Halloysite-Zinc Oxide Nanocomposites as Potential Photocatalysts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Prepared Composites
2.2. Analytical Methods
2.2.1. Investigation of the Photocatalytic Activity
2.2.2. Investigation of Nanocomposite Stability by Zinc Dissolution
2.2.3. Toxicology of Nanocomposites
3. Results and Discussion
3.1. Synthesis and Characterization of Nanocomposites
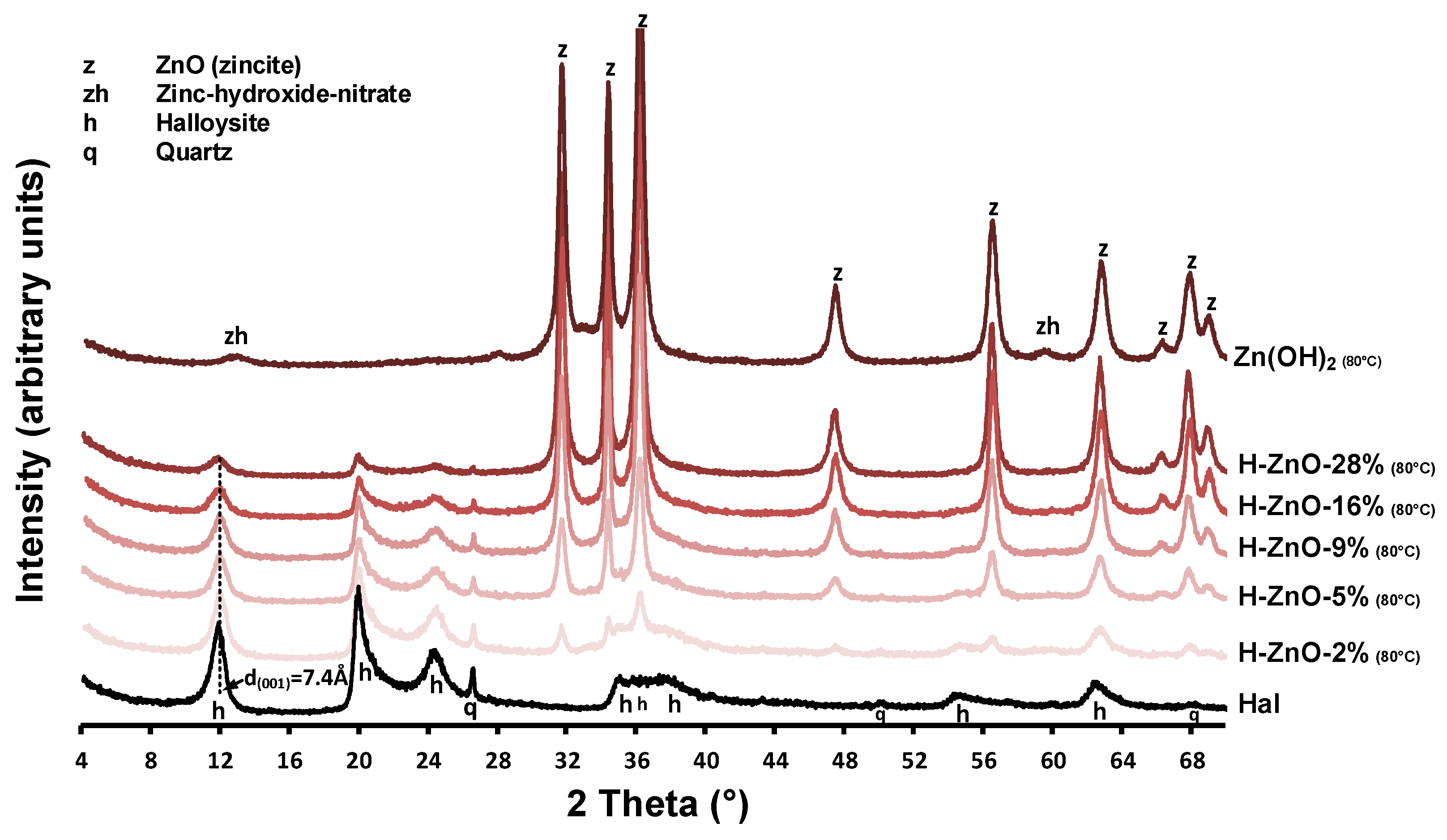
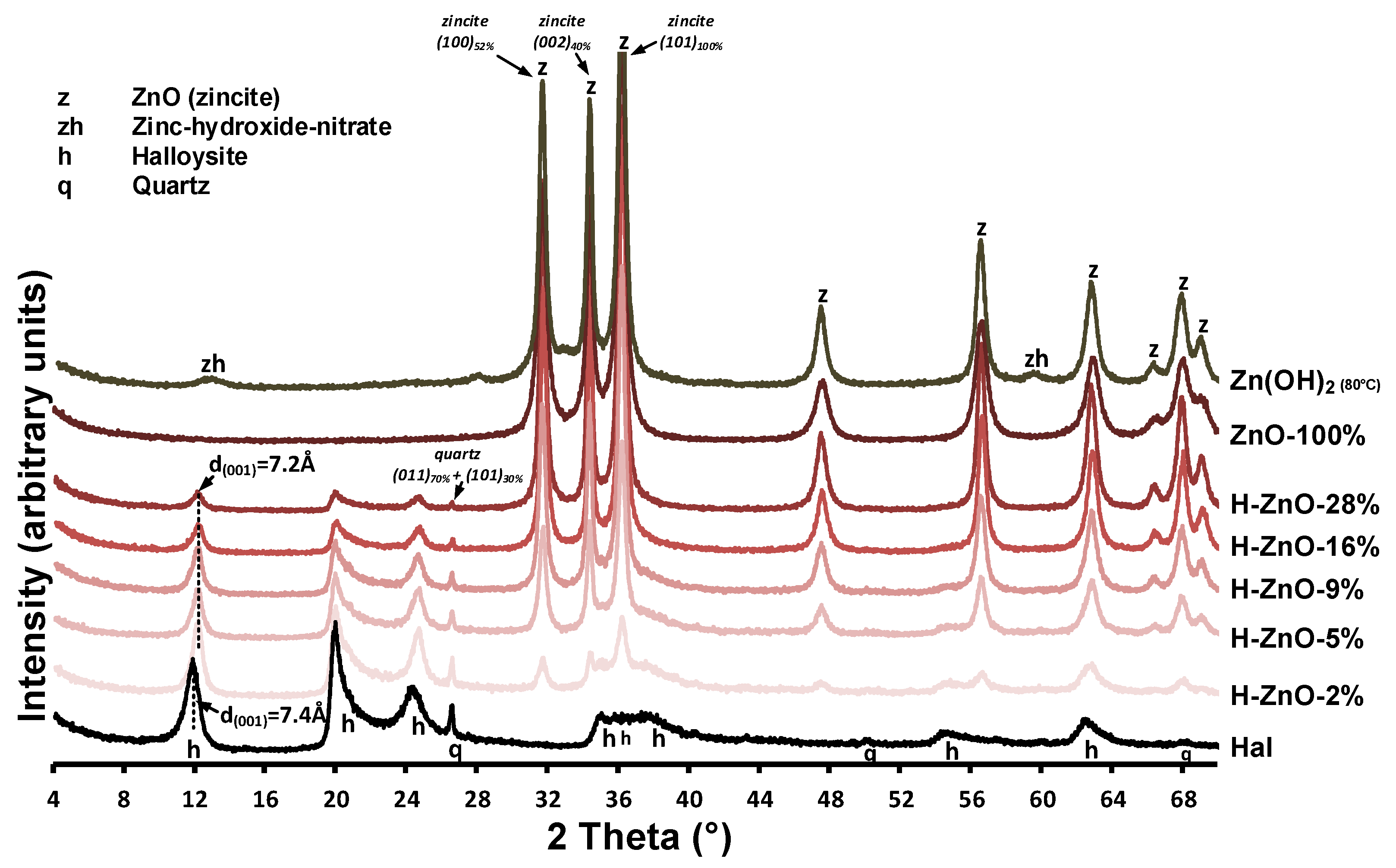
3.1.1. X-ray Diffraction
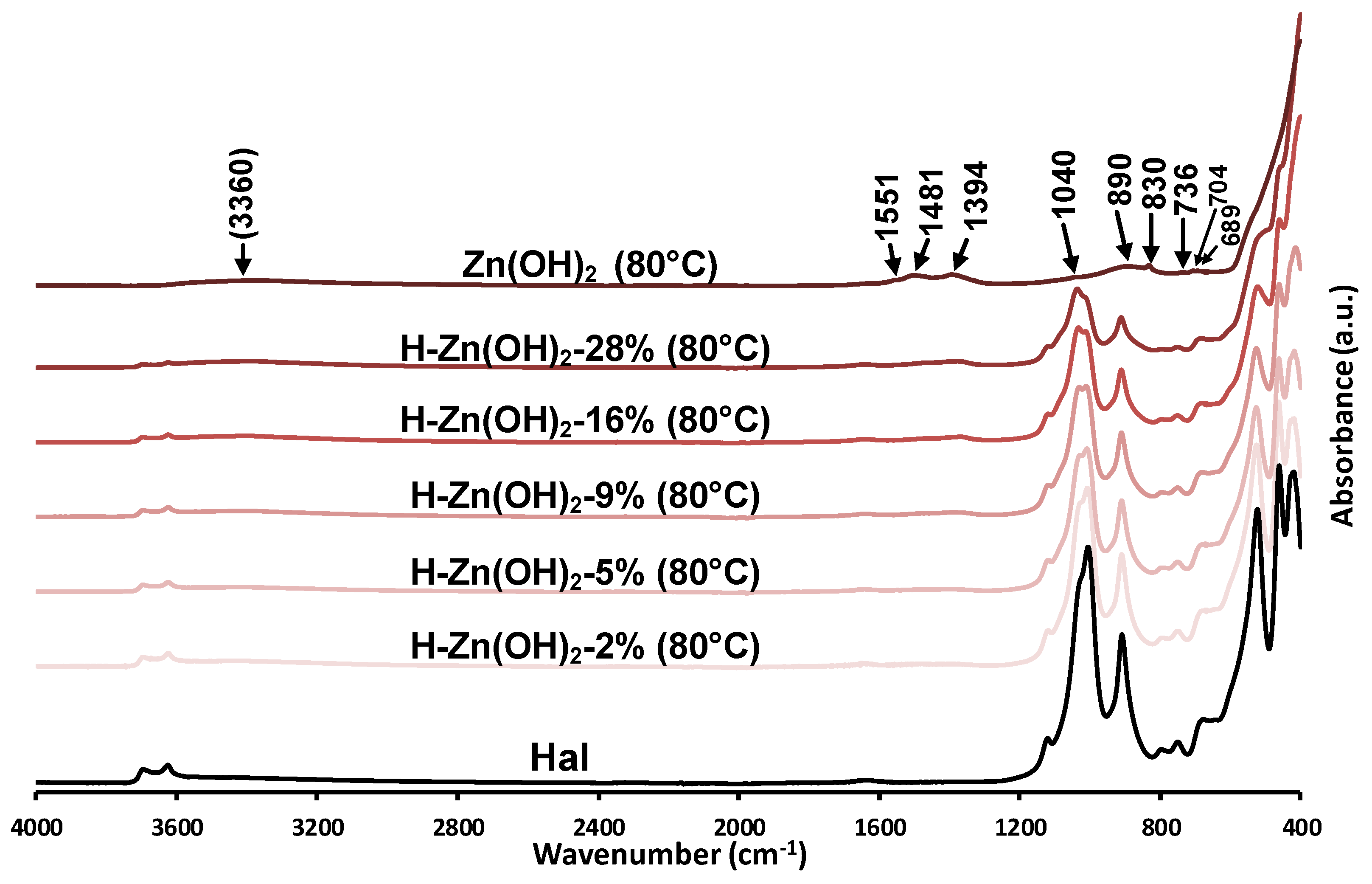
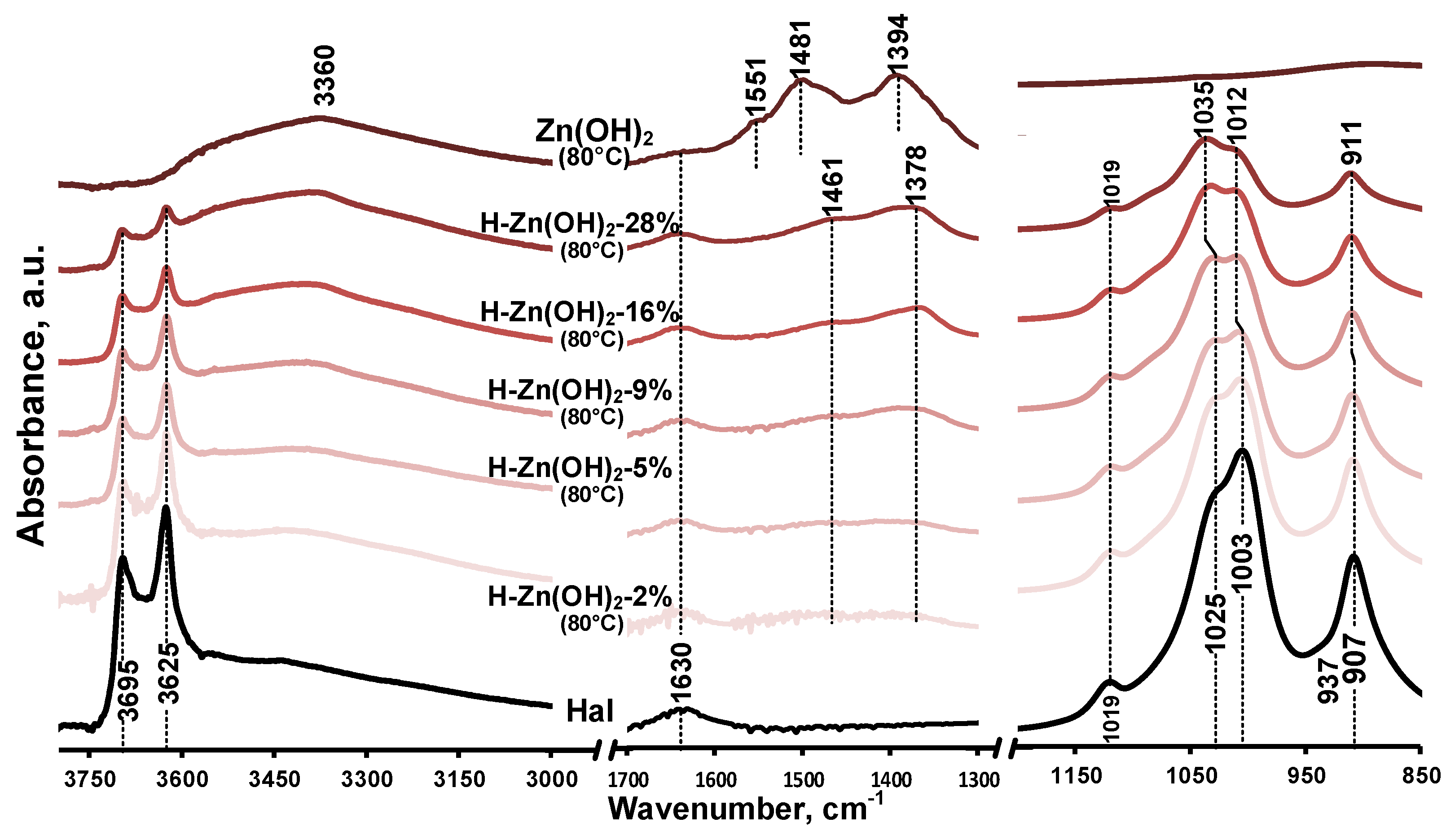
3.1.2. Infrared Spectroscopy (FTIR-ATR)
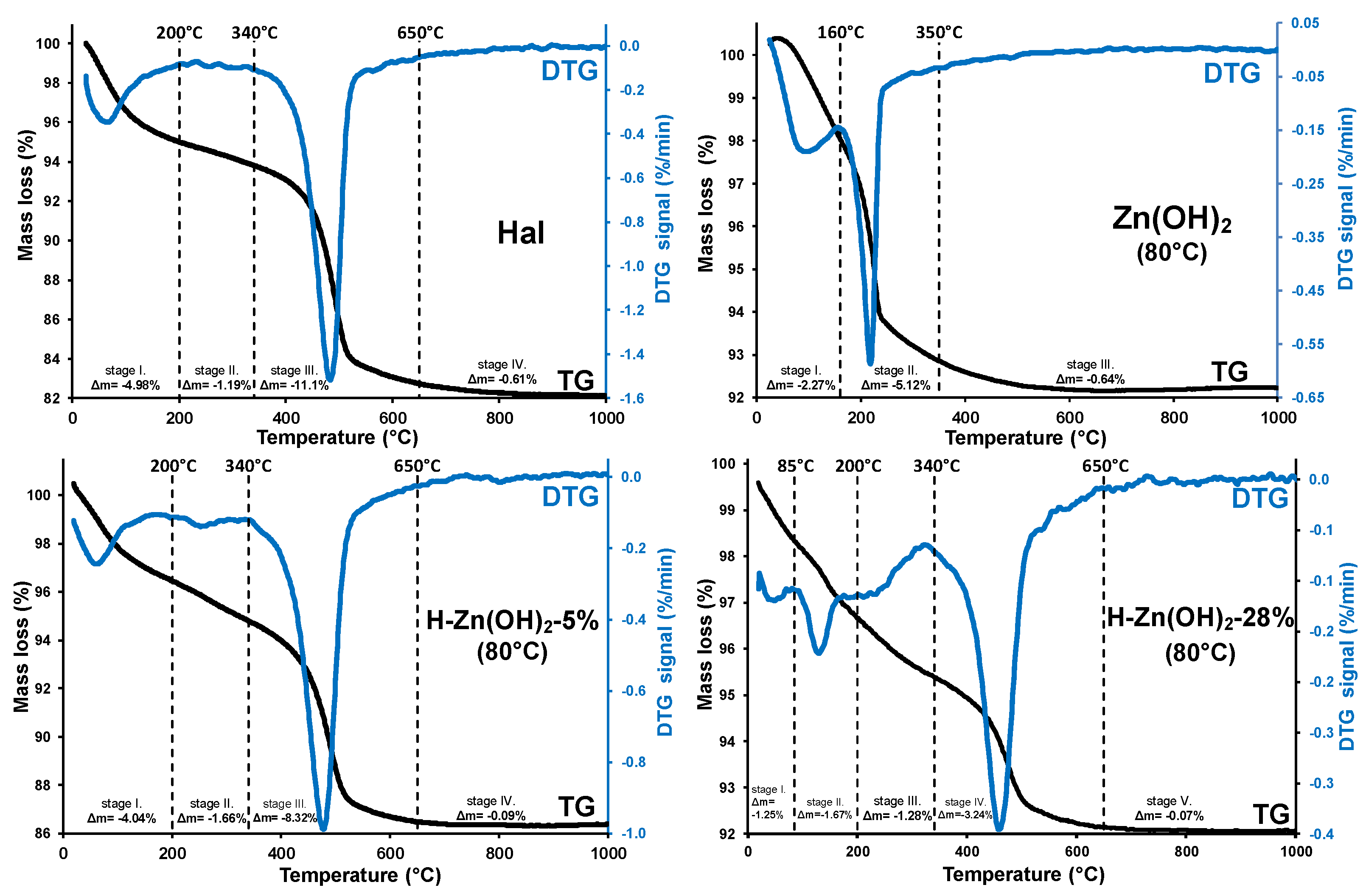
3.1.3. Thermal Analysis (TG/DTG)
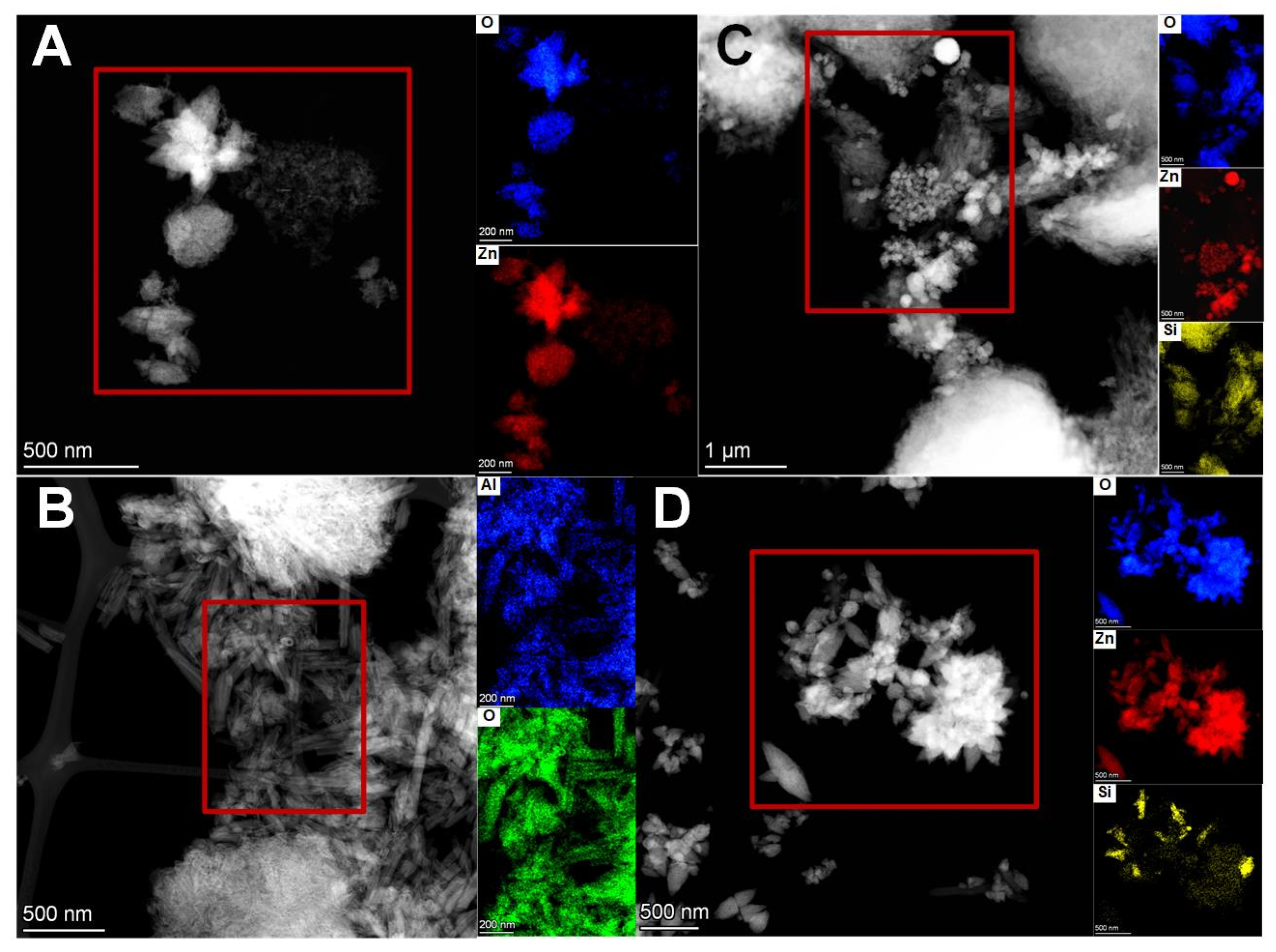
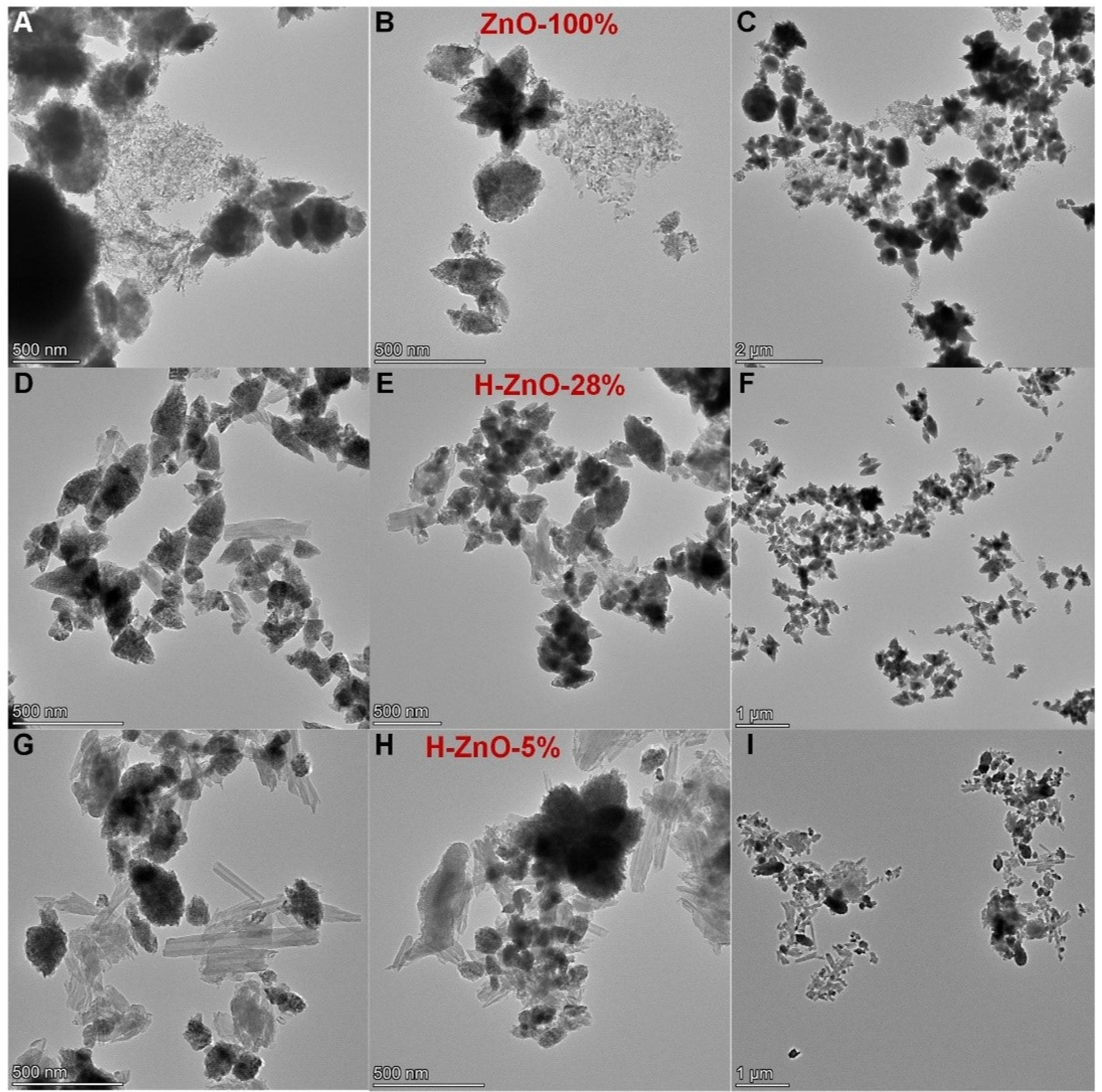
3.1.4. Porosity and Morphology
3.2. Photochemical Characterization of Nanocomposites
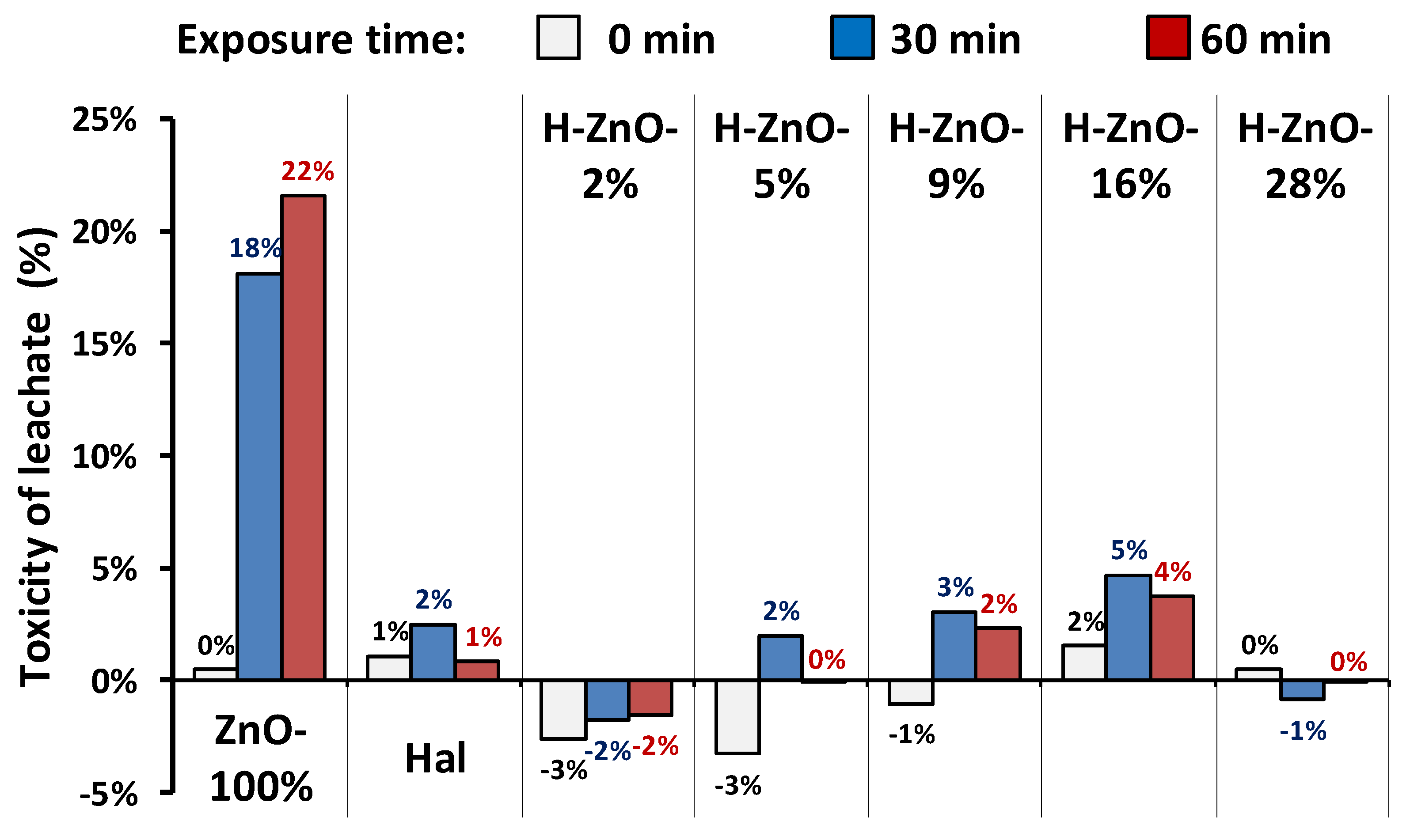
3.3. Stability of the Nanocomposites and Their Toxicity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Sample | Halloysite (001) | Zincite (100) | Zincite (102) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2θ (°) | FWHM (°) | d (nm) | N | 2θ (°) | FWHM (°) | d (nm) | 2θ (°) | FWHM (°) | d (nm) | |
| Hal | 11.91 | 0.842 | 12.2 | 16.4 | - | - | - | - | - | - |
| Hal-ZnO-2% | 12.20 | 0.695 | 15.7 | 21.7 | 31.76 | 0.510 | 26.6 | 47.56 | 0.666 | 20.0 |
| Hal-ZnO-5% | 12.19 | 0.710 | 15.2 | 21.2 | 31.77 | 0.470 | 30.7 | 47.56 | 0.721 | 17.8 |
| Hal-ZnO-9% | 12.18 | 0.689 | 15.8 | 22.0 | 31.75 | 0.450 | 33.4 | 47.55 | 0.707 | 18.3 |
| Hal-ZnO-16% | 12.24 | 0.699 | 15.6 | 21.6 | 31.82 | 0.408 | 40.4 | 47.61 | 0.699 | 18.6 |
| Hal-ZnO-28% | 12.20 | 0.688 | 15.9 | 22.1 | 31.77 | 0.398 | 42.5 | 47.56 | 0.668 | 19.9 |
| ZnO-100% | - | - | - | - | 31.75 | 0.599 | 20.5 | 47.59 | 0.902 | 13.0 |
| Sample | Stage | Tinitial (°C) | Tend (°C) | Mass Loss Step |
|---|---|---|---|---|
| Hal | I. | 20 | 200 | 0.96 mg (4.98%) |
| II. | 200 | 340 | 0.23 mg (1.19%) | |
| III. | 340 | 650 | 2.13 mg (11.1%) | |
| IV. | 650 | 1000 | 0.12 mg (0.61%) | |
| Initial mass: 19.224 mg | ||||
| Total mass loss: 3.44 mg (17.88%) | ||||
| Zn(OH)2-100% (80 °C) | I. | 20 | 160 | 1.31 mg (2.27%) |
| II. | 160 | 350 | 2.95 mg (5.12%) | |
| III. | 350 | 1001 | 0.37 mg (0.64%) | |
| Initial mass: 57.534 mg | ||||
| Total mass loss: 4.63 mg (8.03%) | ||||
| H-Zn(OH)2-5% (80 °C) | I. | 20 | 200 | 0.81 mg (4.04%) |
| II. | 200 | 340 | 0.33 mg (1.66%) | |
| III. | 340 | 650 | 1.67 mg (8.32%) | |
| IV. | 650 | 1000 | 0.02 mg (0.09%) | |
| Initial mass: 20.062 mg | ||||
| Total mass loss: 2.83 mg (14.11%) | ||||
| H-Zn(OH)2-28% (80 °C) | I. | 20 | 85 | 0.25 mg (1.25%) |
| II. | 85 | 200 | 0.34 mg (1.67%) | |
| III. | 200 | 341 | 0.26 mg (1.29%) | |
| IV. | 341 | 1000 | 0.65 mg (3.24%) | |
| V. | 650 | 1001 | 0.01 mg (0.07%) | |
| Initial mass: 20.06 mg | ||||
| Total mass loss: 1.51 mg (7.52%) | ||||
Appendix B. Thermoanalytical Calculations

| Sample | Element | Atom% | Mass% |
|---|---|---|---|
| Hal | O | 69.97 ± 6.43 | 57.43 ± 3.34 |
| Al | 15.26 ± 3.32 | 21.12 ± 4.35 | |
| Si | 14.54 ± 3.1 | 20.95 ± 4.21 | |
| Ti | 0.01 ± 0 | 0.02 ± 0 | |
| Fe | 0.11 ± 0.02 | 0.31 ± 0.05 | |
| Zn | 0 ± 0 | 0 ± 0 | |
| ZnO-100% | O | 54.31 ± 8.56 | 22.65 ± 2.34 |
| Al | 0.07 ± 0.02 | 0.05 ± 0.01 | |
| Si | 0.47 ± 0.12 | 0.35 ± 0.08 | |
| Ti | 0.03 ± 0.01 | 0.04 ± 0.01 | |
| Fe | 0.06 ± 0.01 | 0.08 ± 0.01 | |
| Zn | 45.06 ± 9.29 | 76.83 ± 12.93 | |
| H-ZnO-28% | O | 51.61 ± 8.2 | 21.08 ± 2.18 |
| Al | 0.84 ± 0.21 | 0.58 ± 0.13 | |
| Si | 1.08 ± 0.27 | 0.77 ± 0.17 | |
| Ti | 0 ± 0 | 0 ± 0 | |
| Fe | 0.06 ± 0.01 | 0.08 ± 0.01 | |
| Zn | 46.42 ± 9.63 | 77.49 ± 13.08 | |
| H-ZnO-5% | O | 61.89 ± 5.72 | 38.4 ± 2.28 |
| Al | 12.34 ± 2.69 | 12.91 ± 2.66 | |
| Si | 11.45 ± 2.49 | 12.47 ± 2.56 | |
| Ti | 0.01 ± 0 | 0.01 ± 0 | |
| Fe | 0.17 ± 0.03 | 0.36 ± 0.05 | |
| Zn | 14.13 ± 2.29 | 35.84 ± 5.24 |

References
- Brigatti, M.F.; Galan, E.; Theng, B.K.G. Structures and Mineralogy of Clay Minerals. In Developments in Clay Science, Handbook of Caly Science; Bergaya, F., Lagaly, G., Vayer, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 6, pp. 19–33, 35–48. ISBN 9780080441832. [Google Scholar]
- Schoomheydt, R.A.; Johnston, C.T. Surface and Interface Chemistry of Clay Minerals. In Developments in Clay Science, Handbook of Caly Science; Bergaya, F., Lagaly, G., Vayer, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 87–99. ISBN 9780080441832. [Google Scholar]
- Joussein, E. Geology and Mineralogy of Nanosized Tubular Halloysite. In Developments in Clay Science (2016); Yuan, P., Thill, A., Bergaya, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 12–48. ISBN 978-0-08-100293-3. [Google Scholar] [CrossRef]
- Guan, H.; Zhao, Y. 9—Decontamination application of nanoclays. In Micro and Nano Technologies; Cavallaro, G., Fakhrullin, R., Pasbakhsh, P.B.T.-C.N., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 203–224. ISBN 978-0-12-816783-0. [Google Scholar]
- Kibanova, D.; Trejo, M.; Destaillats, H.; Cervini-Silva, J. Photocatalytic activity of kaolinite. Catal. Commun. 2011, 12, 698–702. [Google Scholar] [CrossRef]
- Szabó, P.; Zsirka, B.; Fertig, D.; Horváth, E.; Csizmadia, T.; Kristóf, J. Delaminated kaolinites as potential photocatalysts: Tracking degradation of Na-benzenesulfonate test compound adsorbed on the dry surface of kaolinite nanostructures. Catal. Today 2017, 287, 37–44. [Google Scholar] [CrossRef]
- Shawky, A.; El-Sheikh, S.M.; Rashed, M.N.; Abdo, S.M.; El-Dosoqy, T.I. Exfoliated kaolinite nanolayers as an alternative photocatalyst with superb activity. J. Environ. Chem. Eng. 2019, 7, 103174. [Google Scholar] [CrossRef]
- Zsirka, B.; Vágvölgyi, V.; Győrfi, K.; Horváth, E.; Szilágyi, R.K.; Szabó-Bárdos, E.; Balogh, S.; Kristóf, J. Compositional, structural, and surface characterization of heat-treated halloysite samples: Influence of surface treatment on photochemical activity. Appl. Clay Sci. 2021, 212, 106222. [Google Scholar] [CrossRef]
- Menager, M.; Sarakha, M. Simulated Solar Light Phototransformation of Organophosphorus Azinphos Methyl at the Surface of Clays and Goethite. Environ. Sci. Technol. 2013, 47, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Győrfi, K.; Vágvölgyi, V.; Zsirka, B.; Horváth, E.; Szilágyi, R.K.; Baán, K.; Balogh, S.; Kristóf, J. Kaolins of high iron-content as photocatalysts: Challenges of acidic surface modifications and mechanistic insights. Appl. Clay Sci. 2020, 195, 105722. [Google Scholar] [CrossRef]
- dos Santos, L.R.; Mascarenhas, A.J.S.; Silva, L.A. Preparation and evaluation of composite with a natural red clay and TiO2 for dye discoloration assisted by visible light. Appl. Clay Sci. 2017, 135, 603–610. [Google Scholar] [CrossRef]
- Szczepanik, B. Photocatalytic degradation of organic contaminants over clay-TiO2 nanocomposites: A review. Appl. Clay Sci. 2017, 141, 227–239. [Google Scholar] [CrossRef]
- Lazzara, G.; Cavallaro, G.; Panchal, A.; Fakhrullin, R.; Stavitskaya, A.; Vinokurov, V.; Lvov, Y. An assembly of organic-inorganic composites using halloysite clay nanotubes. Curr. Opin. Colloid Interface Sci. 2018, 35, 42–50. [Google Scholar] [CrossRef]
- Ruiz-Hitzky, E.; Aranda, P.; Akkari, M.; Khaorapapong, N.; Ogawa, M. Photoactive nanoarchitectures based on clays incorporating TiO2 and ZnO nanoparticles. Beilstein J. Nanotechnol. 2019, 10, 1140–1156. [Google Scholar] [CrossRef] [Green Version]
- Papoulis, D. Halloysite based nanocomposites and photocatalysis: A Review. Appl. Clay Sci. 2019, 168, 164–174. [Google Scholar] [CrossRef]
- Li, C.; Zhu, N.; Yang, S.; He, X.; Zheng, S.; Sun, Z.; Dionysiou, D.D. A review of clay based photocatalysts: Role of phyllosilicate mineral in interfacial assembly, microstructure control and performance regulation. Chemosphere 2021, 273, 129723. [Google Scholar] [CrossRef] [PubMed]
- Vágvölgyi, V.; Győrfi, K.; Zsirka, B.; Horváth, E.; Kristóf, J. The role of thermal analysis in the development of high-iron-content kaolinite-based photocatalysts. J. Therm. Anal. Calorim. 2020, 142. [Google Scholar] [CrossRef] [Green Version]
- Srikant, V.; Clarke, D.R. On the optical band gap of zinc oxide. J. Appl. Phys. 1998, 83, 5447–5451. [Google Scholar] [CrossRef]
- Weerathunga, H.; Tang, C.; Brock, A.J.; Sarina, S.; Wang, T.; Liu, Q.; Zhu, H.-Y.; Du, A.; Waclawik, E.R. Nanostructure Shape-Effects in ZnO heterogeneous photocatalysis. J. Colloid Interface Sci. 2022, 606, 588–599. [Google Scholar] [CrossRef]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef]
- Khan, S.H.; Pathak, B. Zinc oxide based photocatalytic degradation of persistent pesticides: A comprehensive review. Environ. Nanotechnology, Monit. Manag. 2020, 13, 100290. [Google Scholar] [CrossRef]
- Majumder, S.; Chatterjee, S.; Basnet, P.; Mukherjee, J. ZnO based nanomaterials for photocatalytic degradation of aqueous pharmaceutical waste solutions—A contemporary review. Environ. Nanotechnology, Monit. Manag. 2020, 14, 100386. [Google Scholar] [CrossRef]
- Arya, S.; Mahajan, P.; Mahajan, S.; Khosla, A.; Datt, R.; Gupta, V.; Young, S.-J.; Oruganti, S.K. Review—Influence of Processing Parameters to Control Morphology and Optical Properties of Sol-Gel Synthesized ZnO Nanoparticles. ECS J. Solid State Sci. Technol. 2021, 10, 023002. [Google Scholar] [CrossRef]
- Uribe-López, M.C.; Hidalgo-López, M.C.; López-González, R.; Frías-Márquez, D.M.; Núñez-Nogueira, G.; Hernández-Castillo, D.; Alvarez-Lemus, M.A. Photocatalytic activity of ZnO nanoparticles and the role of the synthesis method on their physical and chemical properties. J. Photochem. Photobiol. A Chem. 2021, 404, 112866. [Google Scholar] [CrossRef]
- Sansenya, T.; Masri, N.; Chankhanittha, T.; Senasu, T.; Piriyanon, J.; Mukdasai, S.; Nanan, S. Hydrothermal synthesis of ZnO photocatalyst for detoxification of anionic azo dyes and antibiotic. J. Phys. Chem. Solids 2022, 160, 110353. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Nguyen, V.A. Synthesis, Characterization, and Photocatalytic Activity of ZnO Nanomaterials Prepared by a Green, Nonchemical Route. J. Nanomater. 2020, 2020, 1–8. [Google Scholar] [CrossRef]
- Mohd Yusof, H.; Abdul Rahman, N.; Mohamad, R.; Zaidan, U.H.; Samsudin, A.A. Biosynthesis of zinc oxide nanoparticles by cell-biomass and supernatant of Lactobacillus plantarum TA4 and its antibacterial and biocompatibility properties. Sci. Rep. 2020, 10, 19996. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Nikhil, S.K.; Nair, R.G. Influence of surface morphology on photocatalytic performance of zinc oxide: A review. Nano-Structures & Nano-Objects 2019, 19, 100353. [Google Scholar] [CrossRef]
- Davis, K.; Yarbrough, R.; Froeschle, M.; White, J.; Rathnayake, H. Band gap engineered zinc oxide nanostructures via a sol–gel synthesis of solvent driven shape-controlled crystal growth. RSC Adv. 2019, 9, 14638–14648. [Google Scholar] [CrossRef] [Green Version]
- Shaba, E.Y.; Jacob, J.O.; Tijani, J.O.; Suleiman, M.A.T. A critical review of synthesis parameters affecting the properties of zinc oxide nanoparticle and its application in wastewater treatment. Appl. Water Sci. 2021, 11, 48. [Google Scholar] [CrossRef]
- Freitas, W.A.; Soares, B.E.C.F.; Rodrigues, M.S.; Trigueiro, P.; Honorio, L.M.C.; Peña-Garcia, R.; Alcântara, A.C.S.; Silva-Filho, E.C.; Fonseca, M.G.; Furtini, M.B.; et al. Facile synthesis of ZnO-clay minerals composites using an ultrasonic approach for photocatalytic performance. J. Photochem. Photobiol. A Chem. 2022, 429, 113934. [Google Scholar] [CrossRef]
- Zou, Y.; Hu, Y.; Shen, Z.; Yao, L.; Tang, D.; Zhang, S.; Wang, S.; Hu, B.; Zhao, G.; Wang, X. Application of aluminosilicate clay mineral-based composites in photocatalysis. J. Environ. Sci. 2022, 115, 190–214. [Google Scholar] [CrossRef]
- Weldegebrieal, G.K. Synthesis method, antibacterial and photocatalytic activity of ZnO nanoparticles for azo dyes in wastewater treatment: A review. Inorg. Chem. Commun. 2020, 120, 108140. [Google Scholar] [CrossRef]
- Long, Z.; Wu, Y.-P.; Gao, H.-Y.; Zhang, J.; Ou, X.; He, R.-R.; Liu, M. In vitro and in vivo toxicity evaluation of halloysite nanotubes. J. Mater. Chem. B 2018, 6, 7204–7216. [Google Scholar] [CrossRef]
- Gkouma, E.; Gianni, E.; Avgoustakis, K.; Papoulis, D. Applications of halloysite in tissue engineering. Appl. Clay Sci. 2021, 214, 106291. [Google Scholar] [CrossRef]
- Vergaro, V.; Abdullayev, E.; Lvov, Y.M.; Zeitoun, A.; Cingolani, R.; Rinaldi, R.; Leporatti, S. Cytocompatibility and Uptake of Halloysite Clay Nanotubes. Biomacromolecules 2010, 11, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, H.; Khan, E.; Ilahi, I. Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. J. Chem. 2019, 2019, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Wang, A. Recent researches on antimicrobial nanocomposite and hybrid materials based on sepiolite and palygorskite. Appl. Clay Sci. 2022, 219, 106454. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Chisholm, J. Comparison of Quartz Standards for X-ray Diffraction Analysis: HSE A9950 (Sikron F600) and NIST SRM 1878. Ann. Occup. Hyg. 2005, 49, 351–358. [Google Scholar] [CrossRef] [Green Version]
- Aguzzi, C.; Donnadio, A.; Quaglia, G.; Latterini, L.; Viseras, C.; Ambrogi, V. Halloysite-Doped Zinc Oxide for Enhanced Sunscreening Performance. ACS Appl. Nano Mater. 2019, 2, 6575–6584. [Google Scholar] [CrossRef]
- Hua, G.; Zhang, L.; Dai, J.; Hu, L.; Dai, S. Synthesis of dimension-tunable ZnO nanostructures via the design of zinc hydroxide precursors. Appl. Phys. A 2011, 102, 275–280. [Google Scholar] [CrossRef]
- Li, P.; Xu, Z.P.; Hampton, M.A.; Vu, D.T.; Huang, L.; Rudolph, V.; Nguyen, A. V Control Preparation of Zinc Hydroxide Nitrate Nanocrystals and Examination of the Chemical and Structural Stability. J. Phys. Chem. C 2012, 116, 10325–10332. [Google Scholar] [CrossRef]
- Nicholas, N.J.; Franks, G.V.; Ducker, W.A. The mechanism for hydrothermal growth of zinc oxide. CrystEngComm 2012, 14, 1232–1240. [Google Scholar] [CrossRef]
- Patterson, A.L. The Scherrer Formula for X-ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Santagata, M.; Johnston, C.T. A study of nanoconfined water in halloysite. Appl. Clay Sci. 2022, 221, 106467. [Google Scholar] [CrossRef]
- Christidis, G.; Makri, P. Determination of kaolinite and halloysite crystallite size with X-Ray diffraction: Implications for industrial applications. Bull. Geol. Soc. Greece 2001, 34, 1163. [Google Scholar] [CrossRef] [Green Version]
- Matusik, J.; Wisła-Walsh, E.; Gaweł, A.; Bielańska, E.; Bahranowski, K. Surface Area and Porosity of Nanotubes Obtained from Kaolin Minerals of Different Structural Order. Clays Clay Miner. 2011, 59, 116–135. [Google Scholar] [CrossRef]
- Táborosi, A.; Szilagyi, R.K.; Zsirka, B.; Fónagy, O.; Horváth, E.; Kristóf, J. Molecular treatment of nano-kaolinite generations. Inorg. Chem. 2018, 57, 7151–7167. [Google Scholar] [CrossRef]
- Müller, C.M.; Pejcic, B.; Esteban, L.; Piane, C.D.; Raven, M.; Mizaikoff, B. Infrared Attenuated Total Reflectance Spectroscopy: An Innovative Strategy for Analyzing Mineral Components in Energy Relevant Systems. Sci. Rep. 2015, 4, 6764. [Google Scholar] [CrossRef] [Green Version]
- Viñes, F.; Iglesias-Juez, A.; Illas, F.; Fernández-García, M. Hydroxyl Identification on ZnO by Infrared Spectroscopies: Theory and Experiments. J. Phys. Chem. C 2014, 118, 1492–1505. [Google Scholar] [CrossRef]
- Srivastava, O.K.; Secco, E.A. Studies on metal hydroxy compounds. II. Infrared spectra of zinc derivatives ϵ-Zn(OH)2, β-ZnOHCl, ZnOHF, Zn5(OH)8Cl2, and Zn5(OH)8Cl2 ·H2O. Can. J. Chem. 1967, 45, 585–588. [Google Scholar] [CrossRef]
- Gordeeva, A.; Hsu, Y.-J.; Jenei, I.Z.; Brant Carvalho, P.H.B.; Simak, S.I.; Andersson, O.; Häussermann, U. Layered Zinc Hydroxide Dihydrate, Zn5(OH)10 ·2H2O, from Hydrothermal Conversion of ε-Zn(OH)2 at Gigapascal Pressures and its Transformation to Nanocrystalline ZnO. ACS Omega 2020, 5, 17617–17627. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.S.; Lockwood, D.J.; Poirier, S.; Bock, C.; MacDougall, B.R. Raman and Infrared Spectroscopy of α and β Phases of Thin Nickel Hydroxide Films Electrochemically Formed on Nickel. J. Phys. Chem. A 2012, 116, 6771–6784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zsirka, B.; Táborosi, A.; Szabó, P.; Szilágyi, R.K.; Horváth, E.; Juzsakova, T.; Fertig, D.; Kristóf, J. Surface Characterization of Mechanochemically Modified Exfoliated Halloysite Nanoscrolls. Langmuir 2017, 33, 3534–3547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heller-Kallai, L. Thermally modified clay minerals. In Developments in Clay Science, Handbook of Caly Science; Bergaya, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 411–433. ISBN 9780080982588. [Google Scholar]
- Cheng, H.; Liu, Q.; Yang, J.; Ma, S.; Frost, R.L. The thermal behavior of kaolinite intercalation complexes-A review. Thermochim. Acta 2012, 545, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Podlogar, M.; Rečnik, A.; Yilmazoglu, G.; Özer, I.Ö.; Mazaj, M.; Suvaci, E.; Bernik, S. The role of hydrothermal pathways in the evolution of the morphology of ZnO crystals. Ceram. Int. 2016, 42, 15358–15366. [Google Scholar] [CrossRef]
- Biswick, T.; Jones, W.; Pacuła, A.; Serwicka, E.; Podobinski, J. The role of anhydrous zinc nitrate in the thermal decomposition of the zinc hydroxy nitrates Zn5(OH)8(NO3)2·2H2O and ZnOHNO3·H2O. J. Solid State Chem. 2007, 180, 1171–1179. [Google Scholar] [CrossRef]
- Moezzi, A.; Lee, P.-S.; McDonagh, A.M.; Cortie, M.B. On the thermal decomposition of zinc hydroxide nitrate, Zn5(OH)8(NO3)2⋅2H2O. J. Solid State Chem. 2020, 286, 121311. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, J.; Ouyang, P.; Fu, L.; Yang, H. The relation between nanotube diameter, length and surface area and pore volume of multi-walled spiral halloysite nanotubes: A theoretical study. Appl. Clay Sci. 2021, 215, 106303. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Y.; Ouyang, J. Physicochemical Properties of Halloysite. In Developments in Clay Science; Yuan, P., Thill, A., Bergaya, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 67–91. ISBN 978-0-08-100293-3. [Google Scholar] [CrossRef]
- Thirumavalavan, M.; Huang, K.-L.; Lee, J.-F. Preparation and Morphology Studies of Nano Zinc Oxide Obtained Using Native and Modified Chitosans. Materials 2013, 6, 4198–4212. [Google Scholar] [CrossRef]
- Zhang, Q.P.; Xu, X.N.; Liu, Y.T.; Xu, M.; Deng, S.H.; Chen, Y.; Yuan, H.; Yu, F.; Huang, Y.; Zhao, K.; et al. A feasible strategy to balance the crystallinity and specific surface area of metal oxide nanocrystals. Sci. Rep. 2017, 7, 46424. [Google Scholar] [CrossRef] [Green Version]
- Demoisson, F.; Piolet, R.; Bernard, F. Hydrothermal Synthesis of ZnO Crystals from Zn(OH) 2 Metastable Phases at Room to Supercritical Conditions. Cryst. Growth Des. 2014, 14, 5388–5396. [Google Scholar] [CrossRef]
- Zhou, D.; Keller, A.A. Role of morphology in the aggregation kinetics of ZnO nanoparticles. Water Res. 2010, 44, 2948–2956. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Hu, H.; Hu, Y.H. Role of ZnO morphology in its reduction and photocatalysis. Appl. Surf. Sci. 2020, 502, 144202. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, X.; An, T.; Song, Z.; Fu, J.; Sheng, G.; Cui, M. Kinetics, degradation pathway and reaction mechanism of advanced oxidation of 4-nitrophenol in water by a UV/H2O2 process. J. Chem. Technol. Biotechnol. 2003, 78, 788–794. [Google Scholar] [CrossRef]
- Strachan, J.; Barnett, C.; Masters, A.F.; Maschmeyer, T. 4-Nitrophenol Reduction: Probing the Putative Mechanism of the Model Reaction. ACS Catal. 2020, 10, 5516–5521. [Google Scholar] [CrossRef]
- Pozun, Z.D.; Rodenbusch, S.E.; Keller, E.; Tran, K.; Tang, W.; Stevenson, K.J.; Henkelman, G. A Systematic Investigation of p -Nitrophenol Reduction by Bimetallic Dendrimer Encapsulated Nanoparticles. J. Phys. Chem. C 2013, 117, 7598–7604. [Google Scholar] [CrossRef]
- Dieckmann, M.S.; Gray, K.A. A comparison of the degradation of 4-nitrophenol via direct and sensitized photocatalysis in TiO2 slurries. Water Res. 1996, 30, 1169–1183. [Google Scholar] [CrossRef]
- Farooq, M.; Shujah, S.; Tahir, K.; Nazir, S.; Ullah Khan, A.; Almarhoon, Z.M.; Jevtovic, V.; Al-Shehri, H.S.; Tasleem Hussain, S.; Ullah, A. Ultra efficient 4-Nitrophenol reduction, dye degradation and Cr(VI) adsorption in the presence of phytochemical synthesized Ag/ZnO nanocomposite: A view towards sustainable chemistry. Inorg. Chem. Commun. 2022, 136, 109189. [Google Scholar] [CrossRef]
- Kadam, V.V.; Shanmugam, S.D.; Ettiyappan, J.P.; Balakrishnan, R.M. Photocatalytic degradation of p-nitrophenol using biologically synthesized ZnO nanoparticles. Environ. Sci. Pollut. Res. 2021, 28, 12119–12130. [Google Scholar] [CrossRef]
- Khatamian, M.; Divband, B.; Jodaei, A. Degradation of 4-nitrophenol (4-NP) using ZnO nanoparticles supported on zeolites and modeling of experimental results by artificial neural networks. Mater. Chem. Phys. 2012, 134, 31–37. [Google Scholar] [CrossRef]
- Avramescu, M.-L.; Rasmussen, P.E.; Chénier, M.; Gardner, H.D. Influence of pH, particle size and crystal form on dissolution behaviour of engineered nanomaterials. Environ. Sci. Pollut. Res. 2017, 24, 1553–1564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fatehah, M.O.; Aziz, H.A.; Stoll, S. Stability of ZnO Nanoparticles in Solution. Influence of pH, Dissolution, Aggregation and Disaggregation Effects. J. Colloid Sci. Biotechnol. 2014, 3, 75–84. [Google Scholar] [CrossRef]
- Mudunkotuwa, I.A.; Rupasinghe, T.; Wu, C.-M.; Grassian, V.H. Dissolution of ZnO Nanoparticles at Circumneutral pH: A Study of Size Effects in the Presence and Absence of Citric Acid. Langmuir 2012, 28, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Jennings, V.L.K.; Rayner-Brandes, M.H.; Bird, D.J. Assessing chemical toxicity with the bioluminescent photobacterium (vibrio fischeri): A comparison of three commercial systems. Water Res. 2001, 35, 3448–3456. [Google Scholar] [CrossRef]
- Scheerer, S.; Gomez, F.; Lloyd, D. Bioluminescence of Vibrio fischeri in continuous culture: Optimal conditions for stability and intensity of photoemission. J. Microbiol. Methods 2006, 67, 321–329. [Google Scholar] [CrossRef]
- Heinlaan, M.; Ivask, A.; Blinova, I.; Dubourguier, H.-C.; Kahru, A. Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus. Chemosphere 2008, 71, 1308–1316. [Google Scholar] [CrossRef]
- Groen, J.C.; Peffer, L.A.; Pérez-Ramıírez, J. Pore size determination in modified micro- and mesoporous materials. Pitfalls and limitations in gas adsorption data analysis. Microporous Mesoporous Mater. 2003, 60, 1–17. [Google Scholar] [CrossRef]

| Sample Designation | Halloysite (mg) | ZnO (mg) | ZnO (m/m%) |
|---|---|---|---|
| ZnO-100% | - | 200 | 100% |
| H-ZnO-28% | 500 | 191 | 28% |
| H-ZnO-16% | 500 | 96 | 16% |
| H-ZnO-9% | 500 | 48 | 9% |
| H-ZnO-5% | 500 | 24 | 5% |
| H-ZnO-2% | 500 | 12 | 2% |
| Hal | 500 | - | - |
| Sample Designation | SSABET (m2/g) | SSABJH (m2/g) | SSAmicro1 (m2/g) | Vmicro1 (cm3/g) | Vmicro+meso1 (cm3/g) | Daverage (nm) |
|---|---|---|---|---|---|---|
| ZnO-100% | 13 | 13 | 2.0 | 0.0009 | 0.0562 | 17.4 |
| H-ZnO-28% | 27 | 30 | 3.4 | 0.0014 | 0.0932 | 12.5 |
| H-ZnO-16% | 35 | 38 | 4.4 | 0.0018 | 0.1344 | 14.0 |
| H-ZnO-9% | 41 | 48 | 4.4 | 0.0017 | 0.1550 | 12.8 |
| H-ZnO-5% | 44 | 53 | 4.7 | 0.0018 | 0.1811 | 13.6 |
| H-ZnO-2% | 47 | 59 | 5.7 | 0.0023 | 0.1968 | 13.4 |
| Hal | 94 | 94 | 14 | 0.0058 | 0.2203 | 9.4 |
| Composite Designation | pH = 1.9 Zn2+ (mg/mL) | pH = 12.4 Zn2+ (mg/mL) | pH = 5.6 Zn2+ (mg/mL) |
|---|---|---|---|
| ZnO-100% | 317 | 0.73 | <0.46 |
| H-ZnO-28% | 320 | 0.71 | <0.46 |
| H-ZnO-16% | 308 | 1.03 | <0.46 |
| H-ZnO-9% | 316 | 0.66 | <0.46 |
| H-ZnO-5% | 305 | 0.94 | <0.46 |
| H-ZnO-2% | 320 | 1.11 | <0.46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zsirka, B.; Vágvölgyi, V.; Horváth, E.; Juzsakova, T.; Fónagy, O.; Szabó-Bárdos, E.; Kristóf, J. Halloysite-Zinc Oxide Nanocomposites as Potential Photocatalysts. Minerals 2022, 12, 476. https://doi.org/10.3390/min12040476
Zsirka B, Vágvölgyi V, Horváth E, Juzsakova T, Fónagy O, Szabó-Bárdos E, Kristóf J. Halloysite-Zinc Oxide Nanocomposites as Potential Photocatalysts. Minerals. 2022; 12(4):476. https://doi.org/10.3390/min12040476
Chicago/Turabian StyleZsirka, Balázs, Veronika Vágvölgyi, Erzsébet Horváth, Tatjána Juzsakova, Orsolya Fónagy, Erzsébet Szabó-Bárdos, and János Kristóf. 2022. "Halloysite-Zinc Oxide Nanocomposites as Potential Photocatalysts" Minerals 12, no. 4: 476. https://doi.org/10.3390/min12040476