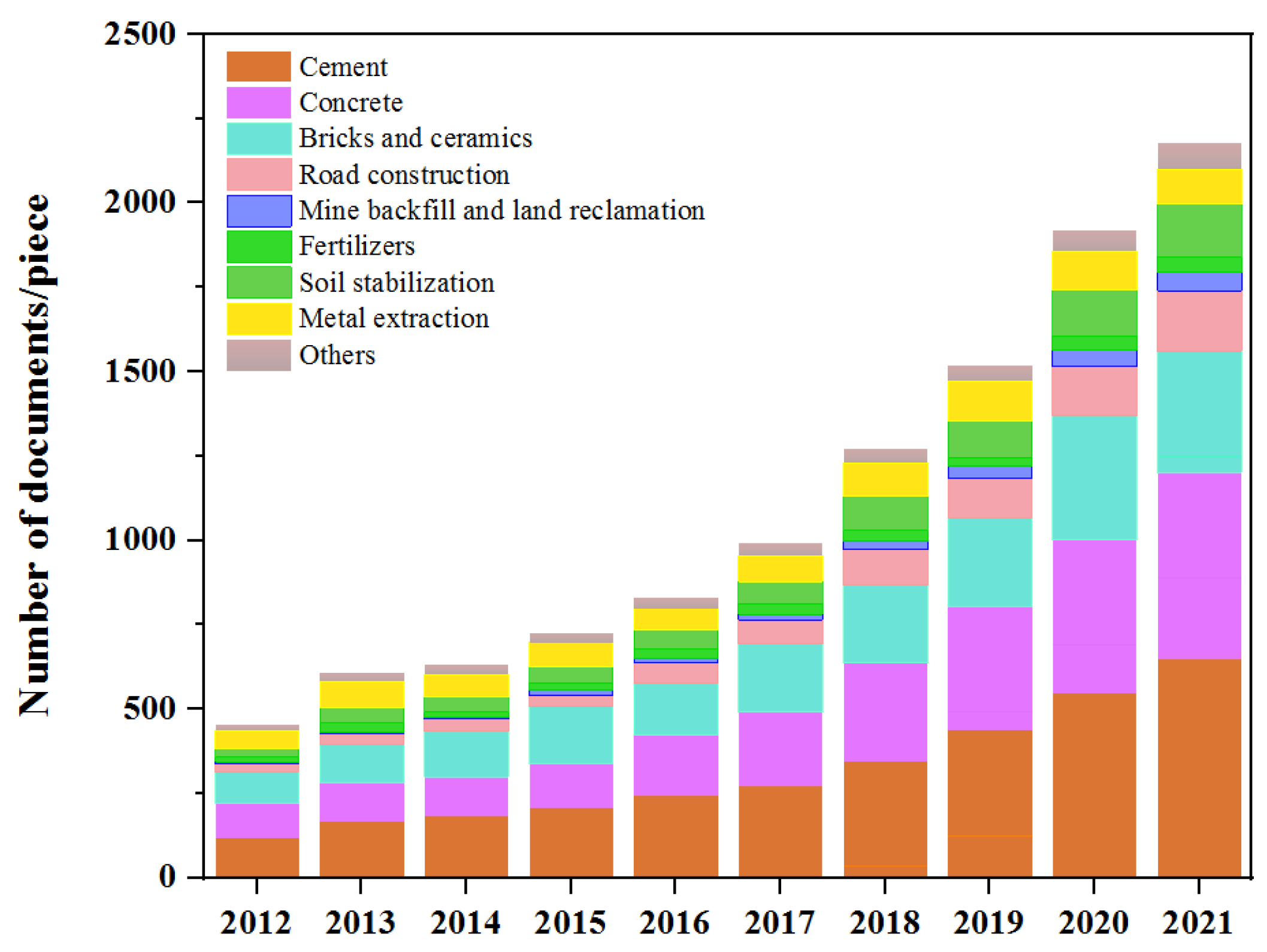
:1. Introduction
2. Properties, Classification and Hazard Assessment of CFA
2.1. Properties of CFA
2.2. Classification of CFA
2.3. Hazard Assessment of CFA
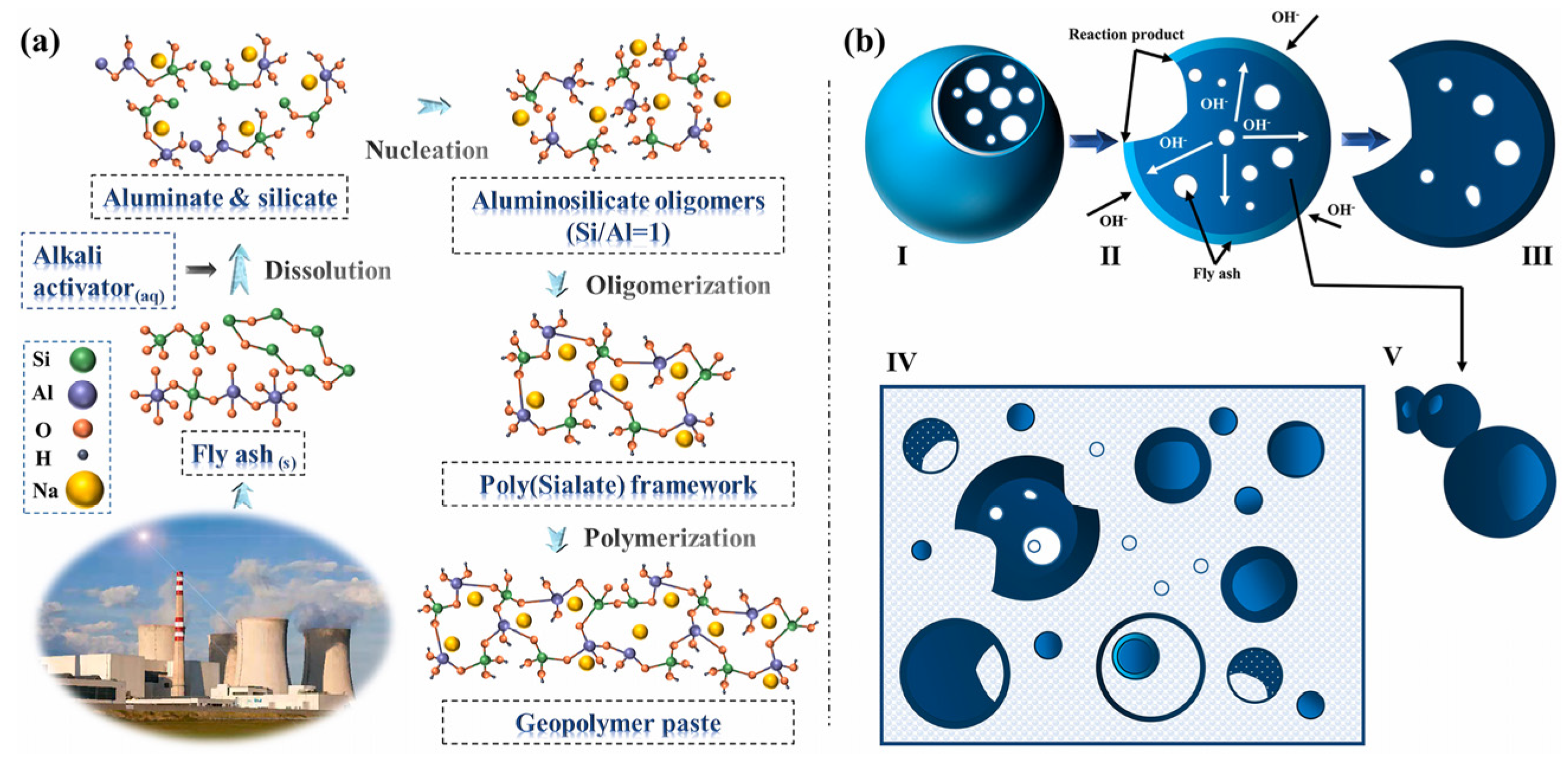
3. Application of CFA in Building Materials
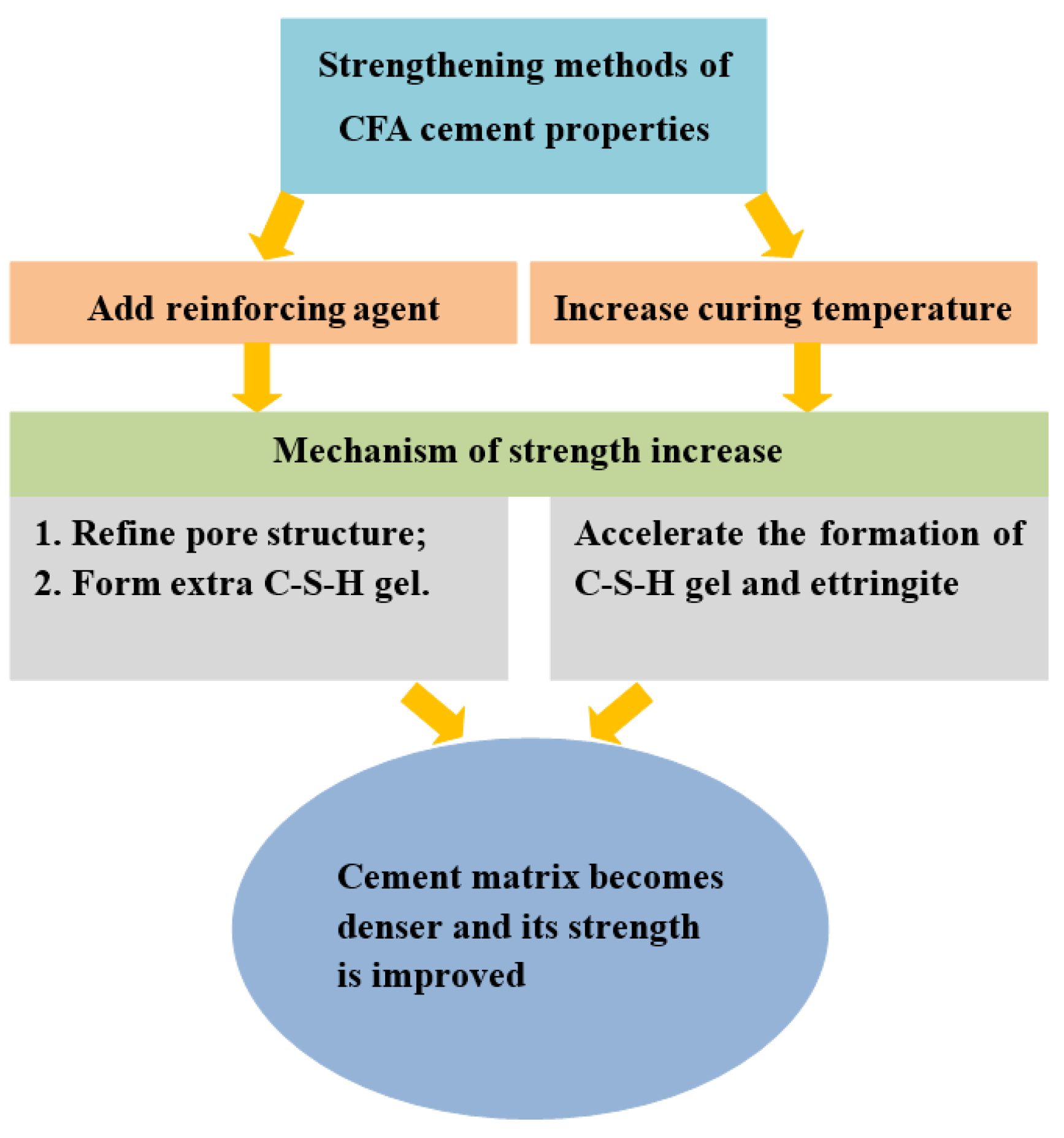
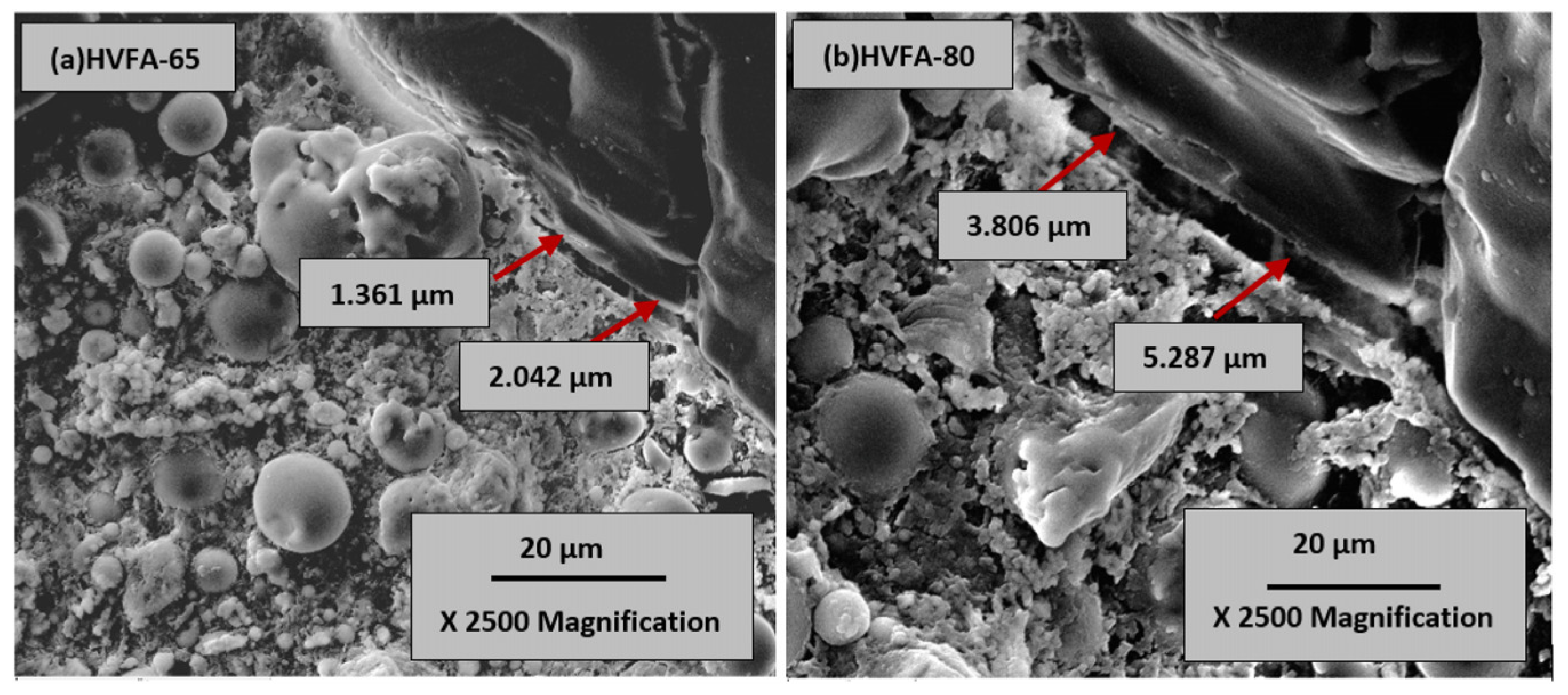
3.1. CFA Cement
3.2. CFA Concrete
| Class of Samples | Raw Materials and Activators | Curing | L/Sa | Compressive Strength [MPa] | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| T[°C] | RH[%] | 7-d | 14-d | 28-d | ||||
| Cement | CFA, PC, Slag | Str. | 0.5 | ~28 | n.r. | ~45 | [47] | |
| CFA, OPC | Str. | n.r. | n.r. | n.r. | ~45 | [48] | ||
| Concrete | CFA, OPC, Fine and coarse aggregate, | n.r. | 0.4 | 30.23 | n.r. | 48.69 | [55] | |
| CFA, PC, Sand | Str. | 0.47 | 54.2 | n.r. | 57.5 | [56] | ||
| CFA, OPC, Sand | Room. | n.r. | 0.4 | ~21 | n.r. | ~33 | [64] | |
| CFA, PC, Sand, Coarse aggregate | Room. | n.r. | n.r. | n.r. | n.r. | 52.3 | [65] | |
| CFA, OPC, Sand, Coarse aggregate | Str. | 0.32 | ~31 | n.r. | ~49 | [66] | ||
| Geopolymers | CFA, CCR | 801; 602 | n.r. | 0.4 | 9 | ~10.3 | 18 | [69] |
| CFA, NaOH, Na2SiO3 | Room. | n.r. | n.r. | ~11.7 | n.r. | ~13.7 | [70] | |
| CFA, Clay, NaOH, Na2SiO3 | 2001; Room.2 | n.r. | 0.25 | 34 | n.r. | n.r. | [71] | |
| CFA, GBFS, NaOH, Na2SiO3 | Room.1; 652; Room.3 | 95 | 0.4 | ~31 | n.r. | ~32.5 | [72] | |
| CFA, MK, Sand, NaOH, Na2SiO3 | 20 ± 2 | 95 | 0.65 | n.r. | n.r. | ~65 | [73] | |
3.3. Ceramics from CFA
3.4. Geopolymers from CFA
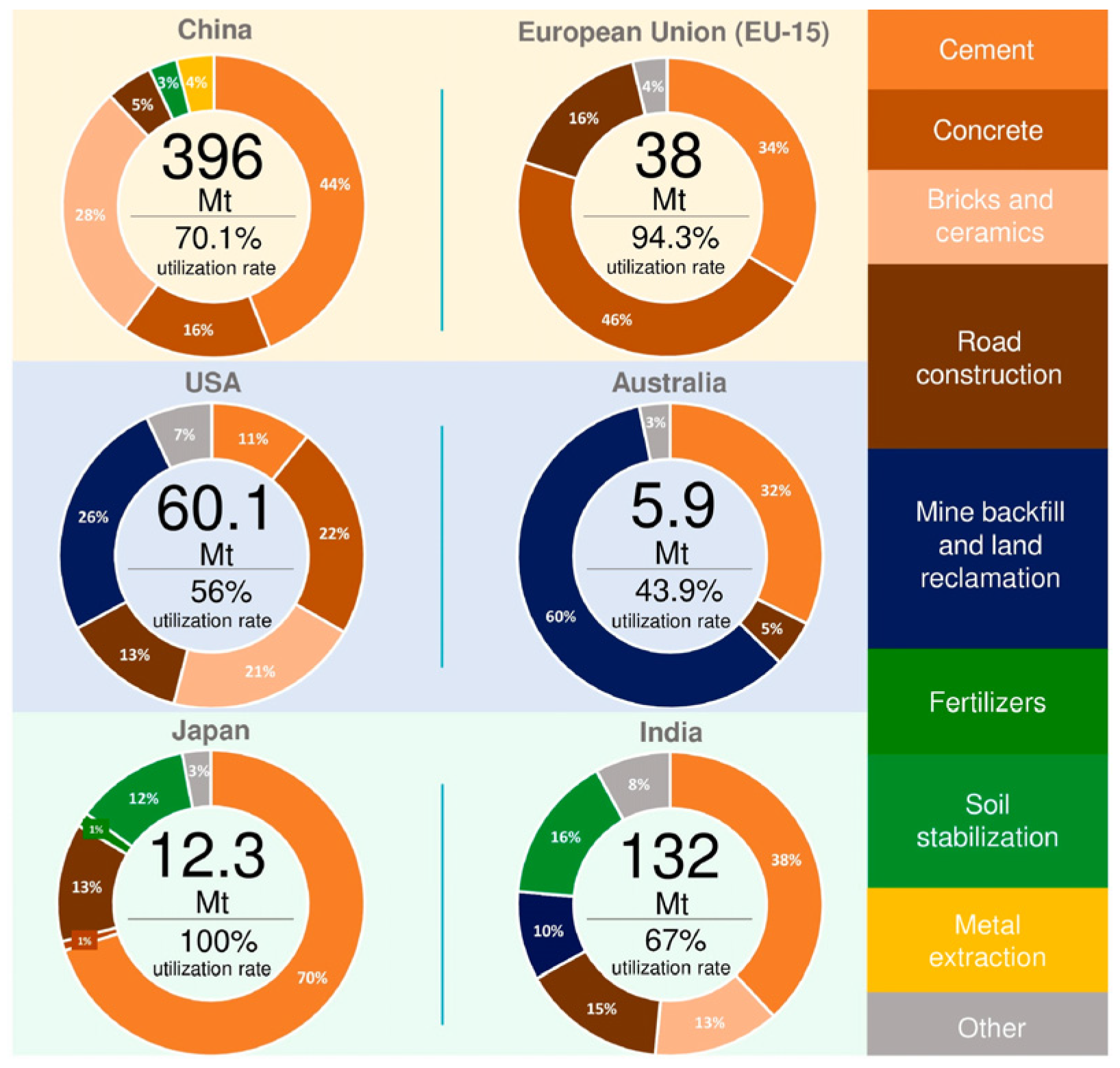
4. Management Policy of CFA
5. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Supelano, G.I.; Cuaspud, J.A.G.; Moreno-Aldana, L.C.; Ortiz, C.; Trujillo, C.A.; Palacio, C.A.; Vargas, C.A.P.; Gómez, J.A.M. Synthesis of magnetic zeolites from recycled fly ash for adsorption of methylene blue. Fuel 2020, 263, 116800. [Google Scholar] [CrossRef]
- Yu, X.; Cui, Y.; Chen, Y.; Chang, I.-S.; Wu, J. The drivers of collaborative innovation of the comprehensive utilization technologies of coal fly ash in China: A network analysis. Environ. Sci. Pollut. Res. Int. 2022, 29, 56291–56308. [Google Scholar] [CrossRef]
- Khairuddin, N.W.A.; Zahari, A.K.; Phillip, E.; Sujan, M.F. Coal Power Plant Fly Ash Characterization Assessment for Geopolymerization Process. Key Eng. Mater. 2022, 908, 678–684. [Google Scholar] [CrossRef]
- Shi, Y.; Jiang, K.-X.; Zhang, T.-A. Cleaner extraction of alumina from coal fly ash: Baking-electrolysis method. Fuel 2020, 273, 117697. [Google Scholar] [CrossRef]
- Qi, X.; Wu, M.; Zheng, J.; Chen, Q.; Chai, L. Rapid identification of reactivity for the efficient recycling of coal fly ash: Hybrid machine learning modeling and interpretation. J. Clean. Prod. 2022, 343, 130958. [Google Scholar] [CrossRef]
- Yao, Z.T.; Ji, X.S.; Sarker, P.K.; Tang, J.H.; Ge, L.Q.; Xia, M.S.; Xi, Y.Q. A comprehensive review on the applications of coal fly ash. Earth-Sci. Rev. 2015, 141, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Basu, M.; Pande, M.; Bhadoria, P.B.S.; Mahapatra, S.C. Potential fly-ash utilization in agriculture: A global review. Prog. Nat. Sci. 2009, 19, 1173–1186. [Google Scholar] [CrossRef]
- Gupta, D.K.; Rai, U.N.; Tripathi, R.D.; Inouhe, M. Impacts of fly-ash on soil and plant responses. J. Plant Res. 2002, 115, 401–409. [Google Scholar] [CrossRef]
- Kaur, R.; Goyal, D. Mineralogical Studies of Coal Fly Ash for Soil Application in Agriculture. Particul. Sci. Technol. 2015, 33, 76–80. [Google Scholar] [CrossRef]
- Bhattacharya, T.; Pandey, S.K.; Pandey, V.C.; Kumar, A. Potential and safe utilization of Fly ash as fertilizer for Pisum sativum L. Grown in phytoremediated and non-phytoremediated amendments. Environ. Sci. Pollut. Res. Int. 2021, 28, 50153–50166. [Google Scholar] [CrossRef]
- Kumar, K.; Kumar, A. A case study of fly ash utilization for enhancement of growth and yield of cowpea (Vigna unguiculata L.) to sustainable agriculture. Biomass Conv. Bioref. 2021, 11, 1–14. [Google Scholar] [CrossRef]
- Khan, I.; Umar, R. Environmental risk assessment of coal fly ash on soil and groundwater quality, Aligarh, India. Groundw. Sustain. Dev. 2019, 8, 346–357. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, Z.; Zhao, Q.; Song, R.; Liu, J. Mechanical properties and microstructure of binding material using slag-fly ash synergistically activated by wet-basis soda residue-carbide slag. Constr. Build. Mater. 2020, 269, 121301. [Google Scholar] [CrossRef]
- Huang, X.; Li, J.-S.; Xue, Q.; Chen, Z.; Du, Y.-J.; Wan, Y.; Liu, L.; Poon, C.S. Use of self-hardening slurry for trench cutoff wall: A review. Constr. Build. Mater. 2021, 286, 122959. [Google Scholar] [CrossRef]
- Eliche-Quesada, D.; Sandalio-Pérez, J.A.; Martínez-Martínez, S.; Pérez-Villarejo, L.; Sánchez-Soto, P.J. Investigation of use of coal fly ash in eco-friendly construction materials: Fired clay bricks and silica-calcareous non fired bricks. Ceram. Int. 2018, 44, 4400–4412. [Google Scholar] [CrossRef]
- Rafieizonooz, M.; Mirza, J.; Salim, M.R.; Hussin, M.W.; Khankhaje, E. Investigation of coal bottom ash and fly ash in concrete as replacement for sand and cement. Constr. Build. Mater. 2016, 116, 15–24. [Google Scholar] [CrossRef]
- Prasad, B.V.; Anand, N.; Arumairaj, P.D.; Kumar, M.S.; Dhilip, D.; Srikanth, G. Studies on Mechanical properties of High Calcium Fly ash based sustainable Geopolymer concrete. J. Phys. Conf. Ser. 2021, 2070, 012184. [Google Scholar] [CrossRef]
- Valeev, D.; Bobylev, P.; Osokin, N.; Zolotova, I.; Rodionov, I.; Salazar-Concha, C.; Verichev, K. A review of the alumina production from coal fly ash, with a focus in Russia. J. Clean. Prod. 2022, 363, 132360. [Google Scholar] [CrossRef]
- Shaheen, S.M.; Hooda, P.S.; Tsadilas, C.D. Opportunities and challenges in the use of coal fly ash for soil improvements—A review. Environ. Manag. 2014, 145, 49–267. [Google Scholar] [CrossRef] [Green Version]
- Palomo, A.; Fernández-Jiménez, A.; Kovalchuk, G.; Ordoñez, L.M.; Naranjo, M.C. Opc-fly ash cementitious systems: Study of gel binders produced during alkaline hydration. J. Mater. Sci. 2007, 42, 2958–2966. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Donatello, S.; Fernández-Jiménez, A.; Palomo, Á. Hydration of Hybrid Alkaline Cement Containing a Very Large Proportion of Fly Ash: A Descriptive Model. Materials 2016, 9, 605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Xu, G.; Shi, X. Reactivity of coal fly ash used in cementitious binder systems: A state-of-the-art overview. Fuel 2021, 301, 121031. [Google Scholar] [CrossRef]
- Kinomura, K.; Ishida, T. Enhanced hydration model of fly ash in blended cement and application of extensive modeling for continuous hydration to pozzolanic micro-pore structures. Cem. Concr. Comp. 2020, 114, 103733. [Google Scholar] [CrossRef]
- Liu, X.; Ni, C.; Meng, K.; Zhang, L.; Liu, D.; Sun, L. Strengthening mechanism of lightweight cellular concrete filled with fly ash. Constr. Build. Mater. 2020, 251, 118954. [Google Scholar] [CrossRef]
- ASTM C618-19; Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete. ASTM International: West Conshohocken, PA, USA, 2019.
- Gollakota, A.R.K.; Volli, V.; Shu, C.-M. Progressive utilisation prospects of coal fly ash: A review. Sci. Total Environ. 2019, 672, 951–989. [Google Scholar] [CrossRef]
- GB/T 1596-2017; Fly Ash Used for Cement and Concrete. Standardization Administration of the People’s Republic of China: Beijing, China, 2017.
- Zhang, J.; Su, P.; Wen, K.; Li, Y.; Li, L. Mechanical Performance and Environmental Effect of Coal Fly Ash on MICP-Induced Soil Improvement. KSCE J. Civ. Eng. 2020, 24, 3189–3201. [Google Scholar] [CrossRef]
- Zhao, L.; Dai, S.; Finkelman, R.B.; French, D.; Graham, I.T.; Yang, Y.; Li, J.; Yang, P. Leaching behavior of trace elements from fly ashes of five Chinese coal power plants. Int. J. Coal Geol. 2020, 219, 103381. [Google Scholar] [CrossRef]
- Gupta, N.; Gedam, V.V.; Moghe, C.; Labhasetwar, P. Investigation of characteristics and leaching behavior of coal fly ash, coal fly ash bricks and clay bricks. Environ. Technol. Innov. 2017, 7, 152–159. [Google Scholar] [CrossRef]
- Longos, A.J.; Tigue, A.A.; Dollente, I.J.; Malenab, R.A.; Bernardo-Arugay, I.; Hinode, H.; Kurniawan, W.; Promentilla, M.A. Optimization of the Mix Formulation of Geopolymer Using Nickel-Laterite Mine Waste and Coal Fly Ash. Minerals 2020, 10, 1144. [Google Scholar] [CrossRef]
- Lu, X.; Li, L.Y.; Wang, F.; Wang, L.; Zhang, X. Radiological hazards of coal and ash samples collected from Xi’an coal-fired power plants of China. Environ. Earth Sci. 2012, 66, 1925–1932. [Google Scholar] [CrossRef]
- Sanjuán, M.Á.; Suarez-Navarro, J.A.; Argiz, C.; Estévez, E. Radiation dose calculation of fine and coarse coal fly ash used for building purposes. J. Radioanal. Nucl. Chem. 2021, 327, 1045–1054. [Google Scholar] [CrossRef]
- Singh, L.M.; Sing, K.Y.; Mahur, A.K. Study of environmental radioactivity and radon measurement associated health effect due to coal and fly ash samples. IOP Conf. Ser. Earth Environ. Sci. 2021, 822, 012026. [Google Scholar] [CrossRef]
- Mahur, A.K.; Kumar, R.; Mishra, M.; Sengupta, D.; Prasad, R. An investigation of radon exhalation rate and estimation of radiation doses in coal and fly ash samples. Appl. Radiat. Isotopes. 2008, 66, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wen, K.; Li, L. Bio-modification of coal fly ash using urease-producing bacteria. Fuel 2021, 286, 119386. [Google Scholar] [CrossRef]
- Turner, L.K.; Collins, F.G. Carbon dioxide equivalent (CO2-e) emissions: A comparison between geopolymer and OPC cement concrete. Constr. Build. Mater. 2013, 43, 125–130. [Google Scholar] [CrossRef]
- Prakasan, S.; Palaniappan, S.; Gettu, R. Study of Energy Use and CO2 Emission s in the Manufacturing of Clinker and Cement. J. Inst. Eng. India Ser. A 2020, 101, 221–232. [Google Scholar] [CrossRef]
- Agrawal, V.M.; Savoikar, P.P. Sustainable use of normal and ultra-fine fly ash in mortar as partial replacement to ordinary Portland cement in ternary combinations. Mater. Today Proc. 2022, 51, 1593–1597. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Li, Z.; Ren, Y.; Wang, Y.; Zhang, W. Preparation, characterization and application of red mud, fly ash and desulfurized gypsum based eco-friendly road base materials. J. Clean. Prod. 2021, 284, 124777. [Google Scholar] [CrossRef]
- Chethan, B.A.; Shankar, A.U.R. Effect of Flash Flood and Weather Changes on Unconfined Compressive Strength of Cement- and Fly Ash-Stabilized Black Cotton Soil Used as Road Materials. Int. J. Pavement Res. Technol. 2021, 14, 1–17. [Google Scholar] [CrossRef]
- Elmrabet, R.; Harfi, A.E.; Youbi, M.S.E. Study of properties of fly ash cements. Mater. Today Proc. 2019, 13, 850–856. [Google Scholar] [CrossRef]
- Hanehara, S.; Tomosawa, F.; Kobayakawa, M.; Hwang, K. Effects of water/powder ratio, mixing ratio of fly ash, and curing temperature on pozzolanic reaction of fly ash in cement paste. Cem. Concr. Res. 2001, 31, 31–39. [Google Scholar] [CrossRef]
- Wang, Q.; Feng, J.J.; Yan, P.Y. An explanation for the negative effect of elevated temperature at early ages on the late-age strength of concrete. J. Mater. Sci. 2011, 46, 7279–7288. [Google Scholar] [CrossRef]
- Kong, Y.; Wang, P.; Liu, S. Compressive strength development and hydration of cement–fly ash composite treated with microwave irradiation. J. Therm. Anal. Calorim. 2019, 138, 123–133. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, S.; Yang, L.; Ding, Y. Microwave curing cement-fly ash blended paste. Constr. Build. Mater. 2021, 282, 122685. [Google Scholar] [CrossRef]
- Wang, J.; Liu, M.; Wang, Y.; Zhou, Z.; Xu, D.; Du, P.; Cheng, X. Synergistic effects of nano-silica and fly ash on properties of cement-based composites. Constr. Build. Mater. 2020, 262, 120737. [Google Scholar] [CrossRef]
- Huang, Q.; Tang, S.; You, Y.; Chen, Y.; Deng, H.; Tian, R. Reduction of heavy metals leaching and pore volume in high-volume fly ash cement pastes by adding nano-SiO2. Environ. Sci. Pollut. Res. 2020, 27, 23369–23373. [Google Scholar] [CrossRef]
- Sun, X.; Zhao, Y.; Tian, Y.; Wu, P.; Guo, Z.; Qiu, J.; Xing, J.; Xiaowei, G. Modification of high-volume fly ash cement with metakaolin for its utilization in cemented paste backfill: The effects of metakaolin content and particle size. Powder Technol. 2021, 393, 539–549. [Google Scholar] [CrossRef]
- Davis, R.E.; Carlson, R.W.; Kelly, J.W.; Davis, H.E. Properties of cements and concretes containing fly ash. J. Proc. 1937, 33, 577–612. [Google Scholar]
- Behl, V.; Singh, V.; Dahiya, V.; Kumar, A. Characterization of physico-chemical and functional properties of fly ash concrete mix. Mater. Today Proc. 2022, 50, 941–945. [Google Scholar] [CrossRef]
- Saha, A.K. Effect of class F fly ash on the durability properties of concrete. Sustain. Environ. Res. 2018, 28, 25–31. [Google Scholar] [CrossRef]
- Uthaman, S.; Vishwakarma, V.; George, R.P.; Ramachandran, D.; Kumari, K.; Preetha, R.; Premila, M.; Rajaraman, R.; Mudali, U.K.; Amarendra, G. Enhancement of strength and durability of fly ash concrete in seawater environments: Synergistic effect of nanoparticles. Constr. Build. Mater. 2018, 187, 448–459. [Google Scholar] [CrossRef]
- Matos, P.R.d.; Junckes, R.; Graeff, E.; Jr, L.R.P. Effectiveness of fly ash in reducing the hydration heat release of mass concrete. J. Build. Eng. 2020, 28, 101063. [Google Scholar] [CrossRef]
- Kumar, M.H.; Mohanta, N.R.; Patel, N.; Samantaray, S.; Reddy, S.V.B. Impact of Fly Ash and Metakaoline on the Crack Resistance and Shrinkage of Concrete. Iran. J. Sci. Technol. Trans. Civ. Eng. 2022, 4, 2011–2026. [Google Scholar] [CrossRef]
- Nagrockienė, D.; Rutkauskas, A. The effect of fly ash additive on the resistance of concrete to alkali silica reaction. Constr. Build. Mater. 2019, 201, 599–609. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, C.; Tan, Y.; Wang, Y.; Li, Q. Chloride binding capacity of green concrete mixed with fly ash or coal gangue in the marine environment. Constr. Build. Mater. 2020, 242, 118006. [Google Scholar] [CrossRef]
- Mao, M.; Ai, Q.; Zhang, D.; Li, S.; Li, J. Durability Performance of Concrete with Fly Ash as Fine Aggregate Eroded by Chloride Salt. Adv. Mater. Sci. Eng. 2022, 2022, 6760385. [Google Scholar] [CrossRef]
- Swathi, V.; Asadi, S. An experimental investigation on mechanical, durability and Microstructural Properties of high-volume fly ash based concrete. J. Build. Rehabil. 2022, 7, 1–10. [Google Scholar] [CrossRef]
- Chahal, N.; Siddique, R.; Rajor, A. Influence of bacteria on the compressive strength, water absorption and rapid chloride permeability of fly ash concrete. Constr. Build. Mater. 2012, 28, 351–356. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, J.; Cao, Q.; Gao, X. Effect of calcium formate on the compressive strength, and hydration process of cement composite containing fly ash and slag. J. Build. Eng. 2022, 50, 104133. [Google Scholar] [CrossRef]
- Herath, C.; Gunasekara, C.; Law, D.W.; Setunge, S. Long term mechanical performance of nano-engineered high volume fly ash concrete. J. Build. Eng. 2021, 43, 103168. [Google Scholar] [CrossRef]
- Kumar, M.; Sinha, A.K.; Kujur, J. Mechanical and Durability Studies on High-volume fly-ash concrete. Struct. Concr. 2020, 22, e1036–e1049. [Google Scholar] [CrossRef]
- Shaikh, F.U.A.; Supit, S.W.M.; Sarker, P.K. A study on the effect of nano silica on compressive strength of high volume fly ash mortars and concretes. Mater. Design 2014, 60, 433–442. [Google Scholar] [CrossRef]
- Zhang, P.; Sha, D.; Li, Q.; Zhao, S.; Ling, Y. Effect of Nano Silica Particles on Impact Resistance and Durability of Concrete Containing Coal Fly Ash. Nanomaterials 2021, 11, 1296. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Shi, J.; He, Z.; Zhang, B.; Peng, Y.; Lu, J. Evaluation of high-volume fly ash (HVFA) concrete modified by metakaolin: Technical, economic and environmental analysis. Powder Technol. 2022, 397, 117121. [Google Scholar] [CrossRef]
- Ghafoor, M.T.; Khan, Q.S.; Qazi, A.U.; Sheikh, M.N.; Hadi, M.N.S. Influence of alkaline activators on the mechanical properties of fly ash based geopolymer concrete cured at ambient temperature. Constr. Build. Mater. 2021, 273, 121752. [Google Scholar] [CrossRef]
- Huseien, G.F.; Shah, K.W. Durability and life cycle evaluation of self-compacting concrete containing fly ash as GBFS replacement with alkali activation. Constr. Build. Mater. 2020, 235, 117458. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, H.; Yu, T.; Yuan, P.; Deng, L.; Zhang, B. Utilization of Calcium Carbide Residue as Solid Alkali for Preparing Fly Ash-Based Geopolymers: Dependence of Compressive Strength and Microstructure on Calcium Carbide Residue, Water Content and Curing Temperature. Materials 2022, 15, 973. [Google Scholar] [CrossRef]
- Ahmad, M.; Rashid, K.; Hameed, R.; Haq, E.U.; Farooq, H.; Ju, M. Physico-mechanical performance of fly ash based geopolymer brick: Influence of pressure- Temperature- Time. J. Build. Eng. 2022, 50, 104161. [Google Scholar] [CrossRef]
- Ahmad, M.; Rashid, K. Novel approach to synthesize clay-based geopolymer brick: Optimizing molding pressure and precursors’ proportioning. Constr. Build. Mater. 2022, 322, 126472. [Google Scholar] [CrossRef]
- Cai, J.; Jiang, J.; Gao, X.; Ding, M. Improving the Mechanical Properties of Fly Ash-Based Geopolymer Composites with PVA Fiber and Powder. Materials 2022, 15, 2363. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, K.; Wang, J.; Guo, J.; Ling, Y. Macroscopic and microscopic analyses on mechanical performance of metakaolin/fly ash based geopolymer mortar. J. Clean. Prod. 2021, 294, 126193. [Google Scholar] [CrossRef]
- Jiang, F.; Qi, Z.; Cang, D.; Zhang, L.; Jin, Y. Recycling coal fly ash rich in CaO to prepare novel ceramics: Role of alkali activation. Mater. Lett. 2022, 311, 131548. [Google Scholar] [CrossRef]
- Luo, Y.; Wu, Y.-h.; Ma, S.-h.; Zheng, S.-l.; Chu, P.K. An eco-friendly and cleaner process for preparing architectural ceramics from coal fly ash: Pre-activation of coal fly ash by a mechanochemical method. J. Clean. Prod. 2019, 214, 419–428. [Google Scholar] [CrossRef]
- Tabit, K.; Hajjou, H.; Waqif, M.; Saâdi, L. Effect of CaO/SiO2 Ratio On Phase Transformation and Properties of Anorthite-based Ceramics From Coal Fly Ash and Steel Slag. J. Technol. Sci. 2020, 46, 7550–7558. [Google Scholar] [CrossRef]
- Nguyen, M.; Sokolář, R. Corrosion Resistance of Novel Fly Ash-Based Forsterite-Spinel Refractory Ceramics. Materials 2022, 15, 1363. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, H.; Zhang, Y.; Liang, D.; Chen, H. Recycle coal fly ash for preparing tubular ceramic membranes applied in transport membrane condenser. Sep. Purif. Technol. 2022, 282, 119972. [Google Scholar] [CrossRef]
- Wang, S.; Wang, H.; Chen, Z.; Ji, R.; Liu, L.; Wang, X. Fabrication and characterization of porous cordierite ceramics prepared from fly ash and natural minerals. Ceram. Int. 2019, 45, 18306–18314. [Google Scholar] [CrossRef]
- Fu, M.; Liu, J.; Dong, X.; Zhu, L.; Dong, Y.; Hampshire, S. Waste recycling of coal fly ash for design of highly porous whisker-structured mullite ceramic membranes. J. Eur. Ceram. Soc. 2019, 39, 5320–5331. [Google Scholar] [CrossRef]
- Li, C.; Zhou, Y.; Tian, Y.; Zhao, Y.; Wang, K.; Li, G.; Chai, Y. Preparation and characterization of mullite whisker reinforced ceramics made from coal fly ash. Ceram. Int. 2019, 45, 5613–5616. [Google Scholar] [CrossRef]
- Fan, W.; Zou, D.; Xu, J.; Chen, X.; Qiu, M.; Fan, Y. Enhanced Performance of Fly Ash-Based Supports for Low-Cost Ceramic Membranes with the Addition of Bauxite. Membranes 2021, 11, 711. [Google Scholar] [CrossRef]
- Jagadeep, R.; Vignesh, R.V.; Sumanth, P.; Sarathi, V.; Govindaraju, M. Fabrication of fly-ash based tiles using liquid phase sintering technology. Mater. Proc. 2021, 46, 7224–7229. [Google Scholar] [CrossRef]
- Krasnyi, B.L.; Ikonnikov, K.I.; Lemeshev, D.O.; Galganova, A.L.; Sizova, A.S. Investigation of the Possibility of Using Light Aluminosilicate Components of Fly Ash for the Production of Refractory Heat-Insulating Materials. Glass Ceram. 2021, 78, 323–327. [Google Scholar] [CrossRef]
- Wan, Z.; Sang, S.; Ma, Y.; Zhu, T. Preparation of high strength and low thermal conductivity mullite refractories based on reconstruction of fly ash. Int. J. Appl. Ceram. Tec. 2022, 19, 2749–2760. [Google Scholar] [CrossRef]
- Payakaniti, P.; Chuewangkam, N.; Yensano, R.; Pinitsoontorn, S.; Chindaprasirt, P. Changes in compressive strength, micro structure and magnetic properties of a high-calcium fly ash geopolymer subjected to high temperatures. Constr. Build. Mater. 2020, 265, 120650. [Google Scholar] [CrossRef]
- Liu, F.; Tang, R.; Wang, B.; Yuan, X. Experimental Study on Solidification of Pb2+ in Fly Ash-Based Geopolymers. Sustainability 2021, 13, 12621. [Google Scholar] [CrossRef]
- Fan, C.; Wang, B.; Ai, H.; Qi, Y.; Liu, Z. A comparative study on solidification/stabilization characteristics of coal fly ash-based geopolymer and Portland cement on heavy metals in MSWI fly ash. J. Clean. Prod. 2021, 319, 128790. [Google Scholar] [CrossRef]
- Li, X.; Bai, C.; Qiao, Y.; Wang, X.; Yang, K.; Colombo, P. Preparation, properties and applications of fly ash-based porous geopolymers: A review. J. Clean. Prod. 2022, 359, 132043. [Google Scholar] [CrossRef]
- Li, S.; Huang, X.; Muhammad, F.; Yu, L.; Xia, M.; Zhao, J.; Jiao, B.; Shiau, Y.; Li, D. Waste solidification/stabilization of lead–zinc slag by utilizing fly ash based geopolymers. Rsc. Adv. 2018, 8, 32956–32965. [Google Scholar] [CrossRef] [Green Version]
- Murmu, A.L.; Dhole, N.; Patel, A. Stabilisation of black cotton soil for subgrade application using fly ash geopolymer. Road Mate. Pavement 2020, 21, 867–885. [Google Scholar] [CrossRef]
- Lin, W.Y.; Prabhakar, A.K.; Mohan, B.C.; Wang, C.-H. A factorial experimental analysis of using wood fly ash as an alkaline activator along with coal fly ash for production of geopolymer-cementitious hybrids. Sci. Total Environ. 2020, 718, 135289. [Google Scholar] [CrossRef]
- Shao, N.; Wei, X.; Monasterio, M.; Dong, Z.; Zhang, Z. Performance and mechanism of mold-pressing alkali-activated material from MSWI fly ash for its heavy metals solidification. Waste Manag. 2021, 126, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, N.; Mehrali, M.; Maheri, M.; Mehrali, M. Hot-pressed geopolymer. Cem. Concr. Res. 2017, 100, 14–22. [Google Scholar] [CrossRef]
- Alomayri, T. Performance evaluation of basalt fiber-reinforced geopolymer composites with various contents of nano CaCO3. Ceram. Int. 2021, 47, 29949–29959. [Google Scholar] [CrossRef]
- Ouni, M.H.E.; Raza, A.; Haider, H.; Arshad, M.; Ali, B. Enhancement of mechanical and toughness properties of carbon fiber-reinforced geopolymer pastes comprising nano calcium oxide. J. Aust. Ceram. Soc. 2022, 58, 1375–1387. [Google Scholar] [CrossRef]
- Lees, H.; Järvik, O.; Konist, A.; Siirde, A.; Maaten, B. Comparison of the ecotoxic properties of oil shale industry by-products to those of coal ash. Oil Shale 2022, 39, 1–19. [Google Scholar] [CrossRef]
- Prasetia, I.; Syauqi, M.; Aini, A.S. Application of central kalimantan coal ash as a sustainable construction material. IOP Conf. Ser. Earth Environ. Sci. 2021, 758, 12011. [Google Scholar] [CrossRef]
- [1994] No. 14; Administrative Measures for the Comprehensive Utilization of Coal Fly Ash. State Economic and Trade Commission of the People’s Republic of China: Beijing, China, 1994.
- [2021] No. 40; Announcement on Improving the Value Added Tax Policy. Ministry of Finance of the People’s Republic of China; State Taxation Administration of the People’s Republic of China: Beijing, China, 2021.
- Act No. 97 of June 10, 1968; Air Pollution Control Act. Ministry of the Environment Government of Japan: Tokyo, Japan, 1968.
- Law No.91 of 1993; The Basic Environment Law. Ministry of the Environment Government of Japan: Tokyo, Japan, 1993.
- [1991] No. 48; Resource Active Utilization Promotion Law. Ministry of Economy, Trade and Industry of Japan: Tokyo, Japan, 1991.
| Chemical Compound (%) | Class (America) | Class (China) | |||
|---|---|---|---|---|---|
| N | F | C | F | C | |
| SiO2 + Al2O3 + Fe2O3, min | 70 | 50 | 50 | - | - |
| CaO | Negligible (report only) | 18 (maximum) | >18 | <10 | ≥10 |
| SO3, max | 4 | 5 | 5 | - | - |
| Moisture, max | 3 | 3 | 3 | - | - |
| LOI, max | 10 | 6A | 6 | - | - |
| No. | Cd | Cr | Pb | Ba | Hg | As | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | n.d. | n.d. | n.d. | 0.01 | n.d. | n.d. | [28] |
| 2 | n.d. | n.d. | n.d. | 0.03 | n.d. | n.d. | |
| 3 | <0.01 | <0.01 | n.d. | 3.8–4.7 | <0.00001 | <0.01 | [29] |
| 4 | <0.01 | <0.001 | n.d. | 0.5–0.9 | <0.000001 | <0.1 | |
| 5 | <0.01 | <0.001 | n.d. | 0.7–0.9 | <0.1 | <0.00001 | |
| 6 | <0.01 | <0.01 | n.d. | 0.2–0.3 | <0.1 | <0.00001 | |
| 7 | <0.01 | <0.01 | n.d. | 3.3–3.9 | <0.00001 | <0.01 | |
| 8 | <0.0001 | <0.1 | <0.01 | - | n.d. | <0.1 | [30] |
| 9 | <0.0001 | <0.1 | <0.01 | 1.5–1.6 | n.d. | <0.1 | |
| 10 | 0.00042 | 0.035 | 0.027 | 2.544 | 0.00085 | 0.069 | [31] |
| TCLP limit | 1 | 5 | 5 | 100 | 0.2 | 5 | [36] |
| Raw Materials | Sintering Condition | Main Phase | Sample Properties | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| T[°C] | Time[h] | [MPa] 1 | [%] 2 | [g·cm−3] 3 | [%] 4 | |||
| CFA | 1175 | 2 | anorthite, albite | 77.6 ± 2.31 | ~18 | n.r. | 0.42 ± 0.09 | [74] |
| >CFA, Feldspar, High plastic clay | >1100 | 1 | mullite | 32.58 | 11.25 | 2.26 | 0.89 | [75] |
| CFA, ladle furnace slag | 1188 | - | anorthite | - | - | 2.49 | - | [76] |
| CFA, CCM, Olivine, Talc, Kaolin | 1550 | 2 | Forsterite, Spinel | 15.5–17.3 | 5.9– 11.3 | 2.365–2.510 | 16.3– 24.2 | [77] |
| CFA, dextrin, carboxymethy cellulose | 1200 | 2 | mullite, anorthite | 29.05 | - | 1.42 | 44.76 | [78] |
| CFA, Quartz, Magnesite | 1300 | 2 | cordierite | 23.92 | - | 1.61 | 33.16 | [79] |
| CFA, Al(OH)3 | 1300 | 2 | mullite | 40.8 ± 1.9 | ~1.6 | ~1.4 | 55.71 ± 0.42 | [80] |
| CFA, Al2O3 | 1200 | 2 | mullite | 59.1 | n.r. | 1.31 | 28.05 | [81] |
| CFA, Bauxite | 1300 | 2 | mullite | 69.6 | ~6.5 | n.r. | ~29 | [82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.; Liu, B.; Zhang, Q.; Wen, Q.; Wang, S.; Xiao, K.; Zhang, S. Recycling of Coal Fly Ash in Building Materials: A Review. Minerals 2023, 13, 25. https://doi.org/10.3390/min13010025
Lu X, Liu B, Zhang Q, Wen Q, Wang S, Xiao K, Zhang S. Recycling of Coal Fly Ash in Building Materials: A Review. Minerals. 2023; 13(1):25. https://doi.org/10.3390/min13010025
Chicago/Turabian StyleLu, Xuhang, Bo Liu, Qian Zhang, Quan Wen, Shuying Wang, Kui Xiao, and Shengen Zhang. 2023. "Recycling of Coal Fly Ash in Building Materials: A Review" Minerals 13, no. 1: 25. https://doi.org/10.3390/min13010025





