Synthesis, Characterization, and Application of Geopolymer/TiO2 Nanoparticles Composite for Efficient Removal of Cu(II) and Cd(II) Ions from Aqueous Media
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of TiO2 Nanoparticles
2.3. Synthesis of Geopolymer and Geopolymer-NanoTiO2 Composites
2.4. Characterization
2.5. Adsorption Experiments
3. Results and Discussion
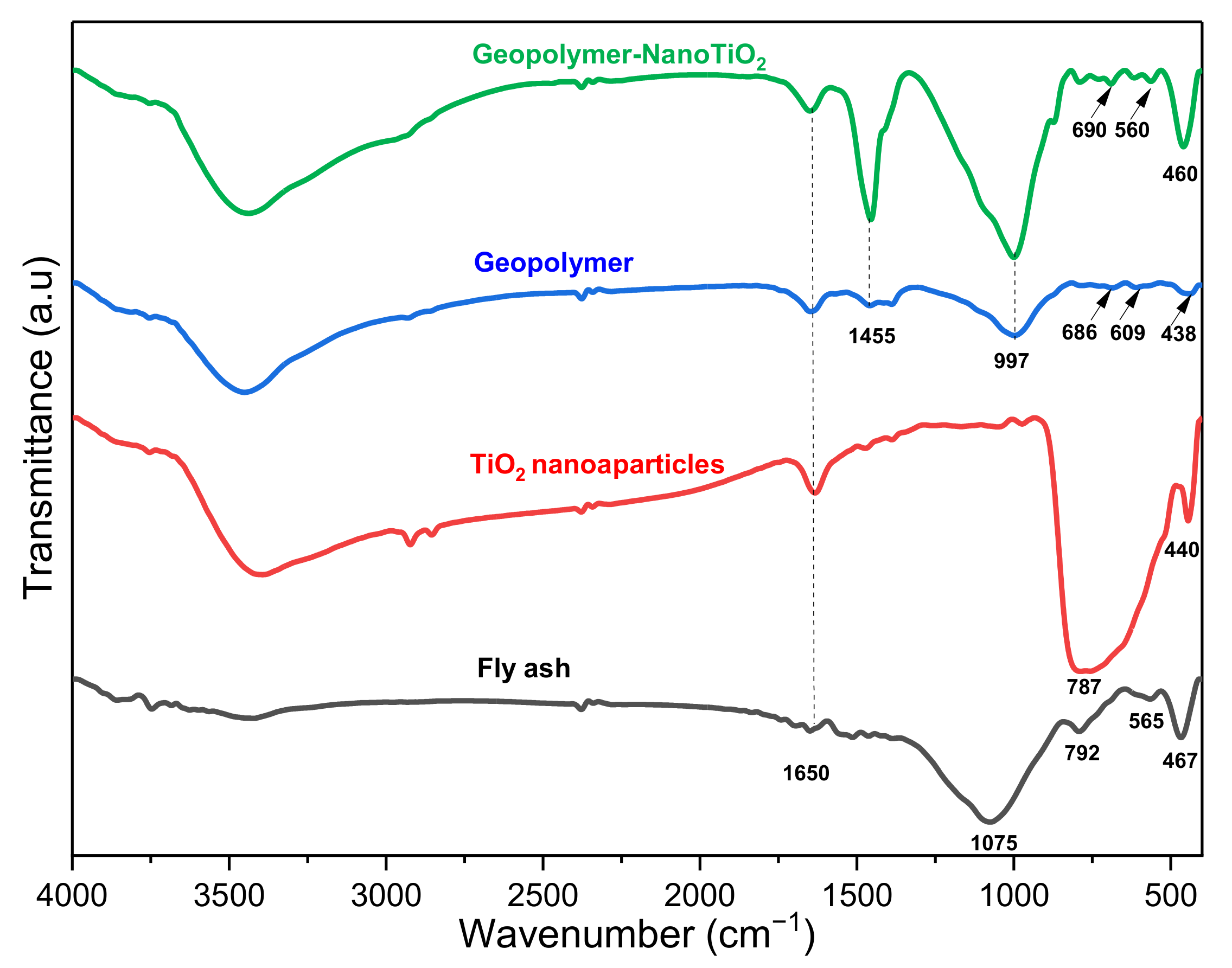
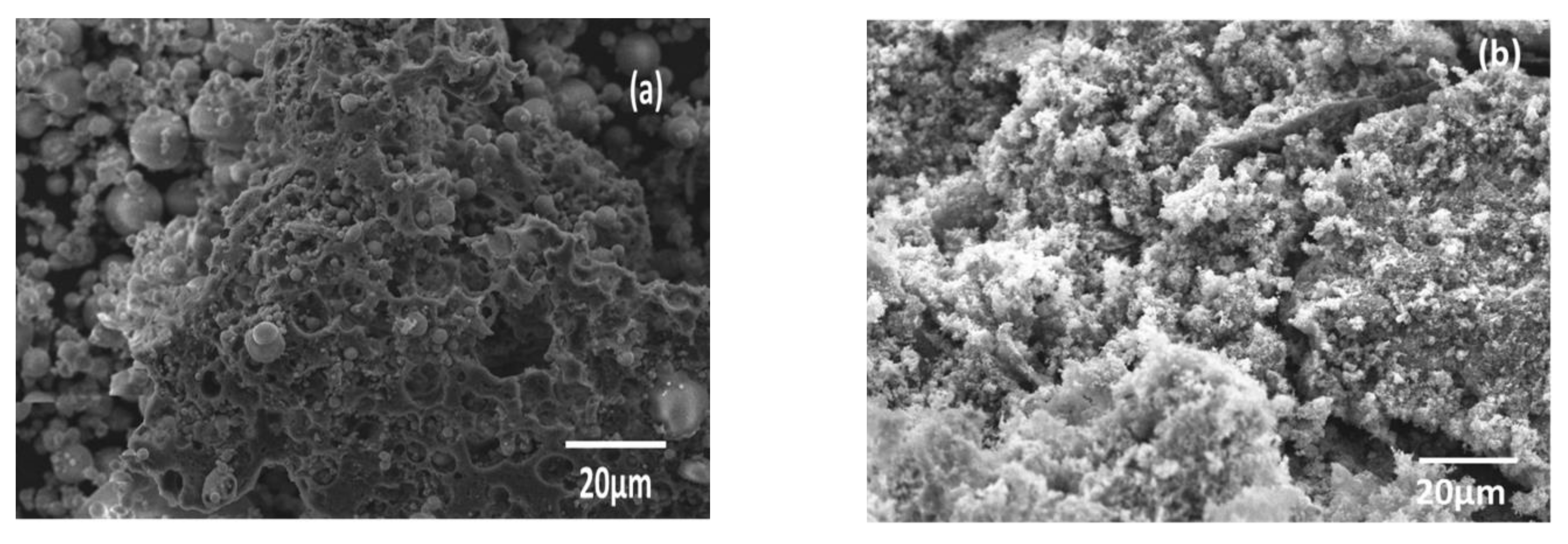
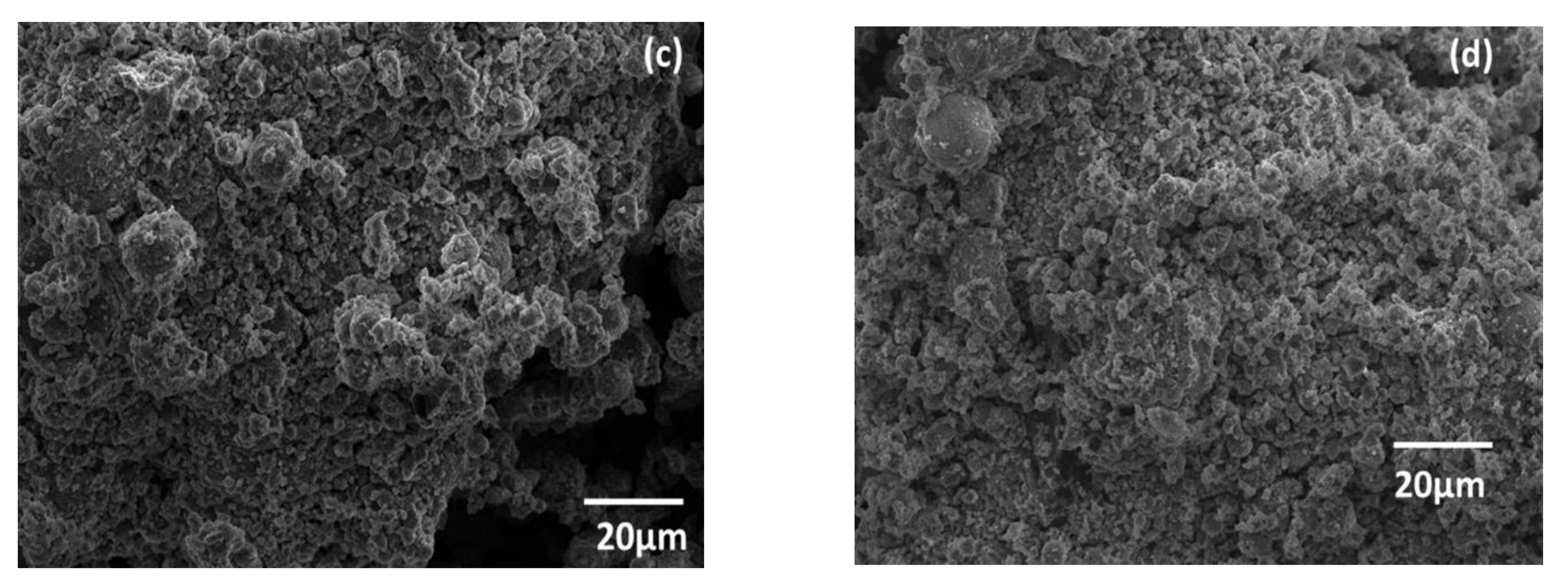
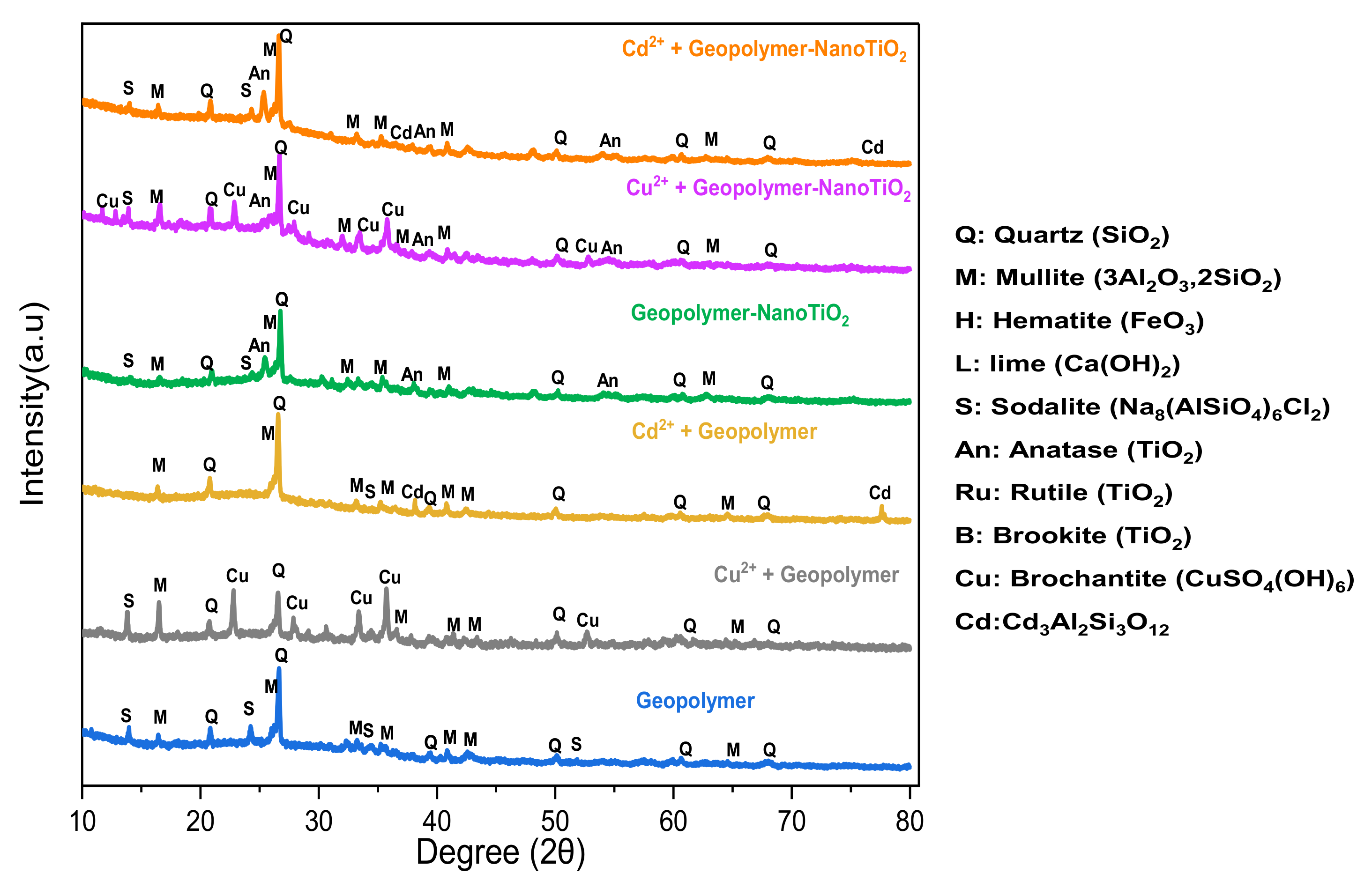
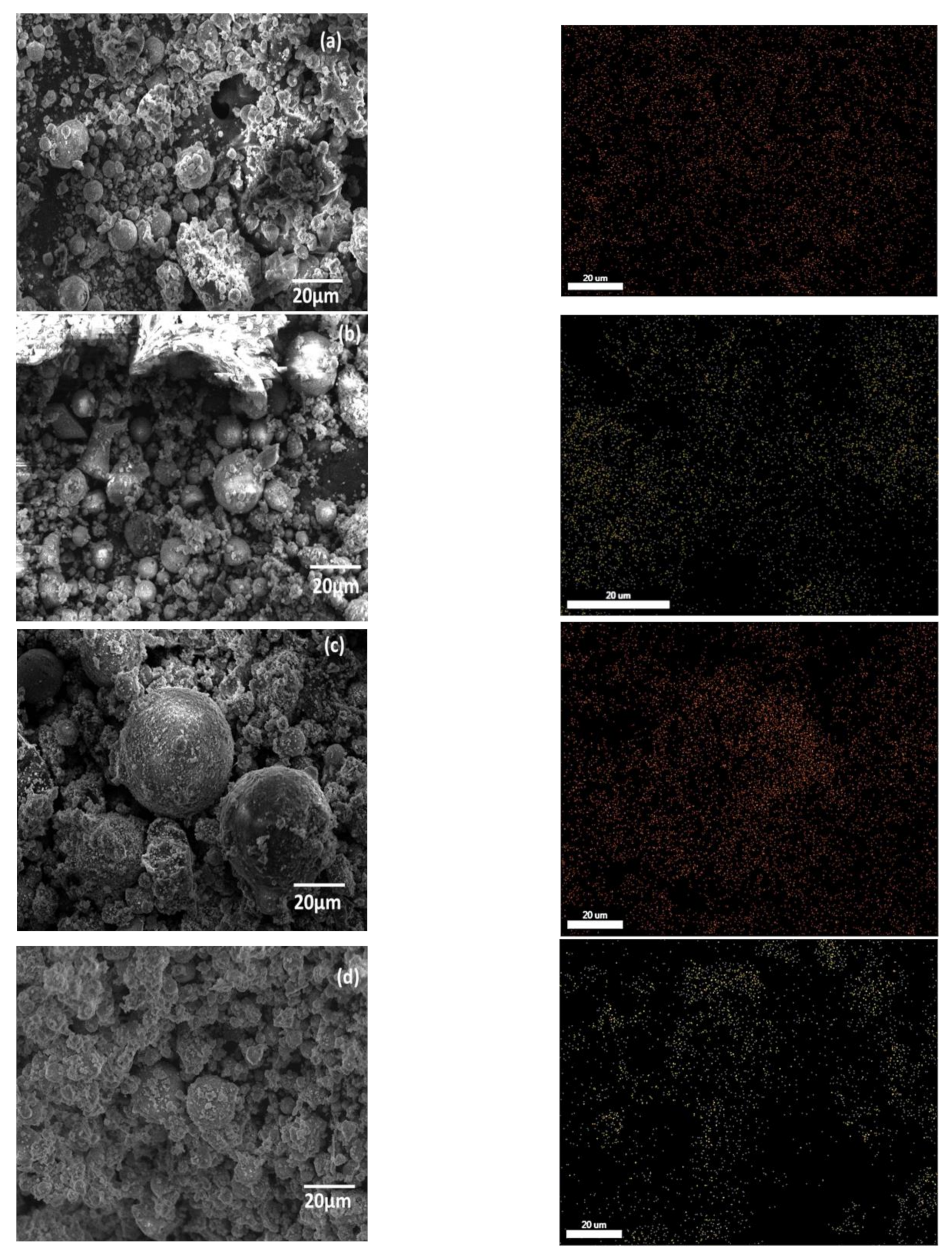
3.1. Sample Characterization
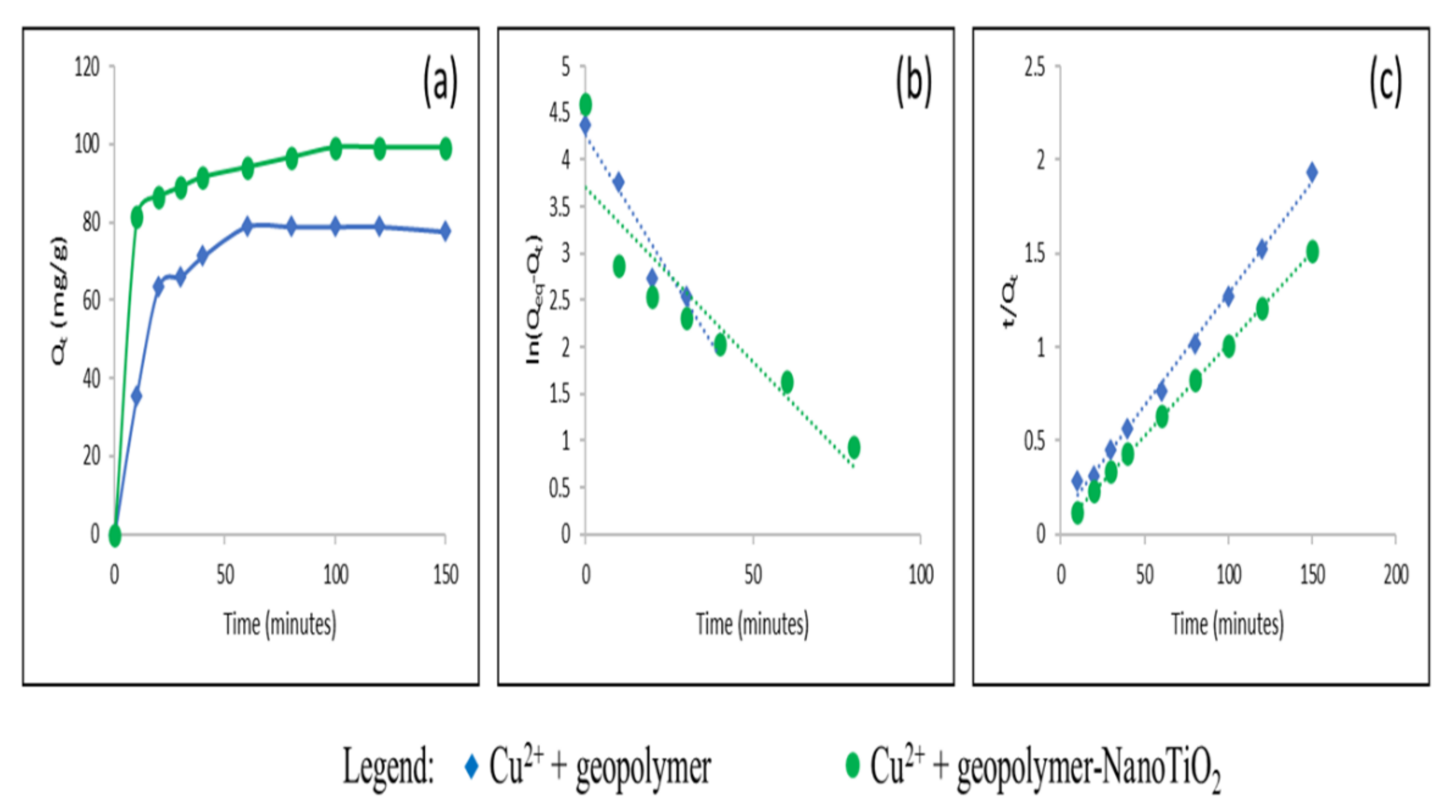
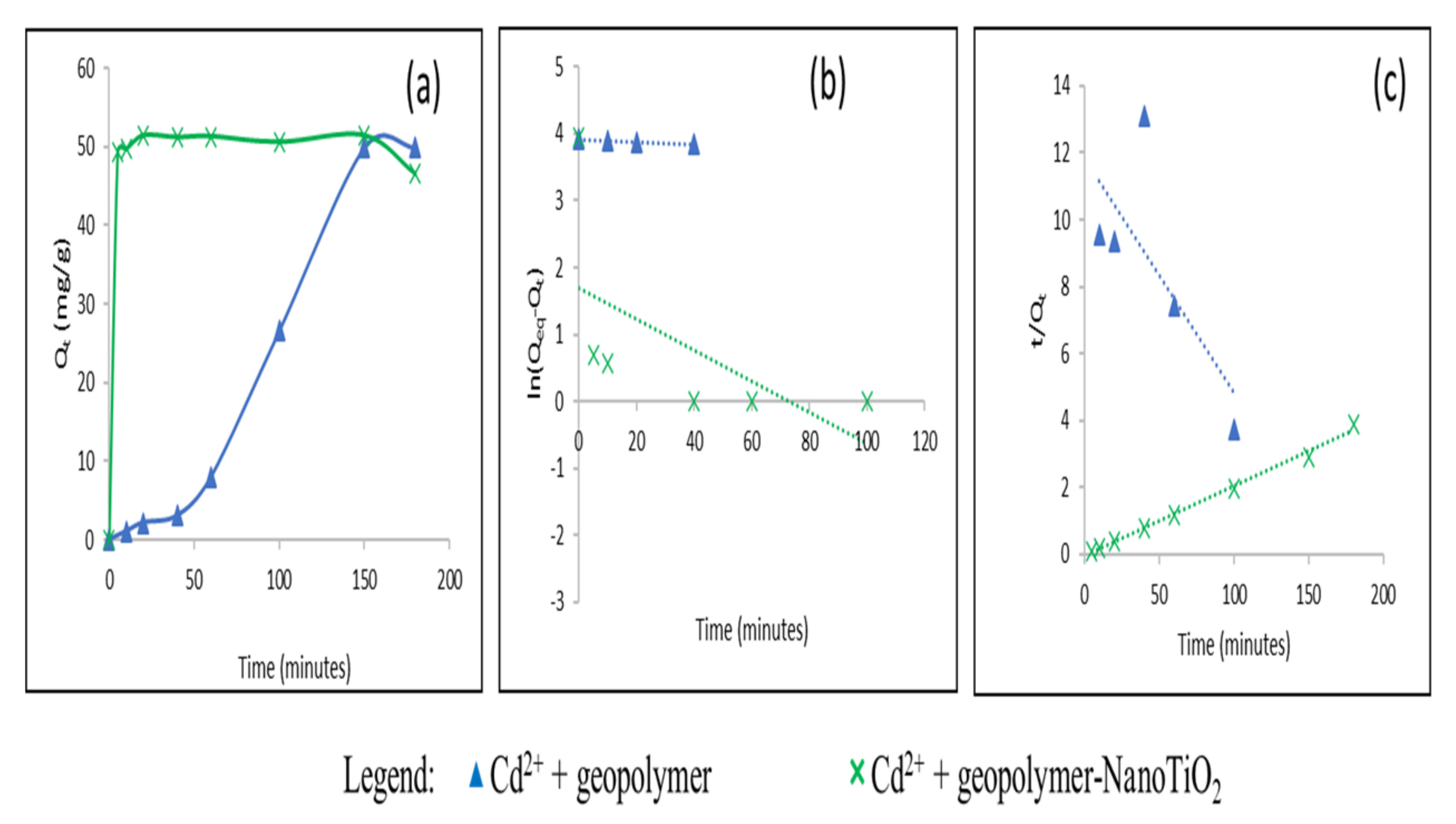
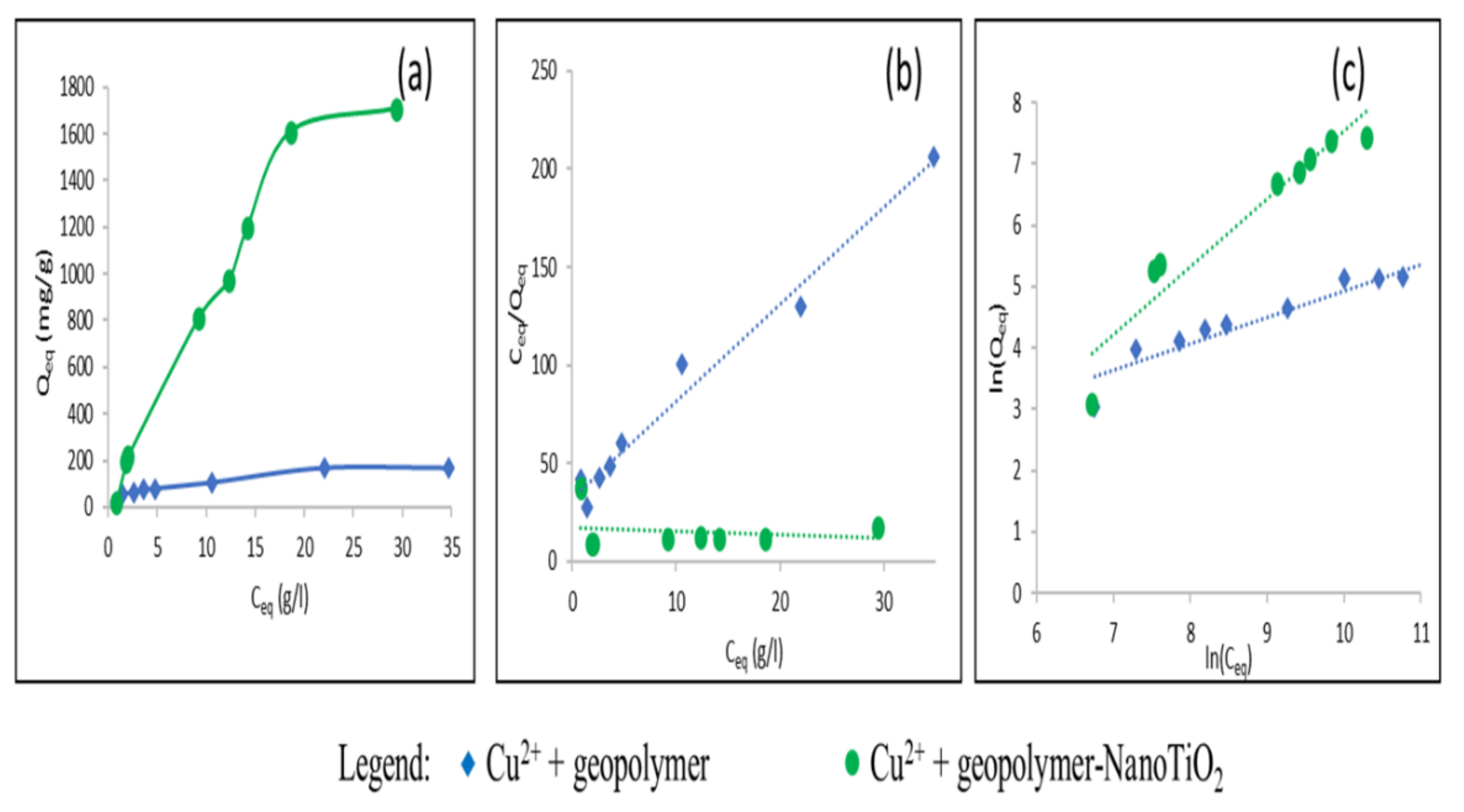
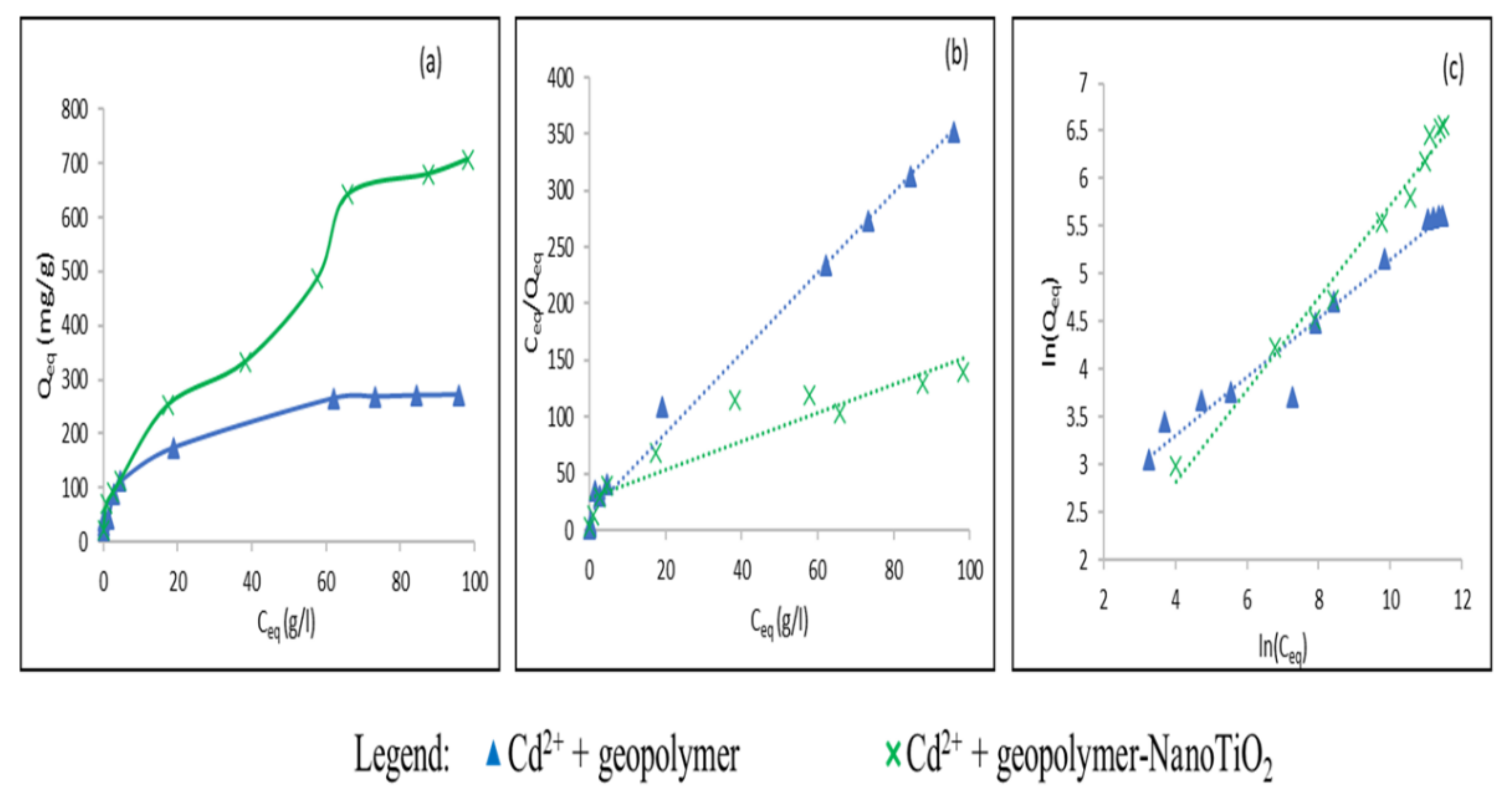
3.2. Study of the Adsorption of Cu2+ and Cd2+ from an Aqueous Solution
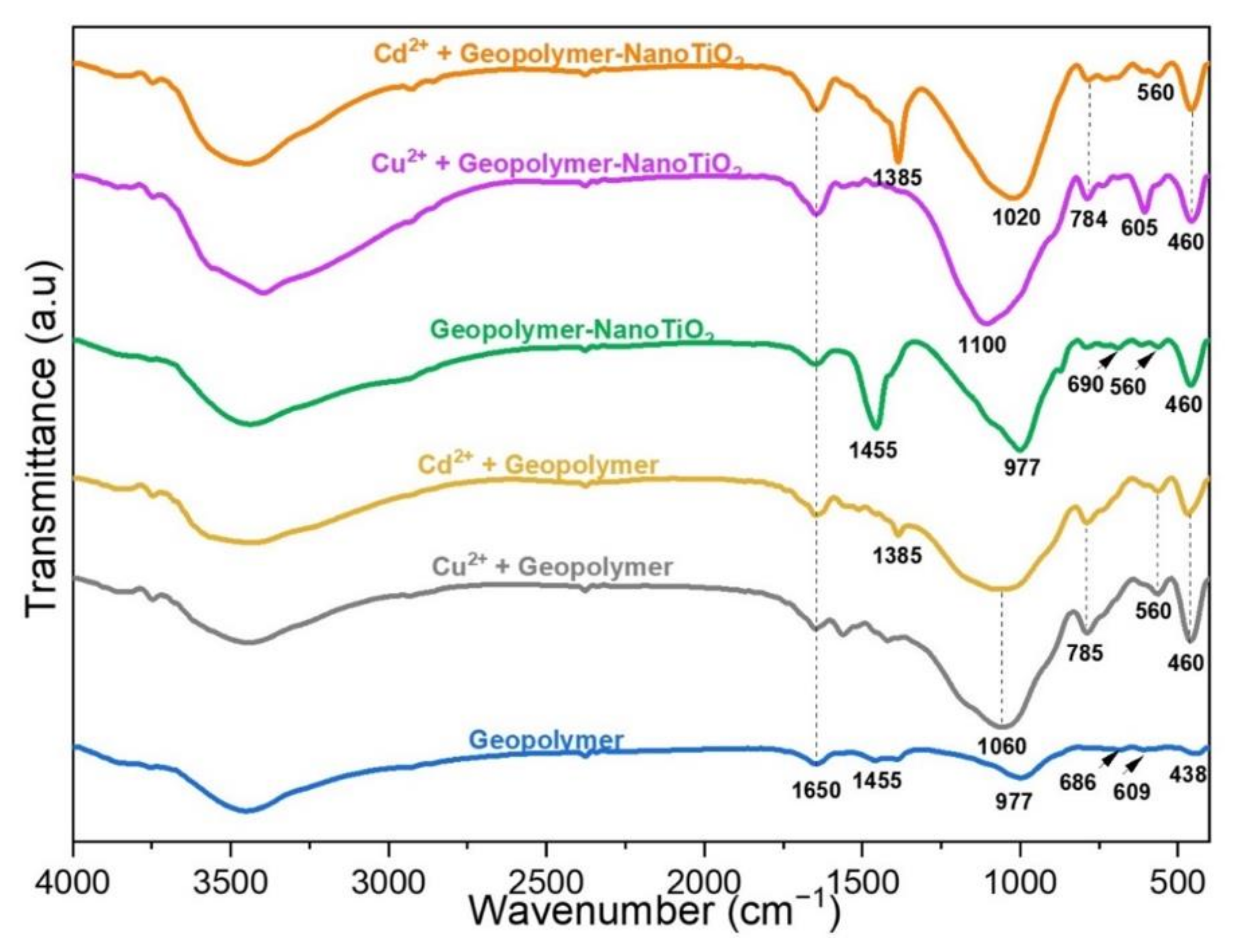
3.3. Characterization after Adsorption of Cu2+ and Cd2+ from an Aqueous Solution
3.4. Comparative Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baby, J.; Raj, J.S.; Biby, E.T.; Sankarganesh, P.; Jeevitha, M.V.; Ajisha, S.U.; Rajan, S.S. Toxic Effect of Heavy Metals on Aquatic Environment. Int. J. Biol. Chem. Sci. 2011, 4, 939–952. [Google Scholar] [CrossRef] [Green Version]
- Crini, G. Recent Developments in Polysaccharide-Based Materials Used as Adsorbents in Wastewater Treatment. Prog. Polym. Sci. 2005, 30, 38–70. [Google Scholar] [CrossRef]
- Mohammed, A.S.; Kapri, A.; Goel, R. Heavy Metal Pollution: Source, Impact, and Remedies. In Biomanagement of Metal-Contaminated Soils; Springer: Dordrecht, The Netherlands, 2011; Volume 20, pp. 1–28. [Google Scholar] [CrossRef]
- Maleki, A.; Hajizadeh, Z.; Sharifi, V.; Emdadi, Z. A Green, Porous and Eco-Friendly Magnetic Geopolymer Adsorbent for Heavy Metals Removal from Aqueous Solutions. J. Clean. Prod. 2019, 215, 1233–1245. [Google Scholar] [CrossRef]
- Darmayanti, L.; Kadja, G.T.M.; Notodarmojo, S.; Damanhuri, E.; Mukti, R.R. Structural Alteration within Fly Ash-Based Geopolymers Governing the Adsorption of Cu2+ from Aqueous Environment: Effect of Alkali Activation. J. Hazard. Mater. 2019, 377, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Ratnarathorn, N.; Chailapakul, O.; Henry, C.S.; Dungchai, W. Simple Silver Nanoparticle Colorimetric Sensing for Copper by Paper-Based Devices. Talanta 2012, 99, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Cui, X.; Liao, C.; Li, Z. Facile Fabrication of Green Geopolymer/Alginate Hybrid Spheres for Efficient Removal of Cu(II) in Water: Batch and Column Studies. Chem. Eng. J. 2017, 311, 126–134. [Google Scholar] [CrossRef]
- Yu, Z.; Dang, Q.; Liu, C.; Cha, D.; Zhang, H.; Zhu, W.; Zhang, Q.; Fan, B. Preparation and Characterization of Poly(Maleic Acid)-Grafted Cross-Linked Chitosan Microspheres for Cd(II) Adsorption. Carbohydr. Polym. 2017, 172, 28–39. [Google Scholar] [CrossRef]
- Meng, J.; Cui, J.; Yu, J.; Huang, W.; Wang, P.; Wang, K.; Liu, M.; Song, C.; Chen, P. Preparation of Green Chelating Fibers and Adsorption Properties for Cd(II) in Aqueous Solution. J. Mater. Sci. 2017, 53, 2277–2289. [Google Scholar] [CrossRef]
- Wang, X.S.; Miao, H.H.; He, W.; Shen, H.L. Competitive Adsorption of Pb(II), Cu(II), and Cd(II) Ions on Wheat-Residue Derived Black Carbon. J. Chem. Eng. Data 2011, 56, 444–449. [Google Scholar] [CrossRef]
- Lan, T.; Li, P.; Rehman, F.U.; Li, X.; Yang, W.; Guo, S. Efficient Adsorption of Cd2+ from Aqueous Solution Using Metakaolin Geopolymers. Environ. Sci. Pollut. Res. 2019, 26, 33555–33567. [Google Scholar] [CrossRef]
- Cheng, Q.; Huang, Q.; Khan, S.; Liu, Y.; Liao, Z.; Li, G.; Ok, Y.S. Adsorption of Cd by Peanut Husks and Peanut Husk Biochar from Aqueous Solutions. Ecol. Eng. 2016, 87, 240–245. [Google Scholar] [CrossRef]
- Kutchko, B.G.; Kim, A.G. Fly Ash Characterization by SEM–EDS. Fuel 2006, 85, 2537–2544. [Google Scholar] [CrossRef]
- Li, L.; Wang, S.; Zhu, Z. Geopolymeric Adsorbents from Fly Ash for Dye Removal from Aqueous Solution. J. Colloid Interface Sci. 2006, 300, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Gaya, U.I.; Abdullah, A.H. Heterogeneous Photocatalytic Degradation of Organic Contaminants over Titanium Dioxide: A Review of Fundamentals, Progress and Problems. J. Photochem. Photobiol. C Photochem. Rev. 2008, 9, 1–12. [Google Scholar] [CrossRef]
- Cho, H.; Oh, D.; Kim, K. A Study on Removal Characteristics of Heavy Metals from Aqueous Solution by Fly Ash. J. Hazard. Mater. 2005, 127, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Aboulayt, A.; Jaafri, R.; Samouh, H.; Cherki El Idrissi, A.; Roziere, E.; Moussa, R.; Loukili, A. Stability of a New Geopolymer Grout: Rheological and Mechanical Performances of Metakaolin-Fly Ash Binary Mixtures. Constr. Build. Mater. 2018, 181, 420–436. [Google Scholar] [CrossRef]
- Kai, M.F.; Dai, J.G. Understanding Geopolymer Binder-Aggregate Interfacial Characteristics at Molecular Level. Cem. Concr. Res. 2021, 149, 106582. [Google Scholar] [CrossRef]
- Sumesh, M.; Alengaram, U.J.; Jumaat, M.Z.; Mo, K.H.; Alnahhal, M.F. Incorporation of Nano-Materials in Cement Composite and Geopolymer Based Paste and Mortar—A Review. Constr. Build. Mater. 2017, 148, 62–84. [Google Scholar] [CrossRef]
- Maiti, M.; Sarkar, M.; Maiti, S.; Malik, M.A.; Xu, S. Modification of Geopolymer with Size Controlled TiO2 Nanoparticle for Enhanced Durability and Catalytic Dye Degradation under UV Light. J. Clean. Prod. 2020, 255, 120183. [Google Scholar] [CrossRef]
- Sivasakthi, M.; Jeyalakshmi, R.; Rajamane, N.P. Investigation of Microstructure and Thermomechanical Properties of Nano-TiO2 Admixed Geopolymer for Thermal Resistance Applications. J. Mater. Eng. Perform. 2021, 30, 3642–3653. [Google Scholar] [CrossRef]
- Jézéquel, H.; Chu, K. Removal of Arsenate from Aqueous Solution by Adsorption onto Titanium Dioxide Nanoparticles. J. Environ. Sci. Health Part A 2007, 41, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Hung, W.C.; Fu, S.H.; Tseng, J.J.; Chu, H.; Ko, T.H. Study on Photocatalytic Degradation of Gaseous Dichloromethane Using Pure and Iron Ion-Doped TiO2 Prepared by the Sol–Gel Method. Chemosphere 2007, 66, 2142–2151. [Google Scholar] [CrossRef] [PubMed]
- Hema, M.; Arasi, A.Y.; Tamilselvi, P.; Anbarasan, R. Titania Nanoparticles Synthesized by Sol-Gel Technique. Chem Sci Trans 2013, 2, 239–245. [Google Scholar] [CrossRef] [Green Version]
- Sindhunata; Van Deventer, J.S.J.; Lukey, G.C.; Xu, H. Effect of Curing Temperature and Silicate Concentration on Fly-Ash-Based Geopolymerization. Ind. Eng. Chem. Res. 2006, 45, 3559–3568. [Google Scholar] [CrossRef]
- Khatib, K.; Kerroumi, H.; El Azhari, M. Synthesis, Characterization and Optimization of New Adsorbent Materials Based on Industrial Discharges for the Decontamination of Liquid Effluents. Mater. Today Proc. 2020, 22, 120–125. [Google Scholar] [CrossRef]
- Sastry, K.V.S.G.K.; Sahitya, P.; Ravitheja, A. Influence of Nano TiO2 on Strength and Durability Properties of Geopolymer Concrete. Mater. Today Proc. 2021, 45, 1017–1025. [Google Scholar] [CrossRef]
- Van Jaarsveld, J.G.S.; Van Deventer, J.S.J. Effect of the Alkali Metal Activator on the Properties of Fly Ash-Based Geopolymers. Ind. Eng. Chem. Res. 1999, 38, 3932–3941. [Google Scholar] [CrossRef]
- Nath, S.K.; Maitra, S.; Mukherjee, S.; Kumar, S. Microstructural and Morphological Evolution of Fly Ash Based Geopolymers. Constr. Build. Mater. 2016, 111, 758–765. [Google Scholar] [CrossRef]
- Yang, L.Y.; Jia, Z.J.; Zhang, Y.M.; Dai, J.G. Effects of Nano-TiO2 on Strength, Shrinkage and Microstructure of Alkali Activated Slag Pastes. Cem. Concr. Compos. 2015, 57, 1–7. [Google Scholar] [CrossRef]
- Karanac, M.; Đolić, M.; Veličković, Z.; Kapidžić, A.; Ivanovski, V.; Mitrić, M.; Marinković, A. Efficient Multistep Arsenate Removal onto Magnetite Modified Fly Ash. J. Environ. Manage. 2018, 224, 263–276. [Google Scholar] [CrossRef]
- Vijayalakshmi, R.; Rajendran, V. Synthesis and Characterization of Nano-Tio2 via Different Methods. Arch. Appl. Sci. Res. 2012, 4, 1183–1190. [Google Scholar]
- Nikolić, V.; Komljenović, M.; Marjanović, N.; Baščarević, Z.; Petrović, R. Lead Immobilization by Geopolymers Based on Mechanically Activated Fly Ash. Ceram. Int. 2014, 40, 8479–8488. [Google Scholar] [CrossRef]
- Khataee, R.; Heydari, V.; Moradkhannejhad, L.; Safarpour, M.; Joo, S.W. Self-Cleaning and Mechanical Properties of Modified White Cement with Nanostructured TiO2. J. Nanosci. Nanotechnol. 2013, 13, 5109–5114. [Google Scholar] [CrossRef]
- Zailan, S.N.; Mahmed, N.; Abdullah, M.M.A.B.; Rahim, S.Z.A.; Halin, D.S.C.; Sandu, A.V.; Vizureanu, P.; Yahya, Z. Potential Applications of Geopolymer Cement-Based Composite as Self-Cleaning Coating: A Review. Coatings 2022, 12, 133. [Google Scholar] [CrossRef]
- Ono, Y.; Amano, Y.; Aikawa, M.; Machida, M. Zur Theorie Der Sogenannten Adsorption Geloster Stoffe. K. Sven. Vetenskapsakademiens. Handl. 1898, 24, 1–39. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Sorption of Dye from Aqueous Solution by Peat. Chem. Eng. J. 1998, 70, 115–124. [Google Scholar] [CrossRef]
- Tang, Q.; Wang, K.; Yaseen, M.; Tong, Z.; Cui, X. Synthesis of Highly Efficient Porous Inorganic Polymer Microspheres for the Adsorptive Removal of Pb2+from Wastewater. J. Clean. Prod. 2018, 193, 351–362. [Google Scholar] [CrossRef]
- Langmuir, I. The Constitution and Fundamental Properties of Solids and Liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef] [Green Version]
- Al-Harahsheh, M.S.; Al Zboon, K.; Al-Makhadmeh, L.; Hararah, M.; Mahasneh, M. Fly Ash Based Geopolymer for Heavy Metal Removal: A Case Study on Copper Removal. J. Environ. Chem. Eng. 2015, 3, 1669–1677. [Google Scholar] [CrossRef]
- Onutai, S.; Kobayashi, T.; Thavorniti, P.; Jiemsirilers, S. The Adsorption of Cadmium Ions on Fly Ash Based Geopolymer Particles. Key Eng. Mater. 2018, 766, 65–70. [Google Scholar] [CrossRef]
- Balamurugan, S.; Balu, A.; Usharani, K.; Suganya, M.; Anitha, S.; Prabha, D.L.; Ilangovan, S. Synthesis of CdO Nanopowders by a Simple Soft Chemical Method and Evaluation of Their Antimicrobial Activities. Pac. Sci. Rev. A Nat. Sci. Eng. 2016, 18, 228–232. [Google Scholar] [CrossRef]
- Pandey, S.; Tiwari, S. Facile Approach to Synthesize Chitosan Based Composite—Characterization and Cadmium(II) Ion Adsorption Studies. Carbohydr. Polym. 2015, 134, 646–656. [Google Scholar] [CrossRef]
- Ge, Y.; Cui, X.; Kong, Y.; Li, Z.; He, Y.; Zhou, Q. Porous Geopolymeric Spheres for Removal of Cu(II) from Aqueous Solution: Synthesis and Evaluation. J. Hazard. Mater. 2015, 283, 244–251. [Google Scholar] [CrossRef]
- Yu, Z.; Song, W.; Li, J.; Li, Q. Improved Simultaneous Adsorption of Cu(II) and Cr(VI) of Organic Modified Metakaolin-Based Geopolymer. Arab. J. Chem. 2020, 13, 4811–4823. [Google Scholar] [CrossRef]
- Taamneh, Y.; Sharadqah, S. The Removal of Heavy Metals from Aqueous Solution Using Natural Jordanian Zeolite. Appl. Water Sci. 2016, 7, 2021–2028. [Google Scholar] [CrossRef]

| Component | SiO2 | Al2O3 | Fe2O3 | CaO | K2O | TiO2 | MgO | Na2O | P2O5 | MnO | Loss Amount |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Content (wt%) | 33.83 | 13.12 | 5.58 | 4.27 | 2.18 | 0.95 | 0.93 | 0.83 | 0.45 | 0.06 | 37.76 |
| Adsorbate | Adsorbents | Qeq (mg/g) Experimental | Pseudo First Order | Pseudo Second Order | ||||
|---|---|---|---|---|---|---|---|---|
| Qeq (mg/g) | K1 (min−1) | R2 | Qeq (mg/g) | K2 (g.mg−1. min−1) | R2 | |||
| Cu2+ | Geopolymer | 78.87 | 37.62 | 0.0244 | 0.86 | 84.03 | 1.5.10−3 | 0.99 |
| Geopolymer-NanoTiO2 | 99.23 | 40.28 | 0.0372 | 0.83 | 102 | 2.62 | 0.99 | |
| Cd2+ | Geopolymer | 49.9 | 56.68 | 7.5.10−3 | 0.81 | 16.72 | 2.8.10−4 | 0.68 |
| Geopolymer-NanoTiO2 | 51.43 | 1.79 | 0.031 | 0.009 | 48.07 | 0.01 | 0.99 | |
| Adsorbate | Adsorbents | Qmax (mg/g) Experimental | Langmuir Model | Freundlich Model | |||||
|---|---|---|---|---|---|---|---|---|---|
| Qmax (mg/g) | KL (L/mg) | RL | R2 | n | KF (mg/g)(L/mg)1/n | R2 | |||
| Cu2+ | Geopolymer | 172.83 | 188.67 | 1.7.10−4 | 0.02 < RL < 0.52 | 0.99 | 2.32 | 1.86 | 0.89 |
| Geopolymer-NanoTiO2 | 1708.2 | 5000 | 1.17.10−5 | 0.25 < RL < 0.94 | 0.033 | 0.9 | 0.029 | 0.91 | |
| Cd2+ | Geopolymer | 271.66 | 285.71 | 2.3.10−4 | 0.006 < RL < 0.77 | 0.99 | 3.25 | 8.03 | 0.95 |
| Geopolymer-NanoTiO2 | 706.9 | 833.33 | 3.78.10−5 | 0.07 < RL < 0.95 | 0.84 | 2.03 | 2.18 | 0.97 | |
| Fly Ash | Nano TiO2 | Geopolymer | Cu2++ Geopolymer | Cd2++ Geopolymer | Geopolymer-NanoTiO2 | Cu2++ Geopolymer-NanoTiO2 | Cd2++ Geopolymer-NanoTiO2 | |
|---|---|---|---|---|---|---|---|---|
| Element | Weight% | Weight% | Weight% | Weight% | Weight% | Weight% | Weight% | Weight% |
| C | 61.48 | 3.07 | 2.31 | 14.5 | 24.54 | 12.59 | 22.42 | 22.78 |
| O | 19.29 | 39.59 | 36.72 | 38.74 | 43.32 | 39.24 | 36.43 | 39.40 |
| Fe | 0.08 | - | 0.51 | 0.05 | 2.38 | 0.00 | 3.08 | 3.33 |
| Na | 0.19 | - | 16.10 | - | 0.84 | 13.84 | - | 2.04 |
| Mg | 0.39 | - | 0.84 | 0.11 | 0.55 | 0.11 | 0.77 | 0.72 |
| Al | 4.87 | - | 7.16 | 5.43 | 8.22 | 6.30 | 6.22 | 7.29 |
| Si | 10.28 | - | 19.40 | 9.87 | 16.42 | 17.20 | 9.81 | 12.13 |
| S | 0.61 | - | - | 4.53 | - | - | 1.5 | - |
| K | 0.98 | - | 6.31 | 0.89 | 1.09 | 1.30 | 0.96 | 1.02 |
| Ca | 1.84 | - | 10.64 | 1.13 | 1.03 | 3.83 | 1.13 | 2.83 |
| N | - | 0.08 | - | 0.38 | - | - | - | - |
| Br | - | - | - | - | - | - | - | |
| Ti | - | 57.26 | - | - | - | 5.60 | 3.71 | 3.05 |
| Cu | - | - | - | 24.36 | - | - | 13.97 | - |
| Cd | - | - | - | - | 1.61 | - | - | 5.41 |
| Adsorbent | Adsorbates | pH | Time (min) | Temperature °C | Q(mg/g) | Ref. |
|---|---|---|---|---|---|---|
| Porous geopolymer | Cu2+ | 5 | 2500 | 25 | 52.63 | [44] |
| Geopolymer/alginate hybrid spheres | Cu2+ | 5 | 2500 | 25 | 60.8 | [7] |
| CTAB/geopolymer | Cu2+ | 5 | 60 | 30 | 147.2 | [45] |
| Jordanian zeolite | Cu2+ | 6 | 20 | - | 14.3 | [46] |
| Cd2+ | 6 | 20 | - | 25.9 | ||
| Metakaolin geopolymer powder | Cd2+ | 5 | 400 | 25 | 70.3 | [11] |
| Fly ash-chitosan | Cd2+ | 8 | 180 | - | 87.72 | [43] |
| Geopolymer | Cu2+ | 6.8 | 60 | 25 | 172.83 | This work |
| Cd2+ | 6.8 | 100 | 25 | 271.66 | ||
| Geopolymer-NanoTiO2 | Cu2+ | 6.8 | 150 | 25 | 1708.2 | |
| Cd2+ | 6.8 | 20 | 25 | 706.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khatib, K.; Lahmyed, L.; El Azhari, M. Synthesis, Characterization, and Application of Geopolymer/TiO2 Nanoparticles Composite for Efficient Removal of Cu(II) and Cd(II) Ions from Aqueous Media. Minerals 2022, 12, 1445. https://doi.org/10.3390/min12111445
Khatib K, Lahmyed L, El Azhari M. Synthesis, Characterization, and Application of Geopolymer/TiO2 Nanoparticles Composite for Efficient Removal of Cu(II) and Cd(II) Ions from Aqueous Media. Minerals. 2022; 12(11):1445. https://doi.org/10.3390/min12111445
Chicago/Turabian StyleKhatib, Khalid, Loubna Lahmyed, and Mohamed El Azhari. 2022. "Synthesis, Characterization, and Application of Geopolymer/TiO2 Nanoparticles Composite for Efficient Removal of Cu(II) and Cd(II) Ions from Aqueous Media" Minerals 12, no. 11: 1445. https://doi.org/10.3390/min12111445





