A Sustainable Bioleaching of a Low-Grade Chalcopyrite Ore
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ore Samples
2.2. Bacterial Strains and Growth Conditions
2.3. Chalcopyrite Column Leaching
3. Results
3.1. Enrichment of Strain
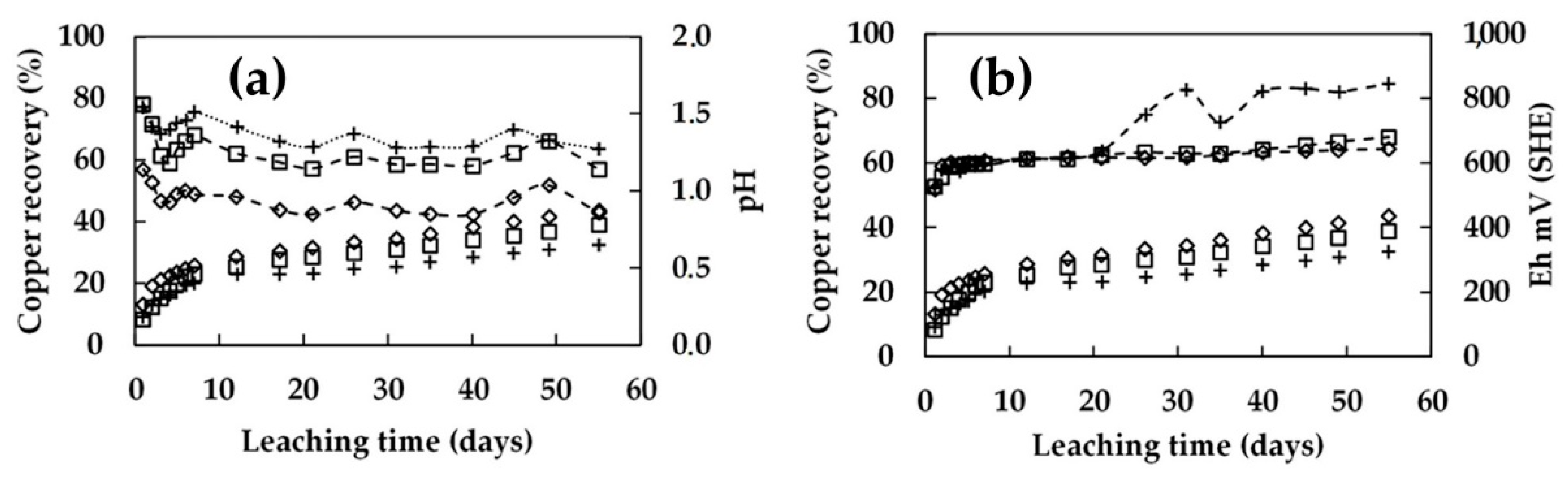
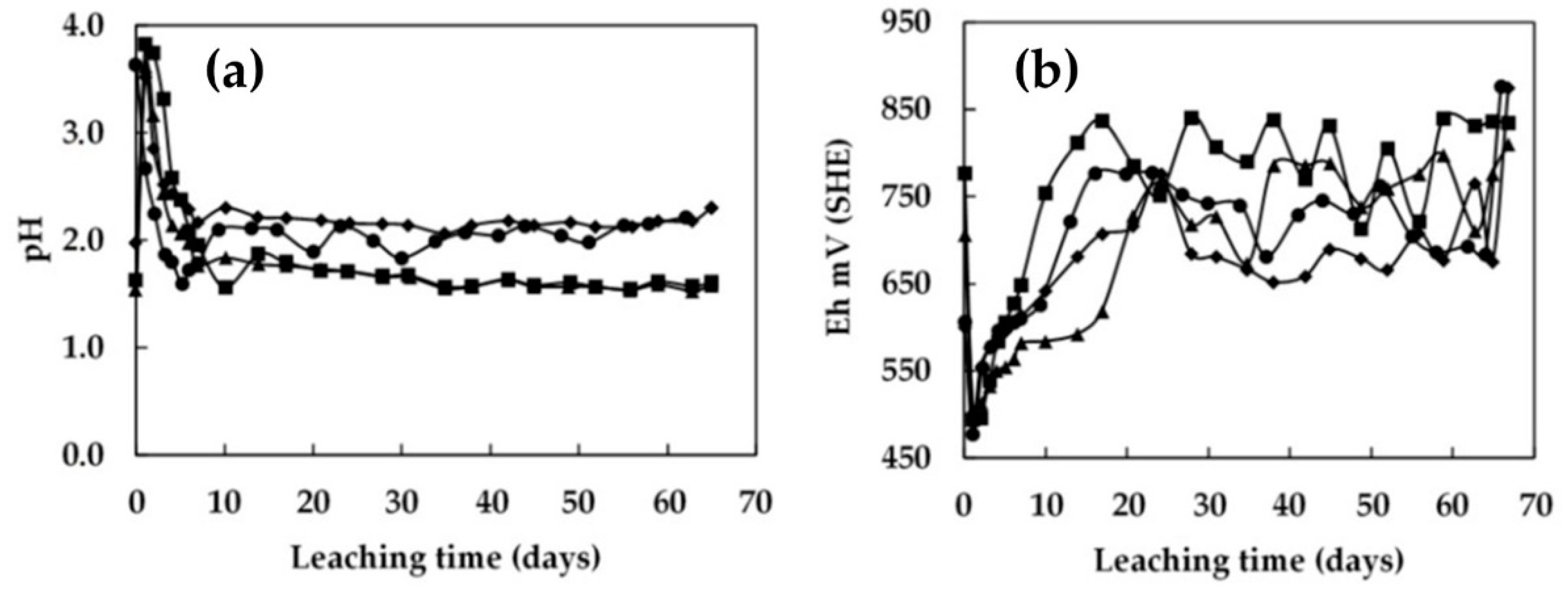
3.2. Leaching in Columns 0.45 m in Height with Continuous Irrigation
3.3. Bioleaching in Columns 0.45 m in Height with Intermittent Irrigation
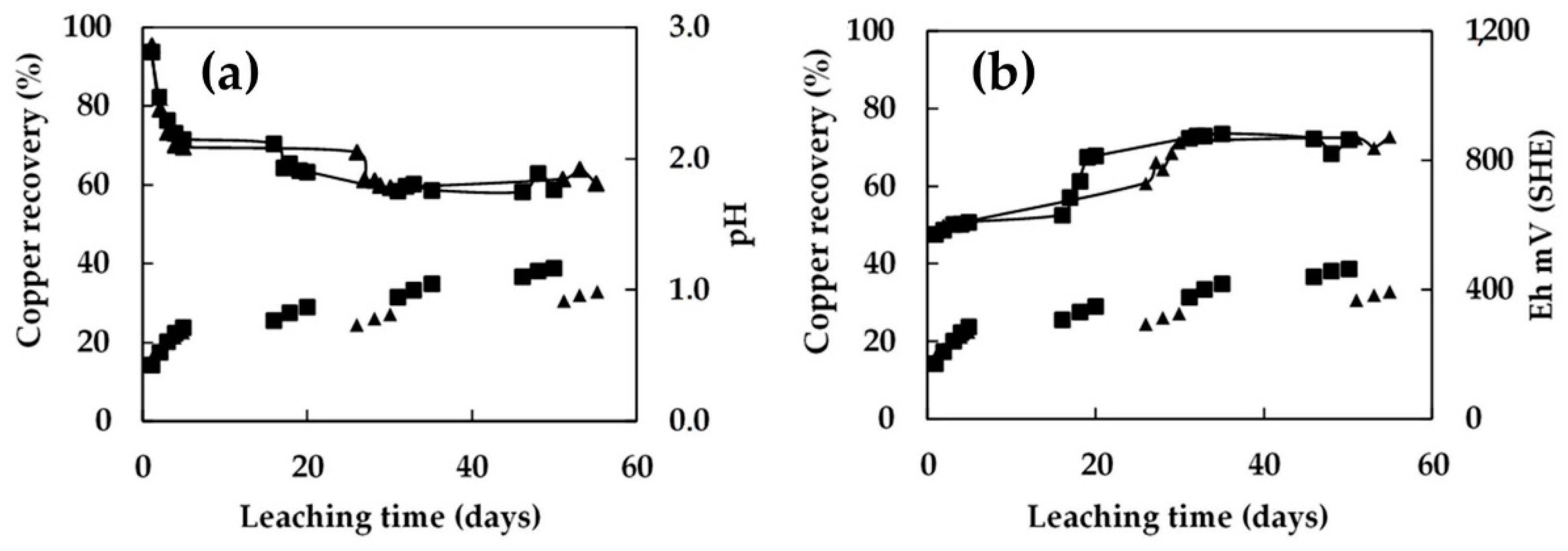
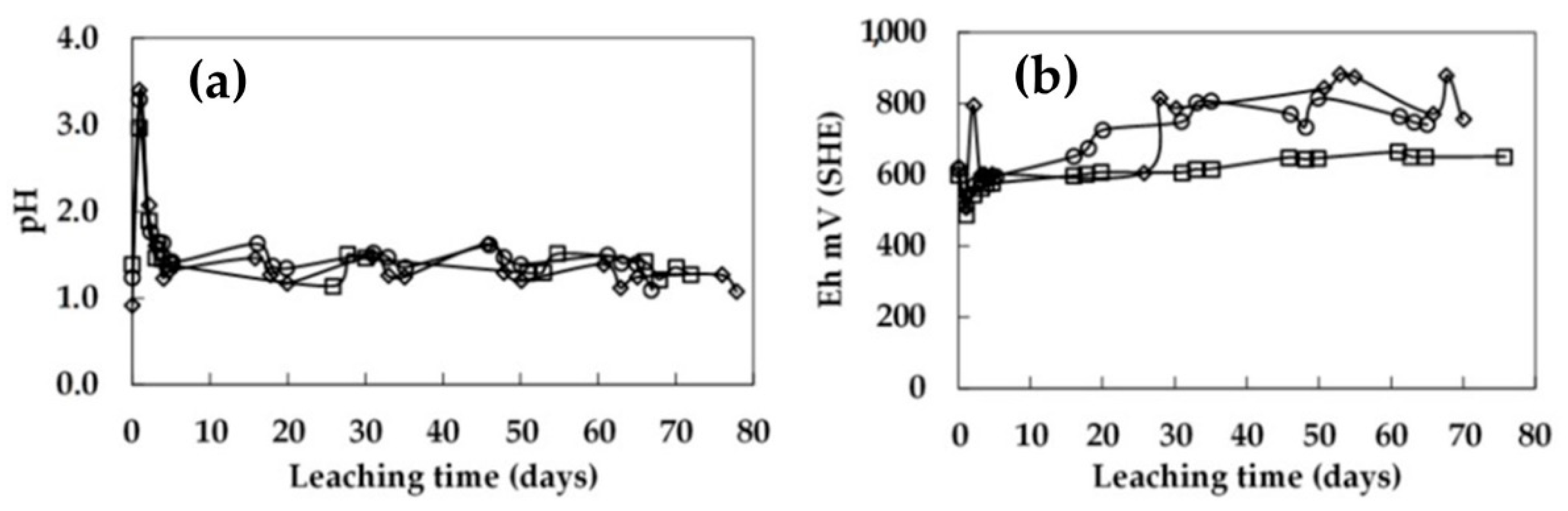
3.4. Bioleaching in Columns 1.0 m in Height with Continuous Irrigation
3.5. Bioleaching in Columns 1.0 m in Height with Intermittent Irrigation
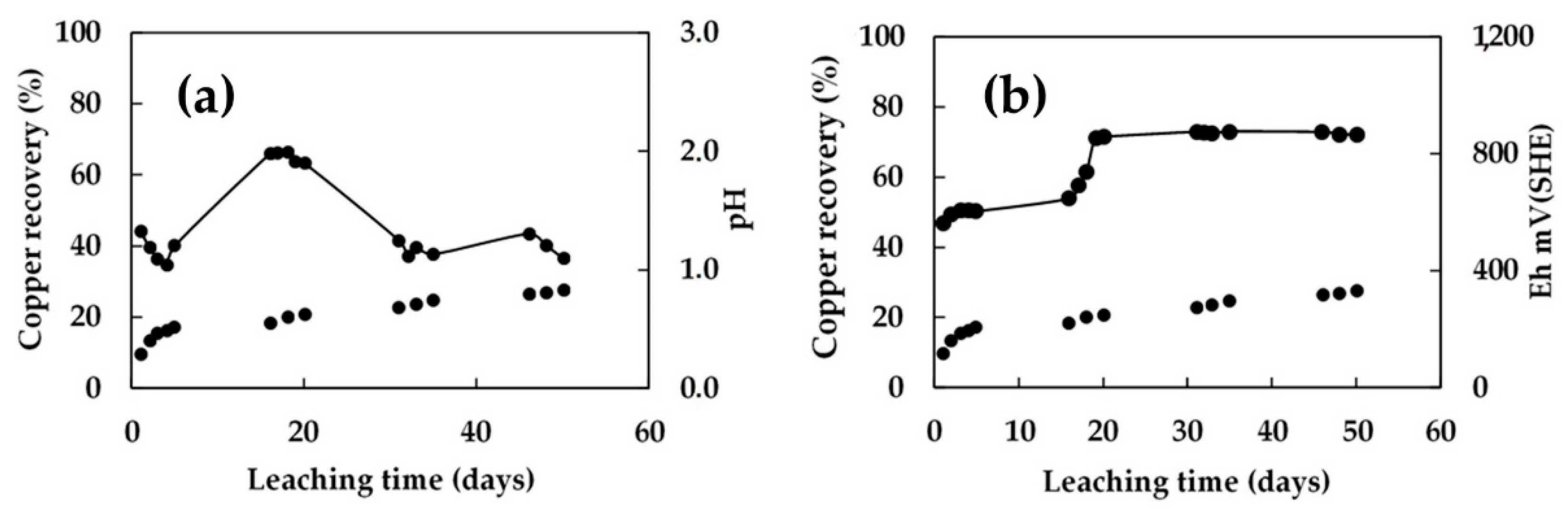
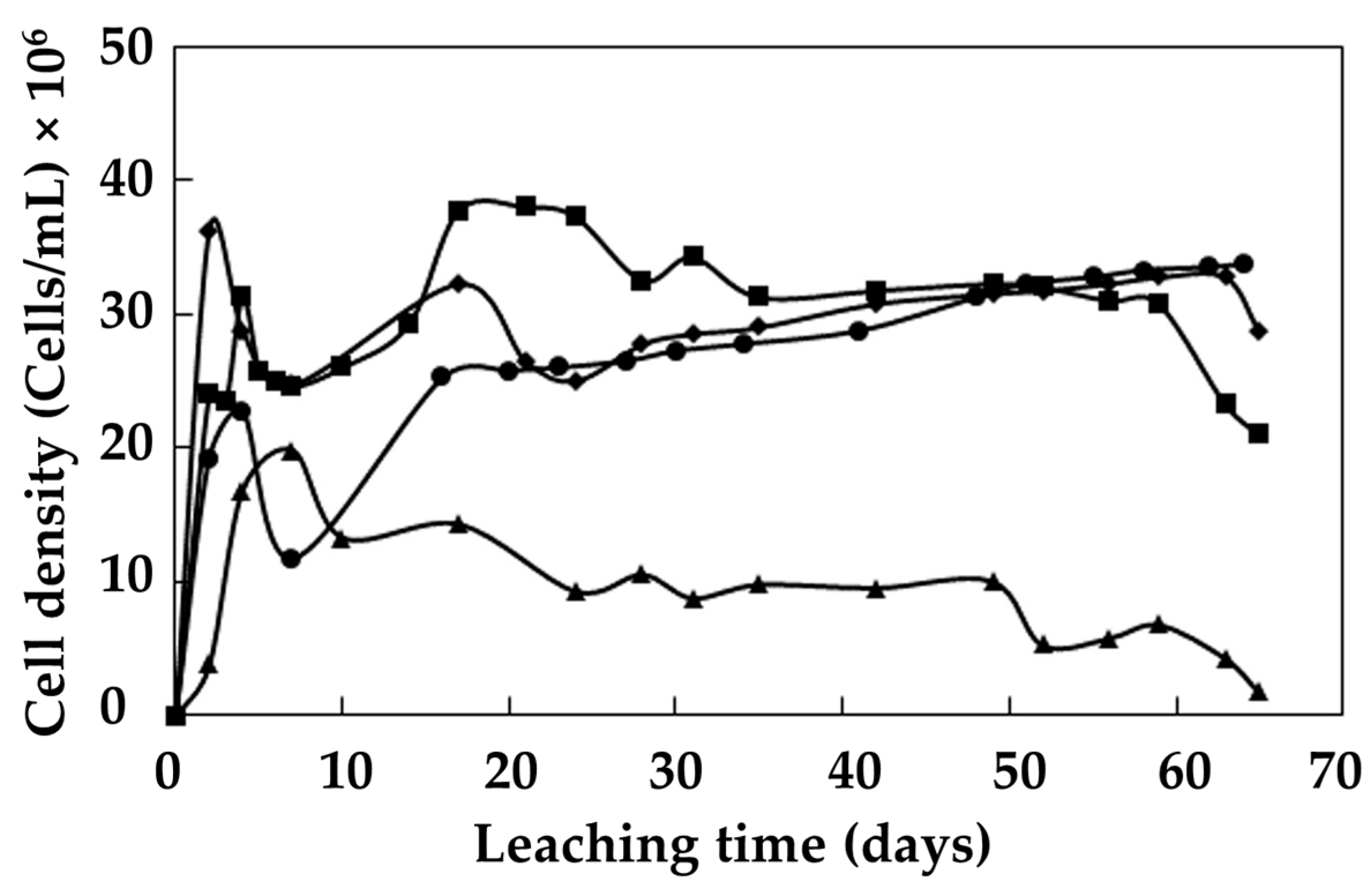
3.6. Evolution of Bacterial Growth
4. Conclusions
- The adaptation stage of an Acidiphilium strain from an ore occurred within the first 20 days after inoculation. Up to 52 days after inoculation, the bacterial population grew continuously, with the microbial density stabilizing at 3.7 × 108 cells/mL. At this point, 60 days after cultivation, a microorganism-enriched solution was obtained.
- The recovery of low-grade chalcopyrite ore was enhanced by increasing the microbial cell density. The addition of 1.5 × 108 cells/mL to the 0.45 m column and 5.0 × 107 cells/mL to the 1 m column resulted in a higher extraction. As a result, the copper dissolution increased from 32% to 44% in the 0.45 m column and from 30% to 40% in the 1.0 m column in 70 days of leaching. Under these conditions, the pH level was stable at around 1.8, and the redox potential was around 840 mV vs. SHE.
- A shorter rest cycle allowed the redox potential to increase during the irrigation. By contrast, this was not observed after long rest cycles, indicating the bioleaching culture became dormant during these long rest periods. It was suggested that continuous irrigation constantly washed the bacteria out of the column, while the rest cycles allowed a better local accumulation of the bacteria.
- It was demonstrated that intermittent irrigation enhanced the dissolution of the chalcopyrite ore, but excessively long rest periods negatively affected chemical and bacterial activity due to a shortage of acid and/or nutrients for the microorganisms. Five days of irrigation followed by ten days of rest period facilitated management of the pH and redox potential levels for the development of bacterial activity. This can be beneficial in an industrial-scale dump-leach operation as reducing the leaching cycle with less irrigation and promoting the continuity of hydrometallurgy plants (LX-SX-EW) would be both more economical and sustainable for a natural microbe-assisted dump-leach operation.
- The bacterial population obtained in the metal-rich solution of the column irrigated using a 5/10-day cycle increased steadily after the resting cycles. In this process, no microorganism adaptation period was observed. The transition stage occurred during the rest period, allowing better management of the pH and redox potential for the development of bacterial activity. The metal-rich solution in the column that received a raffinate solution with pH 1.5 adjusted to 2.0 favored the growth of microorganisms, and was affected in the initial stage by the constant irrigation with solution.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Habashi, F. Chalcopyrite: Its Chemistry and Metallurgy; McGraw-Hill: New York, NY, USA, 1978; Volume 43. [Google Scholar]
- Ghorbani, Y.; Franzidis, J.P.; Petersen, J. Heap leaching technology—Current State, innovations, and future directions: A review. Miner. Process. Extr. Metall. Rev. 2016, 37, 73–119. [Google Scholar] [CrossRef] [Green Version]
- Veloso, T.C.; Peixoto, J.J.M.M.; Pereira, M.S.; Leao, V.A. Kinetics of chalcopyrite leaching in either ferric sulphate or cupric sulphate media in the presence of NaCl. Int. J. Miner. Process. 2016, 148, 147–154. [Google Scholar] [CrossRef]
- Debernardi, G.; Gentina, J.C.; Albistur, P.; Slanzi, G. Evaluation of processing options to avoid the passivation of chalcopyrite. Int. J. Miner. Process. 2013, 125, 1–4. [Google Scholar] [CrossRef]
- Li, Y.; Kawashima, N.; Li, J.; Chandra, A.P.; Gerson, A.R. A review of the structure, and fundamental mechanisms and kinetics of the leaching of chalcopyrite. Adv. Colloid Interface Sci. 2013, 197, 1–32. [Google Scholar] [CrossRef]
- Viramontes-Gamboa, G.; Peña-Gomar, M.M.; Dixon, D.G. Electrochemical hysteresis and bistability in chalcopyrite pas-sivation. Hydrometallurgy 2010, 105, 140–147. [Google Scholar] [CrossRef]
- Córdoba, E.M.; Muñoz, J.A.; Blázquez, M.L.; González, F.; Ballester, A. Leaching of chalcopyrite with ferric ion. Part IV: The role of redox potential in the presence of mesophilic and thermophilic bacteria. Hydrometallurgy 2008, 93, 106–115. [Google Scholar] [CrossRef]
- Velásquez-Yévenes, L.; Quezada-Reyes, V. Influence of seawater and discard brine on the dissolution of copper ore and copper concentrate. Hydrometallurgy 2018, 180, 88–95. [Google Scholar] [CrossRef]
- Nicol, M.J.; Lázaro, I. The role of EH measurements in the interpretation of the kinetics and mechanisms of the oxidation and leaching of sulphide minerals. Hydrometallurgy 2002, 63, 15–22. [Google Scholar] [CrossRef]
- Nicol, M.; Miki, H.; Velásquez-Yévenes, L. The dissolution of chalcopyrite in chloride solutions: Part 3. Mechanisms. Hydrometallurgy 2010, 103, 86–95. [Google Scholar] [CrossRef]
- Nicol, M.; Basson, P. The anodic behaviour of covellite in chloride solutions. Hydrometallurgy 2017, 172, 60–68. [Google Scholar] [CrossRef]
- Velásquez-Yévenes, L.; Miki, H.; Nicol, M. The dissolution of chalcopyrite in chloride solutions: Part 2: Effect of various parameters on the rate. Hydrometallurgy 2010, 103, 80–85. [Google Scholar] [CrossRef]
- Velásquez-Yévenes, L.; Nicol, M.; Miki, H. The dissolution of chalcopyrite in chloride solutions: Part 1. The effect of solution potential. Hydrometallurgy 2010, 103, 108–113. [Google Scholar] [CrossRef]
- Velásquez Yévenes, L. The Kinetics of the Dissolution of Chalcopyrite in Chloride Media. Doctoral Dissertation, Murdoch University, Murdoch, Australia, 2009. [Google Scholar]
- Petersen, J.; Dixon, D.G. Thermophilic heap leaching of a chalcopyrite concentrate. Miner. Eng. 2002, 15, 777–785. [Google Scholar] [CrossRef] [Green Version]
- Yu, R.L.; Zhong, D.L.; Miao, L.; Wu, F.D.; Qiu, G.Z.; Gu, G.H. Relationship and effect of redox potential, jarosites and extra-cellular polymeric substances in bioleaching chalcopyrite by acidithiobacillus ferrooxidans. Trans. Nonferrous Met. Soc. China Engl. Ed. 2011, 21, 1634–1640. [Google Scholar] [CrossRef]
- Feng, S.; Yang, H.; Xin, Y.; Gao, K.; Yang, J.; Liu, T.; Zhang, L.; Wang, W. A novel and highly efficient system for chalcopyrite bioleaching by mixed strains of Acidithiobacillus. Bioresour. Technol. 2013, 129, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Yang, H.; Zhan, X.; Wang, W. Novel integration strategy for enhancing chalcopyrite bioleaching by Acidithiobacillus sp. in a 7-L fermenter. Bioresour. Technol. 2014, 161, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Yang, H.; Wang, W. Improved chalcopyrite bioleaching by Acidithiobacillus sp. via direct step-wise regulation of microbial community structure. Bioresour. Technol. 2015, 192, 75–82. [Google Scholar] [CrossRef]
- Figueroa-Estrada, J.C.; Neria-González, M.I.; Rodríguez Vázquez, R.; Tec-Caamal, E.N.; Aguilar-López, R. Controlling a continuous stirred tank reactor for zinc leaching. Miner. Eng. 2020, 157, 106549. [Google Scholar] [CrossRef]
- Srichandan, H.; Mohapatra, R.K.; Singh, P.K.; Mishra, S.; Parhi, P.K.; Naik, K. Column Bioleaching Applications, Process Development, Mechanism, Parametric Effect and Modelling: A Review. J. Ind. Eng. Chem. 2020, 90, 1–16. [Google Scholar] [CrossRef]
- Rawlings, D.E. Microbially assisted dissolution of minerals and its use in the mining industry. Pure Appl. Chem. 2004, 76, 847–859. [Google Scholar] [CrossRef]
- Acar, S.; Brierley, J.A.; Wan, R.Y. Conditions for bioleaching a covellite-bearing ore. Hydrometallurgy 2005, 77, 239–246. [Google Scholar] [CrossRef]
- Watling, H.R. The bioleaching of sulphide minerals with emphasis on copper sulphides—A review. Hydrometallurgy 2006, 84, 81–108. [Google Scholar] [CrossRef]
- Panda, S.; Parhi, P.K.; Pradhan, N.; Mohapatra, U.B.; Sukla, L.B.; Park, K.H. Extraction of copper from bacterial leach liquor of a low grade chalcopyrite test heap using LIX 984N-C. Hydrometallurgy 2012, 121–124, 116–119. [Google Scholar] [CrossRef]
- Ojumu, T.V.; Petersen, J.; Searby, G.E.; Hansford, G.S. A review of rate equations proposed for microbial ferrous-iron oxidation with a view to application to heap bioleaching. Hydrometallurgy 2006, 83, 21–28. [Google Scholar] [CrossRef]
- Zhou, S.; Gan, M.; Zhu, J.; Li, Q.; Jie, S.; Yang, B.; Liu, X. Catalytic effect of light illumination on bioleaching of chalcopyrite. Bioresour. Technol. 2015, 182, 345–352. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, J.; Gan, X.; Zheng, X.; Tao, L.; Hu, M.; Li, Y.; Qin, W.; Qiu, G. Effects of pyrite and bornite on bioleaching of two different types of chalcopyrite in the presence of Leptospirillum ferriphilum. Bioresour. Technol. 2015, 194, 28–35. [Google Scholar] [CrossRef]
- Pradhan, N.; Nathsarma, K.C.; Srinivasa Rao, K.; Sukla, L.B.; Mishra, B.K. Heap bioleaching of chalcopyrite: A review. Miner. Eng. 2008, 21, 355–365. [Google Scholar] [CrossRef]
- Panda, S.; Akcil, A.; Pradhan, N.; Deveci, H. Current scenario of chalcopyrite bioleaching: A review on the recent advances to its heap-leach technology. Bioresour. Technol. 2015, 196, 694–706. [Google Scholar] [CrossRef]
- Abhilash; Mehta, K.D.; Pandey, B.D. Bacterial Leaching Kinetics for Copper Dissolution from a Low- Grade Indian Chalcopyrite Ore. Rev. Esc. Minas 2013, 66, 245–250. [Google Scholar] [CrossRef] [Green Version]
- Remonsellez, F.; Galleguillos, F.; Moreno-Paz, M.; Parro, V.; Acosta, M.; Demergasso, C. Dynamic of active microorganisms inhabiting a bioleaching industrial heap of low-grade copper sulfide ore monitored by real-time PCR and oligonucleotide prokaryotic acidophile microarray. Microb. Biotechnol. 2009, 2, 613–624. [Google Scholar] [CrossRef]
- Zhou, H.B.; Zeng, W.M.; Yang, Z.F.; Xie, Y.J.; Qiu, G.Z. Bioleaching of chalcopyrite concentrate by a moderately thermophilic culture in a stirred tank reactor. Bioresour. Technol. 2009, 100, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Donati, E.; Sand, W. Microbial Processing of Metal Sulfides; Springer: Dordrecht, The Netherlands, 2007; ISBN 9781119130536. [Google Scholar]
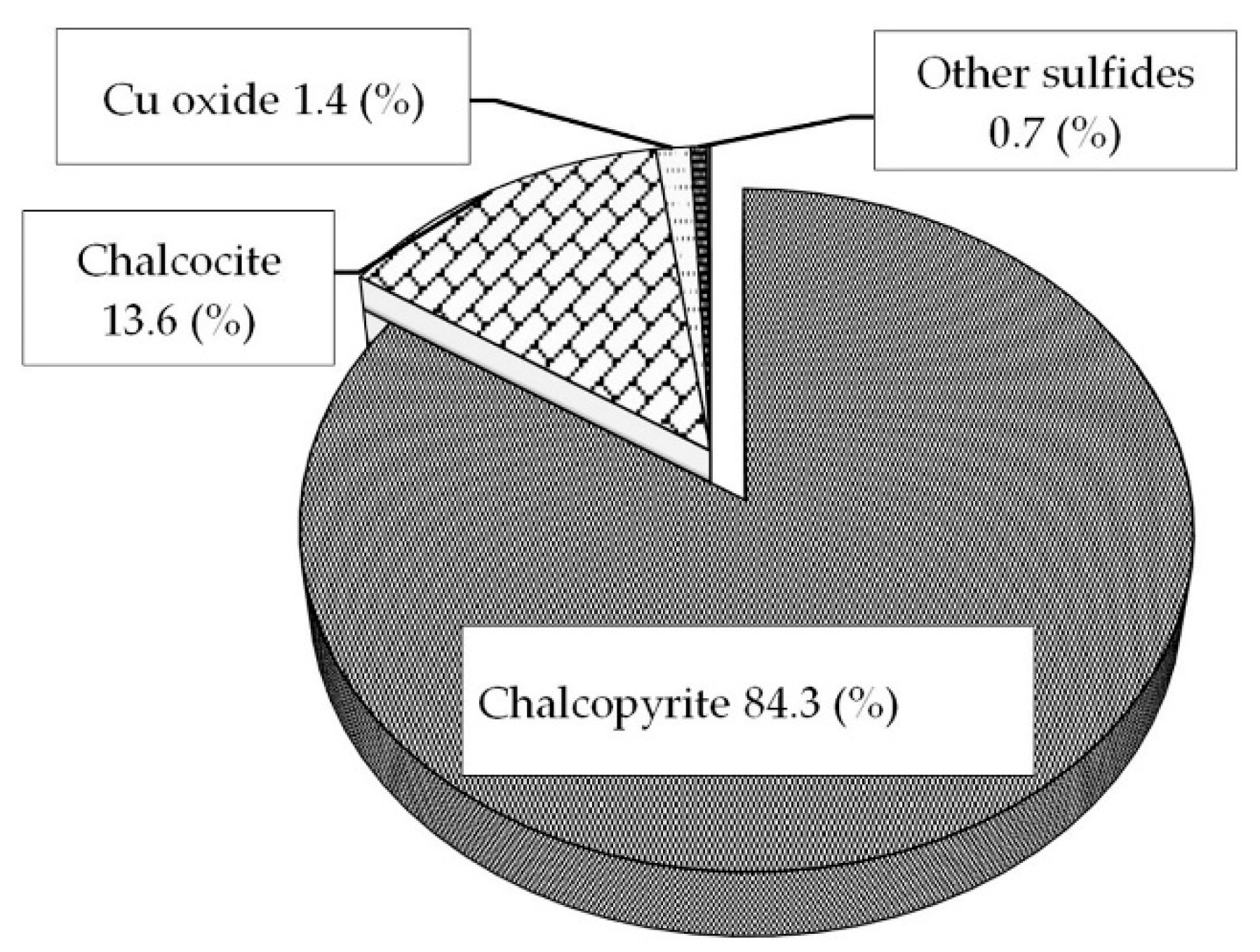








| Mineral | Amount (wt%) |
|---|---|
| Chalcopyrite | 0.62 |
| Chalcocite/digenite | 0.07 |
| Cu oxide minerals | 0.01 |
| Other sulfides | 0.04 |
| Pyrite | 3.35 |
| Quartz | 34.2 |
| Plagioclase | 5.30 |
| Albite | 14.2 |
| Feldspars-K | 15.0 |
| Biotite/phlogopite | 0.97 |
| Sericite/muscovite | 18.1 |
| Chlorite | 5.53 |
| Tourmaline | 1.28 |
| Fe oxides | 1.33 |
| Total | 100 |
| Gene Abundance % | Sample 1 | Sample 2 | Industrial Raffinate |
|---|---|---|---|
| Asticcacaulis | 0 | 0 | 44.4 |
| Acidiphilium | 100 | 100 | 36.8 |
| Mycobacterium | 0 | 0 | 11.9 |
| Test | pH | H2SO4 (g/L) | Cu2+ (g/L) | FeT (g/L) | Cell Concentration (×106 cell/mL) | Irrigation/Rest (day) |
|---|---|---|---|---|---|---|
| 1 | - | 6.0 | 0.5 | 1.0 | Not added | Continuous |
| 2 | - | 10.0 | 0.5 | 1.0 | Not added | Continuous |
| 3 | - | 20.0 | 0.5 | 1.0 | Not added | Continuous |
| 4 | 2.0 | 1.0 | 0.5 | 1.0 | 150 | Continuous |
| 5 | 1.8 | 2.0 | 0.5 | 1.0 | 150 | Continuous |
| 6 | 1.5 | 3.0 | 0.5 | 1.0 | 150 | Continuous |
| 7 | 1.8 | 2.0 | 0.5 | 1.0 | 150 | 5/10 |
| 8 | 1.8 | 2.0 | 0.5 | 1.0 | 150 | 5/20 |
| 9 | 1.0 | 10.0 | 0.5 | 1.0 | 150 (from second cycle) | 5/10 |
| Test | pH | H2SO4 (g/L) | Cu2+ (g/L) | FeT (g/L) | Cell Concentration (×106 cell/mL) | Irrigation/Rest (day) |
|---|---|---|---|---|---|---|
| 10 | - | 3.0 | 0.5 | 1.0 | 10 | Continuous |
| 11 | - | 3.0 | 0.5 | 1.0 | 50 | Continuous |
| 12 | 2.0 | - | 0.5 | 1.0 | 50 | Continuous |
| 13 | 1.5 | - | 0.5 | 1.0 | 50 | Continuous |
| 14 | - | 12 | 0.5 | 1.0 | 50 | 5/10 |
| 15 | - | 12 | 0.5 | 1.0 | 50 | 5/10 |
| 16 | - | 12 | 0.5 | 1.0 | 50 | 5/20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velásquez-Yévenes, L.; Malverde, S.; Quezada, V. A Sustainable Bioleaching of a Low-Grade Chalcopyrite Ore. Minerals 2022, 12, 487. https://doi.org/10.3390/min12040487
Velásquez-Yévenes L, Malverde S, Quezada V. A Sustainable Bioleaching of a Low-Grade Chalcopyrite Ore. Minerals. 2022; 12(4):487. https://doi.org/10.3390/min12040487
Chicago/Turabian StyleVelásquez-Yévenes, Lilian, Sebastián Malverde, and Víctor Quezada. 2022. "A Sustainable Bioleaching of a Low-Grade Chalcopyrite Ore" Minerals 12, no. 4: 487. https://doi.org/10.3390/min12040487






