Adsorption of Selected Heavy and Precious Metals from Simulated Wastewater Using Fabricated Polyacrylonitrile (PAN) and Poly(4-Vinylpyridine) (P4VP) Monoliths
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Solvents
2.2. Optimization of Fabrication of Mesoporous Neat PAN Monoliths
2.3. Optimization of Fabrication of Composite Mesoporous Polymer Monoliths
2.4. Characterization of the Fabricated Polymer Monoliths
2.4.1. Scanning Electron Microscopy and Energy-Dispersive X-ray Spectroscopy Analysis
2.4.2. Brunauer–Emmett–Teller and Barret–Joyner–Halenda Analysis
2.4.3. Thermogravimetric Analysis
2.5. Adsorption Studies
2.6. Spectroscopic Determination
2.7. Figures of Merit
2.7.1. Limit of Detection, the Limit of Quantification, and Coefficients of Determination
2.7.2. Percent Relative Standard Deviation
3. Results and Discussion
3.1. Optimization of Fabrication of Mesoporous Neat PAN Monoliths
3.2. Optimization of Fabrication of Composite Mesoporous Polymer Monoliths
3.3. Common Findings for Both Fabrication Optimizations
3.4. SEM and EDS Analysis
3.4.1. SEM Analysis of Raw PAN
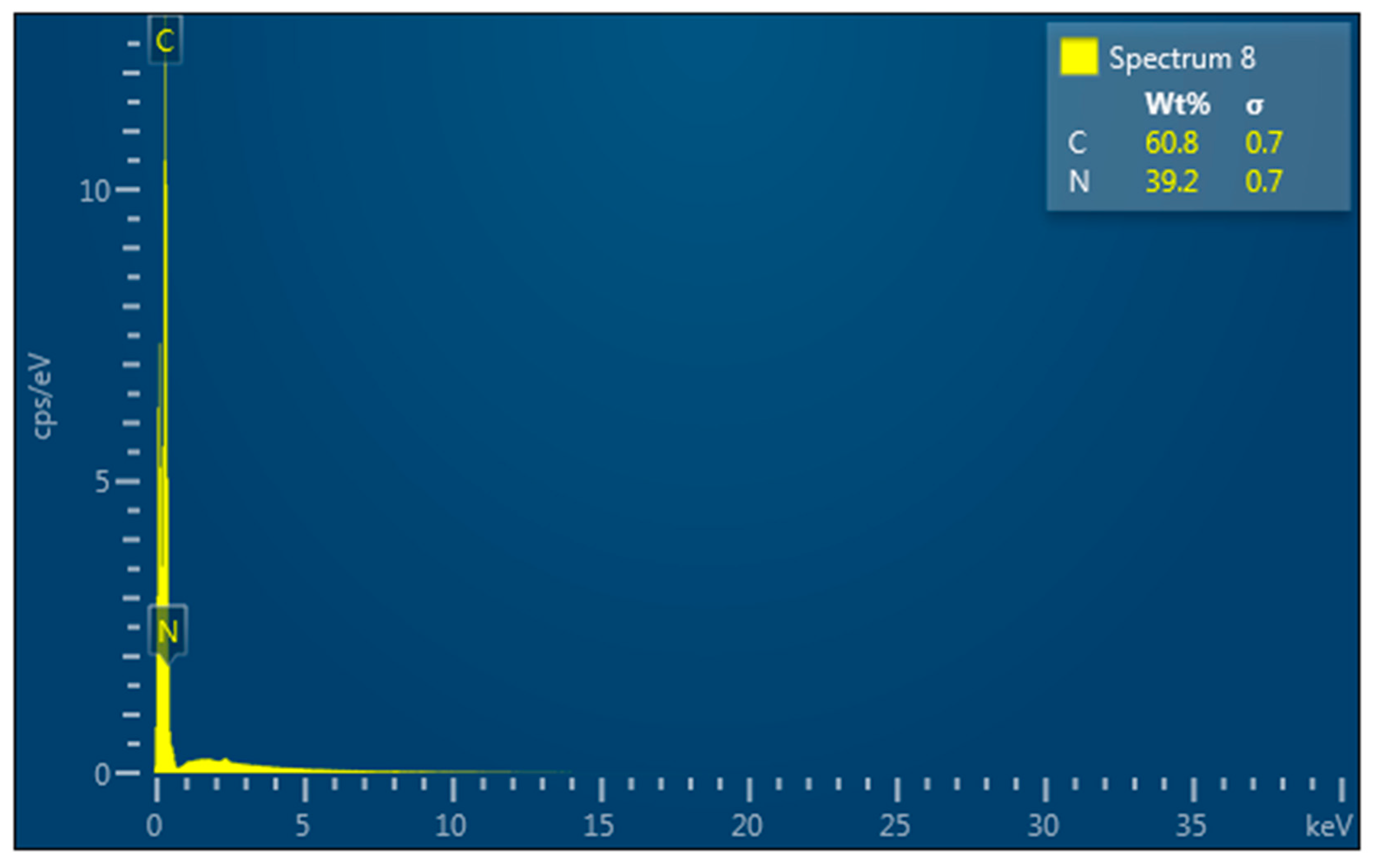
3.4.2. EDS Analysis of Raw PAN
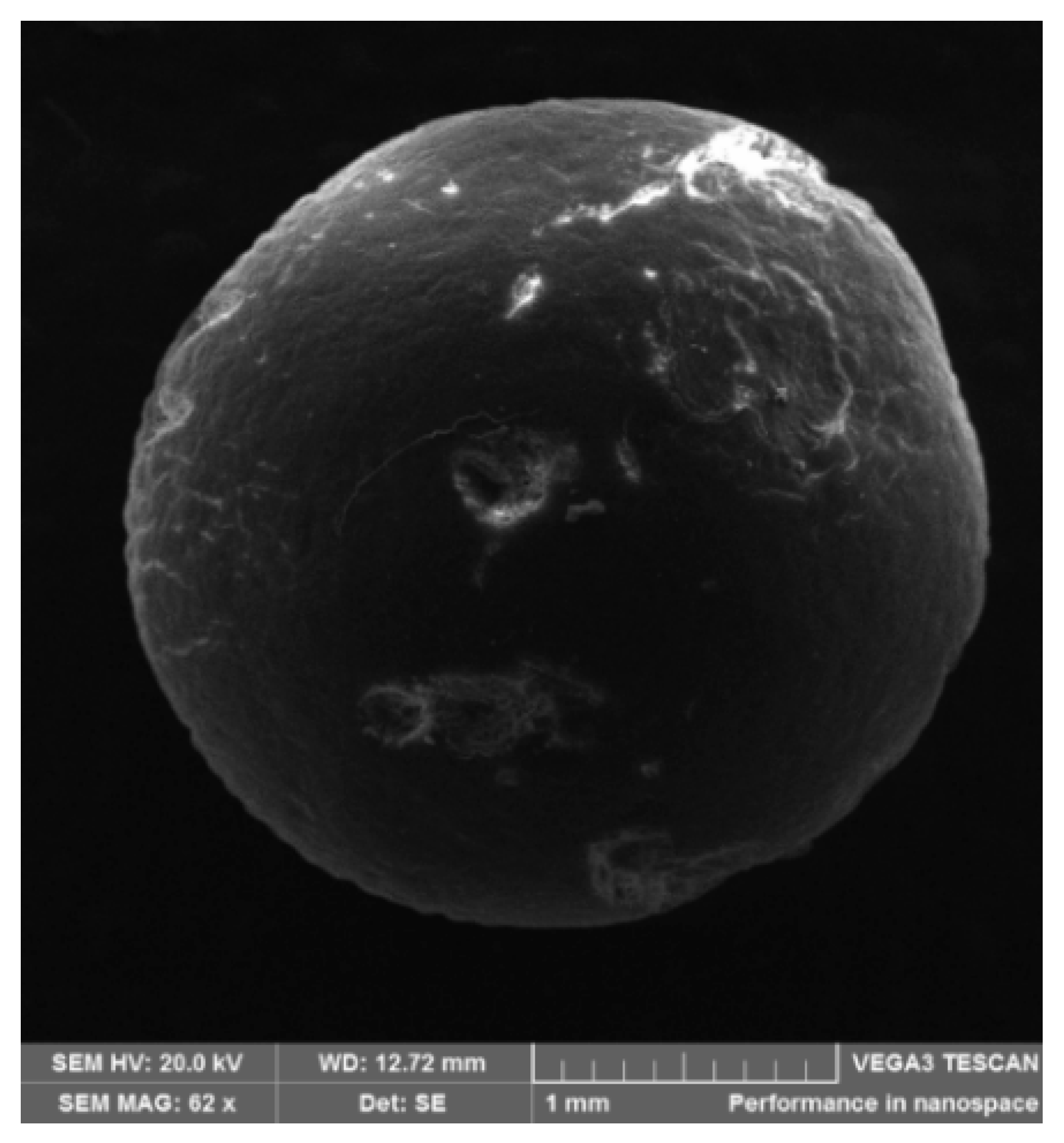
3.4.3. SEM Analysis of Mesoporous Neat PAN Monoliths
3.4.4. EDS Analysis of Mesoporous Neat PAN Monoliths
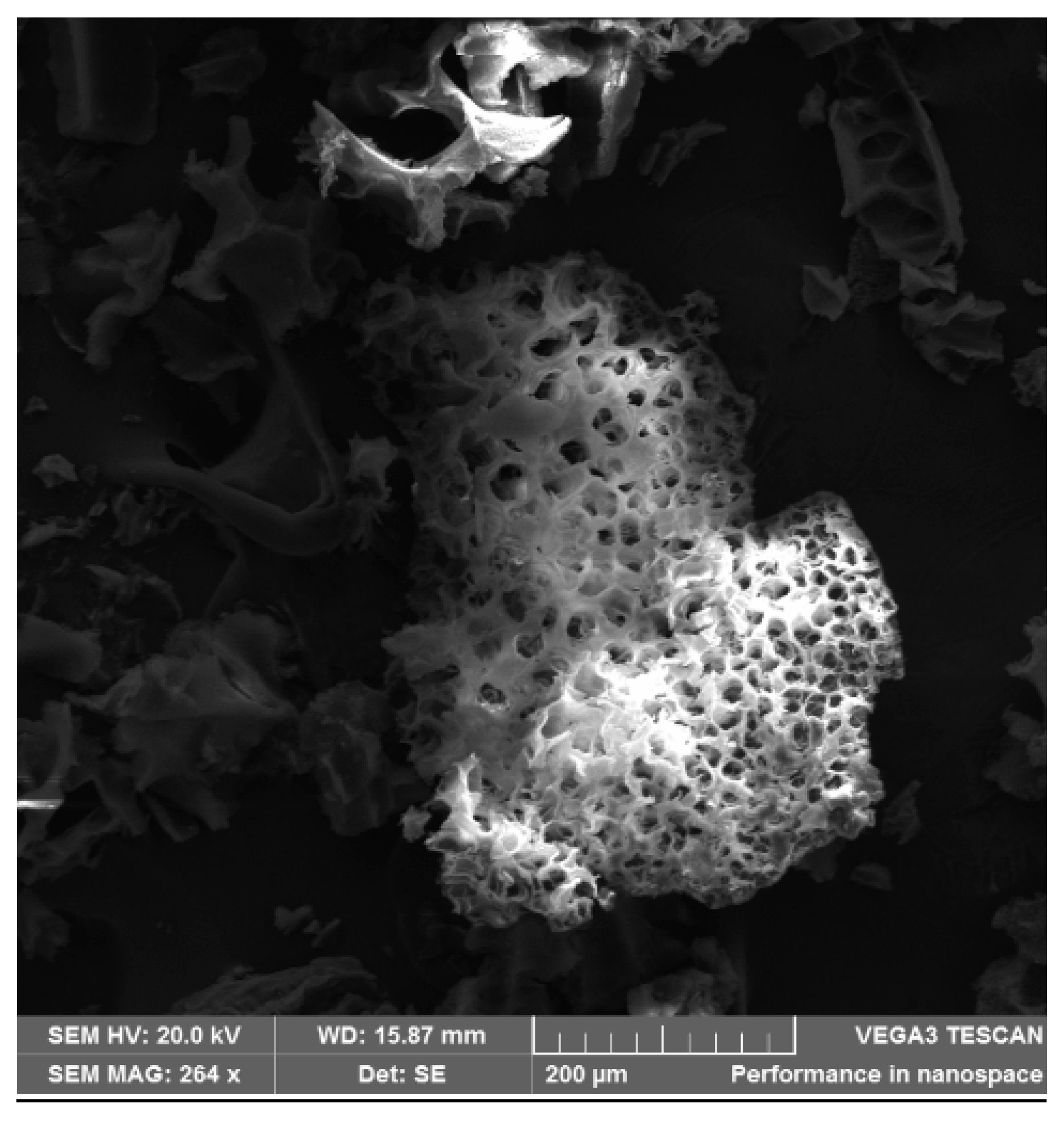
3.4.5. SEM Analysis of Crushed Mesoporous Neat PAN Monoliths
3.4.6. EDS Analysis of Crushed Mesoporous Neat PAN Monoliths
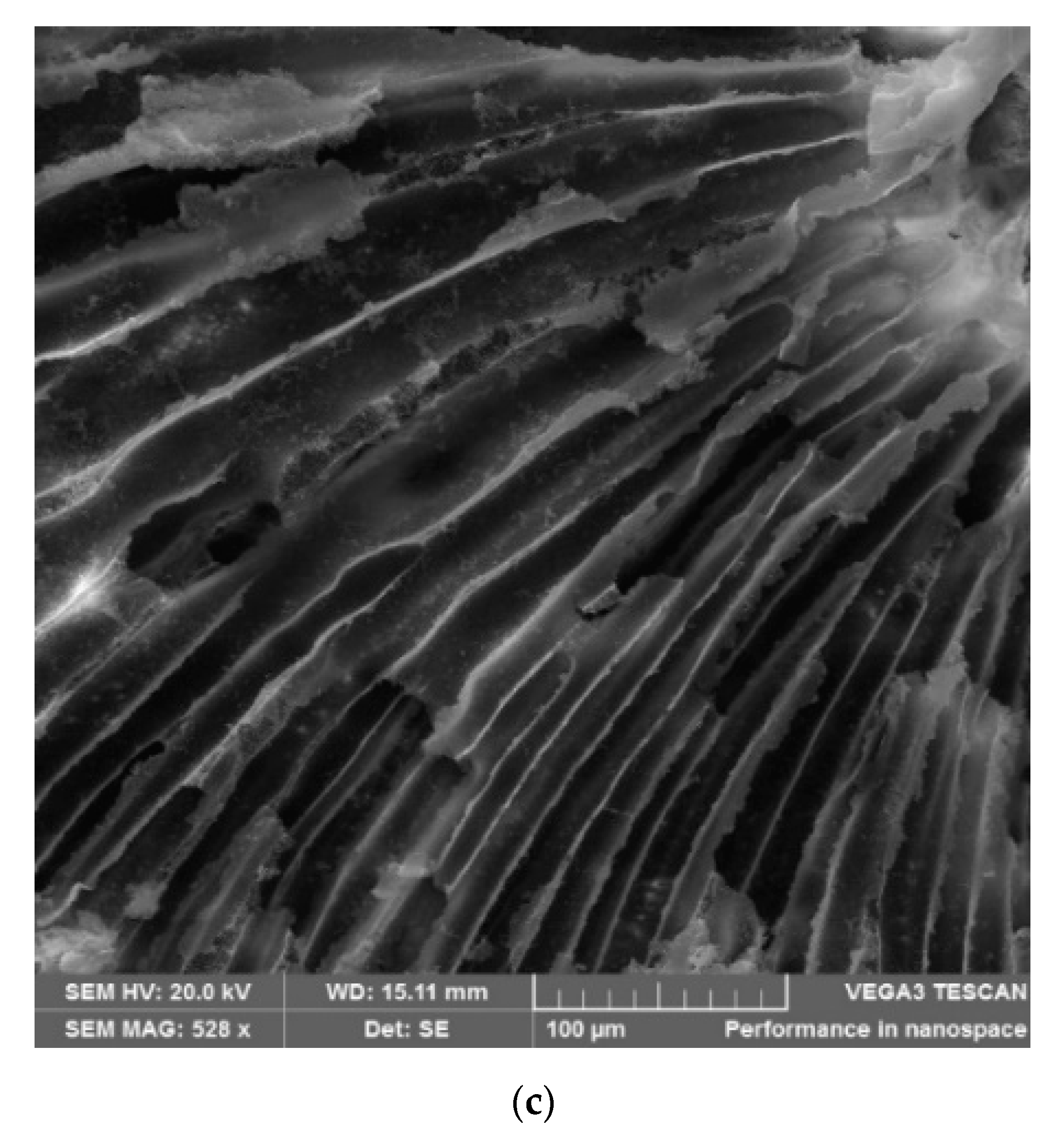
3.4.7. SEM Analysis of Mesoporous Composite Polymer Monoliths
3.4.8. EDS Analysis of Mesoporous Composite Polymer Monoliths
3.4.9. SEM and EDS Analysis of Crushed Mesoporous Composite Polymer Monoliths
3.4.10. BET and BJH Analysis
3.4.11. Thermogravimetric Analysis (TGA)
3.5. Sorption Studies
3.5.1. Limit of Detection and Limit of Quantification
3.5.2. Percent Relative Standard Deviation
3.5.3. Adsorption of the PGMs
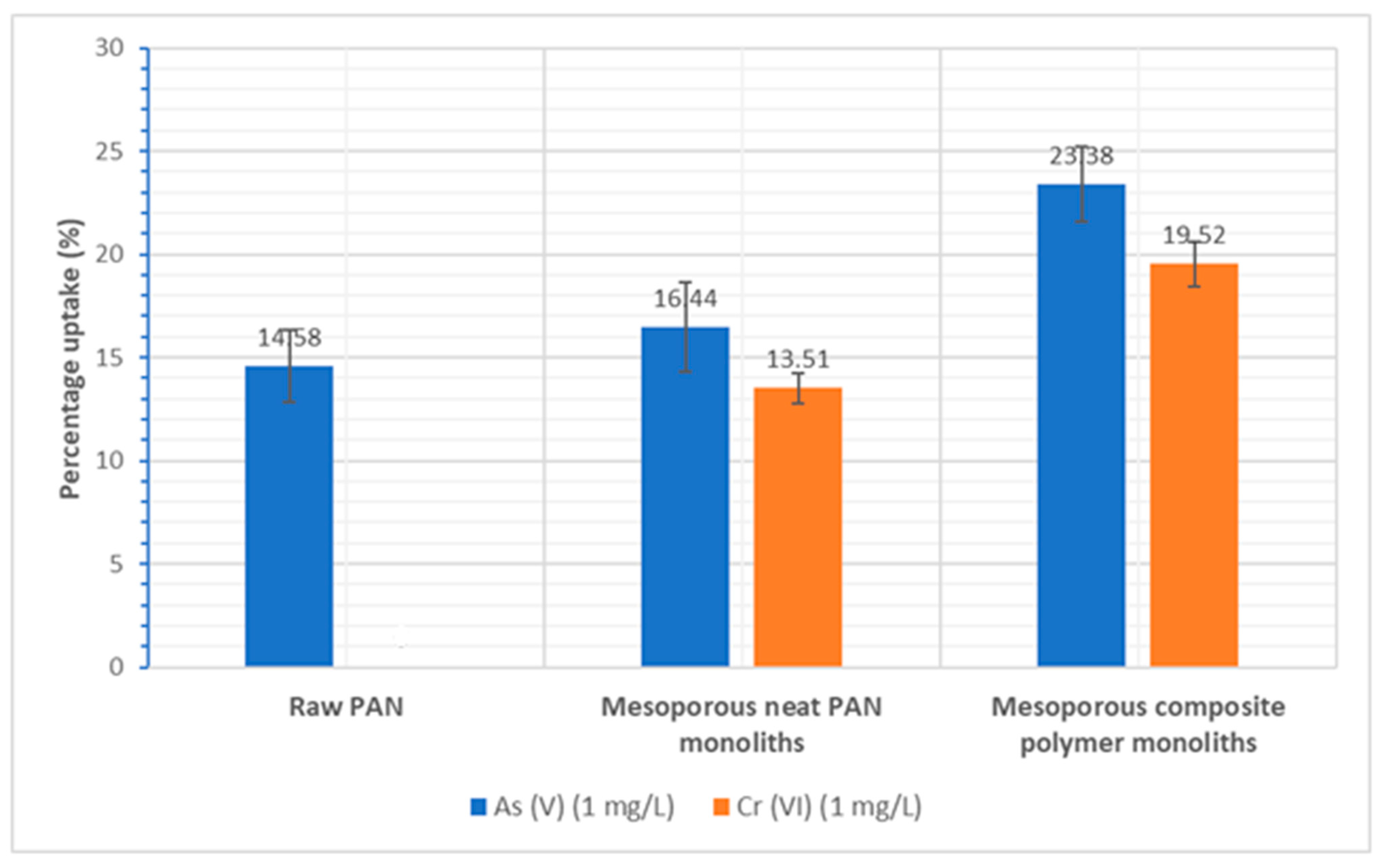
3.5.4. Adsorption of Arsenic(V) and Chromium(VI)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meissner, R.; Steyn, M.; Moyo, E.; Shadung, J.; Masangane, W.; Nohayi, N.; Jacobs-Mata, I. South African local government perceptions of the state of water security. Environ. Sci. Policy 2018, 87, 112–127. [Google Scholar] [CrossRef]
- Nthunya, L.N.; Masheane, M.L.; Malinga, S.P.; Nxumalo, E.N.; Mamba, B.; Mhlanga, S.D. Determination of toxic metals in drinking water sources in the Chief Albert Luthuli Local Municipality in Mpumalanga, South Africa. Phys. Chem. Earth 2017, 100, 94–100. [Google Scholar] [CrossRef]
- Carolin, C.F.; Kumar, P.S.; Saravanan, A.; Joshiba, G.J.; Naushad, M. Efficient techniques for the removal of toxic heavy metals from aquatic environment: A review. J. Environ. Chem. Eng. 2017, 5, 2782–2799. [Google Scholar] [CrossRef]
- Won, S.W.; Kotte, P.; Wei, W.; Lim, A.; Yun, Y.-S. Biosorbents for recovery of precious metals. Bioresour. Technol. 2014, 160, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Nancharaiah, Y.; Mohan, S.V.; Lens, P.N.L. Biological and Bioelectrochemical Recovery of Critical and Scarce Metals. Trends Biotechnol. 2016, 34, 137–155. [Google Scholar] [CrossRef] [PubMed]
- Musina, A.; Bocokic, V.; Lavric, V.; van Zutphen, S. Phosphorus-Based Polymers for Selective Capture of Platinum Group Metals. Ind. Eng. Chem. Res. 2014, 53, 13362–13369. [Google Scholar] [CrossRef]
- Dodson, J.; Parker, H.L.; García, A.M.; Hicken, A.; Asemave, K.; Farmer, T.; He, H.; Clark, J.H.; Hunt, A.J. Bio-derived materials as a green route for precious & critical metal recovery and re-use. Green Chem. 2015, 17, 1951–1965. [Google Scholar] [CrossRef]
- Morcali, M.H.; Zeytuncu, B. Investigation of adsorption parameters for platinum and palladium onto a modified polyacrylonitrile-based sorbent. Int. J. Miner. Process. 2015, 137, 52–58. [Google Scholar] [CrossRef]
- Ehrlich, H.V.; Buslaeva, T.M.; Maryutina, T.A. Trends in Sorption Recovery of Platinum Metals: A Critical Survey. Russ. J. Inorg. Chem. 2017, 62, 1797–1818. [Google Scholar] [CrossRef]
- Hsini, A.; Benafqir, M.; Naciri, Y.; Laabd, M.; Bouziani, A.; Ez-Zahery, M.; Lakhmiri, R.; El Alem, N.; Albourine, A. Synthesis of an arginine-functionalized polyaniline@FeOOH composite with high removal performance of hexavalent chromium ions from water: Adsorption behavior, regeneration and process capability studies. Colloids Surf. A 2021, 617, 126274. [Google Scholar] [CrossRef]
- Lim, A.; Song, M.-H.; Cho, C.-W.; Yun, Y.-S. Development of Surface-Modified Polyacrylonitrile Fibers and Their Selective Sorption Behavior of Precious Metals. Appl. Sci. 2016, 6, 378. [Google Scholar] [CrossRef] [Green Version]
- Pan, G.R.G.Y.; Li, M.; Hoek, E.M.V. Preparation and Characterization of Membranes Formed by Nonsolvent In-duced Phase Separation: A Review. Ind. Eng. Chem. Res 2011, 50, 3798–3817. [Google Scholar] [CrossRef]
- Mphanje, K.; Zinyemba, O.; Darkwa, J. Fabrication of nitrogen donor macro- and meso-porous materials for group 11 metal ions sorption. S. Afr. J. Chem. 2018, 71, 94–102. [Google Scholar] [CrossRef] [Green Version]
- da Silva, I.J.; Lavorante, A.F.; Paim, A.P.; da Silva, M.J. Microwave-assisted digestion employing diluted nitric acid for mineral determination in rice by ICP OES. Food Chem. 2020, 319, 126435. [Google Scholar] [CrossRef]
- Skoog, D.A.; West, D.M.; Holler, F.J.; Crouch, S.R. Fundamentals of Analytical Chemistry; Brooks/Cole Cengage Learning: Belmont, CA, USA, 2014; pp. 109, 186–187. [Google Scholar]
- Armbruster, D.A.; Pry, T. Limit of Blank, Limit of Detection and Limit of Quantitation. Clin. Biochem. Rev. 2008, 29, S49. [Google Scholar]
- Schwarz, G.; Bäumler, S.; Block, A.; Felsenstein, F.G.; Wenzel, G. Determination of detection and quantification limits for SNP allele frequency estimation in DNA pools using real time PCR. Nucleic Acids Res. 2004, 32, 24e. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xin, Y.; Fujimoto, T.; Uyama, H. Facile fabrication of polycarbonate monolith by non-solvent induced phase separation method. Polymer 2012, 53, 2847–2853. [Google Scholar] [CrossRef]
- Shao, J.; Chen, C.; Wang, Y.; Chen, X.; Du, C. Structure and surface nanomechanics of poly(l-lactide) from thermally in-duced phase separation process. Appl. Surf. Sci. 2012, 258, 6665–6671. [Google Scholar] [CrossRef]
- Li, W.; Wu, Z.; Wang, J.; Elzatahry, A.A.; Zhao, D. A Perspective on Mesoporous TiO2 Materials. Chem. Mater 2014, 26, 287–298. [Google Scholar] [CrossRef]
- Nguyen, A.M.; Nordborg, A.; Shchukarev, A.; Irgum, K. Thermally induced dissolution/precipitation—A simple approach for the preparation of macroporous monoliths from linear aliphatic polyamides. J. Sep. Sci. 2009, 32, 2619–2628. [Google Scholar] [CrossRef]
- Vettegren, V.I.; Kulik, V.B.; Savitskii, A.V.; Fetisov, O.I.; Usov, V.V. Molecular mechanism of gelation upon the addition of water to a solution of poly(acrylonitrile) in dimethylsulfoxide. Tech. Phys. 2010, 55, 743–746. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity. IUPAC Commission on Colloid and Surface Chemistry Including Catalysis. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Alothman, Z.A. A Review: Fundamental Aspects of Silicate Mesoporous Materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef] [Green Version]
- Mphanje, K. Polymeric Nitrogen Donor Macro(meso)porous Sorption Materials for Selected Transition Metals. Master’s Thesis, University of Johannesburg, Johannesburg, South Africa, 2014. [Google Scholar]
- Pearson, R.G. Hard and soft acids and bases, HSAB, part 1: Fundamental principles. J. Chem. Educ. 1968, 45. [Google Scholar] [CrossRef]
- Koshcheeva, I.Y.; Kubrakova, I.V.; Korsakova, N.V.; Tyutyunnik, O.A. Solubility and migration ability of rhodium in natural conditions: Model experimental data. Geochem. Int. 2016, 54, 624–632. [Google Scholar] [CrossRef]
- Mavhungu, A.; Mbaya, R.K.K.; Moropeng, M.L. Recovery of Platinum and Palladium Ions from Aqueous Solution Using Grape Stalk Waste. Int. J. Chem. Eng. Appl. 2013, 46, 354–358. [Google Scholar] [CrossRef] [Green Version]
- Gonte, R.; Balasubramanian, K. Heavy and toxic metal uptake by mesoporous hypercrosslinked SMA beads: Isotherms and kinetics. J. Saudi Chem. Soc. 2016, 20, S579–S590. [Google Scholar] [CrossRef] [Green Version]






| Parameter | Crushed Raw PAN | Crushed Mesoporous Neat PAN Monoliths | Crushed Mesoporous Composite Polymer Monoliths |
|---|---|---|---|
| BJH avg. pore diameter (nm) | 43.1 ± 0.3 | 28.8 ± 0.05 | 9.96 ± 0.09 |
| BET surface area (m2/g) | 36.9 ± 0.1 | 1.43 ± 0.21 | 1.52 ± 0.07 |
| Most occurring pore diameter (BJH PSD) (nm) | 38.04 ± 0.4 | 25.7 ± 0.1 | 2.99 ± 0.06 |
| Isotherm type | Type IV | Type IV | Type IV |
| Hysteresis loop type | Type H1 | Type H1 | Type H1 |
| Porosity category | Mesoporous | Mesoporous | Mesoporous |
| Pore geometry | Cylindrical | Cylindrical | Cylindrical |
| Analyte | Limit of Detection (LOD) (mg·L−1) | Limit of Quantification (LOQ) (mg·L−1) | Coefficient of Determination (R2) |
|---|---|---|---|
| As(V) | 0.0126 | 0.0421 | 1.00 |
| Cr(VI) | 0.00460 | 0.0153 | 0.9999 |
| Ru(III) | 0.00966 | 0.0322 | 0.9994 |
| Rh(III) | 0.0170 | 0.0563 | 1.00 |
| Pd(II) | 0.0120 | 0.0401 | 0.9998 |
| Analyte | Adsorbent Type | Average Concentration Following Adsorption (mg·L−1) (Triplicate Analysis) | Standard Deviation | %RSD |
|---|---|---|---|---|
| As(V) | Raw PAN | 0.112 | 0.00349 | 3.12% |
| Neat PAN monolith | 0.0837 | 0.00506 | 6.02% | |
| Composite polymer monolith | 0.0820 | 0.00711 | 8.67% | |
| Cr(VI) | Raw PAN | 0.120 | 0.00346 | 2.88% |
| Neat PAN monolith | 0.0962 | 0.00350 | 3.64% | |
| Composite polymer monolith | 0.0952 | 0.00731 | 7.69% | |
| Rh(III) | Raw PAN | 0.0257 | 0.00223 | 8.58% |
| Neat PAN monolith | 0.0423 | 0.00204 | 4.86% | |
| Composite polymer monolith | <0.0170 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liebenberg, K.E.; Ambushe, A.A.; Zinyemba, O. Adsorption of Selected Heavy and Precious Metals from Simulated Wastewater Using Fabricated Polyacrylonitrile (PAN) and Poly(4-Vinylpyridine) (P4VP) Monoliths. Minerals 2021, 11, 884. https://doi.org/10.3390/min11080884
Liebenberg KE, Ambushe AA, Zinyemba O. Adsorption of Selected Heavy and Precious Metals from Simulated Wastewater Using Fabricated Polyacrylonitrile (PAN) and Poly(4-Vinylpyridine) (P4VP) Monoliths. Minerals. 2021; 11(8):884. https://doi.org/10.3390/min11080884
Chicago/Turabian StyleLiebenberg, Keziah E., Abayneh A. Ambushe, and Orpah Zinyemba. 2021. "Adsorption of Selected Heavy and Precious Metals from Simulated Wastewater Using Fabricated Polyacrylonitrile (PAN) and Poly(4-Vinylpyridine) (P4VP) Monoliths" Minerals 11, no. 8: 884. https://doi.org/10.3390/min11080884






