Field Studies on the Effect of Bioaugmentation with Bacillus amyloliquefaciens FZB42 on Plant Accumulation of Rare Earth Elements and Selected Trace Elements
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Site Characterization
2.2. Plant Growth Experiment and Soil Inoculation
2.3. Incubation Experiment
2.4. Sample Preparation and Analysis
2.4.1. Soil Samples
2.4.2. Plant Samples
2.5. Determination of Concentration of Elements in Soil and Plant Samples
2.6. Statistical Analysis
3. Results
3.1. Effect of Inoculation on Element Bioavailability in Soil
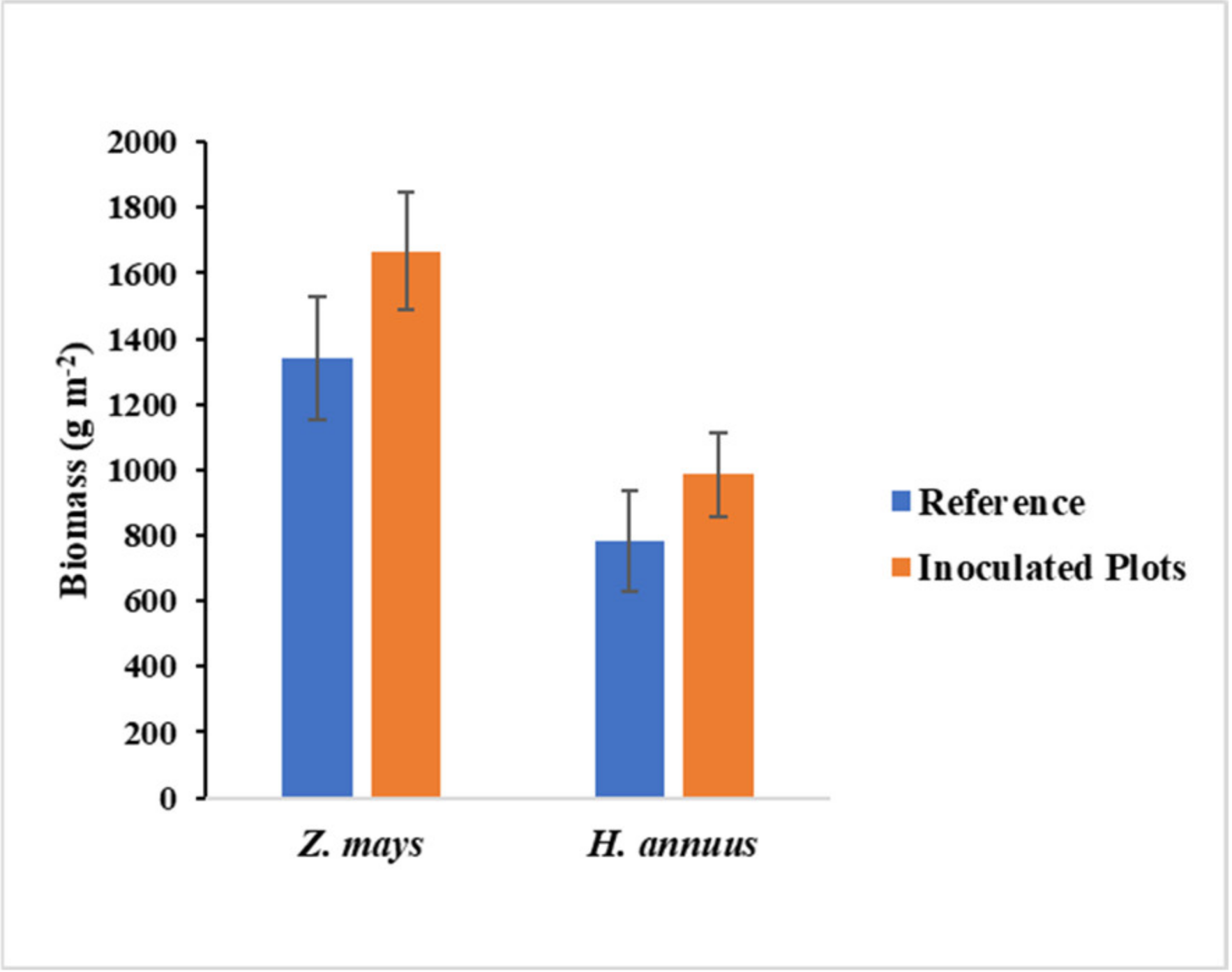
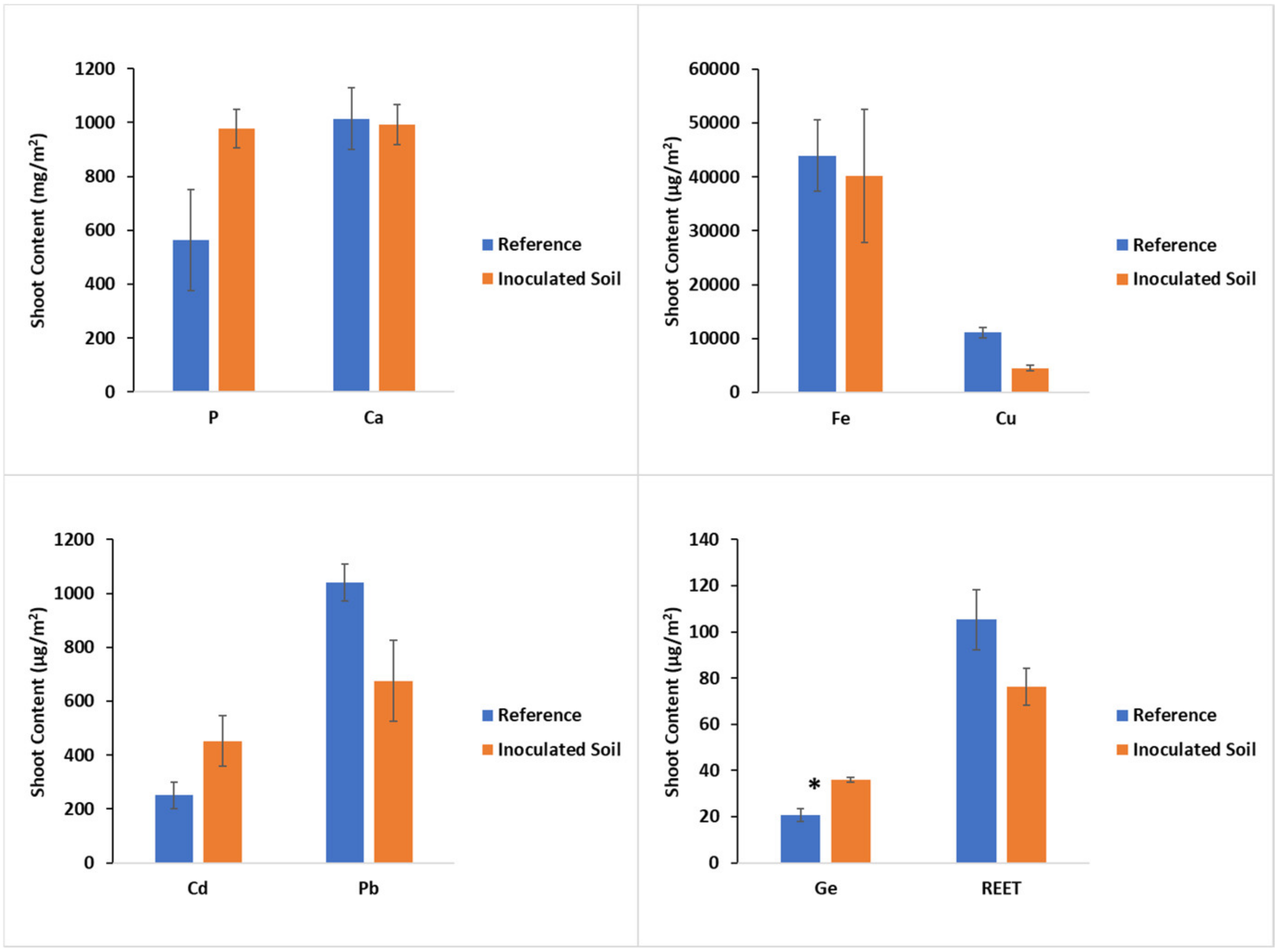
3.2. Effects of Inoculation on Biomass, Concentration, and Accumulation of Investigated Elements by Plants
4. Discussion
4.1. Effect of Soil Inoculation on Biomass Production
4.2. Effect of Soil Inoculation on Bioavailability of Elements in Root Soils
4.3. Effect of Soil Inoculation on Plant Concentration and Accumulation of Investigated Elements
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jordan, G. Sustainable Mineral Resources Management: From Regional Mineral Resources Exploration to Spatial Contamination Risk Assessment of Mining. Environ. Geol. 2009, 58, 153. [Google Scholar] [CrossRef]
- Aide, M. Review and Assessment of Organic and Inorganic Rare Earth Element Complexation in Soil, Surface Water, and Groundwater. In Rare Earth Elements and Their Minerals; Aide, M., Nakajima, T., Eds.; IntechOpen: London, UK, 2020; ISBN 978-1-78984-740-6. [Google Scholar]
- Rouxel, O.J.; Luais, B. Germanium Isotope Geochemistry. Rev. Mineral. Geochem. 2017, 82, 601–656. [Google Scholar] [CrossRef]
- Belissont, R.; Munoz, M.; Boiron, M.-C.; Luais, B.; Mathon, O. Germanium Crystal Chemistry in Cu-Bearing Sulfides from Micro-XRF Mapping and Micro-XANES Spectroscopy. Minerals 2019, 9, 227. [Google Scholar] [CrossRef] [Green Version]
- Tangahu, B.V.; Sheikh Abdullah, S.R.; Basri, H.; Idris, M.; Anuar, N.; Mukhlisin, M. A Review on Heavy Metals (As, Pb, and Hg) Uptake by Plants through Phytoremediation. Int. J. Chem. Eng. 2011, 2011, 1–31. [Google Scholar] [CrossRef]
- Huang, S.-W.; Jin, J.-Y. Status of Heavy Metals in Agricultural Soils as Affected by Different Patterns of Land Use. Environ. Monit. Assess. 2008, 139, 317–327. [Google Scholar] [CrossRef] [PubMed]
- European Commission Critical Raw Materials for the EU. Report of the Ad Hoc Working Group on Defining Critical Raw Materials; European Commission: Brussels, Belgium, 2014. [Google Scholar]
- Melcher, F.; Buchholz, P. Germanium. In Critical Metals Handbook; Gunn, G., Ed.; John Wiley & Sons: Oxford, UK, 2013; pp. 177–203. ISBN 978-1-118-75534-1. [Google Scholar]
- Sattler, J.A.G.; De-Melo, A.A.M.; do Nascimento, K.S.; de Melo, I.L.P.; Mancini-Filho, J.; Sattler, A.; de Almeida-Muradian, L.B. Essential Minerals and Inorganic Contaminants (Barium, Cadmium, Lithium, Lead and Vanadium) in Dried Bee Pollen Produced in Rio Grande Do Sul State, Brazil. Food Sci. Technol. 2016, 36, 505–509. [Google Scholar] [CrossRef] [Green Version]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. In Molecular, Clinical and Environmental Toxicology; Luch, A., Ed.; Experientia Supplementum; Springer: Basel, Switzerland, 2012; Volume 101, pp. 133–164. ISBN 978-3-7643-8339-8. [Google Scholar]
- Malhotra, N.; Hsu, H.-S.; Liang, S.-T.; Roldan, M.J.M.; Lee, J.-S.; Ger, T.-R.; Hsiao, C.-D. An Updated Review of Toxicity Effect of the Rare Earth Elements (REEs) on Aquatic Organisms. Animals 2020, 10, 1663. [Google Scholar] [CrossRef]
- Thomas, P.J.; Carpenter, D.; Boutin, C.; Allison, J.E. Rare Earth Elements (REEs): Effects on Germination and Growth of Selected Crop and Native Plant Species. Chemosphere 2014, 96, 57–66. [Google Scholar] [CrossRef] [Green Version]
- Jordan, G.; D’Alessandro, M. Mining, Mining Waste and Related Environmental Issues: Problems and Solutions in Central and Eastern European Candidate Countries; Office for Official Publications of the European Communities: Luxembourg, 2004; ISBN 978-92-894-4935-9. [Google Scholar]
- Khan, M.N.; Mobin, M.; Abbas, Z.K.; Alamri, S.A. Fertilizers and Their Contaminants in Soils, Surface and Groundwater. In Encyclopedia of the Anthropocene; Elsevier: Amsterdam, The Netherlands, 2018; pp. 225–240. ISBN 978-0-12-813576-1. [Google Scholar]
- Lacalle, R.G.; Aparicio, J.D.; Artetxe, U.; Urionabarrenetxea, E.; Polti, M.A.; Soto, M.; Garbisu, C.; Becerril, J.M. Gentle Remediation Options for Soil with Mixed Chromium (VI) and Lindane Pollution: Biostimulation, Bioaugmentation, Phytoremediation and Vermiremediation. Heliyon 2020, 6, e04550. [Google Scholar] [CrossRef]
- Manoj, S.R.; Karthik, C.; Kadirvelu, K.; Arulselvi, P.I.; Shanmugasundaram, T.; Bruno, B.; Rajkumar, M. Understanding the Molecular Mechanisms for the Enhanced Phytoremediation of Heavy Metals through Plant Growth Promoting Rhizobacteria: A Review. J. Environ. Manage. 2020, 254, 109779. [Google Scholar] [CrossRef]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy Metals, Occurrence and Toxicity for Plants: A Review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Gupta, D.K.; Sandalio, L.M. Metal Toxicity in Plants: Perception, Signaling and Remediation; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2012; ISBN 978-3-642-22080-7. [Google Scholar]
- Cundy, A.B.; Bardos, R.P.; Puschenreiter, M.; Mench, M.; Bert, V.; Friesl-Hanl, W.; Müller, I.; Li, X.N.; Weyens, N.; Witters, N.; et al. Brownfields to Green Fields: Realising Wider Benefits from Practical Contaminant Phytomanagement Strategies. J. Environ. Manage 2016, 184, 67–77. [Google Scholar] [CrossRef] [Green Version]
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A Promising Approach for Revegetation of Heavy Metal-Polluted Land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef]
- Gerhardt, K.E.; Gerwing, P.D.; Greenberg, B.M. Opinion: Taking Phytoremediation from Proven Technology to Accepted Practice. Plant Sci. 2017, 256, 170–185. [Google Scholar] [CrossRef]
- Fischerová, Z.; Tlustoš, P.; Száková, J.; Šichorová, K. A Comparison of Phytoremediation Capability of Selected Plant Species for given Trace Elements. Environ. Pollut. 2006, 144, 93–100. [Google Scholar] [CrossRef]
- Anderson, C.W.N. Phytoextraction to Promote Sustainable Development. J. Degrad. Min. Lands Manag. 2013, 1, 51–56. [Google Scholar]
- Gupta, P.; Rani, R.; Usmani, Z.; Chandra, A.; Kumar, V. The Role of Plant-Associated Bacteria in Phytoremediation of Trace Metals in Contaminated Soils. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 69–76. ISBN 978-0-444-64191-5. [Google Scholar]
- Efremova, M.; Izosimova, A. Contamination of Agricultural Soils with Heavy Metals. In Ecosystem Health and Sustainable Agriculture; Baltic University Press: Uppsala, Sweden, 2012; Volume 1, pp. 250–252. ISBN 978-91-86189-10-5. [Google Scholar]
- Alvarez, A.; Benimeli, C.S.; Saez, J.M.; Fuentes, M.S.; Cuozzo, S.A.; Polti, M.A.; Amoroso, M.J. Bacterial Bio-Resources for Remediation of Hexachlorocyclohexane. Int. J. Mol. Sci. 2012, 13, 15086–15106. [Google Scholar] [CrossRef]
- Gutiérrez-Corona, J.F.; Romo-Rodríguez, P.; Santos-Escobar, F.; Espino-Saldaña, A.E.; Hernández-Escoto, H. Microbial Interactions with Chromium: Basic Biological Processes and Applications in Environmental Biotechnology. World J. Microbiol. Biotechnol. 2016, 32, 191. [Google Scholar] [CrossRef]
- Braud, A.; Jézéquel, K.; Vieille, E.; Tritter, A.; Lebeau, T. Changes in Extractability of Cr and Pb in a Polycontaminated Soil After Bioaugmentation with Microbial Producers of Biosurfactants, Organic Acids and Siderophores. Water Air Soil Pollut. Focus 2006, 6, 261–279. [Google Scholar] [CrossRef]
- Vivas, A.; Vörös, I.; Biró, B.; Campos, E.; Barea, J.M.; Azcón, R. Symbiotic Efficiency of Autochthonous Arbuscular Mycorrhizal Fungus (G. Mosseae) and Brevibacillus Sp. Isolated from Cadmium Polluted Soil under Increasing Cadmium Levels. Environ. Pollut. 2003, 126, 179–189. [Google Scholar] [CrossRef]
- Borriss, R. Use of Plant-Associated Bacillus Strains as Biofertilizers and Biocontrol Agents in Agriculture. In Bacteria in Agrobiology: Plant Growth Responses; Maheshwari, D.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 41–76. ISBN 978-3-642-20331-2. [Google Scholar]
- Borriss, R. Phytostimulation and Biocontrol by the Plant-Associated Bacillus Amyloliquefaciens FZB42: An Update. In Bacilli and Agrobiotechnology; Islam, M.T., Rahman, M., Pandey, P., Jha, C.K., Aeron, A., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 163–184. ISBN 978-3-319-44408-6. [Google Scholar]
- Kumar, P.; Pandey, P.; Dubey, R.C.; Maheshwari, D.K. Bacteria Consortium Optimization Improves Nutrient Uptake, Nodulation, Disease Suppression and Growth of the Common Bean (Phaseolus Vulgaris) in Both Pot and Field Studies. Rhizosphere 2016, 2, 13–23. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Hashem, A.; Abd_Allah, E.F. Bacillus: A Biological Tool for Crop Improvement through Bio-Molecular Changes in Adverse Environments. Front. Physiol. 2017, 8, 667. [Google Scholar] [CrossRef]
- Khan, A.L.; Bilal, S.; Halo, B.A.; Al-Harrasi, A.; Khan, A.R.; Waqas, M.; Al-Thani, G.S.; Al-Amri, I.; Al-Rawahi, A.; Lee, I.-J. Bacillus Amyloliquefaciens BSL16 Improves Phytoremediation Potential of Solanum Lycopersicum during Copper Stress. J. Plant Interact. 2017, 12, 550–559. [Google Scholar] [CrossRef] [Green Version]
- Schwabe, R.; Dittrich, C.; Kadner, J.; Rudi Senges, C.H.; Bandow, J.E.; Tischler, D.; Schlömann, M.; Levicán, G.; Wiche, O. Secondary Metabolites Released by the Rhizosphere Bacteria Arthrobacter Oxydans and Kocuria Rosea Enhance Plant Availability and Soil-Plant Transfer of Germanium (Ge) and Rare Earth Elements (REEs). Chemosphere 2021, 285, 131466. [Google Scholar] [CrossRef]
- Rajkumar, M.; Freitas, H. Influence of Metal Resistant-Plant Growth-Promoting Bacteria on the Growth of Ricinus Communis in Soil Contaminated with Heavy Metals. Chemosphere 2008, 71, 834–842. [Google Scholar] [CrossRef] [Green Version]
- Ogo, Y.; Kakei, Y.; Itai, R.N.; Kobayashi, T.; Nakanishi, H.; Takahashi, H.; Nakazono, M.; Nishizawa, N.K. Spatial Transcriptomes of Iron-deficient and Cadmium-stressed Rice. New Phytol. 2014, 201, 781–794. [Google Scholar] [CrossRef]
- Xie, Y.; Li, X.; Liu, X.; Amombo, E.; Chen, L.; Fu, J. Application of Aspergillus Aculeatus to Rice Roots Reduces Cd Concentration in Grain. Plant Soil 2018, 422, 409–422. [Google Scholar] [CrossRef]
- Thiem, D.; Złoch, M.; Gadzała-Kopciuch, R.; Szymańska, S.; Baum, C.; Hrynkiewicz, K. Cadmium-Induced Changes in the Production of Siderophores by a Plant Growth Promoting Strain of Pseudomonas fulva. J. Basic Microbiol. 2018, 58, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Bolan, N.S.; Adriano, D.C.; Naidu, R. Role of Phosphorus in (Im)Mobilization and Bioavailability of Heavy Metals in the Soil-Plant System. In Reviews of Environmental Contamination and Toxicology; Reviews of Environmental Contamination and Toxicology; Springer: New York, NY, USA, 2003; Volume 177, pp. 1–44. ISBN 978-0-387-00214-9. [Google Scholar]
- Fahad, S.; Hussain, S.; Bano, A.; Saud, S.; Hassan, S.; Shan, D.; Khan, F.A.; Khan, F.; Chen, Y.; Wu, C.; et al. Potential Role of Phytohormones and Plant Growth-Promoting Rhizobacteria in Abiotic Stresses: Consequences for Changing Environment. Environ. Sci. Pollut. Res. 2015, 22, 4907–4921. [Google Scholar] [CrossRef] [PubMed]
- Jambhulkar, P.P.; Sharma, P.; Yadav, R. Delivery Systems for Introduction of Microbial Inoculants in the Field. In Microbial Inoculants in Sustainable Agricultural Productivity; Singh, D.P., Singh, H.B., Prabha, R., Eds.; Springer: New Delhi, India, 2016; pp. 199–218. ISBN 978-81-322-2642-0. [Google Scholar]
- Tang, A.; Haruna, A.O.; Majid, N.M.Ab.; Jalloh, M.B. Potential PGPR Properties of Cellulolytic, Nitrogen-Fixing, Phosphate-Solubilizing Bacteria in Rehabilitated Tropical Forest Soil. Microorganisms 2020, 8, 442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixon, M.; Simonne, E.; Obreza, T.; Liu, G. Crop Response to Low Phosphorus Bioavailability with a Focus on Tomato. Agronomy 2020, 10, 617. [Google Scholar] [CrossRef]
- Li, W.; Lan, P. The Understanding of the Plant Iron Deficiency Responses in Strategy I Plants and the Role of Ethylene in This Process by Omic Approaches. Front. Plant Sci. 2017, 8, 40. [Google Scholar] [CrossRef] [Green Version]
- Morrissey, J.; Guerinot, M.L. Iron Uptake and Transport in Plants: The Good, the Bad, and the Ionome. Chem. Rev. 2009, 109, 4553–4567. [Google Scholar] [CrossRef] [Green Version]
- Robinson, B.; Bolan, N.; Mahimairaja, S.; Clothier, B. Solubility, Mobility, and Bioaccumulation of Trace Elements: Abiotic Processes in the Rhizosphere. In Trace elements in the environment: Biogeochemistry, Biotechnology and Bioremediation; Prasad, M.N.V., Sajwan, K.S., Naidu, R., Eds.; CRC/Taylor and Francis: Boca Raton, FL, USA, 2005; ISBN 978-1-4200-3204-8. [Google Scholar]
- Baldovinos, F.; Thomas, G.W. The Effect of Soil Clay Content on Phosphorus Uptake. Soil Sci. Soc. Am. J. 1967, 31, 680–682. [Google Scholar] [CrossRef]
- Okoroafor, P.U.; Ogunkunle, C.O.; Heilmeier, H.; Wiche, O. Phytoaccumulation Potential of Nine Plant Species for Selected Nutrients, Rare Earth Elements (REEs), Germanium (Ge), and Potentially Toxic Elements (PTEs) in Soil. Int. J. Phytoremediat. 2022, 24, 1–11. [Google Scholar] [CrossRef]
- Marschner, H.; Römheld, V. Strategies of Plants for Acquisition of Iron. Plant Soil 1994, 165, 261–274. [Google Scholar] [CrossRef]
- Naranjo-Arcos, M.A.; Bauer, P. Iron Nutrition, Oxidative Stress, and Pathogen Defense. In Nutritional Deficiency; Erkekoglu, P., Kocer-Gumusel, B., Eds.; InTech: London, UK, 2016; ISBN 978-953-51-2437-5. [Google Scholar]
- Fernandez, M.C.; Rubio, G. Root Morphological Traits Related to Phosphorus-uptake Efficiency of Soybean, Sunflower, and Maize. J. Plant Nutr. Soil Sci. 2015, 178, 807–815. [Google Scholar] [CrossRef]
- Wiche, O.; Dreier, F.; Ehrhardt, A.; Gerisch, M.K.; Jodoin, R.; Kessler, S.; Missfeldt, T.; Röder, M.; Rumberg, C.; Schulte, M.G.; et al. Mobilität von Potentiell Toxischen Spurenelementen in Oberflächennahen Spülsanden Der Spülhalde Davidschacht, Freiberg Und Deren Verlagerung in Umliegende Flächen. Freib. Ecol. Online 2018, 4, 1–19. [Google Scholar]
- Cao, X.; Chen, Y.; Wang, X.; Deng, X. Effects of Redox Potential and PH Value on the Release of Rare Earth Elements from Soil. Chemosphere 2001, 44, 655–661. [Google Scholar] [CrossRef]
- Olaniran, A.; Balgobind, A.; Pillay, B. Bioavailability of Heavy Metals in Soil: Impact on Microbial Biodegradation of Organic Compounds and Possible Improvement Strategies. Int. J. Mol. Sci. 2013, 14, 10197–10228. [Google Scholar] [CrossRef] [Green Version]
- Tóth, G.; Hermann, T.; Da Silva, M.R.; Montanarella, L. Heavy Metals in Agricultural Soils of the European Union with Implications for Food Safety. Environ. Int. 2016, 88, 299–309. [Google Scholar] [CrossRef]
- Okoroafor, P.U.; Mann, L.; Ngu, K.A.; Zaffar, N.; Monei, N.L.; Boldt, C.; Reitz, T.; Heilmeier, H.; Wiche, O. Impact of Soil Inoculation with Bacillus Amyloliquefaciens FZB42 on the Phytoaccumulation of Germanium, Rare Earth Elements, and Potentially Toxic Elements. Plants 2022, 11, 341. [Google Scholar] [CrossRef]
- Uddin, A.H.; Khalid, R.S.; Alaama, M.; Abdualkader, A.M.; Kasmuri, A.; Abbas, S.A. Comparative Study of Three Digestion Methods for Elemental Analysis in Traditional Medicine Products Using Atomic Absorption Spectrometry. J. Anal. Sci. Technol. 2016, 7, 6. [Google Scholar] [CrossRef] [Green Version]
- Petruzzelli, G.; Pedron, F.; Rosellini, I. Bioavailability and Bioaccessibility in Soil: A Short Review and a Case Study. AIMS Environ. Sci. 2020, 7, 208–225. [Google Scholar] [CrossRef]
- Krachler, M.; Mohl, C.; Emons, H.; Shotyk, W. Influence of Digestion Procedures on the Determination of Rare Earth Elements in Peat and Plant Samples by USN-ICP-MS. J. Anal. At. Spectrom. 2002, 17, 844–851. [Google Scholar] [CrossRef] [Green Version]
- Pourret, O.; Hursthouse, A.; Irawan, D.E.; Johannesson, K.; Liu, H.; Poujol, M.; Tartèse, R.; van Hullebusch, E.D.; Wiche, O. Open Access Publishing Practice in Geochemistry: Overview of Current State and Look to the Future. Heliyon 2020, 6, e03551. [Google Scholar] [CrossRef]
- China National Analysis Center for Iron and Steel. Certificate of Certified Reference Materials; LGC Standards: Teddington, UK, 2021. [Google Scholar]
- Kaiser, S.; Wagner, S.; Moschner, C.; Funke, C.; Wiche, O. Accumulation of Germanium (Ge) in Plant Tissues of Grasses Is Not Solely Driven by Its Incorporation in Phytoliths. Biogeochemistry 2020, 148, 49–68. [Google Scholar] [CrossRef] [Green Version]
- Fässler, E.; Robinson, B.H.; Gupta, S.K.; Schulin, R. Uptake and Allocation of Plant Nutrients and Cd in Maize, Sunflower and Tobacco Growing on Contaminated Soil and the Effect of Soil Conditioners under Field Conditions. Nutr. Cycl. Agroecosystems 2010, 87, 339–352. [Google Scholar] [CrossRef] [Green Version]
- Samaniego-Gámez, B.Y.; Garruña, R.; Tun-Suárez, J.M.; Kantun-Can, J.; Reyes-Ramírez, A.; Cervantes-Díaz, L. Bacillus Spp. Inoculation Improves Photosystem II Efficiency and Enhances Photosynthesis in Pepper Plants. Chil. J. Agric. Res. 2016, 76, 409–416. [Google Scholar] [CrossRef] [Green Version]
- Shilev, S. Soil Rhizobacteria Regulating the Uptake of Nutrients and Undesirable Elements by Plants. In Plant Microbe Symbiosis: Fundamentals and Advances; Arora, N.K., Ed.; Springer: New Delhi, India, 2013; pp. 147–167. ISBN 978-81-322-1286-7. [Google Scholar]
- Gowtham, H.G.; Murali, M.; Singh, S.B.; Lakshmeesha, T.R.; Murthy, K.N.; Amruthesh, K.N.; Niranjana, S.R. Plant Growth Promoting Rhizobacteria-Bacillus Amyloliquefaciens Improves Plant Growth and Induces Resistance in Chilli against Anthracnose Disease. Biol. Control 2018, 126, 209–217. [Google Scholar] [CrossRef]
- Alami, Y.; Achouak, W.; Marol, C.; Heulin, T. Rhizosphere Soil Aggregation and Plant Growth Promotion of Sunflowers by an Exopolysaccharide-Producing Rhizobium Sp. Strain Isolated from Sunflower Roots. Appl. Environ. Microbiol. 2000, 66, 3393–3398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbaszadeh-Dahaji, P.; Baniasad-Asgari, A.; Hamidpour, M. The Effect of Cu-Resistant Plant Growth-Promoting Rhizobacteria and EDTA on Phytoremediation Efficiency of Plants in a Cu-Contaminated Soil. Environ. Sci. Pollut. Res. 2019, 26, 31822–31833. [Google Scholar] [CrossRef] [PubMed]
- Breault, R.F.; Colman, J.A.; Aiken, G.R.; McKnight, D. Copper Speciation and Binding by Organic Matter in Copper-Contaminated Streamwater. Environ. Sci. Technol. 1996, 30, 3477–3486. [Google Scholar] [CrossRef]
- Fang, Q.; Fan, Z.; Xie, Y.; Wang, X.; Li, K.; Liu, Y. Screening and Evaluation of the Bioremediation Potential of Cu/Zn-Resistant, Autochthonous Acinetobacter Sp. FQ-44 from Sonchus Oleraceus L. Front. Plant Sci. 2016, 7, 1487. [Google Scholar] [CrossRef] [Green Version]
- Dalvi, A.A.; Bhalerao, S.A. Response of Plants towards Heavy Metal Toxicity: An Overview of Avoidance, Tolerance and Uptake Mechanism. Ann. Plant Sci. 2013, 2, 362–368. [Google Scholar]
- Braud, A.; Jézéquel, K.; Bazot, S.; Lebeau, T. Enhanced Phytoextraction of an Agricultural Cr- and Pb-Contaminated Soil by Bioaugmentation with Siderophore-Producing Bacteria. Chemosphere 2009, 74, 280–286. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of Heavy Metals—Concepts and Applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef]
- Van Belleghem, F.; Cuypers, A.; Semane, B.; Smeets, K.; Vangronsveld, J.; d’Haen, J.; Valcke, R. Subcellular Localization of Cadmium in Roots and Leaves of Arabidopsis thaliana. New Phytol. 2007, 173, 495–508. [Google Scholar] [CrossRef]
- De Oliveira, C.; Ramos, S.J.; Siqueira, J.O.; Faquin, V.; de Castro, E.M.; Amaral, D.C.; Techio, V.H.; Coelho, L.C.; e Silva, P.H.P.; Schnug, E.; et al. Bioaccumulation and Effects of Lanthanum on Growth and Mitotic Index in Soybean Plants. Ecotoxicol. Environ. Saf. 2015, 122, 136–144. [Google Scholar] [CrossRef]
- Hu, Z.; Richter, H.; Sparovek, G.; Schnug, E. Physiological and Biochemical Effects of Rare Earth Elements on Plants and Their Agricultural Significance: A Review. J. Plant Nutr. 2004, 27, 183–220. [Google Scholar] [CrossRef]
- Kashem, M.A.; Singh, B.R.; Kondo, T.; Imamul Huq, S.M.; Kawai, S. Comparison of Extractability of Cd, Cu, Pb and Zn with Sequential Extraction in Contaminated and Non-Contaminated Soils. Int. J. Environ. Sci. Technol. 2007, 4, 169–176. [Google Scholar] [CrossRef] [Green Version]
- Khorasanipour, M.; Tangestani, M.H.; Naseh, R.; Hajmohammadi, H. Hydrochemistry, Mineralogy and Chemical Fractionation of Mine and Processing Wastes Associated with Porphyry Copper Mines: A Case Study from the Sarcheshmeh Mine, SE Iran. Appl. Geochem. 2011, 26, 714–730. [Google Scholar] [CrossRef]
- Novakovic, R.; Giuranno, D.; Lee, J.; Mohr, M.; Delsante, S.; Borzone, G.; Miani, F.; Fecht, H.-J. Thermophysical Properties of Fe-Si and Cu-Pb Melts and Their Effects on Solidification Related Processes. Metals 2022, 12, 336. [Google Scholar] [CrossRef]
- Konieczyński, P.; Moszczyński, J.; Wesołowski, M. Comparison of Fe, Zn, Mn, Cu, Cd and Pb Concentration in Spruce Needles Collected in the Area of Gdansk and Gdynia in Northern Poland. Environ. Prot. Nat. Resour. 2018, 29, 1–9. [Google Scholar] [CrossRef]
- Dietz, S.; Herz, K.; Gorzolka, K.; Jandt, U.; Bruelheide, H.; Scheel, D. Root Exudate Composition of Grass and Forb Species in Natural Grasslands. Sci. Rep. 2020, 10, 10691. [Google Scholar] [CrossRef]
- Grover, M.; Bodhankar, S.; Sharma, A.; Sharma, P.; Singh, J.; Nain, L. PGPR Mediated Alterations in Root Traits: Way Toward Sustainable Crop Production. Front. Sustain. Food Syst. 2021, 4, 618230. [Google Scholar] [CrossRef]


| Soil physicochemical parameters | ||
| Soil physicochemical parameters | Value | |
| pH value in aqueous solution | 5.6 | |
| Conductivity | 351 µS/cm | |
| Organic matter content | 7.3% | |
| Nitrate concentration | 160.0 mg/kg | |
| Ammonium concentration | 1.4 mg/kg | |
| Phosphate concentration | 42.9 mg/kg | |
| Total concentration (µg/g) and water-soluble concentration (mean ± SE, n = 6) | ||
| Total concentration | Water-soluble concentration | |
| P | 477 ± 38 | 1.26 ± 0.41 |
| Ca | 2689 ± 115 | 48 ± 5.6 |
| Fe | 23,570 ± 1937 | 7.40 ± 0.78 |
| Cu | 31 ± 1.8 | 0.19 ± 0.012 |
| Cd | 1.9 ± 0.08 | 0.0052 ± 0.0003 |
| Pb | 228 ± 18 | 0.12 ± 0.007 |
| REET | 109 ± 11 | 0.03 ± 0.002 |
| Ge | 0.63 ± 0.03 | 0.001 ± 0.0001 |
| Species | Treatment | P | Fe | Cu | Cd | Pb | Ge | REET | |
|---|---|---|---|---|---|---|---|---|---|
| Zea mays | NIL | 16.90 ± 6.26 | 1.68 ± 0.21 | 0.49 ± 0.02 | 1.51 ± 0.10 | 1.02 ± 0.52 | 0.0023 ± 0.0006 | 0.60 ± 1.87 | |
| R | 25.62 ± 6.95 | 1.78 ± 0.33 | 0.55 ± 0.07 | 1.97 ± 0.06 | 1.39 ± 0.68 | 0.0029 ± 0.0004 | 0.65 ± 0.27 | ||
| Statistic a | 0.87 | 0.07 | 0.63 | 16.63 | 0.18 | 0.71 | 0.03 | ||
| p value | 0.40 | 0.81 | 0.50 | 0.02 | 0.69 | 0.46 | 0.88 | ||
| Helianthus annuus | |||||||||
| NIL | 24.21 ± 4.90 | 2.74 ± 0.82 | 0.58 ± 0.11 | 1.85 ± 0.10 | 2.29 ± 1.15 | 0.0080 ± 0.0060 | 0.92 ± 0.37 | ||
| R | 21.10 ± 4.47 | 1.51 ± 0.10 | 0.41 ± 0.04 | 1.57 ± 0.07 | 1.08 ± 0.62 | 0.0033 ± 0.0011 | 0.55 ± 0.23 | ||
| Statistic a | 0.22 | 2.222 | 2.205 | 4.951 | 0.844 | 0.585 | 0.722 | ||
| p value | 0.661 | 0.271 | 0.254 | 0.094 | 0.423 | 0.52 | 0.449 | ||
| Species | Treatment | P | Ca | Fe | Cu | Cd | Pb | Ge | REET |
| Zea mays | NIL | 0.96 ± 0.43 | 40.15 ± 7.24 | 5.87 ± 0.56 | 0.17 ± 0.011 | 0.004 ± 0.00014 | 0.104 ± 0.019 | 0.00050 ± 0.000098 | 0.029 ± 0.0022 |
| R | 0.94 ± 0.27 | 41.75 ± 7.90 | 6.73 ± 1.50 | 0.18 ± 0.017 | 0.007 ± 0.0037 | 0.141 ± 0.068 | 0.00064 ± 0.000083 | 0.034 ± 0.0071 | |
| Statistic a | 0.001 | 0.022 | 0.291 | 0.204 | 0.948 | 0.284 | 0.427 | 1.292 | |
| p value | 0.978 | 0.889 | 0.633 | 0.678 | 0.433 | 0.641 | 0.571 | 0.321 | |
| Helianthus annuus | NIL | 0.88 ± 0.18 | 46.27 ± 3.87 | 6.29 ± 0.40 | 0.18 ± 0.00 | 0.0063 ± 0.0024 | 0.13 ± 0.03 | 0.00056 ± 0.000065 | 0.035 ± 0.004 |
| R | 1.34 ± 0.61 | 59.17 ± 6.65 | 5.85 ± 0.75 | 0.16 ± 0.02 | 0.0037 ± 0.0003 | 0.09 ± 0.02 | 0.00051 ± 0.000033 | 0.029 ± 0.001 | |
| Statistic a | 0.537 | 2.811 | 0.275 | 0.627 | 1.192 | 1.618 | 0.422 | 1.795 | |
| p value | 0.509 | 0.16 | 0.625 | 0.482 | 0.385 | 0.283 | 0.562 | 0.297 | |
| Species | Treatment | P | Ca | Fe | Cu | Cd | Pb | Ge | REET |
|---|---|---|---|---|---|---|---|---|---|
| A (SC) | NIL | 2336 ± 286 | 4952 ± 1061 | 172 ± 39 | 64 ± 13.7 | 1.04 ± 0.23 | 4.39 ± 1.04 | 0.07 ± 0.003 | 0.49 ± 0.094 |
| R | 2534 ± 361 | 3985 ± 79 | 138 ± 31 | 18 ± 2.2 | 1.48 ± 0.29 | 2.64 ± 0.60 | 0.11 ± 0.010 | 0.37 ± 0.059 | |
| Statistic a | 0.14 | 0.82 | 0.39 | 10.53 | 1.17 | 1.96 | 6.08 | 1.03 | |
| p value | 0.73 | 0.46 | 0.56 | 0.08 | 0.35 | 0.24 | 0.13 | 0.37 | |
| A (RC) | NIL | 130 ± 14.0 | 1974 ± 164 | 2386 ± 424 | 6.69 ± 1.29 | 1.41 ± 0.08 | 25.4 ± 4.02 | 0.13 ± 0.022 | 13.05 ± 1.87 |
| R | 304 ± 106 | 2916 ± 330 | 1891 ± 316 | 8.18 ± 0.23 | 2.29 ± 0.42 | 22.3 ± 2.82 | 0.12 ± 0.014 | 10.58 ± 1.55 | |
| Statistic a | 2.67 | 6.55 | 0.88 | 1.29 | 4.31 | 0.42 | 0.11 | 1.03 | |
| p value | 0.24 | 0.09 | 0.41 | 0.37 | 0.17 | 0.56 | 0.76 | 0.37 | |
| B (SC) | NIL | 3265 ± 532 | 32,822 ± 1199 | 437 ± 77 | 114 ± 6.5 | 12.0 ± 4.4 | 11.2 ± 2.4 | 0.019 ± 0.002 | 1.4 ± 0.3 |
| R | 2829 ± 84 | 35,253 ± 1568 | 417 ± 85 | 135 ± 33 | 8.6 ± 3.6 | 11.8 ± 2.8 | 0.022 ± 0.005 | 2.0 ± 0.4 | |
| Statistic a | 0.66 | 1.52 | 0.03 | 0.37 | 0.37 | 0.02 | 0.41 | 1.81 | |
| p value | 0.50 | 0.27 | 0.87 | 0.58 | 0.58 | 0.89 | 0.55 | 0.24 | |
| A vs. B (NIL SC) | Statistic a | 2.366 | 302.893 | 9.506 | 11.284 | 6.304 | 6.820 | 7.367 | 10.695 |
| p value | 0.220 | <0.001 | 0.055 | 0.047 | 0.128 | 0.088 | 0.113 | 0.061 | |
| A vs. B (R SC) | Statistic a | 0.516 | 396.341 | 9.206 | 12.357 | 3.782 | 10.175 | 3.090 | 20.161 |
| p value | 0.542 | <0.001 | 0.039 | 0.038 | 0.146 | 0.042 | 0.220 | 0.018 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okoroafor, P.U.; Ikwuka, G.; Zaffar, N.; Ngalle Epede, M.; Mensah, M.K.; Haupt, J.; Golde, A.; Heilmeier, H.; Wiche, O. Field Studies on the Effect of Bioaugmentation with Bacillus amyloliquefaciens FZB42 on Plant Accumulation of Rare Earth Elements and Selected Trace Elements. Minerals 2022, 12, 409. https://doi.org/10.3390/min12040409
Okoroafor PU, Ikwuka G, Zaffar N, Ngalle Epede M, Mensah MK, Haupt J, Golde A, Heilmeier H, Wiche O. Field Studies on the Effect of Bioaugmentation with Bacillus amyloliquefaciens FZB42 on Plant Accumulation of Rare Earth Elements and Selected Trace Elements. Minerals. 2022; 12(4):409. https://doi.org/10.3390/min12040409
Chicago/Turabian StyleOkoroafor, Precious Uchenna, God’sfavour Ikwuka, Nazia Zaffar, Melvice Ngalle Epede, Martin Kofi Mensah, Johann Haupt, Andreas Golde, Hermann Heilmeier, and Oliver Wiche. 2022. "Field Studies on the Effect of Bioaugmentation with Bacillus amyloliquefaciens FZB42 on Plant Accumulation of Rare Earth Elements and Selected Trace Elements" Minerals 12, no. 4: 409. https://doi.org/10.3390/min12040409







