A Study on the Raman Spectral Characteristics of Garnet from the Jiama Copper Polymetallic Deposit in Tibet
Abstract
:1. Introduction
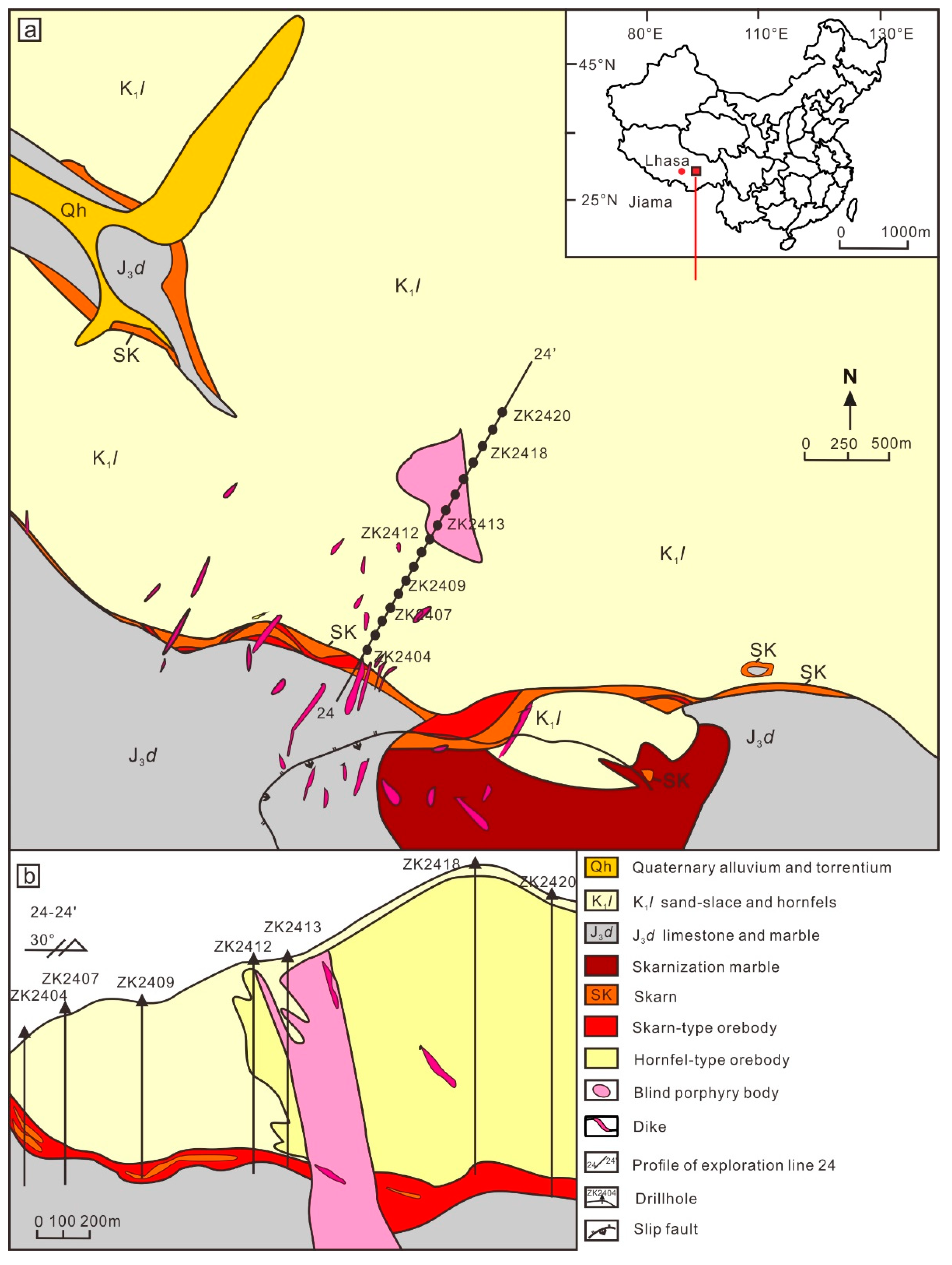
2. Geological Background
3. Measurement Methods
4. Results
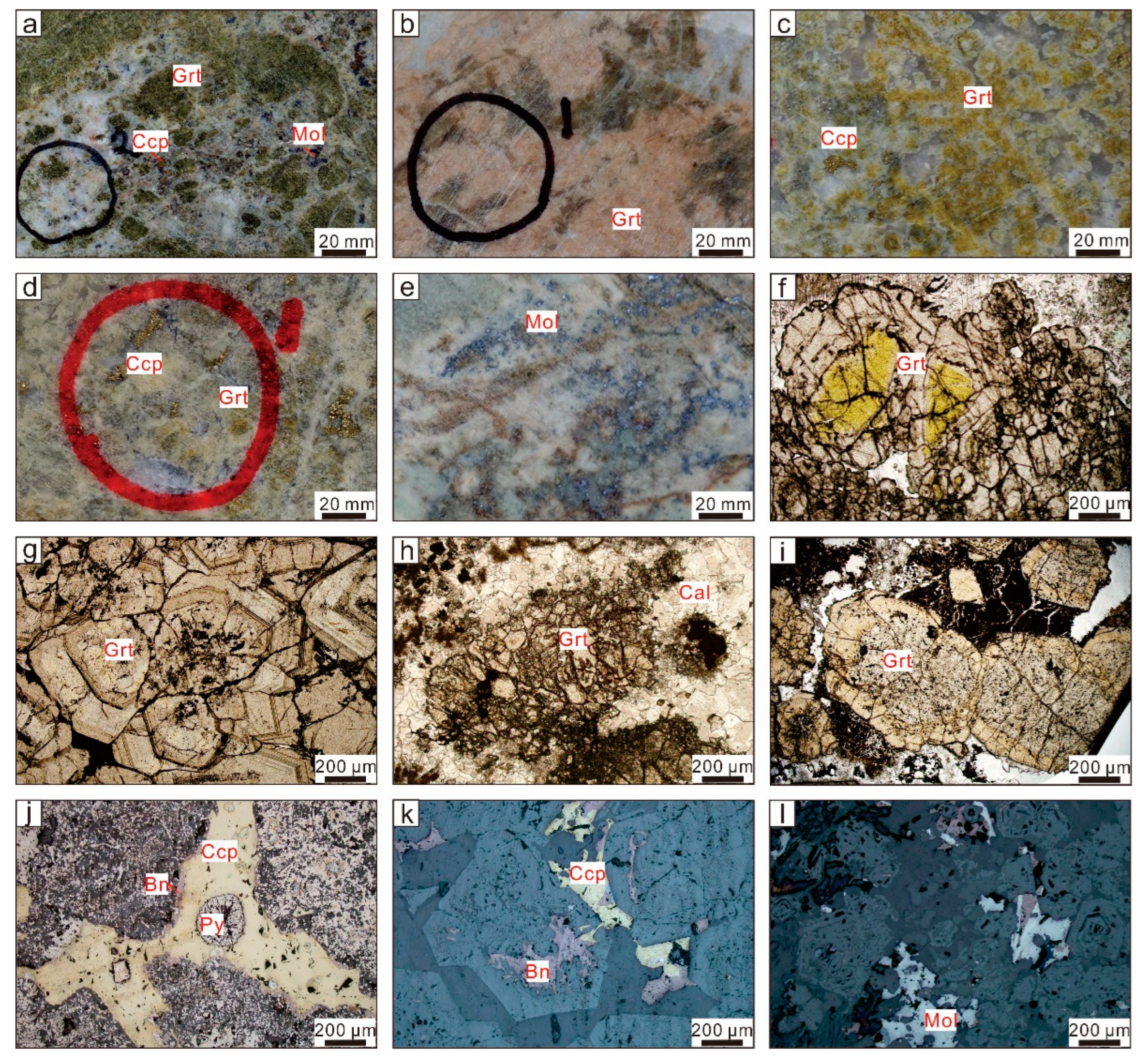
4.1. Electron Probe Results
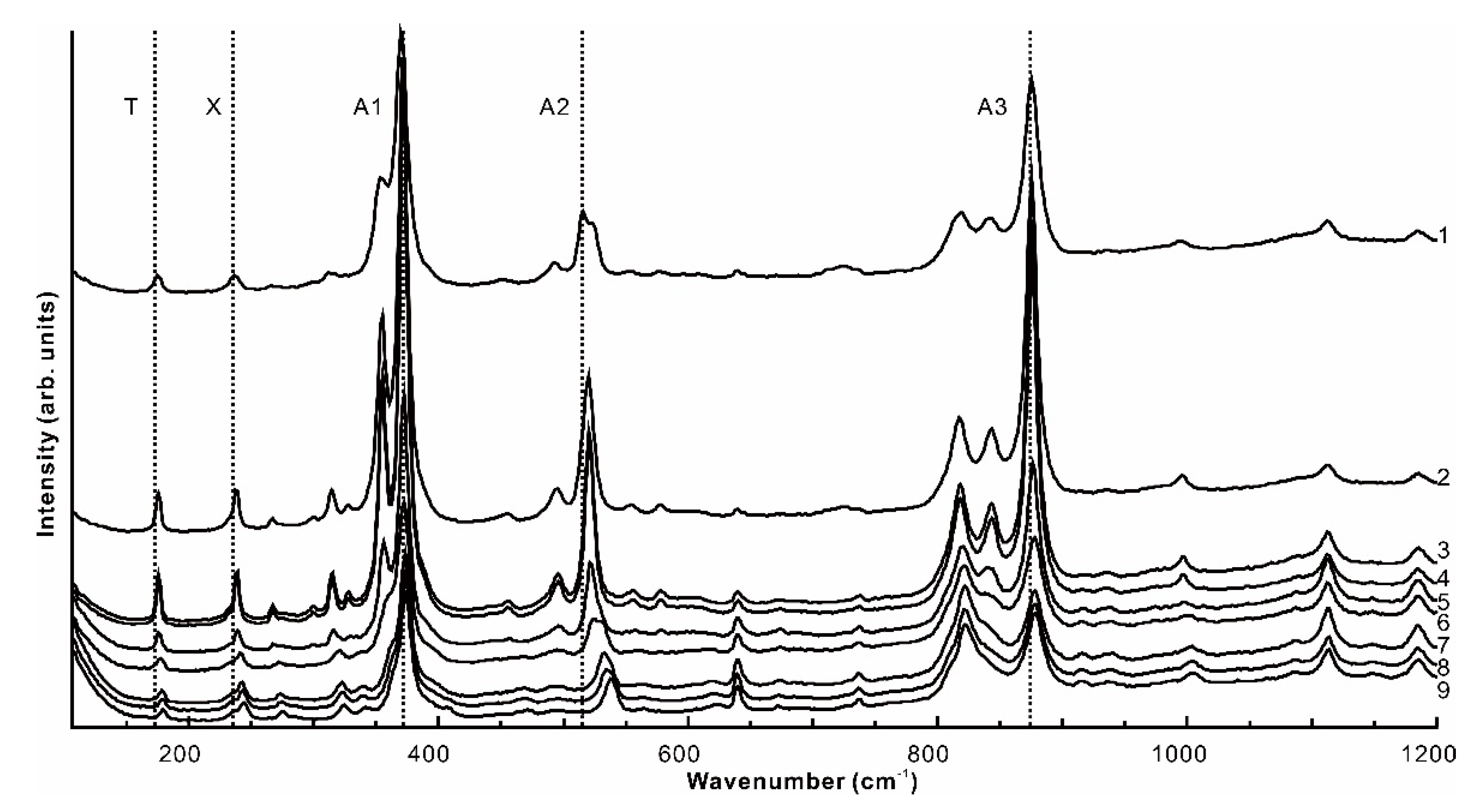
4.2. Raman Spectrum Results
5. Discussion
5.1. Raman Spectra of End Member Garnet
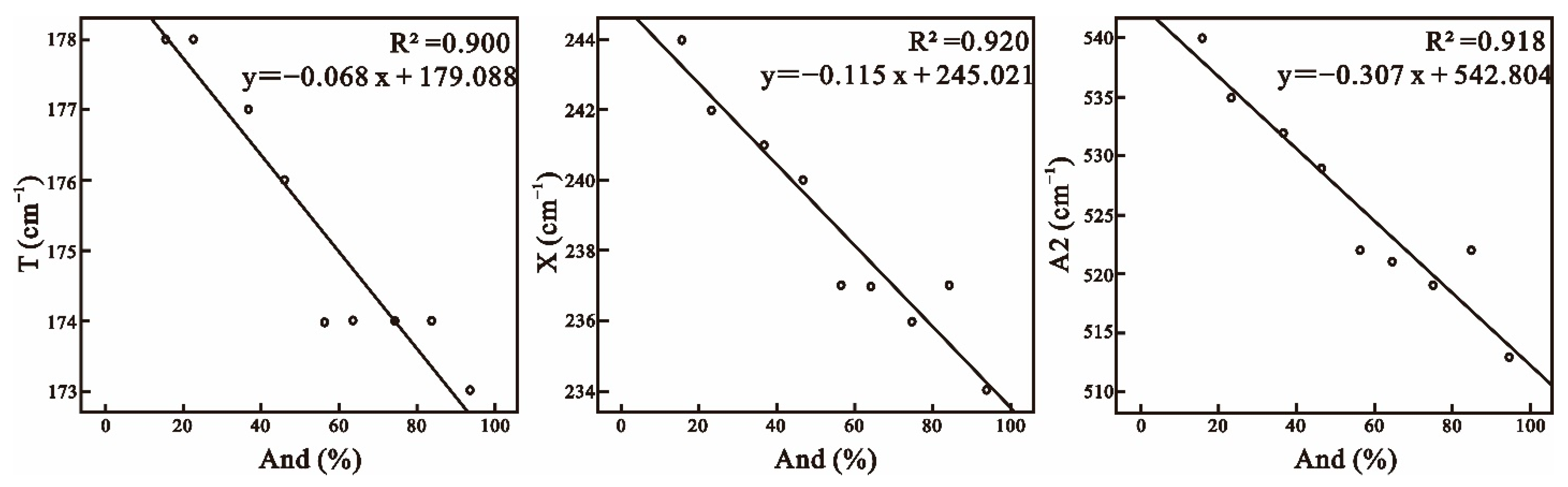
5.2. Raman Spectral Characteristics of Jiama Garnet and Its Relationship with Composition
5.3. Relationship between Garnet and Mineralization
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dai, J.J.; Zhao, L.X.; Jiang, Q.; Wang, H.Y.; Liu, T.Y. Review of thermal-infrared spectroscopy applied in geological ore exploration. Acta Geol. Sin. 2020, 94, 2520–2533. [Google Scholar]
- Bersani, D.; Andò, S.; Vignola, P.; Moltifiori, G.; Marino, I.G.; Lottici, P.P.; Diella, V. Micro-Raman spectroscopy as a routine tool for garnet analysis. Spectrochim. Acta 2009, 73, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Barton, M.D. Compositional characteristics of garnets and pyroxenes in contact-metasomatic skarn deposits and their relationship to metallization. Chin. J. Geochem. 1987, 7, 329–335. [Google Scholar]
- Li, J.X.; Qin, K.Z.; Li, G.M.; Lin, J.D.; Xiao, B.; Jiang, H.Z.; Han, F.J.; Huang, S.F.; Chen, L.; Zhao, J.X. Zircon U-Pb geochronology and garnet composition of the Qiangdui Cu-Mo deposit in the eastern section of Gangdese and their significances. Geol. Explor. 2011, 47, 11–19. [Google Scholar]
- Zhang, W.; Zhang, X.C.; Leng, C.B.; Su, W.C.; Qin, C.J.; Cao, J.L.; Yan, J.H. Zoning and genesis of garnets in the Seleteguole reduced porphyry-skarn deposit of West Tianshan Mountains, Xinjiang. Miner. Depos. 2017, 36, 412–428. [Google Scholar]
- Shi, W.X.; Yi, J.J.; Wang, H.; Tian, R.J. Study on the characteristics of the infrared spectrum and the alteration zoning of drill core in the Makeng iron deposit. Rock Miner. Anal. 2020, 39, 934–943. [Google Scholar]
- Laukamp, C.; Legras, M.; Montenegro, V.; Windle, S.; Mcfarlane, A. Grandite-based resource characterization of the skarn-hosted Cu-Zn-Mo deposit of Antamina, Peru. Miner. Depos. 2021, 57, 107–128. [Google Scholar] [CrossRef]
- Kos, S.; Dolenec, M.; Lux, J.; Dolenec, S. Raman Microspectroscopy of Garnets from S-Fibulae from the Archaeological Site Lajh (Slovenia). Minerals 2020, 10, 325. [Google Scholar] [CrossRef] [Green Version]
- Cai, X.C.; Fu, Y.D.; Kan, X.M.; Peng, W.S.; Liu, G.K.; Tang, R.B. Mineral spectroscopy and genetic mineralogy. J. Mineral. Petrol. 1981, Z1, 1–19. [Google Scholar]
- Zhang, H.F. Lecture 20 of modern mineralogy application of laser Raman spectroscopy in mineralogical research. Earth Environ. 1984, 12, 59–64+58. [Google Scholar]
- Hofmeister, A.M.; Chopelas, A. Vibrational spectroscopy of end-member silicate garnets. Phys. Chem. Miner. 1991, 17, 503–526. [Google Scholar] [CrossRef]
- Xie, X.D.; Cha, F.B. Vibrational spectral study of borates—I: Raman spectroscopy. Acta Miner. Sin. 1993, 2, 130–136. [Google Scholar]
- Cha, F.B.; Xie, X.D.; Peng, W.S. A vibrational spectral study of borates—II: Infrared spectroscopy. Acta Miner. Sin. 1993, 3, 230–236. [Google Scholar]
- Kolesov, B.A.; Geiger, C.A. Raman scattering in silicate garnets: An investigation of their resonance intensities. J. Raman Spectrosc. 1997, 28, 659–662. [Google Scholar] [CrossRef]
- Kolesov, B.A.; Geiger, C.A. Raman spectra of silicate garnets. Phys. Chem. Miner. 1998, 25, 142–151. [Google Scholar] [CrossRef]
- Wu, L.; Ouyang, Z.H.; Cao, S.C.; Yi, D.L.; Sun, S.X.; Liu, X. Research development and application of Raman scattering technology. J. Light Scatt. 2005, 2, 180–186. [Google Scholar]
- Makreski, P.; Runcevski, T.; Jovanovski, G. Minerals from Macedonia. XXVI. Characterization and spectra-structure correlations for grossular and uvarovite. Raman study supported by IR spectroscopy. J. Raman Spectrosc. 2011, 42, 72–77. [Google Scholar] [CrossRef]
- Wu, F.; Zhang, X.C.; Zhu, Z.L. Quantitative relation between Raman shift and metal ion content in garnets. J. Light Scatt. 2015, 27, 350–354. [Google Scholar]
- Fan, J.L.; Liu, X.L.; Guo, S.G.; Yang, M.Y. Study on Raman spectra of garnets and relative identification. Appl. Laser 2007, 27, 310–313+299. [Google Scholar]
- Zhu, Q.Q.; Xie, G.Q.; Li, W.; Zhang, F.; Wang, J.; Zhang, P.; Yu, B.F. In situ analysis of garnets from the Jingshandian iron skarn deposit, Hubei Province, and its geological implications. Geol. China 2014, 41, 1944–1963. [Google Scholar]
- Liu, J.; Yang, M.X.; Di, J.R.; He, C. Spectra characterization of the uvarovite in anorthitic jade. Spectrosc. Spectr. Anal. 2018, 38, 1758–1762. [Google Scholar]
- Wang, Y.C. Raman scattering of grossular-andradite solid solution. Chin. J. High Pressure Phys. 2020, 34, 3–11. [Google Scholar]
- Chen, Y.M.; Yu, X.Y.; Yang, Y.; Ruan, C.T. A study of gemological and mineralogical characteristics and color zonation of garnets from Jinan, Shandong Province. Acta Mineral. Petrol. 2021, 40, 581–592. [Google Scholar]
- Tang, J.X.; Wang, Q.; Yang, H.H.; Gao, X.; Zhang, Z.B.; Zou, B. Mineralization, exploration and resource potential of porphyry-skarn-epithermal copper polymetallic deposits in Tibet. Acta Geosci. Sin. 2017, 38, 571–613. [Google Scholar]
- Ying, L.J.; Tang, J.X.; Wang, D.H.; Wang, W.P. Features of garnet in the Jiama super-large Cu polymetallic deposit and its genetic significance. Acta Geol. Sin. 2012, 86, 1735–1747. [Google Scholar]
- Tang, X.Q.; Wang, G.Z.; Qin, Z.P.; Yao, X.F.; Zhou, Y.X. Mineralogical characteristics and genesis of garnet in the Jiama (Gyama) copper-polymetallic deposit of Tibet. Acta Geosci. Sin. 2012, 33, 633–640. [Google Scholar]
- Jia, Y.H.; Qian, J.P. Study on REE distribution and mineralogical characteristics of different garnets by electron probe and inductively coupled plasma-mass spectrometry. Rock Miner. Anal. 2020, 39, 886–895. [Google Scholar]
- Qin, Z.P.; Duo, J.; Wang, X.W.; Liu, H.F.; Zhou, Y.; Peng, H.J. Characteristics and significance of magmatic-hydrothermal transition in Jiama (Gyama) monzonite granite-porphyry, Tibet. Acta Geosci. Sin. 2012, 33, 501–509. [Google Scholar]
- Leng, Q.F. Skarn Diagenesis and Mineralization of Jiama Copper Polymetallic Deposit in Tibet. Ph.D. Thesis, Chengdu University of Technology, Chengdu, China, 2015. [Google Scholar]
- Muhling, J.R.; Griffin, B.J. On recasting garnet analyses into end-member molecules-revisited. Comput. Geosci. 1991, 17, 161–170. [Google Scholar] [CrossRef]
- Peng, M.S.; Mao, H.K.; Li, D.E.; Chao, E.C.T. Raman spectra of garnet-group minerals. Spectrosc. Spectr. Anal. 1991, 5, 16–21. [Google Scholar]
- Liu, G.K.; Peng, W.S. Infrared spectral study of cookeite. Acta Miner. Sin. 1987, 1, 52–57. [Google Scholar]
- Li, J.; Lu, L.N.; Cui, Y.J.; Xie, C.; Du, J.G.; Si, Z.S. Raman spectra features of the garnet in elcogite from the Dabie mountain and its geological significances. J. Miner. Petrol. 2016, 36, 17–21. [Google Scholar]
- Enami, M. Influence of garnet hosts on the Raman spectra of quartz inclusions. J. Miner. Pet. Sci. 2012, 107, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Laukamp, C.; Rodger, A.; Legras, M.; Lampinen, H.; Lau, I.C.; Pejcic, B.; Stromberg, J.; Francis, N.; Ramanaidou, E. Mineral physicochemistry underlying feature-based extraction of mineral abundance and composition from shortwave, mid and thermal infrared reflectance spectra. Minerals 2021, 11, 347. [Google Scholar] [CrossRef]
- Maschio, L.; Kirtman, B.; Salustro, S.; Zicovich-Wilson, C.M.; Orlando, R.; Dovesi, R. Raman Spectrum of Pyrope Garnet. A Quantum Mechanical Simulation of Frequencies, Intensities, and Isotope Shifts. J. Phys. Chem. A 2013, 117, 11464–11471. [Google Scholar] [CrossRef]
- Ai, Y.F.; Jin, L.N. Preliminary study on the relationship between garnet composition and mineralization. Acta Sci. Nat. Univ. Pekin. 1981, 1, 83–90. [Google Scholar]
- Zhao, B.; Li, T.J.; Li, Z.P. Experimental study of physico-chemical conditions of the formation of skarns. Geochimica 1983, 3, 256–267+331. [Google Scholar] [CrossRef]
- Liang, X.J. Characteristics and metasomatic mechanism of grossular-andradite series garnet. Acta Petrol. Miner. 1994, 4, 342–352. [Google Scholar]
- Zhao, H.J.; Xie, G.Q.; Wei, K.T.; Ke, Y.F. Skarn mineral and stable isotopic characteristics of Tonglushan Cu-Fe deposit in Hubei Province. Geol. Rev. 2012, 58, 379–395. [Google Scholar]
- Zheng, W.; Chen, M.H.; Zhao, H.J.; Xu, L.G.; Zhang, D.Y.; Ling, S.B.; Yao, L.; Yu, M.; Chang, L.Z. Skarn mineral characteristics of the Tiantang Cu-Pb-Zn polymetallic deposit in Guangdong Province and their geological significance. Acta Petrol. Mineral. 2013, 32, 23–40. [Google Scholar]

| No. | 2404-1 | 2404-2 | 2404-3 | 2404-4 | 2404-5 | 2407-1 | 2407-2 | 2407-3 | 2407-4 | 2407-5 | 2407-6 | 2407-7 |
| SiO2 | 37.691 | 38.506 | 38.798 | 38.545 | 38.909 | 39.164 | 38.393 | 36.709 | 38.457 | 36.675 | 37.006 | 37.194 |
| Al2O3 | 0.601 | 0.394 | 10.094 | 12.102 | 9.115 | 11.786 | 12.15 | 6.919 | 7.377 | 0.179 | 2.316 | 3.011 |
| FeO | 26.818 | 27.865 | 14.556 | 12.867 | 15.48 | 13.472 | 13.03 | 18.387 | 17.187 | 26.281 | 23.221 | 22.763 |
| MnO | 0.126 | 0.103 | 0.362 | 0.367 | 0.17 | 0.371 | 0.526 | 0.292 | 0.412 | 0.076 | 0.084 | 0.095 |
| MgO | 0.058 | 0.079 | 0.094 | 0.081 | 0.021 | 0.111 | 0.088 | 0.072 | 0.062 | 0.09 | 0.108 | 0.061 |
| CaO | 32.234 | 32.201 | 33.262 | 34.848 | 33.006 | 33.012 | 32.984 | 31.93 | 32.13 | 31.572 | 31.483 | 31.969 |
| Total | 97.528 | 99.148 | 97.166 | 98.81 | 96.701 | 97.916 | 97.171 | 94.309 | 95.625 | 94.873 | 94.218 | 95.093 |
| Number of ions on the basis of 12O | ||||||||||||
| Si | 3.145 | 3.174 | 3.123 | 3.032 | 3.163 | 3.114 | 3.07 | 3.075 | 3.158 | 3.153 | 3.176 | 3.155 |
| Al | 0.059 | 0.038 | 0.958 | 1.122 | 0.874 | 1.105 | 1.146 | 0.683 | 0.714 | 0.018 | 0.234 | 0.301 |
| Fe2+ | 0.046 | 0.063 | 0.045 | 0.014 | 0.055 | 0.056 | 0.044 | 0.042 | 0.056 | 0.041 | 0.05 | 0.044 |
| Fe3+ | 0.164 | 0.162 | 0.079 | 0.082 | 0.08 | 0.066 | 0.069 | 0.107 | 0.092 | 0.162 | 0.137 | 0.137 |
| Mn | 0.016 | 0.013 | 0.043 | 0.043 | 0.021 | 0.044 | 0.063 | 0.036 | 0.05 | 0.01 | 0.011 | 0.012 |
| Mg | 0.004 | 0.006 | 0.006 | 0.005 | 0.001 | 0.007 | 0.006 | 0.005 | 0.004 | 0.007 | 0.008 | 0.004 |
| Ca | 2.882 | 2.844 | 2.869 | 2.937 | 2.875 | 2.813 | 2.826 | 2.866 | 2.827 | 2.909 | 2.895 | 2.906 |
| Gro | 0 | 0 | 43.98 | 54.36 | 39.9 | 49.45 | 51.96 | 30.04 | 31.23 | 0 | 8.37 | 12.16 |
| And | 82.26 | 80.38 | 38.11 | 39.05 | 39.34 | 31.46 | 33.15 | 53.92 | 45.34 | 83.85 | 70.61 | 69.68 |
| No. | 2407-8 | 2407-9 | 2409-1 | 2409-2 | 2409-3 | 2409-4 | 2409-5 | 2412-1 | 2412-2 | 2412-3 | 2412-4 | 2412-5 |
| SiO2 | 36.107 | 37.421 | 39.4 | 38.261 | 38.464 | 37.703 | 38.126 | 38.591 | 38.056 | 38.116 | 39.037 | 40.148 |
| Al2O3 | 0.031 | 0.829 | 5.295 | 0.132 | 1.529 | 2.1 | 2.548 | 6.593 | 0.258 | 5.797 | 12.5 | 16.974 |
| FeO | 27.369 | 26.846 | 22.049 | 27.579 | 26.427 | 24.927 | 25.238 | 20.668 | 28.462 | 21.231 | 12.923 | 7.929 |
| MnO | 0.201 | 0.046 | 0.191 | 0.177 | 0.127 | 0.198 | 0.119 | 0.43 | 0.213 | 0.345 | 0.314 | 0.472 |
| MgO | 0.038 | 0.029 | 0.053 | 0.052 | 0.092 | 0.045 | 0.091 | 0 | 0.005 | 0.026 | 0.014 | 0.015 |
| CaO | 31.273 | 32.129 | 33.33 | 32.418 | 32.6 | 32.368 | 32.663 | 32.742 | 32.057 | 32.695 | 34.725 | 34.781 |
| Total | 95.019 | 97.3 | 100.318 | 98.619 | 99.239 | 97.341 | 98.785 | 99.024 | 99.051 | 98.21 | 99.513 | 100.319 |
| Number of ions on the basis of 12O | ||||||||||||
| Si | 3.032 | 3.134 | 3.141 | 3.166 | 3.148 | 3.138 | 3.126 | 3.1 | 3.141 | 3.073 | 3.049 | 3.058 |
| Al | 0.003 | 0.082 | 0.498 | 0.013 | 0.148 | 0.206 | 0.246 | 0.624 | 0.025 | 0.551 | 1.151 | 1.524 |
| Fe2+ | 0 | 0.045 | 0.058 | 0.052 | 0.052 | 0.046 | 0.048 | 0.054 | 0.057 | 0.056 | 0.023 | 0.042 |
| Fe3+ | 0.19 | 0.164 | 0.124 | 0.166 | 0.158 | 0.15 | 0.151 | 0.117 | 0.17 | 0.12 | 0.078 | 0.034 |
| Mn | 0.025 | 0.006 | 0.023 | 0.022 | 0.015 | 0.025 | 0.015 | 0.051 | 0.026 | 0.041 | 0.037 | 0.054 |
| Mg | 0.003 | 0.002 | 0.004 | 0.004 | 0.006 | 0.003 | 0.006 | 0 | 0 | 0.002 | 0.001 | 0.001 |
| Ca | 2.814 | 2.883 | 2.847 | 2.874 | 2.859 | 2.887 | 2.87 | 2.818 | 2.835 | 2.825 | 2.906 | 2.839 |
| Gro | 0 | 0.51 | 20.04 | 0 | 3 | 6.7 | 8.03 | 25.59 | 0 | 22.28 | 54.98 | 71.52 |
| And | 94.17 | 82.66 | 59.68 | 82.48 | 77.72 | 75.28 | 74.63 | 56.52 | 84.34 | 58.25 | 36.79 | 15.79 |
| No. | 2412-6 | 2412-7 | 2412-8 | 2412-9 | 2412-10 | 2412-11 | 2412-12 | 2413-1 | 2413-2 | 2413-3 | 2413-4 | 2413-5 |
| SiO2 | 39.242 | 39.754 | 39.322 | 37.026 | 38.137 | 36.011 | 38.444 | 37.347 | 36.907 | 39.633 | 39.706 | 39.863 |
| Al2O3 | 21.086 | 14.93 | 8.323 | 0.202 | 4.354 | 0.083 | 1.954 | 0.404 | 0.149 | 8.487 | 6.929 | 8.671 |
| FeO | 1.784 | 10.698 | 18.573 | 29.433 | 22.269 | 29.327 | 25.254 | 27.469 | 27.474 | 18.443 | 19.364 | 17.922 |
| MnO | 0.359 | 0.342 | 0.284 | 0.298 | 0.132 | 0.217 | 0.157 | 0.077 | 0.154 | 0.171 | 0.171 | 0.269 |
| MgO | 0.211 | 0.009 | 0.064 | 0.036 | 0.038 | 0 | 0.038 | 0.118 | 0.144 | 0.112 | 0.131 | 0.144 |
| CaO | 35.505 | 34.158 | 32.915 | 31.033 | 32.554 | 31.312 | 32.935 | 32.398 | 32.06 | 33.47 | 33.411 | 33.367 |
| Total | 98.187 | 99.891 | 99.481 | 98.028 | 97.484 | 96.95 | 98.782 | 97.813 | 96.888 | 100.316 | 99.712 | 100.236 |
| Number of ions on the basis of 12O | ||||||||||||
| Si | 3.005 | 3.068 | 3.124 | 3.094 | 3.144 | 3.045 | 3.154 | 3.119 | 3.109 | 3.128 | 3.167 | 3.143 |
| Al | 1.904 | 1.358 | 0.78 | 0.02 | 0.423 | 0.008 | 0.189 | 0.04 | 0.015 | 0.79 | 0.652 | 0.806 |
| Fe2+ | 0.009 | 0.049 | 0.064 | 0.057 | 0.051 | 0.038 | 0.049 | 0.039 | 0.037 | 0.058 | 0.06 | 0.061 |
| Fe3+ | 0.008 | 0.05 | 0.097 | 0.176 | 0.129 | 0.185 | 0.151 | 0.172 | 0.173 | 0.099 | 0.105 | 0.094 |
| Mn | 0.041 | 0.039 | 0.034 | 0.037 | 0.016 | 0.027 | 0.019 | 0.01 | 0.019 | 0.02 | 0.02 | 0.032 |
| Mg | 0.014 | 0.001 | 0.004 | 0.003 | 0.003 | 0 | 0.003 | 0.008 | 0.01 | 0.007 | 0.009 | 0.01 |
| Ca | 2.914 | 2.824 | 2.802 | 2.779 | 2.876 | 2.837 | 2.895 | 2.9 | 2.894 | 2.831 | 2.855 | 2.819 |
| Gro | 93.15 | 62.53 | 32.73 | 0 | 17.11 | 0 | 6.15 | 0 | 0 | 33.98 | 27.84 | 34.42 |
| And | 3.69 | 23.18 | 46.57 | 88.67 | 64.1 | 94.14 | 74.7 | 86.12 | 87.52 | 47.24 | 50.32 | 44.62 |
| No. | 2418-1 | 2418-2 | 2418-3 | 2418-4 | 2418-5 | 2418-6 | 2418-7 | 2420-1 | 2420-2 | 2420-3 | 2420-4 | 2420-5 |
| SiO2 | 37.592 | 38.447 | 37.403 | 37.665 | 38.478 | 37.417 | 36.7 | 37.71 | 37.974 | 38.151 | 37.78 | 37.2 |
| Al2O3 | 2.135 | 11.133 | 6.528 | 4.99 | 10.054 | 0.518 | 0.051 | 0 | 1.933 | 1.985 | 0.064 | 0.01 |
| FeO | 25.709 | 14.562 | 20.179 | 21.606 | 16.059 | 27.066 | 28.267 | 28.311 | 26.167 | 25.577 | 27.841 | 28.43 |
| MnO | 0.134 | 0.506 | 0.361 | 0.299 | 0.494 | 0.206 | 0.278 | 0.334 | 0.175 | 0.118 | 0.357 | 0.315 |
| MgO | 0.02 | 0.069 | 0.042 | 0 | 0.046 | 0.039 | 0.022 | 0.042 | 0.103 | 0.074 | 0.027 | 0.03 |
| CaO | 32.103 | 34.564 | 32.579 | 32.545 | 34.159 | 31.792 | 31.563 | 31.583 | 32.375 | 32.537 | 31.141 | 31.24 |
| Total | 97.693 | 99.281 | 97.092 | 97.105 | 99.29 | 97.038 | 96.881 | 97.98 | 98.727 | 98.442 | 97.21 | 97.225 |
| Number of ions on the basis of 12O | ||||||||||||
| Si | 3.124 | 3.009 | 3.065 | 3.096 | 3.029 | 3.143 | 3.099 | 3.149 | 3.124 | 3.136 | 3.177 | 3.131 |
| Al | 0.209 | 1.027 | 0.631 | 0.484 | 0.933 | 0.051 | 0.005 | 0 | 0.187 | 0.192 | 0.006 | 0.001 |
| Fe2+ | 0.051 | 0.017 | 0.035 | 0.041 | 0.027 | 0.047 | 0.044 | 0.059 | 0.049 | 0.042 | 0.066 | 0.055 |
| Fe3+ | 0.153 | 0.093 | 0.123 | 0.13 | 0.098 | 0.165 | 0.175 | 0.168 | 0.158 | 0.157 | 0.161 | 0.171 |
| Mn | 0.017 | 0.059 | 0.044 | 0.037 | 0.058 | 0.026 | 0.035 | 0.042 | 0.021 | 0.014 | 0.045 | 0.04 |
| Mg | 0.001 | 0.005 | 0.003 | 0 | 0.003 | 0.003 | 0.002 | 0.003 | 0.007 | 0.005 | 0.002 | 0.002 |
| Ca | 2.859 | 2.898 | 2.861 | 2.866 | 2.882 | 2.861 | 2.855 | 2.826 | 2.854 | 2.866 | 2.806 | 2.817 |
| Gro | 5.85 | 48.77 | 27.31 | 20.37 | 43.36 | 0 | 0 | 0 | 4.64 | 5.98 | 0 | 0 |
| And | 76.73 | 43.74 | 60.59 | 64.34 | 46.7 | 83.39 | 88.92 | 84.34 | 77.97 | 77.53 | 81.36 | 86.41 |
| Samples | Eg + F2g | F2g | A1g + Eg + F2g | A1g | A1g + Eg | Samples | Eg + F2g | F2g | A1g + Eg + F2g | A1g | A1g + Eg |
| T | X | A1 | A2 | A3 | T | X | A1 | A2 | A3 | ||
| 2404-1 | 174 | 236 | 370 | 518 | 874 | 2412-6 | 177 | 240 | 370 | 543 | 877 |
| 2404-2 | 173 | 236 | 369 | 517 | 872 | 2412-7 | 178 | 242 | 372 | 535 | 876 |
| 2404-3 | 177 | 241 | 372 | 531 | 876 | 2412-8 | 176 | 240 | 372 | 529 | 876 |
| 2404-4 | 177 | 241 | 373 | 533 | 876 | 2412-9 | 173 | 235 | 369 | 516 | 873 |
| 2404-5 | 177 | 241 | 371 | 530 | 876 | 2412-10 | 174 | 237 | 370 | 521 | 876 |
| 2407-1 | 177 | 241 | 372 | 533 | 876 | 2412-11 | 173 | 234 | 365 | 513 | 873 |
| 2407-2 | 177 | 241 | 372 | 531 | 876 | 2412-12 | 174 | 236 | 371 | 519 | 874 |
| 2407-3 | 175 | 239 | 371 | 527 | 876 | 2413-1 | 173 | 236 | 367 | 516 | 873 |
| 2407-4 | 175 | 240 | 372 | 528 | 875 | 2413-2 | 173 | 236 | 371 | 515 | 873 |
| 2407-5 | 173 | 236 | 369 | 517 | 872 | 2413-3 | 176 | 240 | 372 | 527 | 875 |
| 2407-6 | 174 | 237 | 370 | 519 | 874 | 2413-4 | 176 | 239 | 371 | 525 | 875 |
| 2407-7 | 174 | 237 | 371 | 522 | 875 | 2413-5 | 176 | 240 | 372 | 528 | 876 |
| 2407-8 | 173 | 235 | 369 | 516 | 874 | 2418-1 | 173 | 236 | 369 | 517 | 874 |
| 2407-9 | 173 | 236 | 371 | 517 | 873 | 2418-2 | 174 | 237 | 369 | 523 | 875 |
| 2409-1 | 176 | 239 | 371 | 523 | 875 | 2418-3 | 173 | 235 | 369 | 517 | 874 |
| 2409-2 | 174 | 237 | 371 | 520 | 875 | 2418-4 | 174 | 237 | 370 | 522 | 875 |
| 2409-3 | 174 | 236 | 371 | 518 | 874 | 2418-5 | 176 | 240 | 367 | 532 | 876 |
| 2409-4 | 176 | 239 | 371 | 523 | 875 | 2418-6 | 173 | 236 | 370 | 517 | 875 |
| 2409-5 | 174 | 237 | 371 | 522 | 875 | 2418-7 | 173 | 235 | 369 | 516 | 873 |
| 2412-1 | 174 | 237 | 369 | 522 | 876 | 2420-1 | 173 | 235 | 369 | 515 | 874 |
| 2412-2 | 174 | 237 | 370 | 522 | 875 | 2420-2 | 174 | 236 | 371 | 518 | 874 |
| 2412-3 | 174 | 237 | 370 | 523 | 876 | 2420-3 | 174 | 236 | 371 | 518 | 874 |
| 2412-4 | 177 | 241 | 372 | 532 | 876 | 2420-4 | 173 | 235 | 369 | 516 | 873 |
| 2412-5 | 178 | 244 | 373 | 540 | 877 | 2420-5 | 173 | 235 | 369 | 516 | 873 |
| No. | SiO2 | TiO2 | Al2O3 | FeO | MnO | MgO | CaO | Gro | And | T | X | A1 | A2 | A3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 36.011 | 0.038 | 0.083 | 29.327 | 0.217 | 0 | 31.312 | 0 | 94.14% | 173 | 234 | 365 | 513 | 873 |
| 2 | 38.056 | 0.023 | 0.258 | 28.462 | 0.213 | 0.005 | 32.057 | 0 | 84.34% | 174 | 237 | 370 | 522 | 875 |
| 3 | 38.444 | 0.03 | 1.954 | 25.254 | 0.157 | 0.038 | 32.935 | 6.15% | 74.70% | 174 | 236 | 371 | 519 | 874 |
| 4 | 38.137 | 0 | 4.354 | 22.269 | 0.132 | 0.038 | 32.554 | 17.11% | 64.10% | 174 | 237 | 370 | 521 | 876 |
| 5 | 38.591 | 0.187 | 6.593 | 20.668 | 0.43 | 0 | 32.742 | 25.59% | 56.52% | 174 | 237 | 369 | 522 | 876 |
| 6 | 39.322 | 0.163 | 8.323 | 18.573 | 0.284 | 0.064 | 32.915 | 32.73% | 46.57% | 176 | 240 | 372 | 529 | 876 |
| 7 | 39.037 | 0.041 | 12.5 | 12.923 | 0.314 | 0.014 | 34.725 | 54.98% | 36.79% | 177 | 241 | 372 | 532 | 876 |
| 8 | 39.754 | 0.228 | 14.93 | 10.698 | 0.342 | 0.009 | 34.158 | 62.53% | 23.18% | 178 | 242 | 372 | 535 | 876 |
| 9 | 40.148 | 0.176 | 16.974 | 7.929 | 0.472 | 0.015 | 34.781 | 71.52% | 15.79% | 178 | 244 | 373 | 540 | 877 |
| SiO2 | TiO2 | Al2O3 | FeO | MnO | MgO | CaO | Gro | And | |
|---|---|---|---|---|---|---|---|---|---|
| T | 0.856 ** | 0.621 | 0.958 ** | −0.953 ** | 0.625 | 0.065 | 0.915 ** | 0.959 ** | −0.948 ** |
| X | 0.903 ** | 0.616 | 0.954 ** | −0.953 ** | 0.674 * | 0.097 | 0.909 ** | 0.952 ** | −0.959 ** |
| A1 | 0.948 ** | 0.385 | 0.725 * | −0.757 * | 0.355 | 0.420 | 0.832 ** | 0.715 * | −0.796 * |
| A2 | 0.912 ** | 0.626 | 0.951 ** | −0.950 ** | 0.686 * | 0.074 | 0.911 ** | 0.949 ** | −0.958 ** |
| A3 | 0.856 ** | 0.542 | 0.788 * | −0.803 ** | 0.619 | 0.166 | 0.728* | 0.776 * | −0.843 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, M.; Dai, J.; Zhao, L. A Study on the Raman Spectral Characteristics of Garnet from the Jiama Copper Polymetallic Deposit in Tibet. Minerals 2022, 12, 1578. https://doi.org/10.3390/min12121578
Fu M, Dai J, Zhao L. A Study on the Raman Spectral Characteristics of Garnet from the Jiama Copper Polymetallic Deposit in Tibet. Minerals. 2022; 12(12):1578. https://doi.org/10.3390/min12121578
Chicago/Turabian StyleFu, Minghai, Jingjing Dai, and Longxian Zhao. 2022. "A Study on the Raman Spectral Characteristics of Garnet from the Jiama Copper Polymetallic Deposit in Tibet" Minerals 12, no. 12: 1578. https://doi.org/10.3390/min12121578





