Flotation of Carbonaceous Matter from a Double Refractory Gold Ore: The Effect of MIBC on Flotation Performance and Kinetics
Abstract
:1. Introduction
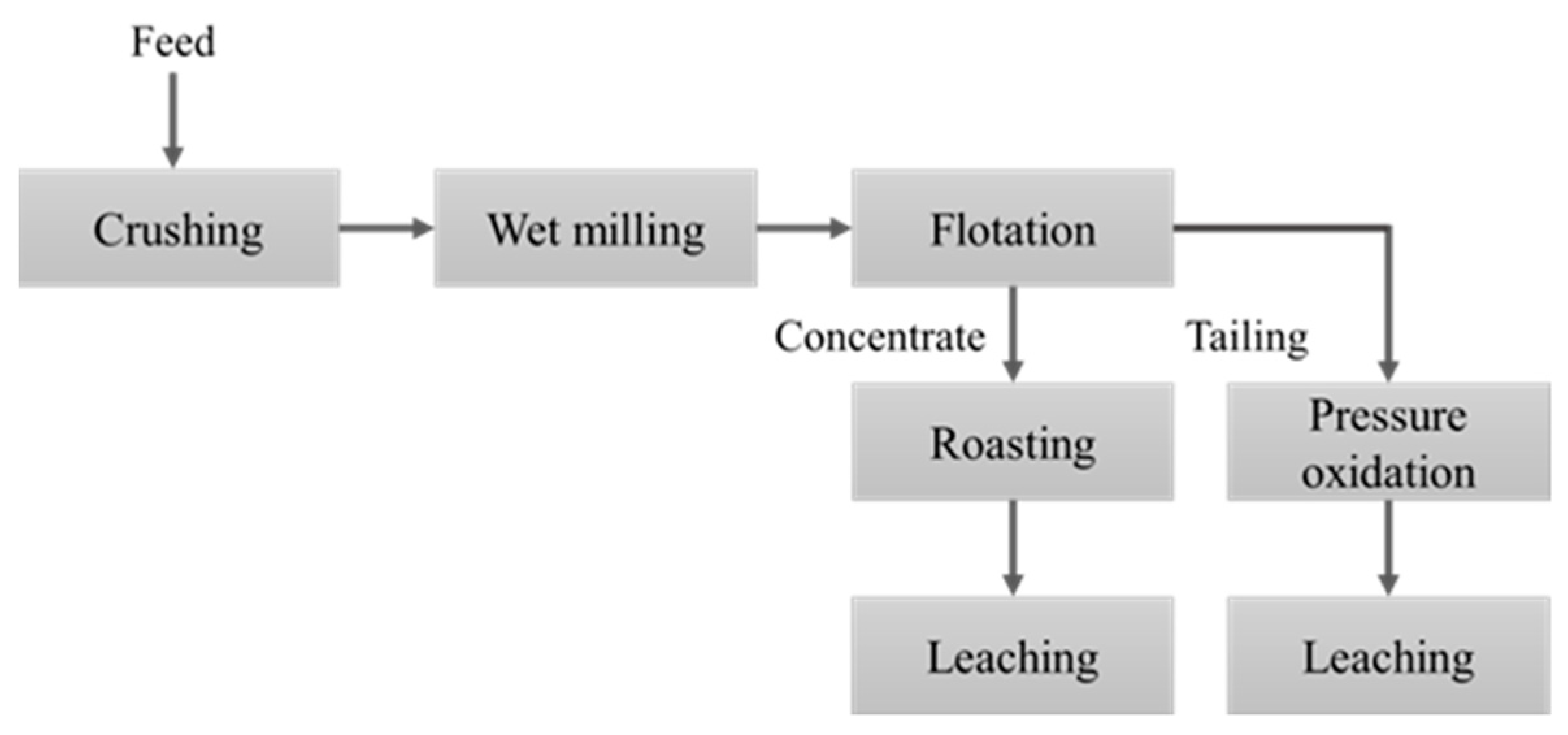
2. Materials and Methods
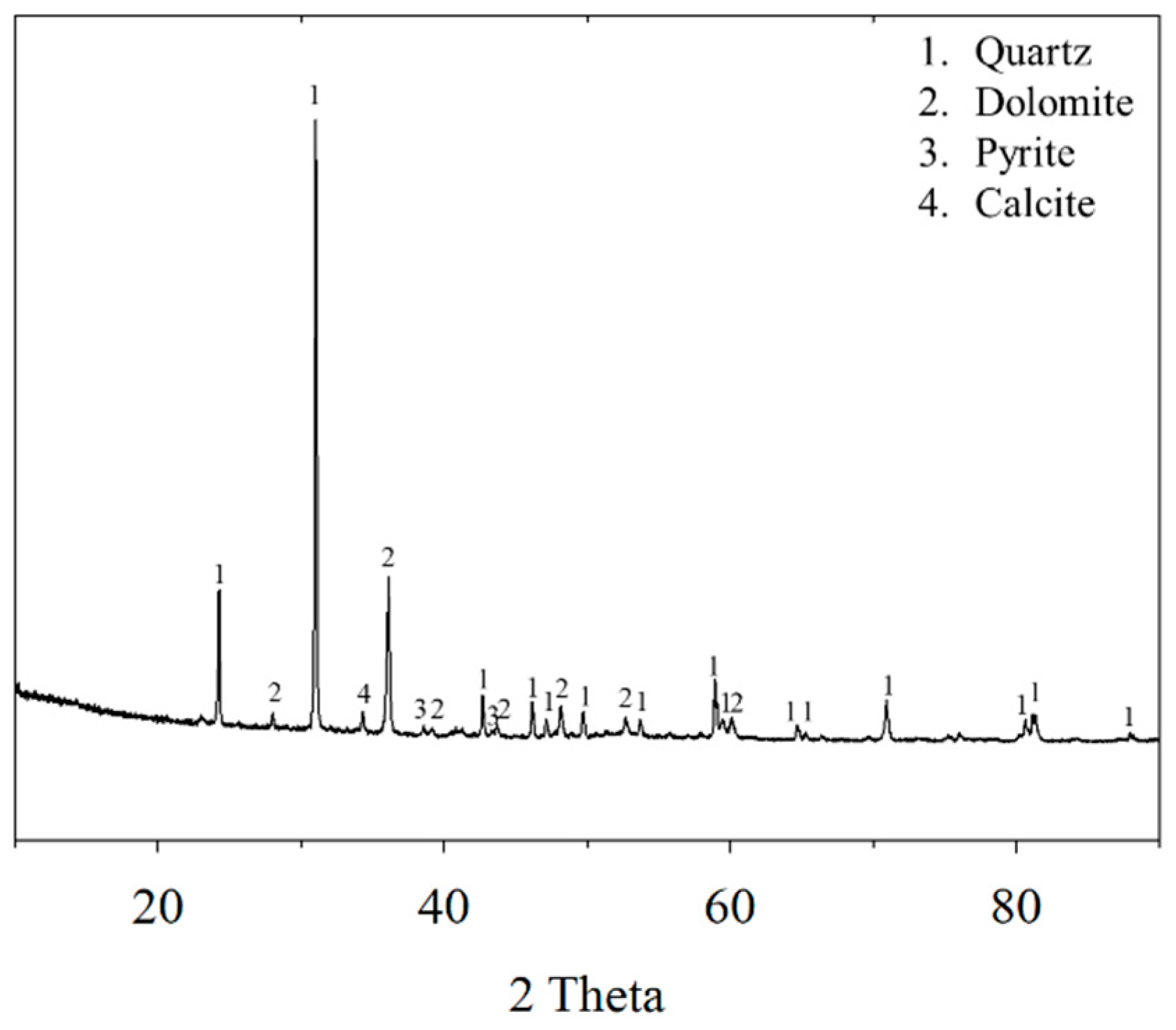
2.1. Materials
2.2. Methods
3. Results and Discussion
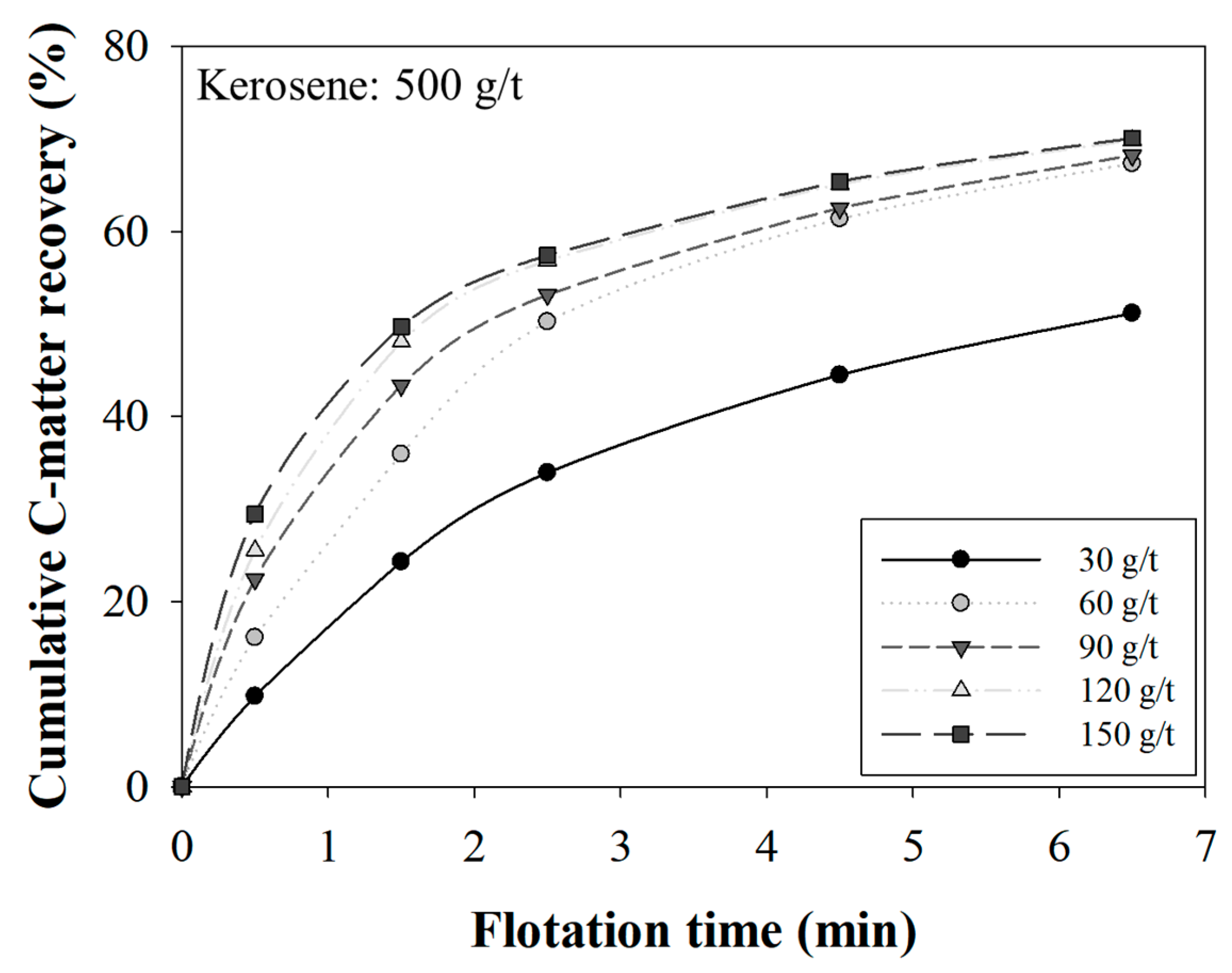
3.1. The Effect of MIBC Dosage on Recovery
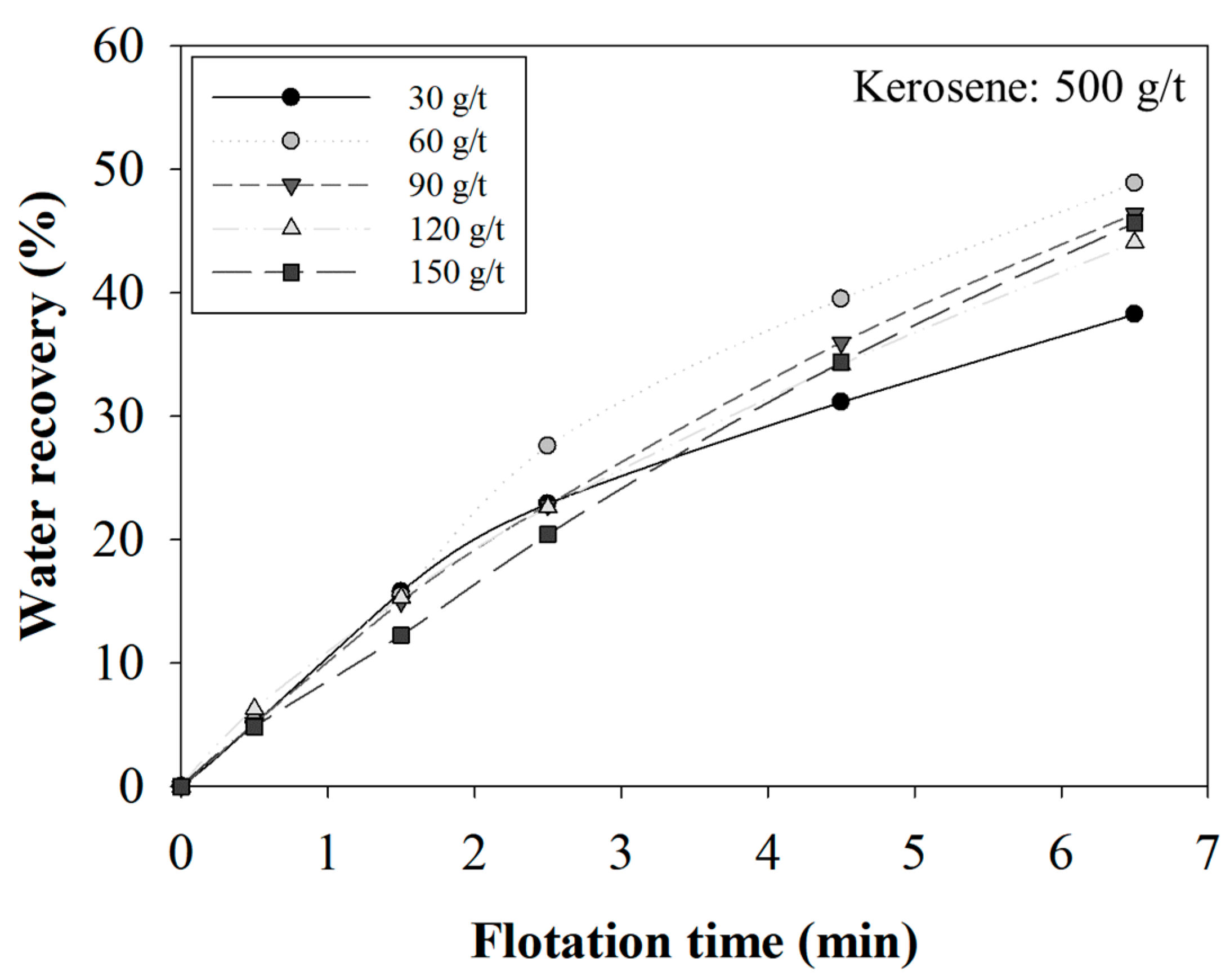
3.2. The Effect of MIBC Dosage on the Selectivity
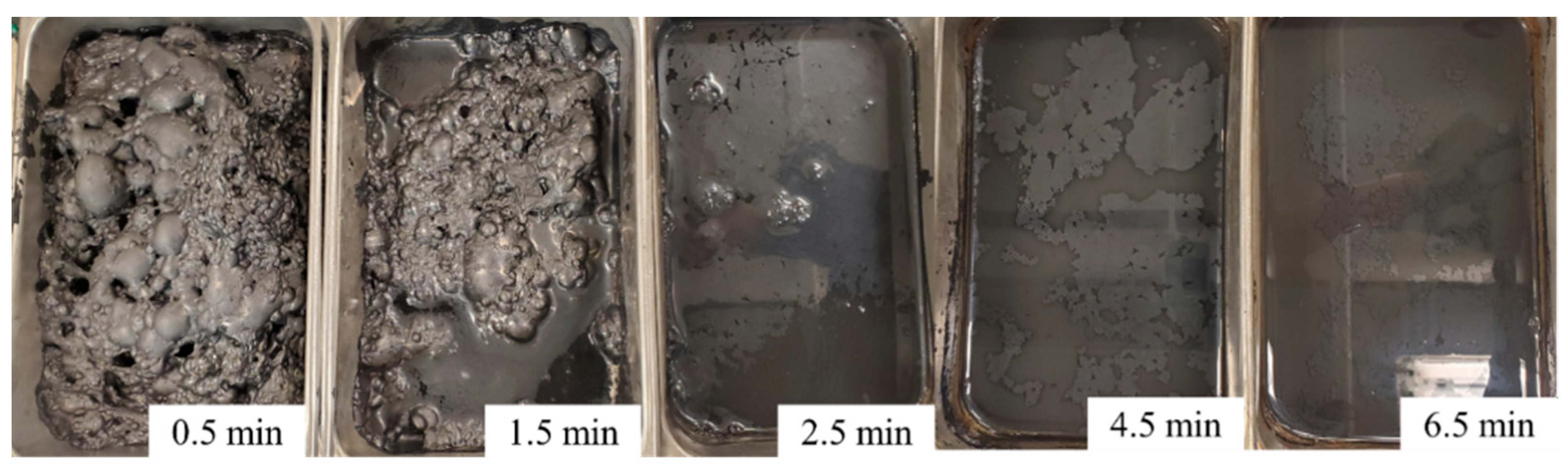
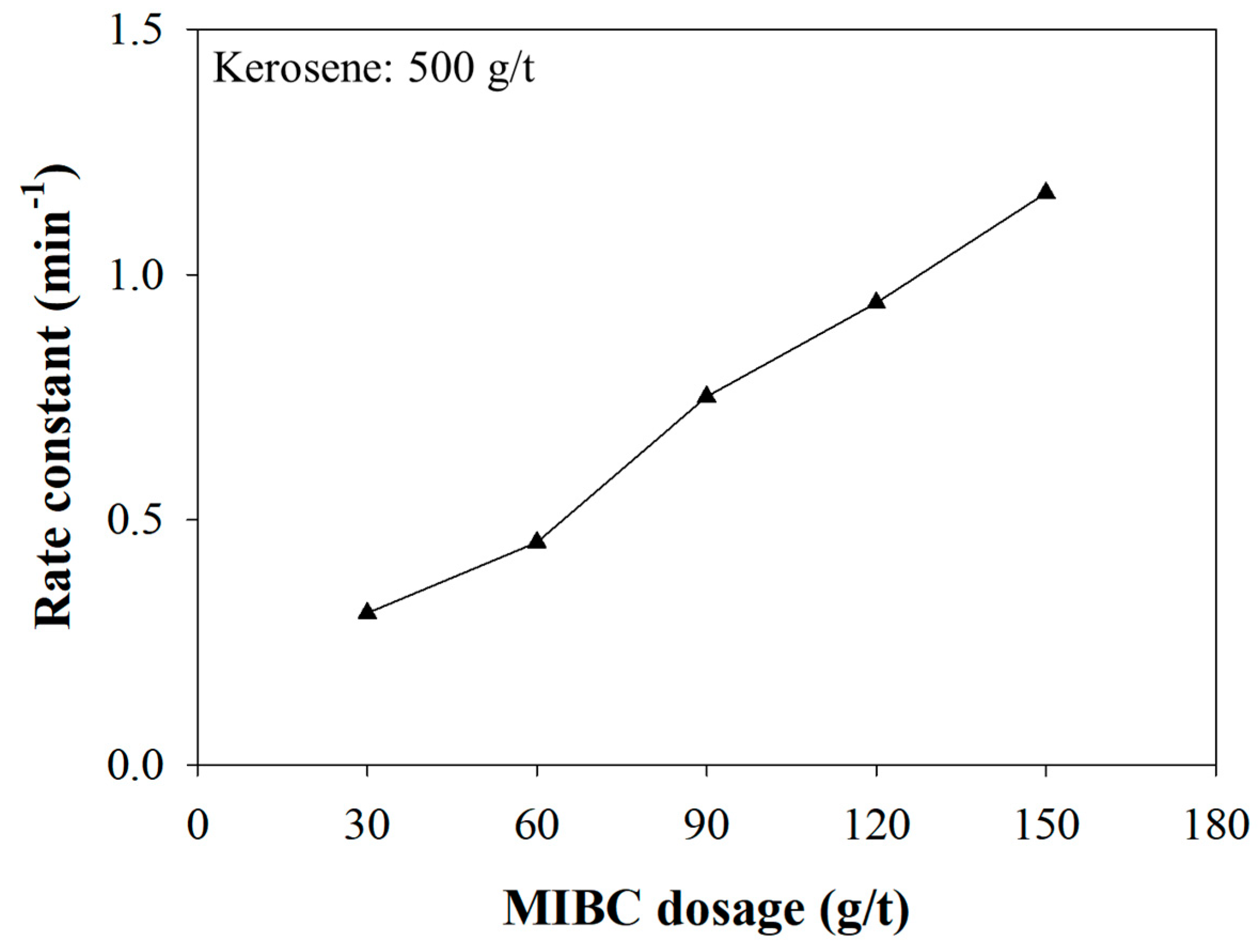
3.3. The Effect of the MIBC Dosage on Kinetics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Komnitsas, C.; Pooley, F.D. Mineralogical characteristics and treatment of refractory gold ores. Miner. Eng. 1989, 2, 449–457. [Google Scholar] [CrossRef]
- La Brooy, S.R.; Linge, H.G.; Walker, G.S. Review of gold extraction from ores. Miner. Eng. 1994, 7, 1213–1241. [Google Scholar] [CrossRef]
- Chen, T.T.; Cabri, L.J.; Dutrizac, J.E. Characterizing gold in refractory sulfide gold ores and residues. JOM 2002, 54, 20–22. [Google Scholar] [CrossRef]
- Adams, M.D. (Ed.) Advances in Gold Ore Processing; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Thomas, K.G.; Pearson, M.S. Pressure oxidation overview. In Gold Ore Processing; Elsevier: Amsterdam, The Netherlands, 2016; pp. 341–358. [Google Scholar]
- Aylmore, M.G.; de Klerk, L.W. Conditions and design considerations for maximising recoverable gold in roasting of refractory gold ores. In Proceedings of the World Gold Conference/Brisbane, Brisbane, QLD, Australia, 26–29 September 2013; pp. 26–29. [Google Scholar]
- Cho, Y.S.; Laskowski, J.S. Effect of flotation frothers on bubble size and foam stability. Int. J. Miner. Process. 2002, 64, 69–80. [Google Scholar] [CrossRef] [Green Version]
- Grau, R.A.; Laskowski, J.S.; Heiskanen, K. Effect of frothers on bubble size. Int. J. Miner. Process. 2005, 76, 225–233. [Google Scholar] [CrossRef]
- Laskowski, J.S. Testing flotation frothers. Physicochem. Probl. Miner. Process. 2004, 38, 13–22. [Google Scholar]
- Gupta, A.K.; Banerjee, P.K.; Mishra, A.; Satish, P. Effect of alcohol and polyglycol ether frothers on foam stability, bubble size and coal flotation. Int. J. Miner. Process. 2007, 82, 126–137. [Google Scholar] [CrossRef]
- Guven, O.; Batjargal, K.; Ozdemir, O.; Karakashev, S.I.; Grozev, N.A.; Boylu, F.; Çelik, M.S. Experimental procedure for the determination of the critical coalescence concentration (CCC) of simple frothers. Minerals 2020, 10, 617. [Google Scholar] [CrossRef]
- Finch, J.A.; Nesset, J.E.; Acuña, C. Role of frother on bubble production and behaviour in flotation. Miner. Eng. 2008, 21, 949–957. [Google Scholar] [CrossRef]
- Finch, J.A.; Gelinas, S.; Moyo, P. Frother-related research at McGill University. Miner. Eng. 2006, 19, 726–733. [Google Scholar] [CrossRef]
- Kracht, W.; Finch, J.A. Bubble break-up and the role of frother and salt. Int. J. Miner. Process. 2009, 92, 153–161. [Google Scholar] [CrossRef]
- Zhou, Z.A.; Egiebor, N.O.; Plitt, L.R. Frother effects on single bubble motion in a water column. Can. Metall. Q. 1992, 31, 11–16. [Google Scholar] [CrossRef]
- Sam, A.; Gomez, C.O.; Finch, J.A. Axial velocity profiles of single bubbles in water/frother solutions. Int. J. Miner. Process. 1996, 47, 177–196. [Google Scholar] [CrossRef]
- Rafiei, A.A.; Robbertze, M.; Finch, J.A. Gas holdup and single bubble velocity profile. Int. J. Miner. Process. 2011, 98, 89–93. [Google Scholar] [CrossRef]
- Zhou, Z.A.; Egiebor, N.O.; Plitt, L.R. Frother effects on bubble motion in a swarm. Can. Metall. Q. 1993, 32, 89–96. [Google Scholar] [CrossRef]
- Wu, M.; Gharib, M. Experimental studies on the shape and path of small air bubbles rising in clean water. Phys. Fluids 2002, 14, L49–L52. [Google Scholar] [CrossRef] [Green Version]
- Kracht, W.; Finch, J.A. Effect of frother on initial bubble shape and velocity. Int. J. Miner. Process. 2010, 94, 115–120. [Google Scholar] [CrossRef]
- Tsatouhas, G.; Grano, S.R.; Vera, M. Case studies on the performance and characterisation of the froth phase in industrial flotation circuits. Miner. Eng. 2006, 19, 774–783. [Google Scholar] [CrossRef]
- Barbian, N.; Ventura-Medina, E.; Cilliers, J.J. Dynamic froth stability in froth flotation. Miner. Eng. 2003, 16, 1111–1116. [Google Scholar] [CrossRef]
- Laskowski, J.S.; Cho, Y.S.; Ding, K. Effect of frothers on bubble size and foam stability in potash ore flotation systems. Can. J. Chem. Eng. 2003, 81, 63–69. [Google Scholar] [CrossRef]
- Moreno, Y.S.; Bournival, G.; Ata, S. Foam stability of flotation frothers under dynamic and static conditions. Sep. Purif. Technol. 2020, 274, 117822. [Google Scholar] [CrossRef]
- Zheng, X.; Johnson, N.W.; Franzidis, J.P. Modelling of entrainment in industrial flotation cells: Water recovery and degree of entrainment. Miner. Eng. 2006, 19, 1191–1203. [Google Scholar] [CrossRef]
- Małysa, K. Wet foams: Formation, properties and mechanism of stability. Adv. Colloid Interface Sci. 1992, 40, 37–83. [Google Scholar] [CrossRef]
- Melo, F.; Laskowski, J.S. Fundamental properties of flotation frothers and their effect on flotation. Miner. Eng. 2006, 19, 766–773. [Google Scholar] [CrossRef] [Green Version]
- Smith, P.G.; Warren, L.J. Entrainment of particles into flotation froths. Miner. Process. Extr. Metall. Rev. 1989, 5, 123–145. [Google Scholar] [CrossRef]
- Barbian, N.; Hadler, K.; Ventura-Medina, E.; Cilliers, J.J. The froth stability column: Linking froth stability and flotation performance. Miner. Eng. 2005, 18, 317–324. [Google Scholar] [CrossRef]
- Gorain, B.K.; Franzidis, J.P.; Manlapig, E.V. Studies on impeller type, impeller speed and air flow rate in an industrial scale flotation cell. Part 4: Effect of bubble surface area flux on flotation performance. Miner. Eng. 1997, 10, 367–379. [Google Scholar] [CrossRef]
- Laskowski, J.S. Frothers and flotation froth. Miner. Process. Extr. Metall. Rev. 1993, 12, 61–89. [Google Scholar] [CrossRef]
- Yoon, R.H.; Luttrell, G.H. The effect of bubble size on fine particle flotation. Miner. Process. Extr. Metall. Rev. 1989, 5, 101–122. [Google Scholar] [CrossRef]
- Ek, C. Flotation kinetics. In Innovations in Flotation Technology; Springer: Dordrecht, The Netherlands, 1992; pp. 183–210. [Google Scholar]
- Sahoo, S.K.; Suresh, N.; Varma, A.K. Performance evaluation of basic flotation kinetic models using advanced statistical techniques. Int. J. Coal Prep. Util. 2019, 39, 65–87. [Google Scholar] [CrossRef]
- Bu, X.; Xie, G.; Peng, Y.; Ge, L.; Ni, C. Kinetics of flotation. Order of process, rate constant distribution and ultimate recovery. Physicochem. Probl. Miner. Process. 2017, 53, 342–365. [Google Scholar] [CrossRef]
- Gharai, M.; Venugopal, R. Modeling of flotation process—An overview of different approaches. Miner. Process. Extr. Metall. Rev. 2016, 37, 120–133. [Google Scholar] [CrossRef]
- Tao, D. Role of bubble size in flotation of coarse and fine particles—A review. Sep. Sci. Technol. 2005, 39, 741–760. [Google Scholar] [CrossRef]
- Savassi, O.N.; Alexander, D.J.; Franzidis, J.P.; Manlapig, E.V. An empirical model for entrainment in industrial flotation plants. Miner. Eng. 1998, 11, 243–256. [Google Scholar] [CrossRef]
- Boylu, F.; Laskowski, J.S. Rate of water transfer to flotation froth in the flotation of low-rank coal that also requires the use of oily collector. Int. J. Miner. Process. 2007, 83, 125–131. [Google Scholar] [CrossRef]
- Dobby, G.S.; Finch, J. Particle collection in columns—Gas rate and bubble size effects. Can. Metall. Q. 1986, 25, 9–13. [Google Scholar] [CrossRef]
- Yoon, R.H.; Luttrell, G.H. The effect of bubble size on fine coal flotation. Coal Prep. 1986, 2, 179–192. [Google Scholar] [CrossRef]
- Kracht, W.; Orozco, Y.; Acuña, C. Effect of surfactant type on the entrainment factor and selectivity of flotation at laboratory scale. Miner. Eng. 2016, 92, 216–220. [Google Scholar] [CrossRef]
- Neethling, S.J.; Lee, H.T.; Cilliers, J.J. Simple relationships for predicting the recovery of liquid from flowing foams and froths. Miner. Eng. 2003, 16, 1123–1130. [Google Scholar] [CrossRef]
- Laskowski, J.S.; Tlhone, T.; Williams, P.; Ding, K. Fundamental properties of the polyoxypropylene alkyl ether flotation frothers. Int. J. Miner. Process. 2003, 72, 289–299. [Google Scholar] [CrossRef]
- Wang, L.; Peng, Y.; Runge, K. Entrainment in froth flotation: The degree of entrainment and its contributing factors. Powder Technol. 2016, 288, 202–211. [Google Scholar] [CrossRef] [Green Version]
- Diaz-Penafiel, P.; Dobby, G.S. Kinetic studies in flotation columns: Bubble size effect. Miner. Eng. 1994, 7, 465–478. [Google Scholar] [CrossRef]
- Yoon, R.H.; Yordan, J.L. The critical rupture thickness of thin water films on hydrophobic surfaces. J. Colloid Interface Sci. 1991, 146, 565–572. [Google Scholar] [CrossRef]
- Lekki, J.; Laskowski, J. On the dynamic effect of frother-collector joint action in flotation. Trans. Inst. Min. Metall. 1971, 80, C174. [Google Scholar]
- Zhou, Z.A.; Plitt, L.R.; Egiebor, N.O. The effects of solids and reagents on the characteristics of coal flotation in columns. Miner. Eng. 1993, 6, 291–306. [Google Scholar] [CrossRef]

| Model | Flotation Rate Equation |
|---|---|
| Classical first-order | |
| Modified first-order (Agar) | |
| Improved gas/solid adsorption | |
| First-order with rectangular distribution (Klimpel) |
| Ca (%) | Mg (%) | Fe (%) | S (%) | Al (%) | Tot. C (C-Matter %) | Au (g/t) |
|---|---|---|---|---|---|---|
| 7.92 | 3.96 | 1.32 | 1.33 | 2.21 | 5.93 (1.95) | 2.14 |
| (g/t) | 30 | 60 | 90 | 120 | 150 |
|---|---|---|---|---|---|
| (ppm) | 9 | 19 | 28 | 38 | 47 |
| Classical | Agar | Klimpel | Improved Gas/Solid | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (g/t) | R∞ | k | R2 | R∞ | k | b | R2 | R∞ | k | R2 | R∞ | k | R2 |
| 30 | 55.4 | 0.38 | 0.9990 | 56.0 | 0.36 | 0.04 | 0.9992 | 66.0 | 0.66 | 0.9997 | 76.7 | 0.31 | 0.9995 |
| 60 | 69.6 | 0.50 | 0.9986 | 70.1 | 0.48 | 0.04 | 0.9989 | 81.1 | 0.91 | 0.9987 | 91.1 | 0.45 | 0.9969 |
| 90 | 66.8 | 0.70 | 0.9863 | 68.9 | 0.55 | 0.23 | 0.9969 | 75.3 | 1.39 | 0.9958 | 81.7 | 0.75 | 0.9996 |
| 120 | 67.9 | 0.83 | 0.9846 | 69.6 | 0.66 | 0.22 | 0.9957 | 75.6 | 1.69 | 0.9969 | 81.1 | 0.94 | 0.9994 |
| 150 | 67.0 | 0.96 | 0.9696 | 70.0 | 0.63 | 0.40 | 0.9947 | 73.7 | 2.04 | 0.9879 | 78.3 | 1.17 | 0.9978 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Gibson, C.E.; Ghahreman, A. Flotation of Carbonaceous Matter from a Double Refractory Gold Ore: The Effect of MIBC on Flotation Performance and Kinetics. Minerals 2021, 11, 1021. https://doi.org/10.3390/min11091021
Lee S, Gibson CE, Ghahreman A. Flotation of Carbonaceous Matter from a Double Refractory Gold Ore: The Effect of MIBC on Flotation Performance and Kinetics. Minerals. 2021; 11(9):1021. https://doi.org/10.3390/min11091021
Chicago/Turabian StyleLee, Sugyeong, Charlotte E. Gibson, and Ahmad Ghahreman. 2021. "Flotation of Carbonaceous Matter from a Double Refractory Gold Ore: The Effect of MIBC on Flotation Performance and Kinetics" Minerals 11, no. 9: 1021. https://doi.org/10.3390/min11091021
APA StyleLee, S., Gibson, C. E., & Ghahreman, A. (2021). Flotation of Carbonaceous Matter from a Double Refractory Gold Ore: The Effect of MIBC on Flotation Performance and Kinetics. Minerals, 11(9), 1021. https://doi.org/10.3390/min11091021






