Major, Trace, and Rare-Earth Element Geochemistry of Nb-V Rich Andradite-Schorlomite-Morimotoite Garnet from Ambadungar-Saidivasan Alkaline Carbonatite Complex, India: Implication for the Role of Hydrothermal Fluid-Induced Metasomatism
Abstract
:1. Introduction
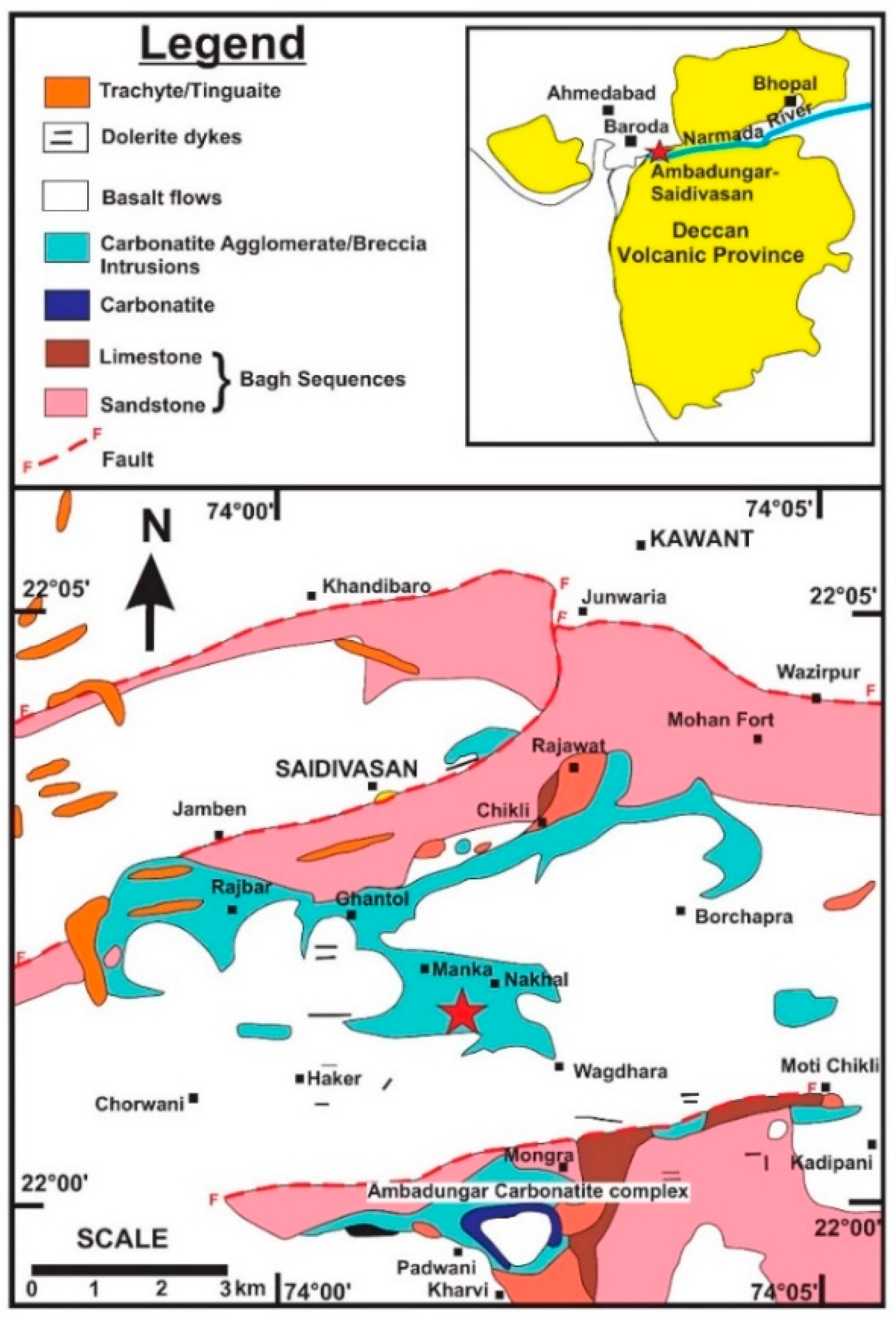
2. Geological Framework
3. Petrography
4. Analytical Techniques
5. Results
Major and Trace Element Geochemistry
6. Discussion
6.1. Site Occupancies of Ti in Garnet and Its Classification
6.2. Genesis of Garnet: Primary vs. Metasomatic
6.3. Nature of Hydrothermal Fluid
7. Conclusions
- The garnets are rich in TiO2 and characterized by andradite, schorolomite, and morimotoite end members in decreasing order.
- The garnets do not display any distinct chemical zonation; only minor variations in Ti content are observed.
- Based on textural evidence, LREE enriched REE pattern with M-type first tetrad and the presence of abnormally high content of Ti, Nb, and V, the role of metasomatic reactions between earlier formed minerals and hydrothermal fluid enriched in Fe, Si, LREE, U, Nb, V, and Ti content is suggested for the genesis of garnets.
- Magnetite, ilmenite, and pyrochlore present in different varieties of carbonatites in the Ambadungar-Saidivasan complex could be the primary source for these elements released during interaction with hydrothermal fluids. The hydrothermal fluid is moderately acidic pH having fluorine and sulfate as primary ligands.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deer, W.A.; Howie, R.A.; Zussman, J. Rock Forming Minerals: Orthosilicates; Geological Society: London, UK, 1997; p. 918. [Google Scholar]
- Grew, E.S.; Locock, A.J.; Mills, S.J.; Galuskina, I.O.; Galuskin, E.V.; Hålenius, U. Nomenclature of the garnet supergroup. Am. Mineral. 2013, 98, 785–811. [Google Scholar] [CrossRef]
- Deer, W.A.; Howie, R.A.; Zussman, J. Rock-Forming Minerals, IA: Orthosilicates; Longman: New York, NY, USA, 1982. [Google Scholar]
- Gaspar, M.; Knaack, C.; Meinert, L.D.; Moretti, R. REE in skarn systems: A LA–ICP-MS study of garnets from the Crown Jewel gold deposit. Geochim. Cosmochim. Acta 2008, 72, 185–205. [Google Scholar] [CrossRef]
- Hickmott, D.D.; Spear, F.S. Major-and trace-element zoning in garnets from calcareous pelites in the NW Shelburne Falls Quadrangle, Massachusetts: Garnet growth histories in retrograded rocks. J. Petrol. 1992, 33, 965–1005. [Google Scholar] [CrossRef]
- Jamtveit, B.; Hervig, R.L. Constraints on transport and kinetics in hydrothermal systems from zoned garnet crystals. Science 1994, 263, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Chernoff, C.B.; Carlson, W.D. Trace element zoning as a record of chemical disequilibrium during garnet growth. Geology 1999, 27, 555–558. [Google Scholar] [CrossRef]
- Smith, M.P.; Henderson, P.; Jeffries, T.E.R.; Long, J.; Williams, C.T. The rare earth elements and uranium in garnets from the Beinn and Dubhaich Aureole, Skye, Scotland, UK: Constraints on processes in a dynamic hydrothermal system. J. Petrol. 2004, 45, 457–484. [Google Scholar] [CrossRef] [Green Version]
- Scheibner, B.; Wörner, G.; Civetta, L.; Stosch, H.G.; Simon, K.; Kronz, A. Rare earth element fractionation in magmatic Ca-rich garnets. Contr. Mineral. Petrol. 2007, 154, 55–74. [Google Scholar] [CrossRef]
- Sukheswala, R.N.; Borges, S.M. The carbonatite-injected sandstones of Siriwasan, Chhota Udaipur, Gujarat. Indian J. Earth Sci. 1975, 2, 1–10. [Google Scholar]
- Viladkar, S.G. The carbonatites of Amba Dongar, Gujarat, India. Bull. Geol. Soc. Finl. 1981, 53, 17–28. [Google Scholar] [CrossRef]
- Gwalani, L.G.; Rock, N.M.S.; Chang, W.J.; Fernandez, S.; Allegre, C.J.; Prinzhofer, A. Alkaline rocks and carbonatites of Amba Dongar and adjacent areas, Deccan Igneous Province, Gujarat, India: 1. Geology, Petrography and Petrochemistry. Mineral. Petrol. 1993, 47, 219–253. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Hall, R.P. Tectonic setting of Indian carbonatites. In Magmatism in Relation to Diverse Tectonic Settings; Oxford & IBH Publishers: New Delhi, India, 1995; pp. 135–154. [Google Scholar]
- Srivastava, R.K. Petrology, geochemistry and genesis of rift-related carbonatites of Ambadungar, India. Mineral. Petrol. 1997, 61, 47–66. [Google Scholar] [CrossRef]
- Viladkar, S.G.; Gittins, J. Trace elements and REE geochemistry of Siriwasan carbonatites. J. Geol. Soc. India 2016, 87, 709–715. [Google Scholar] [CrossRef]
- Krishnamurthy, P. Carbonatites of India. J. Geol. Soc. India 2019, 94, 117–138. [Google Scholar] [CrossRef]
- Srivastava, R.K. Petrology, Petrochemistry and Genesis of the alkaline rocks associated with the Ambdungar carbonatite complex, Baroda district, Gujarat, India. J. Geol. Soc. India 1994, 43, 23–39. [Google Scholar]
- Sukheswala, R.N.; Udas, G.R. Note on the carbonatite of Amba Dongar (Gujarat State) and its economic potentialities. Sci. Cult. 1963, 29, 563–568. [Google Scholar]
- Viladkar, S.G. Geology of the Carbonatite-Alkalic Diatreme of Amba Dongar, Gujarat; Gujarat Mineral Development Corporation: Ahmedabad, India, 1996; p. 74. [Google Scholar]
- Gwalani, L.G.; Rock, N.M.S.; Ramasamy, R.; Griffin, B.J.; Mulai, B.P. Complexly zoned Ti-rich melanite-schorlomite garnets from Ambadungar carbonatite-alkalic complex, Deccan igneous province, Gujarat State, western India. J. Asian Earth Sci. 2000, 18, 163–176. [Google Scholar] [CrossRef]
- Krishnamurthy, P. Carbonatites of India. Explor. Res. At. Miner. 1988, 1, 81–115. [Google Scholar] [CrossRef]
- Karkare, S.G.; Srivastava, R.K. Regional dyke swarms related to the Deccan Trap alkaline province, India. In Mafic Dykes and Emplacement Mechanisms; Parker, A.J., Rickwood, P.C., Tucker, D.I.-I., Eds.; Balkema: Rotterdam, The Netherlands, 1990; pp. 335–347. [Google Scholar]
- Ray, J.S.; Pande, K.; Pattanayak, S.K. Evolution of the Amba Dongar Carbonatite Complex: Constraints from 40Ar-39Ar chronologies of the Inner Basalt and an alkaline plug. Intl. Geol. Rev. 2003, 45, 857–862. [Google Scholar] [CrossRef]
- Ray, J.S.; Shukla, P.N. Trace element geochemistry of Amba Dongar carbonatite complex, India: Evidence for fractional crystallization and silicate-carbonate melt immiscibility. J. Earth Syst. Sci. 2004, 113, 519–531. [Google Scholar] [CrossRef] [Green Version]
- Viladkar, S.G. Ferro-carbonatites in the Amba Dongar Diatreme, Gujarat, India. J. Geol. Soc. India 2018, 92, 141–144. [Google Scholar] [CrossRef]
- Ray, J.S.; Pande, K. Carbonatite-alkaline magmatism associated with continental flood basalts at stratigraphic boundaries: Cause for mass extinctions. Geoph. Res. Lett. 1999, 26, 1917–1920. [Google Scholar] [CrossRef]
- Ray, J.S.; Pande, K.; Venkatesan, T.R. Emplacement of Amba Dongar carbonatite-alkaline complex at Cretaceous/Tertiary boundary: Evidence from Ar40-Ar39 chronology. Proc. Indian Acad. Sci. Earth Planet. Sci. 2000, 109, 39–47. [Google Scholar]
- Fosu, B.R.; Ghosh, P.; Chew, D.M.; Viladkar, S.G. Composition and U-Pb ages of apatite in the Amba Dongar carbonatite-alkaline complex, India. Geol. J. 2019, 54, 3438–3454. [Google Scholar] [CrossRef]
- Basu, A.R.; Renne, P.R.; DasGupta, D.K.; Teichmann, F.; Poreda, R.J. Early and late alkali igneous pulses and a high-3 He plume origin for the Deccan flood basalts. Science 1993, 261, 902–906. [Google Scholar] [CrossRef]
- Chenet, A.L.; Courtillot, V.; Fluteau, F.; Gérard, M.; Quidelleur, X.; Khadri, S.F.R.; Subbarao, K.V.; Thordarson, T. Determination of rapid Deccan eruptions across the Cretaceous-Tertiary boundary using paleomagnetic secular variation: 2. Constraints from analysis of eight new sections and synthesis for a 3500-m-thick composite section. J. Geophys. Res. 2009, 114, B06103. [Google Scholar] [CrossRef]
- Schoene, B.; Eddy, M.P.; Samperton, K.M.; Keller, C.B.; Keller, G.; Adatte, T.; Khadri, S.F.R. U-Pb constraints on pulsed eruption of the Deccan Traps across the end-Cretaceous mass extinction. Science 2019, 363, 862–866. [Google Scholar] [CrossRef] [PubMed]
- Sprain, C.J.; Renne, P.R.; Vanderkluysen, L.; Pande, K.; Self, S.; Mittal, T. The eruptive tempo of Deccan volcanism in relation to the Cretaceous-Paleogene boundary. Science 2019, 363, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Pande, K. Age and duration of the Deccan Traps, India: A review of radiometric and paleomagnetic constraints. J. Earth Syst. Sci. 2002, 111, 115. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Wang, F.; Shi, W. Substantiation of Réunion plume induced prolonged magmatic pulses (ca. 70.5–65.5 Ma) of the Deccan LIP in the Chhotanagpur Gneissic Complex, eastern India: Constraints from 40Ar/39Ar geochronology. J. Earth Syst. Sci. 2020, 129, 1–10. [Google Scholar] [CrossRef]
- Ernst, R.E.; Bell, K. Large igneous provinces (LIPs) and carbonatites. Mineral. Petrol. 2010, 98, 55–76. [Google Scholar] [CrossRef]
- Simmoneti, A.; Bell, K.; Viladkar, S.G. Isotopic data from the Amba Dongar carbonatite complex, West-Central India: Evidence for an enriched mantle source. Chem. Geol. 1995, 122, 185–198. [Google Scholar] [CrossRef]
- Simonetti, A.; Goldstein, S.L.; Schmidberger, S.S.; Viladkar, S.G. Geochemical and Nd, Pb, and Sr isotope data from Deccan alkaline complexes—Inferences for mantle sources and plume-lithosphere interaction. J. Petrol. 1998, 39, 1847–1864. [Google Scholar] [CrossRef]
- Gomes, C.D.B. Electron microprobe analysis of zoned melanites. Am. Mineral. 1969, 54, 1654–1661. [Google Scholar]
- Pearce, T.H. The analcite-bearing volcanic rocks of the Crowsnest Formation, Alberta. Can. J. Earth Sci. 1970, 7, 46–66. [Google Scholar] [CrossRef]
- Dingwell, D.B.; Brearley, M. Mineral chemistry of igneous melanite garnets from analcite-bearing volcanic rocks, Alberta, Canada. Contr. Mineral. Petrol. 1985, 90, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Whitney, D.L.; Evans, B.W. Abbreviations for names of rock-forming minerals. Am. Mineral. 2010, 95, 185–187. [Google Scholar] [CrossRef]
- Griffin, W.L.; Powell, W.J.; Pearson, N.J.; O’Reilly, S.Y. GLITTER: Data reduction software for laser ablation ICP-MS. In Laser Ablation ICP-MS in the Earth Sciences: Current Practices and Outstanding Issues; Short Course Series; Sylvester, P., Ed.; Mineralogical Association of Canada: Québec, QC, Canada, 2008; Volume 40, pp. 307–311. [Google Scholar]
- Locock, A.J. An Excel spreadsheet to recast analyses of garnet into end-member components, and a synopsis of the crystal chemistry of natural silicate garnets. Comput. Geosci. 2008, 34, 1769–1780. [Google Scholar] [CrossRef]
- Antao, S.M.; Mohib, S.; Zaman, M.; Marr, R.A. Ti-rich Andradites: Chemistry, structure, multi-phases, optical anisotropy, and oscillatory zoning. Canad. Mineral. 2015, 53, 133–158. [Google Scholar] [CrossRef]
- McDonough, W.F.; Sun, S.S. The composition of the Earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- Brod, J.A.; Junqueira-Brod, T.C.; Gaspar, J.C.; Gibson, S.A.; Thompson, R.N. Ti-rich and Ti-poor garnet from the Tapira Carbonatite Complex, SE Brazil: Fingerprinting fractional crystallisation and liquid immiscibility. In Proceedings of the 8th International Kimberlite Conference: Extended Abstracts, Victoria, BC, Canada, 22–27 June 2003; Volume 8. [Google Scholar]
- Sun, S.; McDonough, W.F. Chemical and isotopic systematics of oceanic basalts: Implications for mantle composition and processes. In Magmatism in the Ocean Basins; Saunders, A.D., Norry, M.J., Eds.; Geological Society: London, UK, 1989; Volume 42, pp. 313–345. [Google Scholar]
- Chakhmouradian, A.R.; McCammon, C.A. Schorlomite: A discussion of the crystal chemistry, formula, and inter-species boundaries. Phys. Chem. Miner. 2005, 32, 277–289. [Google Scholar] [CrossRef]
- Ito, J.; Frondel, C. Synthetic zirconium and titanium garnets. Am. Mineral. 1967, 52, 773–781. [Google Scholar]
- Zedlitz, O. Lime-iron garnets rich in titanium. Zentralbl Mineral. Geol. A 1933, 225–239. [Google Scholar]
- Manning, P.G.; Harris, D.C. Optical absorption and electron- microprobe studies of some high-Ti andradites. Can. Mineral. 1970, 10, 260–271. [Google Scholar]
- Laverne, C. Unusual occurrence of aegirine-augite, fassaite and melanite in oceanic basalts (DSDP Hole 504B). Lithos 1987, 20, 135–151. [Google Scholar] [CrossRef]
- Melluso, L.; Srivastava, R.K.; Guarino, V.; Zanetti, A.; Sinha, A.K. Mineral compositions and petrogenetic evolution of the ultramafic-alkaline–carbonatitic complex of Sung Valley, northeastern India. Can. Mineral. 2010, 48, 205–229. [Google Scholar] [CrossRef]
- Viladkar, S.G.; Wimmenauer, W. Geochemical and petrological studies on the Amba Dongar carbonatites (Gujarat, India). Chem. Erde. 1992, 52, 277–291. [Google Scholar]
- Viladkar, S.G.; Bismayer, U. Compositional variation in pyrochlores of Amba Dongar carbonatite complex, Gujarat. J. Geol. Soc. India 2010, 75, 495–502. [Google Scholar] [CrossRef]
- Magna, T.; Viladkar, S.; Rapprich, V.; Pour, O.; Hopp, J.; Čejková, B. Nb–V-enriched sövites of the northeastern and eastern part of the Amba Dongar carbonatite ring dike, India–A reflection of post-emplacement hydrothermal overprint? Chem. Erde. 2020, 80, 125534. [Google Scholar] [CrossRef]
- Weber, H.P.; Virgo, D.; Huggins, F.E. A neutron diffraction and 57Fe Mössbauer study of a synthetic Ti-rich garnet. In Cambridge Institute Year Book; Cambridge University: Washington, DC, USA, 1975; Volume 74, pp. 575–579. [Google Scholar]
- Nicolescu, S.; Cornell, D.H.; Sodervall, U.; Odelius, H. Secondary ion mass spectrometry analysis of rare earth elements in grandite garnet and other skarn related silicates. Eur. J. Mineral. 1998, 10, 251–259. [Google Scholar] [CrossRef]
- Whitney, P.R.; Olmsted, J.F. Rare earth element metasomatism in hydrothermal systems: The Willsboro-Lewis wollastonite ores, New York, USA. Geochim. Cosmochim. Acta 1998, 62, 2965–2977. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Q.; Shao, Y.; Li, H. Fingerprinting the hydrothermal fluid characteristics from LA-ICP-MS trace element geochemistry of garnet in the Yongping Cu deposit, SE China. Minerals 2017, 7, 199. [Google Scholar] [CrossRef]
- Zhang, Y.; Shao, Y.J.; Wu, C.D.; Chen, H.Y. LA-ICP-MS trace element geochemistry of garnets: Constraints on hydrothermal fluid evolution and genesis of the Xinqiao Cu–S–Fe–Au deposit, eastern China. Ore Geol. Rev. 2017, 86, 426–439. [Google Scholar] [CrossRef]
- Graunch, R.I. Rare earth elements in metamorphic rocks. In Geochemistry and Mineralogical Rare Earth Elements; Lipin, B.R., Mckay, G.A., Eds.; De Gruyter: Berlin, Germany, 1989; Volume 21, pp. 146–167. [Google Scholar]
- Mckay, G.A. Partition of rare earth elements between major silicate minerals and basaltic melts. In Geochemistry and Mineralogy of Rare Earth Elements; De Gruyter: Berlin, Germany, 1989; Volume 21, pp. 45–78. [Google Scholar]
- Harris, N.B.W.; Gravestock, P.; Inger, S. Ion-microprobe determinations of trace-element concentrations in garnets from anatectic assemblages. Chem. Geol. 1992, 100, 41–49. [Google Scholar] [CrossRef]
- Schwandt, C.S.; Papike, J.J.; Shearer, C.K.; Brearley, A.J. A SIMS investigation of REE chemistry of garnet in garnetite associated with the Broken Hill Pb–Zn–Ag orebodies, Australia. Can. Mineral. 1993, 31, 371–379. [Google Scholar] [CrossRef] [Green Version]
- Bea, F.; Montero, P.; Garuti, G.; Zacharini, F. Pressure-dependece of rare earth element distribution in amphibolite and granulite-grade garnets. A LA-ICP-MS study. Geostandard Newslett. 1997, 21, 253–270. [Google Scholar] [CrossRef]
- Bea, F.; Pereira, M.D.; Stroh, A. Mineral/leucosome trace-element partitioning in a peraluminous migmatite (a laser ablation-ICP-MS study). Chem. Geol. 1994, 117, 291–312. [Google Scholar] [CrossRef]
- Hoal, K.E.O.; Hoal, B.G.; Erlank, A.J.; Shimizu, N. Metasomatism of the mantle lithosphere recorded by rare earth elements in garnets. Earth Planet. Sci. Lett. 1994, 126, 303–313. [Google Scholar] [CrossRef]
- Skublov, S.G.; Drugova, G.M. REE Distribution in Metamorphic Garnets. Herald DGGGMS RAS 2. pp. 60–61. Available online: http://geo.web.ru/conf/khitariada/5-2000.2/magm27.eng.pdf (accessed on 12 July 2021).
- Zhang, H.; Menzies, M.A.; Lu, F.; Zhou, X. Major and trace element studies on garnets from Paleozoic kimberliteborne mantle xenoliths and megacrysts from the North China craton. Sci. China 2000, D43, 423–430. [Google Scholar] [CrossRef]
- Boyd, F.R.; Pearson, D.G.; Hoal, K.O.; Hoal, B.G.; Nixon, P.H.; Kingston, M.J.; Mertzman, S.A. Garnet lherzolites from Louwrensia, Namibia: Bulk composition and P/T relations. Lithos 2004, 77, 573–592. [Google Scholar] [CrossRef]
- Doroshkevich, G.A.; Viladkar, S.G.; Ripp, G.S.; Burtsera, V. Hydrothermal REE Mineralisation in the Amba Dongar Carbonatite Complex, Gujarat, India. Can. Mineral. 2009, 47, 1105–1116. [Google Scholar] [CrossRef]
- Saha, A.; Ray, J.; Ganguly, S.; Chatterjee, N. Occurrence of melanite garnet in syenite and ijolite–melteigite rocks of Samchampi–Samteran alkaline complex, Mikir Hills, Northeastern India. Curr. Sci. 2011, 101, 95–100. [Google Scholar]
- Masuda, A.; Lkeuchi, Y. Lanthanide tetrad effect observed in marine environment. Geochem. J. 1979, 13, 19–22. [Google Scholar] [CrossRef]
- Peretyazhko, I.S.; Savina, E.A. Tetrad effects in the rare earth element patterns of granitoid rocks as an indicator of fluoride-silicate liquid immiscibility in magmatic systems. Petrology 2010, 18, 514–543. [Google Scholar] [CrossRef]
- Doelter, C. Granatgruppe. In Handbuck der Mineralchemie: Bd.II (1915–1917); Springer: Berlin/Heidelberg, Germany, 1916; pp. 878–915. [Google Scholar]
- Badalov, S.T. Vanadium-bearing tourmaline and garnet Zafiski Vsesoyuz. Minerol. Obs. 1951, 80, 212–213. [Google Scholar]
- Mariano, A.N. Nature of economic mineralization in carbonatites and related rocks. In Carbonatites: Genesis and Evolution; Unwin Hyman: London, UK, 1989; pp. 149–176. [Google Scholar]
- Bau, M. Rare-earth element mobility during hydrothermal and metamorphic fluid-rock interaction and the significance of the oxidation state of europium. Chem. Geol. 1991, 93, 219–230. [Google Scholar] [CrossRef]
- Migdisov, A.; Williams-Jones, A.E.; Brugger, J.; Caporuscio, F.A. Hydrothermal transport, deposition, and fractionation of the REE: Experimental data and thermodynamic calculations. Chem. Geol. 2016, 439, 13–42. [Google Scholar] [CrossRef] [Green Version]
- Migdisov, A.A.; Williams-Jones, A.E. Hydrothermal transport and deposition of the rare earth elements by fluorine-bearing aqueous liquids. Miner. Depos. 2014, 49, 987–997. [Google Scholar] [CrossRef]
- Williams-Jones, A.E.; Migdisov, A.A.; Samson, I.M. Hydrothermal mobilisation of the rare earth elements—A tale of “ceria” and “yttria”. Elements 2012, 8, 355–360. [Google Scholar] [CrossRef]
- Allen, D.E.; Seyfried, W.E. REE controls in ultramafic hosted MOR hydrothermal systems: An experimental study at elevated temperature and pressure. Geochim. Cosmochim. Acta 2005, 69, 675–683. [Google Scholar] [CrossRef]
- Roedder, E. Fluid inclusions from the fluorite deposits associated with the curbonatlte of Amba Dongar, India and Okorusu, south west Africa. Trans. Inst. Min. Metal. 1973, 82, B35–B39. [Google Scholar]
- Simonetti, A.; Bell, K. Nd, Pb, and Sr isotope systematics of fluorite at the Amba Dongar carbonatite complex, India: Evidence for hydrothermal and crustal fluid mixing. Econ. Geol. 1995, 90, 2018–2027. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, R.K.; Taylor, L.A. Carbon-and oxygen-isotope variations in Indian carbonatites. Int. Geol. Rev. 1996, 38, 419–429. [Google Scholar] [CrossRef]
- Viladkar, S.G.; Schidlowski, M. Carbon and oxygen isotope geochemistry of the Amba Dongar carbonatite complex, Gujarat, India. Gondwana Res. 2000, 3, 415–424. [Google Scholar] [CrossRef]
- Ray, J.S.; Ramesh, R. Stable carbon and oxygen isotopic compositions of Indian carbonatites. Intl. Geol. Rev. 2006, 48, 17–45. [Google Scholar] [CrossRef] [Green Version]
- Hopp, J.; Viladkar, S.G. Noble gas composition of Indian carbonatites (Amba Dongar, Siriwasan): Implications on mantle source compositions and late-stage hydrothermal processes. Earth Planet. Sci. Lett. 2018, 492, 186–196. [Google Scholar] [CrossRef]







| Oxide (wt. %) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | 28.5 | 28.6 | 28.73 | 28.71 | 28.73 | 28.69 | 29.22 | 29.3 | 28.66 | 29.11 | 28.85 | 29.26 | 29.2 | 29.91 | 29.58 |
| TiO2 | 12.77 | 12.8 | 12.48 | 12 | 12.82 | 12.31 | 12.27 | 12.25 | 12.1 | 12.2 | 12.31 | 13.04 | 12.75 | 11.18 | 11.45 |
| Al2O3 | 2.08 | 2.24 | 2.32 | 2.19 | 2.17 | 2.63 | 2.34 | 2.33 | 2.28 | 2.21 | 2.21 | 1.97 | 1.12 | 1.39 | 0.75 |
| Cr2O3 | 0.09 | 0.10 | 0.08 | 0.10 | 0.08 | 0.1 | 0.10 | 0.07 | 0.10 | 0.09 | 0.10 | 0.11 | 0.12 | 0.11 | 0.08 |
| V2O3 | 0.57 | 0.47 | 0.25 | 0.38 | 0.41 | 0.47 | 0.38 | 0.5 | 0.31 | 0.54 | 0.6 | 0.45 | 0.66 | 0.63 | 0.39 |
| FeOtot | 20.95 | 19.81 | 20.67 | 19.65 | 19.52 | 20.63 | 19.63 | 19.6 | 21.34 | 19.84 | 19.58 | 19.36 | 20.95 | 21.38 | 22.01 |
| MnOtot | 0.29 | 0.23 | 0.25 | 0.34 | 0.2 | 0.44 | 0.42 | 0.32 | 0.29 | 0.38 | 0.29 | 0.42 | 0.34 | 0.27 | 0.41 |
| MgO | 0.92 | 0.83 | 0.8 | 0.88 | 0.83 | 0.77 | 0.87 | 0.79 | 0.91 | 0.75 | 0.81 | 0.72 | 0.51 | 0.58 | 0.57 |
| CaO | 33.33 | 32.8 | 33.26 | 32.74 | 32.54 | 32.74 | 32.93 | 33.14 | 32.69 | 32.74 | 32.72 | 32.94 | 32.65 | 32.95 | 32.62 |
| Na2O | 0.02 | 0.01 | 0.03 | 0.03 | 0.07 | 0.06 | 0.02 | 0.02 | 0.05 | 0.06 | 0.02 | 0.07 | 0.18 | 0.09 | 0.10 |
| Total (calc.) | 99.51 | 97.9 | 98.89 | 97.02 | 97.39 | 98.85 | 98.18 | 98.33 | 98.73 | 97.92 | 97.5 | 98.33 | 98.48 | 98.5 | 97.97 |
| Recalc. (wt. %) | |||||||||||||||
| final FeO | 0.85 | 1.95 | 1.16 | 1.11 | 2.26 | 1.38 | 1.70 | 1.76 | 1.12 | 1.83 | 1.80 | 2.48 | 2.49 | 1.95 | 2 |
| final Fe2O3 | 22.33 | 19.84 | 21.68 | 20.61 | 19.19 | 21.39 | 19.92 | 19.83 | 22.47 | 20.02 | 19.76 | 18.76 | 20.52 | 21.59 | 22.24 |
| final MnO | 0.29 | 0.23 | 0.25 | 0.34 | 0.2 | 0.44 | 0.42 | 0.32 | 0.29 | 0.38 | 0.29 | 0.42 | 0.34 | 0.27 | 0.41 |
| final Mn2O3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ∑(Recalc.) | 101.75 | 99.89 | 101.06 | 99.09 | 99.32 | 100.99 | 100.17 | 100.31 | 100.98 | 99.94 | 99.48 | 100.22 | 100.54 | 100.67 | 100.19 |
| Cations for 12 O atoms | |||||||||||||||
| Mn2+ | 0.020 | 0.016 | 0.018 | 0.024 | 0.014 | 0.031 | 0.030 | 0.023 | 0.020 | 0.027 | 0.021 | 0.030 | 0.024 | 0.019 | 0.030 |
| Mg | 0 | 0.007 | 0 | 0 | 0.011 | 0.020 | 0 | 0 | 0.033 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ca | 2.977 | 2.974 | 2.984 | 2.990 | 2.964 | 2.938 | 2.972 | 2.986 | 2.939 | 2.965 | 2.977 | 2.976 | 2.958 | 2.970 | 2.968 |
| Na | 0.003 | 0.002 | 0.005 | 0.005 | 0.011 | 0.010 | 0.004 | 0.004 | 0.008 | 0.010 | 0.004 | 0.011 | 0.029 | 0.014 | 0.016 |
| ∑X | 3.000 | 3.000 | 3.007 | 3.019 | 3.000 | 3.000 | 3.005 | 3.013 | 3.000 | 3.003 | 3.002 | 3.017 | 3.011 | 3.005 | 3.014 |
| Ti4+ | 0.801 | 0.815 | 0.787 | 0.769 | 0.820 | 0.776 | 0.777 | 0.775 | 0.764 | 0.776 | 0.786 | 0.827 | 0.811 | 0.708 | 0.731 |
| Al3+ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cr3+ | 0.006 | 0.006 | 0.005 | 0.006 | 0.005 | 0.007 | 0.007 | 0.004 | 0.006 | 0.006 | 0.006 | 0.007 | 0.008 | 0.007 | 0.005 |
| Fe2+ | 0.059 | 0.138 | 0.081 | 0.079 | 0.160 | 0.097 | 0.1197 | 0.124 | 0.079 | 0.129 | 0.128 | 0.175 | 0.176 | 0.137 | 0.142 |
| Fe3+ | 0.981 | 0.911 | 1.003 | 0.987 | 0.892 | 1.014 | 0.955 | 0.951 | 1.049 | 0.955 | 0.934 | 0.853 | 0.886 | 1.026 | 1.009 |
| Mn3+ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mg | 0.114 | 0.098 | 0.100 | 0.112 | 0.095 | 0.075 | 0.1096 | 0.099 | 0.081 | 0.094 | 0.102 | 0.090 | 0.064 | 0.073 | 0.072 |
| V | 0.038 | 0.032 | 0.017 | 0.026 | 0.028 | 0.031 | 0.026 | 0.034 | 0.021 | 0.037 | 0.0409 | 0.031 | 0.044 | 0.042 | 0.027 |
| ∑Y | 1.999 | 2.000 | 1.993 | 1.981 | 2.000 | 2.000 | 1.995 | 1.987 | 2.000 | 1.997 | 1.998 | 1.983 | 1.989 | 1.995 | 1.986 |
| Si4+ | 2.376 | 2.421 | 2.406 | 2.446 | 2.443 | 2.403 | 2.461 | 2.464 | 2.405 | 2.461 | 2.45 | 2.467 | 2.469 | 2.519 | 2.512 |
| Al3+ | 0.2041 | 0.223 | 0.229 | 0.2198 | 0.217 | 0.259 | 0.232 | 0.231 | 0.226 | 0.220 | 0.2212 | 0.196 | 0.112 | 0.137 | 0.075 |
| Fe3+ | 0.420 | 0.353 | 0.364 | 0.334 | 0.336 | 0.335 | 0.308 | 0.304 | 0.370 | 0.318 | 0.329 | 0.337 | 0.420 | 0.341 | 0.412 |
| ∑Z | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| End members mol. % | |||||||||||||||
| Andradite | 48.41 | 44.76 | 50.14 | 49.38 | 43.76 | 48.99 | 47.5 | 47.53 | 50.68 | 47.24 | 46.25 | 42.65 | 44.29 | 51.32 | 50.45 |
| Schorlomite | 20.99 | 17.66 | 18.18 | 16.7 | 16.8 | 16.74 | 15.37 | 15.21 | 18.48 | 15.92 | 16.44 | 16.85 | 20.98 | 17.06 | 20.63 |
| Schorlomite-Al | 10.21 | 11.16 | 11.44 | 10.99 | 10.86 | 12.97 | 11.59 | 11.57 | 11.30 | 11.02 | 11.06 | 9.81 | 5.58 | 6.88 | 3.76 |
| Morimotoite | 5.94 | 13.80 | 8.13 | 7.92 | 16.05 | 9.66 | 11.97 | 12.40 | 7.87 | 12.91 | 12.80 | 17.48 | 17.57 | 13.73 | 14.19 |
| Morimotoite-Mg | 11.42 | 9.82 | 10.03 | 11.22 | 9.48 | 7.53 | 10.96 | 9.91 | 8.15 | 9.43 | 10.25 | 9.03 | 6.44 | 7.32 | 7.21 |
| Goldmanite | 1.90 | 1.59 | 0.83 | 1.30 | 1.40 | 1.57 | 1.29 | 1.68 | 1.03 | 1.84 | 2.05 | 1.53 | 2.23 | 2.12 | 1.34 |
| Uvarovite | 0.31 | 0.32 | 0.25 | 0.32 | 0.27 | 0.33 | 0.33 | 0.22 | 0.32 | 0.31 | 0.33 | 0.36 | 0.39 | 0.36 | 0.27 |
| NaTi Garnet | 0.15 | 0.12 | 0.26 | 0.26 | 0.57 | 0.5 | 0.18 | 0.18 | 0.4 | 0.51 | 0.2 | 0.57 | 1.45 | 0.7 | 0.8 |
| Calderite | 0.65 | 0.55 | 0 | 0 | 0.47 | 1.04 | 0.27 | 0 | 0.68 | 0.5 | 0.43 | 0 | 0 | 0 | 0 |
| Khoharite | 0 | 0.13 | 0 | 0 | 0.22 | 0.59 | 0 | 0 | 1.09 | 0 | 0 | 0 | 0 | 0 | 0 |
| Remainder | 0.03 | 0.09 | 0.74 | 1.91 | 0.14 | 0.09 | 0.54 | 1.31 | 0 | 0.30 | 0.20 | 1.72 | 1.07 | 0.50 | 1.36 |
| Total (calc.) | 100.01 | 100 | 100 | 100 | 100.02 | 100.01 | 100 | 100.01 | 100 | 99.98 | 100.01 | 100 | 100 | 99.99 | 100.01 |
| Quality Index | Sup | Sup | Sup | Exc | Sup | Sup | Sup | Exc | Sup | Sup | Sup | Exc | Exc | Sup | Exc |
| Rare-Earth Elements | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elements | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
| La | 72.1 | 67.3 | 52.0 | 50.8 | 39.9 | 52.2 | 46.8 | 64.1 | 47.1 | 33.1 | 65.5 | 51.1 | 48.6 | 51.5 | 49.9 | 29.35 |
| Ce | 379.0 | 366.0 | 315.0 | 283.0 | 248.0 | 247.0 | 276.0 | 339.0 | 231.0 | 202.0 | 346.0 | 295.0 | 275.0 | 285.0 | 209.0 | 162.0 |
| Pr | 64.0 | 63.2 | 55.0 | 48.3 | 46.1 | 45.5 | 47.9 | 61.7 | 42.3 | 37.2 | 63.5 | 52.0 | 51.2 | 46.9 | 38.8 | 29.2 |
| Nd | 335.0 | 340.0 | 295.0 | 256.0 | 238.0 | 234.0 | 245.0 | 323.0 | 225.0 | 199.0 | 329.0 | 281.0 | 281.0 | 234.0 | 198.0 | 154.4 |
| Sm | 87.2 | 91.6 | 71.6 | 60.8 | 58.1 | 58.1 | 64.8 | 86.3 | 55.6 | 51.8 | 88.1 | 75.2 | 75.1 | 59.9 | 61.1 | 45.25 |
| Eu | 29.7 | 32.2 | 24.3 | 19.4 | 19.2 | 19.5 | 22.1 | 29.6 | 18.6 | 17.1 | 31.5 | 26.8 | 25.8 | 20.3 | 21.0 | 16.07 |
| Gd | 79.9 | 81.3 | 61.5 | 50.9 | 49.8 | 49.1 | 55.61 | 74.2 | 46.7 | 42.7 | 79.4 | 68.6 | 63.0 | 50.7 | 50.2 | 38.52 |
| Tb | 12.4 | 13.5 | 9.4 | 7.2 | 7.1 | 6.9 | 8.4 | 12.0 | 7.0 | 6.8 | 12.8 | 11.1 | 10.5 | 8.19 | 8.7 | 6.5 |
| Dy | 72.9 | 78.7 | 48.2 | 39.48 | 39.2 | 37.4 | 48.0 | 70.0 | 39.1 | 37.3 | 75.7 | 65.4 | 58.7 | 47.1 | 51.7 | 39.5 |
| Ho | 12.9 | 14.2 | 8.7 | 7.1 | 6.9 | 6.4 | 8.2 | 12.8 | 6.9 | 6.6 | 13.8 | 12.0 | 10.6 | 8.6 | 9.4 | 7.0 |
| Er | 34.8 | 38.3 | 23.3 | 18.3 | 18.7 | 17.1 | 22.3 | 33.7 | 18.7 | 18.0 | 36.2 | 32.3 | 28.1 | 24.0 | 25.6 | 19.4 |
| Tm | 4.5 | 4.9 | 3.3 | 2.5 | 2.5 | 2.4 | 3.1 | 4.5 | 2.5 | 2.5 | 4.8 | 4.2 | 3.7 | 3.2 | 3.5 | 2.6 |
| Yb | 31.1 | 34.5 | 21.8 | 16.6 | 17.0 | 16.7 | 20.2 | 30.2 | 17.4 | 16.7 | 31.5 | 27.9 | 24.7 | 21.3 | 23.0 | 17.9 |
| Lu | 3.9 | 4.3 | 2.8 | 2.3 | 2.2 | 2.3 | 2.7 | 3.9 | 2.4 | 2.3 | 4.3 | 3.7 | 3.4 | 2.8 | 3.1 | 2.3 |
| ∑LREE | 967.0 | 959.9 | 813.0 | 718.1 | 649.3 | 656.0 | 702.4 | 903.8 | 619.9 | 539.4 | 923.5 | 781.1 | 757.2 | 697.1 | 578.4 | 435.9 |
| ∑HREE | 252.5 | 269.8 | 179.1 | 144.3 | 143.3 | 138.2 | 168.6 | 241.2 | 140.9 | 132.9 | 258.4 | 225.1 | 202.7 | 165.9 | 175.3 | 133.8 |
| ∑REE | 1219.5 | 1229.7 | 992.1 | 862.4 | 792.6 | 794.3 | 871.1 | 1145.1 | 760.73 | 672.3 | 1181.9 | 1006.3 | 959.9 | 863.0 | 753.7 | 569.6 |
| LREE/HREE | 3.83 | 3.56 | 4.54 | 4.97 | 4.53 | 4.75 | 4.16 | 3.75 | 4.40 | 4.06 | 3.57 | 3.47 | 3.74 | 4.20 | 3.30 | 3.26 |
| La/Yb | 1.6 | 1.3 | 1.6 | 2.1 | 1.6 | 2.1 | 1.6 | 1.4 | 1.8 | 1.3 | 1.4 | 1.2 | 1.3 | 1.6 | 1.5 | 1.1 |
| δEu | 1.08 | 1.14 | 1.12 | 1.06 | 1.09 | 1.11 | 1.12 | 1.13 | 1.11 | 1.11 | 1.15 | 1.14 | 1.14 | 1.13 | 1.15 | 1.17 |
| Trace Elements | ||||||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | |
| Sc | 15 | 16 | 14 | 12 | 13 | 12 | 14 | 15 | 14 | 14 | 14 | 14 | 13 | 11 | 13 | 10 |
| V | 1127 | 1083 | 1396 | 1693 | 2027 | 1670 | 1853 | 1114 | 2155 | 1903 | 1359 | 1311 | 1575 | 1533 | 1998 | 1451 |
| Mn | 2694 | 2616 | 2822 | 2629 | 2822 | 2660 | 2705 | 2369 | 3558 | 3197 | 3062 | 2777 | 2757 | 2693 | 2749 | 1863 |
| Co | 11 | 11 | 8 | 7 | 6 | 7 | 7 | 11 | 7 | 9 | 10 | 9 | 8 | 9 | 9 | 7 |
| Ni | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 1 | 2 | 2 | 2 | 2 | 1 |
| Zn | 111 | 106 | 81 | 85 | 86 | 64 | 97 | 82 | 136 | 113 | 121 | 111 | 88 | 107 | 106 | 64 |
| Sr | 61 | 58 | 49 | 51 | 49 | 54 | 55 | 67 | 178 | 93 | 68 | 61 | 62 | 90 | 59 | 45 |
| Y | 335 | 365 | 238 | 194 | 194 | 189 | 231 | 335 | 193 | 191 | 362 | 315 | 290 | 244 | 266 | 195 |
| Zr | 3360 | 3379 | 2762 | 2057 | 2072 | 2082 | 2328 | 3892 | 1904 | 1817 | 3436 | 2876 | 2895 | 2257 | 1888 | 1821 |
| Nb | 2282 | 2134 | 957 | 1274 | 435 | 964 | 1196 | 739 | 1275 | 695 | 1742 | 1218 | 718 | 156 | 695 | 282 |
| Hf | 62 | 60 | 50 | 38 | 40 | 34 | 41 | 80 | 37 | 37 | 63 | 55 | 56 | 46 | 35 | 40 |
| Ta | 78 | 73 | 39 | 33 | 21 | 33 | 48 | 44 | 53 | 28 | 73 | 61 | 36 | 48 | 29 | 15 |
| Th | 22 | 23 | 14 | 13 | 7 | 11 | 12 | 15 | 8 | 6 | 17 | 13 | 10 | 12 | 7 | 5 |
| U | 22 | 21 | 17 | 14 | 10 | 14 | 16 | 16 | 13 | 10 | 19 | 14 | 12 | 14 | 11 | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samal, A.K.; Srivastava, R.K.; Upadhyay, D. Major, Trace, and Rare-Earth Element Geochemistry of Nb-V Rich Andradite-Schorlomite-Morimotoite Garnet from Ambadungar-Saidivasan Alkaline Carbonatite Complex, India: Implication for the Role of Hydrothermal Fluid-Induced Metasomatism. Minerals 2021, 11, 756. https://doi.org/10.3390/min11070756
Samal AK, Srivastava RK, Upadhyay D. Major, Trace, and Rare-Earth Element Geochemistry of Nb-V Rich Andradite-Schorlomite-Morimotoite Garnet from Ambadungar-Saidivasan Alkaline Carbonatite Complex, India: Implication for the Role of Hydrothermal Fluid-Induced Metasomatism. Minerals. 2021; 11(7):756. https://doi.org/10.3390/min11070756
Chicago/Turabian StyleSamal, Amiya K., Rajesh K. Srivastava, and Dewashish Upadhyay. 2021. "Major, Trace, and Rare-Earth Element Geochemistry of Nb-V Rich Andradite-Schorlomite-Morimotoite Garnet from Ambadungar-Saidivasan Alkaline Carbonatite Complex, India: Implication for the Role of Hydrothermal Fluid-Induced Metasomatism" Minerals 11, no. 7: 756. https://doi.org/10.3390/min11070756






