Characterization of Waste Sludge Pigment from Production of ZnCl2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
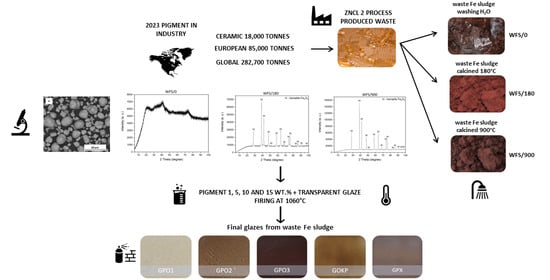
2.2. Sample Preparation
2.3. Characterization Techniques
- Chemical composition of raw materials was determined using energy dispersive fluorescence spectrometer SPECTRO XEPO (SPECTRO Analytical Instruments GmbH, Kleve, Germany), equipped with 50 Watt Pd X-ray tube.
- The phase composition of samples was characterized using a Bruker D8 Advance X-ray powder diffractometer (Bruker AXS GmbH, Karlsruhe, Germany). The diffraction patterns in the range of 5° to 70° 2θ were recorded under CoKα (λ = 1.78897 Å, U = 35 kV, I = 25 mA) irradiation with scanning rate 2°/min using fast position-sensitive detector VÅNTEC1.
- The morphology of the particles was characterized using the scanning electron microscope (SEM) QUANTA 450 FEG, (FEI, Hillsboro, OR, USA); the images were collected using a secondary electron detector.
- The particle size distribution (PSD) was analyzed using Mastersizer (Malvern Panalytical Ltd., Malvern, UK). Measurements were performed in an aquatic environment, and ultrasound was used for homogenization of the suspension.
- The characterization of the thermal behavior was performed on TG/DTAanalyzer STA504 (TA Instruments, New Castle, Delaware, USA). The sample of scale placed in alumina crucible was analyzed in a temperature range from 21 to 1100 °C in the dynamic atmosphere of N2 (5 L⋅h−1), and the heating rate was 10 K·min−1.
- The reflectance spectra of the final glazes in the spectral range 400–700 nm were obtained using MiniScan EZ0828 spectrometer (HunterLab, Reston, VA, USA), model 45°/0°, small observation area. The color of the glazes was expressed using CIE L*a*b* coordinates calculated for 10° observer and D65 illuminant.
- Specific surface area was determined by BET method-equipment (Quantachrome NovaWin Instrument-Acquisition and Reduction for NOVA instrument, analysis gas nitrogen, Graz, Austria).
- Density of samples was determined by Pycnomatic and Pycnomatic ATC (Helium pycnometer) POROTEC GmbH, Coconut Creek, FL, USA.
3. Results and Discussion
3.1. Chemical Composition
3.2. Phase Composition of Commercial Pigments
3.3. Granulometry of Commercial Pigments
3.4. SEM Analysis of Commercial Pigments
3.5. The Treatment of Waste Fe Sludge
- WFS/0—Waste Fe sludge washed by H2O by 1 L H2O/300 g of WFS,
- WFS/180—Waste Fe sludge washed by H2O by 1 L H2O/300 g of WFS and calcined at 180 °C at the mode of 15 °C/min for 10 h/180 °C, and
- WFS/900—Waste Fe sludge washed by H2O by 1 L H2O/300 g of WFS and calcined at 900 °C at the mode of 15 °C/min for 3 h/900 °C.
3.6. Granulometry of Waste Fe Sludge
3.7. SEM Analysis of Waste Fe Sludge
3.8. Phase Analysis of Waste Fe Sludge
3.9. TG and DTA of Waste Fe Sludge
3.10. Color of Glaze
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Future of High-Performance Pigments to 2023, Focus on Pigments. Available online: http://www.smithers.com (accessed on 10 February 2021).
- Monrós, G. Pigment, Ceramic. In Encyclopedia of Color Science and Technology; Luo, R., Ed.; Springer: New York, NY, USA, 2014; pp. 1–15. [Google Scholar]
- Benda, P.; Kalendová, A. Anticorrosion properties of pigments based on ferrite coated zinc particles. Phys. Procedia 2013, 44, 185–194. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Osorio, A.; Rivera, C.E.; Vázquez-Olmos, A.; Chávez, J. Luminescent ceramic nano-pigments based on terbium-doped zinc aluminate: Synthesis, properties and performance. Dyes Pigment. 2015, 119, 22–29. [Google Scholar] [CrossRef]
- Phillips, R.W.; LaGallee, C.R.; Markantes, C.T.; Coombs, P.G. Multi-Layered Magnetic Pigments and Foils. Patents US-6099895-A, 8 August 2000. [Google Scholar]
- Zheng, S.; Li, J. Inorganic-organic sol-gel hybrid coatings for corrosion protection of metal. J. Sol-Gel Sci. Technol. 2010, 54, 174–187. [Google Scholar] [CrossRef]
- Pereira, O.C.; Bernadin, A.M. Ceramic colorant from untreated iron ore residue. J. Hazard. Mater. 2012, 233, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Bondioli, F.; Andreola, F.; Barbieri, L.; Manfredini, T.; Ferrari, A.M. Effect of rice husk ash (RHA) in the synthesis of (Pr,Zr)SiO4 ceramic pigment. J. Eur. Ceram. Soc. 2007, 27, 3483–3488. [Google Scholar] [CrossRef]
- Eppler, R.A. Colorants for Ceramics. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Inc.: New York, NY, USA, 2002. [Google Scholar] [CrossRef]
- Mufti, N.; Atma, T.; Fuad, A.; Datudji, E. Synthesis and characterization of black, red and yellow nanoparticles pigments from the iron sand. AIP Conf. Proc. 2014, 1617, 165. [Google Scholar] [CrossRef]
- Legodi, M.A.; de Waal, D. The preparation of magnetite, goethite, hematite and maghemite of pigment quality from mill scale iron waste. Dyes Pigment. 2007, 74, 161–168. [Google Scholar] [CrossRef]
- Sugimoto, T.; Muramatsu, A.; Sakata, K.; Shindo, D.J. Characterization of hematite particles of different shapes. Colloid Interface Sci. 1993, 158, 420–428. [Google Scholar] [CrossRef]
- Ueda, M.; Shimada, S.; Inaga, M. Synthesis of crystalline ferrites below 60 °C. J. Eur. Ceram. Soc. 1996, 16, 685–686. [Google Scholar] [CrossRef]
- Prim, S.R.; Folgueras, M.V.; De Lima, M.A.; Hotza, D. Synthesis and characterization of hematite pigment obtained from a steel waste industry. J. Hazard. Mater. 2011, 193, 1307–1313. [Google Scholar] [CrossRef]
- Ismail, H.M.; Gadenhead, D.A.; Zaki, M.I. Thermal genesis course of iron oxide pigmentary powders from steel-pickling chemical waste. J. Colloid Interface Sci. 1996, 183, 320–328. [Google Scholar] [CrossRef]
- Khoiroh, L.M.; Nuraini, E.D.; Aini, N. Synthesis of goethite (α-FeOOH) pigment by precipitation method from iron lathe waste. Mater. Sci. 2018, 6, 65–69. [Google Scholar] [CrossRef] [Green Version]
- Müller, M.; Villalba, J.C.; Mariani, F.Q.; Dalpasquale, M.; Lemos, M.Z.; Huila, M.F.G.; Anaissi, F.J. Synthesis and characterization of iron oxide pigments through the method of the forced hydrolysis of inorganic salts. Dyes Pigment. 2015, 120, 271–278. [Google Scholar] [CrossRef]
- Nunez, N.O.; Morales, M.P.; Tatraj, P.; Serna, C.J. Preparation of high acicular and uniform goethite particles by a modified-carbonate route. J. Mater. Chem. 2000, 10, 2561–2565. [Google Scholar] [CrossRef]
- Lu, Y.; Dong, W.; Wang, W.; Ding, J.; Wang, Q.; Hui, A.; Wang, A. Optimal Synthesis of Environment-Friendly Iron Red Pigment from Natural Nanostructured Clay Minerals. Nanomaterials 2018, 11, 925. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Huang, Z.; Liu, Y.; Fang, M.; Ouyang, X.; Hu, M. Controlled synthesis of porous FeCO3 microspheres and the conversion to α-Fe2O3 with unconventional morphology. Ceram. Int. 2014, 14, 11975–11983. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Chen, X.; Leung, Y.H.; Ching, A.M. ZnO nanostructures: Growth, properties and applications. J. Mater. Chem. 2021, 22, 6526–6536. [Google Scholar] [CrossRef]
- Cao, N.J.; Xu, Q.; Chen, L.F. Acid hydrolysis of cellulose in zinc chloride solution. Appl. Biochem. Biotechnol. 1995, 1, 21–28. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Dong, C.; Ding, X.Y.; Xia, Y.K.A. Generalized ZnCl2 activation method to produce nitrogen-containing nanoporous carbon materials for supercapacitor applications. J. Alloys Compd. 2017, 5, 12653–12672. [Google Scholar] [CrossRef]
- Eppler, R.A.; Eppler, D.A. Glaze and Glass Coating; The American Ceramic Society: Westerville, OH, USA, 2000. [Google Scholar]
- Jandová, J.; Lisá, K.; Salátová, Z. Continuous Precipitation of Metals and Its Application in Hydrometallurgical Processes. In Proceedings of the 7th Conference on Environment and Mineral Processing, Ostrava, Czech Republic, 5–6 March 2003. [Google Scholar]
- Widodo, R.D.; Priyono; Rusiyanto; Anis, S.; Ichwani, A.A.; Setiawan, B.; Fitriyana, D.F.; Rochman, L. Synthesis and characterization of iron (III) oxide form natural iron sand of the south coastal area, Purworejo Central Java. J. Phys. Conf. Ser. 2020, 1444, 012043. [Google Scholar] [CrossRef]
- Bedoya, P.A.C.; Botta, P.M.; Bercoff, P.G.; Fanovich, M.A. Magnetic iron oxides nanoparticles obtained by mechanochemical reactions from different solid precursors. J. Alloys Compd. 2021, 860, 157892. [Google Scholar] [CrossRef]
- Yadav, B.S.; Singh, R.; Vishwakarma, A.K.; Kumar, N. Facile synthesis of substantially magnetic hollow nanospheres of maghemite (gamma-Fe2O3) originated from magnetite (Fe3O4) via solvothermal method. J. Supercond. Nov. Magn. 2020, 33, 2199–2208. [Google Scholar] [CrossRef]
- Sankova, N.; Parkhomchuk, E. Pseudomorphism and size stabilization of hematite particles in the organic phase synthesis. J. Solid State Chem. 2020, 282, 121130. [Google Scholar] [CrossRef]
- Novotny, M.; Solc, Z.; Trojan, M. Pigments, Inorganic. In Kirk-Othmer Encyklopedia of Chemical Technology, 5th ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2005. [Google Scholar]
- Meng, F.; Morin, S.A.; Jin, S. Rational solution growth of α-FeOOH nanowires driven by screw dislocations and their conversion to α-Fe2O3 nanowires. J. Am. Chem. Soc. 2011, 133, 8408–8411. [Google Scholar] [CrossRef]
- Bondioli, F.; Ferrari, A.M.; Leonelli, C.; Manfredini, T. Syntheses of Fe2O3/Silica Red Inorganic Inclusion Pigments for Ceramic Application. Mater. Res. Bull. 1998, 33, 723–729. [Google Scholar] [CrossRef]
- Ullrich, A.; Rölle, N.; Horn, S. From wustite to hematite: Thermal transformation of differently sized iron oxide nanoparticles in air. J. Nanopart. Res. 2019, 21, 168. [Google Scholar] [CrossRef]
- Koizumi, H.; Uddin, M.d.A.; Kato, Y. Effect of ultrasonic irradiation on γ-Fe2O3 formation by co-precipitation method with Fe3+ salt and alkaline solution. Inorg. Chem. Commun. 2021, 124, 108400. [Google Scholar] [CrossRef]
- Gregor, C.; Hermanek, M.; Jancík, D.; Pechousek, J.; Filip, J.; Hrbač, J.; Zbořil, R. The Effect of Surface Area and Crystal Structure on the Catalytic Efficiency of Iron(III) Oxide Nanoparticles in Hydrogen Peroxide Decomposition. Eur. J. Inorg. Chem. 2010, 16, 2343–2351. [Google Scholar] [CrossRef]
- Buchner, W.; Schliebs, R.; Winter, G.; Buchel, K.H. Industrial Inorganic Chemistry; Wiley-VCH Verlag GmbH: Los Angeles, CA, USA, 1989. [Google Scholar]
- Schanda, J. Colorimetry: Understanding the CIE System; John Wiley: Hoboken, NJ, USA, 2007. [Google Scholar]
- Gulrajani, M.L. Colour Measurement: Principles, Advances and Industrial Applications; Woodhead Publishing: Philadelphia, PA, USA, 2010. [Google Scholar]
- Buxbuam, G. Industrial Inorganic Pigments; WILET-VCH: Weinheim, Germany, 1998. [Google Scholar]
- Pekkan, K.; Karasu, B. Evaluation of borax solid wastes in production of frits suitable for fast single-fired wall tile opaque glass-ceramic glazes. Bull. Mater. Sci. 2010, 33, 135–144. [Google Scholar] [CrossRef]
- Bernardin, A.M. The influence of particle size distribution on the surface appearance of glazed tile. Dyes Pigment. 2009, 80, 121–124. [Google Scholar] [CrossRef]











| Recipe | Samples | Amount of the Materials (wt.%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Transparent Glaze (TG) | Waste Fe Sludge (WFS) | Standard Pigments (SP) | ||||||
| WFS/0 | WFS/180 | WFS/900 | SP1 | SP2 | SP3 | |||
| R1 | GP01 | 99 | 1 | |||||
| GP02 | 95 | 5 | ||||||
| GP03 | 90 | 10 | ||||||
| GP04 | 85 | 15 | ||||||
| GPX | 90 | 10 | ||||||
| GOKP | 90 | 10 | ||||||
| R2 | GPF1 | 90 | 10 | |||||
| GPF2 | 90 | 10 | ||||||
| GPF3 | 90 | 10 | ||||||
| Amount of Oxides in Raw Materials (wt.%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Oxides | Ceramic Slurry (CS) | Waste Fe Sludge(WFS) | Waste Fe Sludge (WFS/0) | Waste Fe Sludge (WFS/180) | Transparent Glaze (TG) | SP3 (Dark Brown) | SP2 (Light Brown) | SP1 (Red) |
| Na2O | 1.22 | 5.61 | 4.61 | 5.50 | ||||
| MgO | 2.83 | 1.12 | 0.18 | <0.3 | 0.58 | 0.44 | <0.001 | |
| Al2O3 | 12.42 | 0.20 | 0.74 | 0.49 | 9.10 | <0.001 | 0.44 | <0.001 |
| SiO2 | 69.57 | 80.10 | 0.16 | 0.80 | <0.001 | |||
| P2O5 | <0.0012 | 0.06 | 0.16 | <0.01 | 0.022 | 0.073 | <0.001 | |
| SO3 | 0.19 | 0.15 | 0.19 | 0.71 | 0.12 | 0.27 | 0.33 | 0.20 |
| K2O | 2.08 | 0.88 | <0.001 | <0.001 | <0.001 | |||
| CaO | 4.52 | 0.02 | <0.001 | 0.30 | 0.05 | 0.31 | 0.01 | |
| TiO2 | 0.56 | <0.001 | 0.13 | 0.67 | 0.59 | 1.311 | ||
| MnO | 0.07 | <0.001 | 0.006 | 1.06 | 0.75 | 0.13 | ||
| Fe2O3 | 4.09 | 40.92 | 90.09 | 97.56 | 0.22 | 96.97 | 95.22 | 97.807 |
| BaO | 0.05 | 0.05 | ||||||
| Cl | 22.08 | 2.47 | 0.49 | 0.006 | 0.017 | <0.001 | ||
| ZnO | 29.87 | 1.52 | 0.24 | 0.15 | 0.13 | 0.03 | ||
| PbO | 0.05 | 0.12 | <0.001 | |||||
| V2O5 | <0.001 | 0.03 | <0.001 | |||||
| Cr2O3 | 0.14 | <0.001 | 0.12 | <0.001 | ||||
| NiO | 0.028 | 0.10 | <0.001 | |||||
| CuO | <0.001 | 0.047 | <0.001 | |||||
| Samples | CIE Values | ||
|---|---|---|---|
| L* | a* | b* | |
| GPO1 | 82.04 | 6.28 | 17.85 |
| GPO2 | 31.48 | 6.97 | 16.57 |
| GPO3 | 21.28 | 12.23 | 9.71 |
| GPX | 16.98 | 17.69 | 10.93 |
| GOKP | 27.95 | 6.36 | 25.84 |
| GPF1 | 23.44 | 25.41 | 18.88 |
| GPF2 | 20.23 | 21.27 | 15.72 |
| GPF3 | 18.29 | 20.27 | 16.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ovčačíková, H.; Velička, M.; Maierová, P.; Vlček, J.; Tokarský, J.; Čegan, T. Characterization of Waste Sludge Pigment from Production of ZnCl2. Minerals 2021, 11, 313. https://doi.org/10.3390/min11030313
Ovčačíková H, Velička M, Maierová P, Vlček J, Tokarský J, Čegan T. Characterization of Waste Sludge Pigment from Production of ZnCl2. Minerals. 2021; 11(3):313. https://doi.org/10.3390/min11030313
Chicago/Turabian StyleOvčačíková, Hana, Marek Velička, Petra Maierová, Jozef Vlček, Jonáš Tokarský, and Tomáš Čegan. 2021. "Characterization of Waste Sludge Pigment from Production of ZnCl2" Minerals 11, no. 3: 313. https://doi.org/10.3390/min11030313
APA StyleOvčačíková, H., Velička, M., Maierová, P., Vlček, J., Tokarský, J., & Čegan, T. (2021). Characterization of Waste Sludge Pigment from Production of ZnCl2. Minerals, 11(3), 313. https://doi.org/10.3390/min11030313