Invasive and Non-Invasive Analyses of Ochre and Iron-Based Pigment Raw Materials: A Methodological Perspective
Abstract
:1. Introduction
2. A Broad Methodological Review
2.1. Choosing the Dedicated Methods: An Introduction
- What is the archeological question?
- What is the amount of material, the form of the sample (a piece of rock, residues on a bead, a thin pictorial layer, etc.)?
- Is destructive sampling possible, to which extent?
- Is it a short-term or long-term project?
- What are the facilities I have easy access too?
2.2. Non-Invasive Methods Used in Ochre Studies: A Rreview of Their Limits
2.2.1. Raw Material Characterization of Ochre: Macroscopic Examination versus Physico-Chemical Analyses
2.2.2. Ochre Provenance Research
2.2.3. Iron Oxy-Hydroxide Heat Treatment
3. Non-Invasive Analyses: A Case Study from Diepkloof Rock Shelter, South Africa
- XRD: for structural analyses;
- SEM-EDXS: for bulk analyses of major elements; and
- PIXE: for bulk analyses of major and traces elements.
3.1. Material and Methods
3.1.1. Archeological Samples
3.1.2. Geological Samples
3.1.3. Preparation of the Samples
3.1.4. XRD Analyses
3.1.5. SEM-EDXS Analyses
3.1.6. PIXE Analyses
3.2. Results
3.2.1. XRD Analyses
Geological
Archeological
3.2.2. SEM-EDXS Analyses
Geological
Archeological
3.2.3. PIXE
Geological
Archeological
3.3. Discussion
3.3.1. Instrumental Limits
3.3.2. Post-Depositional Alterations
3.3.3. Consequences for Archeological Inferences
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brooks, A.S.; Yellen, J.E.; Potts, R.; Behrensmeyer, A.K.; Deino, A.L.; Leslie, D.E.; Ambrose, S.H.; Ferguson, J.R.; d’Errico, F.; Zipkin, A.M.; et al. Long-Distance Stone Transport and Pigment Use in the Earliest Middle Stone Age. Science 2018, 360, 90–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barham, L.S. Systematic Pigment Use in the Middle Pleistocene of South-Central Africa. Curr. Anthropol. 2002, 43, 181–190. [Google Scholar] [CrossRef]
- Mcbrearty, S.; Brooks, A.S. The Revolution That Wasn’t: A New Interpretation of the Origin of Modern Human Behavior. J. Hum. Evol. 2000, 39, 453–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roebroeks, W.; Sier, M.J.; Nielsen, T.K.; De Loecker, D.; Parés, J.M.; Arps, C.E.S.; Mücher, H.J. Use of Red Ochre by Early Neandertals. Proc. Natl. Acad. Sci. USA 2012, 109, 1889–1894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watts, I.; Chazan, M.; Wilkins, J. Early Evidence for Brilliant Ritualized Display: Specularite Use in the Northern Cape (South Africa) between ∼500 and ∼300 Ka. Curr. Anthropol. 2016, 57, 287–310. [Google Scholar] [CrossRef] [Green Version]
- Audouin, F.; Plisson, H. Les Ocres et Leurs Témoins Au Paléolithique En France: Enquête et Expériences Sur Leur Validité Archéologique. Cah. Cent. Rech. Préhistoriques 1982, 8, 33–80. [Google Scholar]
- Philibert, S. L’ocre et Le Traitement Des Peaux: Révision d’une Conception Traditionnelle Par l’analyse Fonctionnelle Des Grattoirs Ocrés de La Balma Magineda (Andorre). L’Anthropologie 1994, 98, 447–453. [Google Scholar]
- Rifkin, R.F. Assessing the Efficacy of Red Ochre as a Prehistoric Hide Tanning Ingredient. J. Afr. Archaeol. 2011, 9, 131–158. [Google Scholar] [CrossRef]
- Rifkin, R.F. Ethnographic and Experimental Perspectives on the Efficacy of Ochre as a Mosquito Reppellent. S. Afr. Archaeol. Bull. 2015, 70, 64–75. [Google Scholar]
- Rifkin, R.F.; Dayet, L.; Queffelec, A.; Summers, B.; Lategan, M.; d’Errico, F. Evaluating the Photoprotective Effects of Ochre on Human Skin by In Vivo SPF Assessment: Implications for Human Evolution, Adaptation and Dispersal. PLoS ONE 2015, 10, e0136090. [Google Scholar] [CrossRef] [PubMed]
- Rudner, I.E. Khoisan Pigments and Paints and Their Relationship to Rock Paintings; South African Museum: Cape Town, South Africa, 1982. [Google Scholar]
- Wadley, L. Putting Ochre to the Test: Replication Studies of Adhesives That May Have Been Used for Hafting Tools in the Middle Stone Age. J. Hum. Evol. 2005, 49, 587–601. [Google Scholar] [CrossRef] [PubMed]
- Wadley, L.; Williamson, B.; Lombard, M. Ochre in Hafting in Middle Stone Age Southern Africa: A Practical Role. Antiquity 2004, 78, 661–675. [Google Scholar] [CrossRef]
- White, R. Actes de Substance: De La Matière Au Sens Dans La Représentation Paléolithique. Techné 1996, 3, 29–38. [Google Scholar]
- Cornell, R.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses; Wiley: Weinheim, Germany, 2003. [Google Scholar]
- Cavallo, G.; Fontana, F.; Gonzato, F.; Peresani, M.; Riccardi, M.P.; Zorzin, R. Textural, Microstructural, and Compositional Characteristics of Fe-Based Geomaterials and Upper Paleolithic Ocher in the Lessini Mountains, Northeast Italy: Implications for Provenance Studies. Geoarchaeology 2017, 32, 437–455. [Google Scholar] [CrossRef]
- Dayet, L.; Bourdonnec, F.-X.L.; Daniel, F.; Porraz, G.; Texier, P.-J. Ochre Provenance and Procurement Strategies during The Middle Stone Age at Diepkloof Rock Shelter, South Africa. Archaeometry 2016, 58, 807–829. [Google Scholar] [CrossRef]
- MacDonald, B.L.; Hancock, R.G.V.; Cannon, A.; Pidruczny, A. Geochemical Characterization of Ochre from Central Coastal British Columbia, Canada. J. Archaeol. Sci. 2011, 38, 3620–3630. [Google Scholar] [CrossRef]
- MacDonald, B.L.; Fox, W.; Dubreuil, L.; Beddard, J.; Pidruczny, A. Iron Oxide Geochemistry in the Great Lakes Region (North America): Implications for Ochre Provenance Studies. J. Archaeol. Sci. Rep. 2018, 19, 476–490. [Google Scholar] [CrossRef]
- Rifkin, R.F. Processing Ochre in the Middle Stone Age: Testing the Inference of Prehistoric Behaviours from Actualistically Derived Experimental Data. J. Anthropol. Archaeol. 2012, 31, 174–195. [Google Scholar] [CrossRef]
- Watts, I. Ochre in the Middle Stone Age of Southern Africa: Ritualised Display or Hide Preservative? S. Afr. Archaeol. Bull. 2002, 57, 1–14. [Google Scholar] [CrossRef]
- Couraud, C. Pigments Utilisés En Préhistoire, Provenance, Préparation, Mode d’utilisation. L’Anthropologie 1988, 92, 17–28. [Google Scholar]
- Watts, I. The Pigments from Pinnacle Point Cave 13B, Western Cape, South Africa. J. Hum. Evol. 2010, 59, 392–411. [Google Scholar] [CrossRef]
- Hovers, E.; Ilani, S.; Bar-Yosef, O.; Vandermeersch, B. An Early Case of Color Symbolism: Ochre Use by Modern Humans in Qafzeh Cave. Curr. Anthropol. 2003, 44, 491–522. [Google Scholar] [CrossRef] [Green Version]
- Salomon, H. Les Matières Colorantes Au Début Du Paléolithique Supérieur: Sources, Transformations et Fonctions; Université Bordeaux 1: Bordeaux, France, 2009. [Google Scholar]
- Hodgskiss, T. An Investigation into the Properties of the Ochre from Sibudu, KwaZulu-Natal, South Africa. S. Afr. Humanit. 2012, 24, 99–120. [Google Scholar]
- Dayet Bouillot, L.; Wurz, S.; Daniel, F. Ochre Resources, Behavioural Complexity and Regional Patterns in the Howiesons Poort: New Insights from Klasies River Main Site, South Africa. J. Afr. Archaeol. 2017, 15, 20–41. [Google Scholar] [CrossRef]
- Dayet, L.; Texier, P.-J.; Daniel, F.; Porraz, G. Ochre Resources from the Middle Stone Age Sequence of Diepkloof Rock Shelter, Western Cape, South Africa. J. Archaeol. Sci. 2013, 40, 3492–3505. [Google Scholar] [CrossRef]
- Dayet, L.; Faivre, J.-P.; Le Bourdonnec, F.-X.; Discamps, E.; Royer, A.; Claud, E.; Lahaye, C.; Cantin, N.; Tartar, E.; Queffelec, A.; et al. Manganese and Iron Oxide Use at Combe-Grenal (Dordogne, France): A Proxy for Cultural Change in Neanderthal Communities. J. Archaeol. Sci. Rep. 2019, 25, 239–256. [Google Scholar] [CrossRef]
- Rosso, D.E.; d’Errico, F.; Queffelec, A. Patterns of Change and Continuity in Ochre Use during the Late Middle Stone Age of the Horn of Africa: The Porc-Epic Cave Record. PLoS ONE 2017, 12, e0177298. [Google Scholar] [CrossRef]
- Rosso, D.E.; d’Errico, F.; Zilhão, J. Stratigraphic and Spatial Distribution of Ochre and Ochre Processing Tools at Porc-Epic Cave, Dire Dawa, Ethiopia. Quat. Int. 2014, 343, 85–99. [Google Scholar] [CrossRef]
- Cavallo, G.; Fontana, F.; Gialanella, S.; Gonzato, F.; Riccardi, M.P.; Zorzin, R.; Peresani, M. Heat Treatment of Mineral Pigment During the Upper Palaeolithic in North-East Italy. Archaeometry 2018, 60, 1045–1061. [Google Scholar] [CrossRef]
- Mauran, G.; Lebon, M.; Lapauze, O.; Nankela, A.; Détroit, F.; Lesur, J.; Bahain, J.-J.; Pleurdeau, D. Archaeological Ochres of the Rock Art Site of Leopard Cave (Erongo, Namibia): Looking for Later Stone Age Sociocultural Behaviors. Afr. Archaeol. Rev. 2020, 37, 527–550. [Google Scholar] [CrossRef]
- Fiore, D.; Maier, M.; Parera, S.D.; Orquera, L.; Piana, E. Chemical Analyses of the Earliest Pigment Residues from the Uttermost Part of the Planet (Beagle Channel Region, Tierra Del Fuego, Southern South America). J. Archaeol. Sci. 2008, 35, 3047–3056. [Google Scholar] [CrossRef]
- Salomon, H.; Vignaud, C.; Coquinot, Y.; Pagès-Camagna, S.; Pomiès, M.-P.; Geneste, J.-M.; Menu, M.; Julien, M.; David, F. Les matières colorantes au début du Paléolithique supérieur: Caractérisation chimique et structurale, transformation et valeur symbolique. In Techné Hors-Série; Center for Research and Restoration of the Museums of France: Paris, France, 2008; pp. 17–23. [Google Scholar]
- Mathis, F.; Bodu, P.; Dubreuil, O.; Salomon, H. PIXE Identification of the Provenance of Ferruginous Rocks Used by Neanderthals. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2014, 331, 275–279. [Google Scholar] [CrossRef] [Green Version]
- Pradeau, J.-V.; Binder, D.; Vérati, C.; Lardeaux, J.-M.; Dubernet, S.; Lefrais, Y.; Regert, M. Procurement Strategies of Neolithic Colouring Materials: Territoriality and Networks from 6th to 5th Millennia BCE in North-Western Mediterranean. J. Archaeol. Sci. 2016, 71, 10–23. [Google Scholar] [CrossRef]
- Zipkin, A.M.; Hanchar, J.M.; Brooks, A.S.; Grabowski, M.W.; Thompson, J.C.; Gomani-Chindebvu, E. Ochre Fingerprints: Distinguishing among Malawian Mineral Pigment Sources with Homogenized Ochre Chip LA–ICPMS. Archaeometry 2015, 57, 297–317. [Google Scholar] [CrossRef]
- Zipkin, A.M.; Ambrose, S.H.; Lundstrom, C.C.; Bartov, G.; Dwyer, A.; Taylor, A.H. Red Earth, Green Glass, and Compositional Data: A New Procedure for Solid-State Elemental Characterization, Source Discrimination, and Provenience Analysis of Ochres. J. Archaeol. Method Theory 2020, 27, 930–970. [Google Scholar] [CrossRef]
- Watts, I. Red ochre, body-painting, and language: Interpreting the Blombos ochre. In The Cradle of Language; Botha, R., Knight, C., Eds.; Oxford University Press: Oxford, UK, 2009; pp. 62–92. ISBN 978-0-19-954586-5. [Google Scholar]
- D’Errico, F.; Salomon, H.; Vignaud, C.; Stringer, C. Pigments from the Middle Palaeolithic Levels of Es-Skhul (Mount Carmel, Israel). J. Archaeol. Sci. 2010, 37, 3099–3110. [Google Scholar] [CrossRef]
- Henshilwood, C.S.; d’Errico, F.; van Niekerk, K.L.; Coquinot, Y.; Jacobs, Z.; Lauritzen, S.-E.; Menu, M.; García-Moreno, R. A 100,000-Year-Old Ochre-Processing Workshop at Blombos Cave, South Africa. Science 2011, 334, 219–222. [Google Scholar] [CrossRef] [Green Version]
- Gialanella, S.; Belli, R.; Dalmeri, G.; Lonardelli, I.; Mattarelli, M.; Montagna, M.; Toniutti, L. Artificial or Natural Origin of Hematite-Based Red Pigments in Archaeological Contexts: The Case of Riparo Dalmeri (Trento, Italy). Archaeometry 2011, 53, 950–962. [Google Scholar] [CrossRef]
- Moyo, S.; Mphuthi, D.; Cukrowska, E.; Henshilwood, C.S.; van Niekerk, K.; Chimuka, L. Blombos Cave: Middle Stone Age Ochre Differentiation through FTIR, ICP OES, ED XRF and XRD. Quat. Int. 2016, 404, 20–29. [Google Scholar] [CrossRef]
- Garilli, V.; Vita, G.; La Parola, V.; Pinto Vraca, M.; Giarrusso, R.; Rosina, P.; Bonfiglio, L.; Sineo, L. First Evidence of Pleistocene Ochre Production from Bacteriogenic Iron Oxides. A Case Study of the Upper Palaeolithic Site at the San Teodoro Cave (Sicily, Italy). J. Archaeol. Sci. 2020, 123, 105221. [Google Scholar] [CrossRef]
- MacDonald, B.L.; Chatters, J.C.; Reinhardt, E.G.; Devos, F.; Meacham, S.; Rissolo, D.; Rock, B.; Maillot, C.L.; Stalla, D.; Marino, M.D.; et al. Paleoindian Ochre Mines in the Submerged Caves of the Yucatán Peninsula, Quintana Roo, Mexico. Sci. Adv. 2020, 6, eaba1219. [Google Scholar] [CrossRef] [PubMed]
- Goemaere, E.; Salomon, H.; Billard, C.; Querré, G.; MATHIS, F.; Golitko, M.; Dubrulle-Brunaud, C.; Savary, X.; Dreesen, R. Les Hématites Oolithiques Du Néolithique Ancien et Du Mésolithique de Basse-Normandie (France): Caractérisation Physico-Chimique et Recherche Des Provenances. Anthropol. Praehist. 2016, 125, 89–119. [Google Scholar]
- Tortosa, J.E.A.; Gallello, G.; Roldán, C.; Cavallo, G.; Pastor, A.; Murcia-Mascarós, S. Characterization and Sources of Paleolithic–Mesolithic Ochre from Coves de Santa Maira (Valencian Region, Spain). Geoarchaeology 2021, 36, 72–91. [Google Scholar] [CrossRef]
- Matarrese, A.; Di Prado, V.; Poiré, D.G. Petrologic Analysis of Mineral Pigments from Hunter-Gatherers Archaeological Contexts (Southeastern Pampean Region, Argentina). Quat. Int. 2011, 245, 2–12. [Google Scholar] [CrossRef]
- Zilhão, J.; Angelucci, D.E.; Badal-García, E.; d’Errico, F.; Daniel, F.; Dayet, L.; Douka, K.; Higham, T.F.G.; Martínez-Sánchez, M.J.; Montes-Bernárdez, R.; et al. Symbolic Use of Marine Shells and Mineral Pigments by Iberian Neandertals. Proc. Natl. Acad. Sci. USA 2010, 107, 1023–1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darchuk, L.; Tsybrii, Z.; Worobiec, A.; Vázquez, C.; Palacios, O.M.; Stefaniak, E.A.; Gatto Rotondo, G.; Sizov, F.; Van Grieken, R. Argentinean Prehistoric Pigments’ Study by Combined SEM/EDX and Molecular Spectroscopy. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2010, 75, 1398–1402. [Google Scholar] [CrossRef]
- Lebon, M.; Gallet, X.; Bondetti, M.; Pont, S.; Mauran, G.; Walter, P.; Bellot-Gurlet, L.; Puaud, S.; Zazzo, A.; Forestier, H.; et al. Characterization of Painting Pigments and Ochres Associated with the Hoabinhian Archaeological Context at the Rock-Shelter Site of Doi Pha Kan (Thailand). J. Archaeol. Sci. Rep. 2019, 26, 101855. [Google Scholar] [CrossRef]
- Wojcieszak, M.; Wadley, L. Raman Spectroscopy and Scanning Electron Microscopy Confirm Ochre Residues on 71 000-Year-Old Bifacial Tools from Sibudu, South Africa. Archaeometry 2018, 60, 1062–1076. [Google Scholar] [CrossRef]
- Wojcieszak, M.; Wadley, L. A Raman Micro-Spectroscopy Study of 77,000 to 71,000 Year Old Ochre Processing Tools from Sibudu, KwaZulu-Natal, South Africa. Herit. Sci. 2019, 7, 24. [Google Scholar] [CrossRef]
- Rosso, D.E.; Martí, A.P.; d’Errico, F. Middle Stone Age Ochre Processing and Behavioural Complexity in the Horn of Africa: Evidence from Porc-Epic Cave, Dire Dawa, Ethiopia. PLoS ONE 2016, 11, e0164793. [Google Scholar] [CrossRef] [Green Version]
- Popelka-Filcoff, R.S.; Robertson, J.D.; Glascock, M.D.; Descantes, C. Trace Element Characterization of Ochre from Geological Sources. J. Radioanal. Nucl. Chem. 2007, 272, 17–27. [Google Scholar] [CrossRef]
- Popelka-Filcoff, R.S.; Craig, N.; Glascock, M.D.; Robertson, J.D.; Aldenderfer, M.; Speakman, R.J. Instrumental Neutron Activation Analysis of Ochre Artifacts from Jiskairumoko, Peru. In Archaeological Chemistry; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2007; Volume 968, pp. 480–505. ISBN 978-0-8412-7413-6. [Google Scholar]
- Popelka-Filcoff, R.S.; Lenehan, C.E.; Glascock, M.D.; Bennett, J.W.; Stopic, A.; Quinton, J.S.; Pring, A.; Walshe, K. Evaluation of Relative Comparator and K0-NAA for Characterization of Aboriginal Australian Ochre. J. Radioanal. Nucl. Chem. 2012, 291, 19–24. [Google Scholar] [CrossRef]
- Eiselt, B.S.; Popelka-Filcoff, R.S.; Darling, J.A.; Glascock, M.D. Hematite Sources and Archaeological Ochres from Hohokam and O’odham Sites in Central Arizona: An Experiment in Type Identification and Characterization. J. Archaeol. Sci. 2011, 38, 3019–3028. [Google Scholar] [CrossRef]
- Attard Montalto, N.; Shortland, A.; Rogers, K. The Provenancing of Ochres from the Neolithic Temple Period in Malta. J. Archaeol. Sci. 2012, 39, 1094–1102. [Google Scholar] [CrossRef]
- Domingo, I.; García-Borja, P.; Roldán, C. Identification, Processing and Use of Red Pigments (Hematite and Cinnabar) in the Valencian Early Neolithic (Spain). Archaeometry 2012, 54, 868–892. [Google Scholar] [CrossRef]
- Scadding, R.; Winton, V.; Brown, V. An LA-ICP-MS Trace Element Classification of Ochres in the Weld Range Environ, Mid West Region, Western Australia. J. Archaeol. Sci. 2015, 54, 300–312. [Google Scholar] [CrossRef]
- Zarzycka, S.E.; Surovell, T.A.; Mackie, M.E.; Pelton, S.R.; Kelly, R.L.; Goldberg, P.; Dewey, J.; Kent, M. Long-Distance Transport of Red Ocher by Clovis Foragers. J. Archaeol. Sci. Rep. 2019, 25, 519–529. [Google Scholar] [CrossRef]
- Pierce, D.E.; Wright, P.J.; Popelka-Filcoff, R.S. Seeing Red: An Analysis of Archeological Hematite in East Central Missouri. Archaeol. Anthropol. Sci. 2020, 12, 23. [Google Scholar] [CrossRef]
- Trabska, J.; Walanus, A.; Ciesielczuk, J.; Samek, L.; Dutkiewicz, E. Ferruginous Raw Material Sources for Palaeolithic in Poland? Promising Results of Provenance Studies. In Proceedings of the 9th International Conference on NDT of Art 2008, Jerusalem, Israel, 25–30 May 2008; pp. 1–8. [Google Scholar]
- Young, T. Appendix 5, Geochemical analysis of ferruginous materials from Twin Rivers. In The Middle Stone Age of Zambia, Southcentral Africa; Barham, L.S., Ed.; University of Bristol: Bristol, UK, 2000; p. 26572. [Google Scholar]
- Beck, L.; Salomon, H.; Lahlil, S.; Lebon, M.; Odin, G.P.; Coquinot, Y.; Pichon, L. Non-Destructive Provenance Differentiation of Prehistoric Pigments by External PIXE. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2012, 273, 173–177. [Google Scholar] [CrossRef]
- Belli, R.; Lonardelli, I.; Quaranta, A.; Girardi, F.; Cusinato, A.; Dalmeri, G. A methodological approach to discriminate between natural and synthetic haematite used as prehistoric pigments: Preliminary results (Riparo Dalmeri—Trentino, Italy). In Proceedings of the Conference of the Associazione Italiana di Archeometria, Firenze 2007, Patron Editore, Bologna, Italy, 26–29 February 2008; pp. 179–185. [Google Scholar]
- Bernatchez, J.A. Geochemical Characterization of Archaeological Ochre at Nelson Bay Cave (Western Cape Province), South Africa. S. Afr. Archaeol. Bull. 2008, 63, 3–11. [Google Scholar] [CrossRef]
- Cavallo, G.; Fontana, F.; Gonzato, F.; Guerreschi, A.; Riccardi, M.P.; Sardelli, G.; Zorzin, R. Sourcing and Processing of Ochre during the Late Upper Palaeolithic at Tagliente Rock-Shelter (NE Italy) Based on Conventional X-Ray Powder Diffraction Analysis. Archaeol. Anthropol. Sci. 2017, 9, 763–775. [Google Scholar] [CrossRef] [Green Version]
- Cavallo, G.; Riccardi, M.P.; Zorzin, R. Powder Diffraction of Yellow and Red Natural Earths from Lessini Mountains in NE Italy. Powder Diffr. 2015, 30, 122–129. [Google Scholar] [CrossRef] [Green Version]
- Salomon, H.; Vignaud, C.; Coquinot, Y.; Beck, L.; Stringer, C.; Strivay, D.; D’errico, F. Selection and Heating of Colouring Materials in the Mousterian Level of Es-Skhul (ca. 100 000 Years BP, Mount Carmel, Israel). Archaeometry 2012, 54, 698–722. [Google Scholar] [CrossRef]
- D’Errico, F.; García Moreno, R.; Rifkin, R.F. Technological, Elemental and Colorimetric Analysis of an Engraved Ochre Fragment from the Middle Stone Age Levels of Klasies River Cave 1, South Africa. J. Archaeol. Sci. 2012, 39, 942–952. [Google Scholar] [CrossRef]
- Dayet, L.; Daniel, F.; Guibert, P.; Texier, P.-J. Non-Destructive Analysis of Archaeological Ochre: A Preliminary Application to the Middle Stone Age Site of Diepkloof Rock Shelter (South Africa). Open J. Archaeom. 2013, 1, e19. [Google Scholar] [CrossRef] [Green Version]
- Dayet, L.; d’Errico, F.; Garcia-Moreno, R. Searching for Consistencies in Châtelperronian Pigment Use. J. Archaeol. Sci. 2014, 44, 180–193. [Google Scholar] [CrossRef]
- Godfrey-Smith, D.I.; Ilani, S. Past Thermal History of Goethite and Hematite Fragments from Qafzeh Cave Deduced from Thermal Activation Characteristics of the 110 °C TL Peak of Enclosed Quartz Grains. Archéosci. Rev. Archéom. 2004, 28, 185–190. [Google Scholar] [CrossRef]
- Goemaere, E.; Salomon, H.; Querré, G.; MATHIS, F.; Dreesen, R.; Hamon, C.; Constantin, C.; Dominique, B.; Wijnen, J.; Jadin, I. Caractérisation Physico-Chimique et Recherche Des Provenances Des Hématites Oolithiques Des Sites Du Néolithique Ancien de Hesbaye (Province de Liège, Belgique) et Des Sites Néolithiques Des Sources de La Dendre (Province Du Hainaut, Belgique). Anthropol. Praehist. 2016, 125, 153–191. [Google Scholar]
- Hughes, J.C.; Solomon, A. A Preliminary Study of Ochres and Pigmentaceous Materials from KwaZulu-Natal, South Africa: Towards an Understanding of San Pigment and Paint Use. S. Afr. Humanit. 2000, 12, 15–31. [Google Scholar]
- Macdonald, B.L.; Hancock, R.G.V.; Cannon, A.; McNeill, F.; Reimer, R.; Pidruczny, A. Elemental Analysis of Ochre Outcrops in Southern British Columbia, Canada. Archaeometry 2013, 55, 1020–1033. [Google Scholar] [CrossRef]
- Di Prado, V.S.; Scalice, R.; Poire, D.G.; Canalicchio, J.; Gómez Peral, L. Análisis de los Elementos Colorantes Provenientes del Sitio Calera (Sierras Bayas, Región Pampeana). Una Exploración del uso Social y Ritual de los Pigmentos; Sociedad Argentina de Antropología: Buenos Aires, Argentina, 2007; ISBN 978-987-1280-07-0. [Google Scholar]
- Mooney, S.D.; Geiss, C.; Smith, M.A. The Use of Mineral Magnetic Parameters to Characterize Archaeological Ochres. J. Archaeol. Sci. 2003, 30, 511–523. [Google Scholar] [CrossRef]
- Salomon, H.; Vignaud, C.; Lahlil, S.; Menguy, N. Solutrean and Magdalenian Ferruginous Rocks Heat-Treatment: Accidental and/or Deliberate Action? J. Archaeol. Sci. 2015, 55, 100–112. [Google Scholar] [CrossRef] [Green Version]
- San Juan-Foucher, C. Étude typotechnologique, fonctionnelle et spatiale des matières colorantes du Bois-Ragot. Niveaux magdaléniens et aziliens. In Mémoires de la Société Préhistorique Française; Chollet, A., Dujardin, V., Eds.; French Prehistoric Society: Rennes, France, 2005; Volume 38, pp. 261–274. [Google Scholar]
- Pomiès, M.-P.; Vignaud, C. Analyses de pigments provenant du site de Bois-Ragot: Diffraction Rx et observation en MET, La grotte du Bois-Ragot à Gouex (Vienne), Magdalénien et Azilien, essais sur les hommes et leur environnement. In Mémoires de la Société Préhistorique Française; Chollet, A., Dujardin, V., Eds.; French Prehistoric Society: Rennes, France, 2005; Volume 38, pp. 275–278. [Google Scholar]
- Velliky, E.C.; MacDonald, B.L.; Porr, M.; Conard, N.J. First Large-Scale Provenance Study of Pigments Reveals New Complex Behavioural Patterns during the Upper Palaeolithic of South-Western Germany. Archaeometry 2020, 63, 173–193. [Google Scholar] [CrossRef]
- Velliky, E.C.; Barbieri, A.; Porr, M.; Conard, N.J.; MacDonald, B.L. A Preliminary Study on Ochre Sources in Southwestern Germany and Its Potential for Ochre Provenance during the Upper Paleolithic. J. Archaeol. Sci. Rep. 2019, 27, 101977. [Google Scholar] [CrossRef]
- Wadley, L. Cemented Ash as a Receptacle or Work Surface for Ochre Powder Production at Sibudu, South Africa, 58,000 Years Ago. J. Archaeol. Sci. 2010, 37, 2397–2406. [Google Scholar] [CrossRef]
- De Faria, D.L.; Venâncio Silva, S.; De Oliveira, M.T. Raman microspectroscopy of some iron oxides and oxyhydroxides. J. Raman Spectrosc. 1997, 28, 873–878. [Google Scholar] [CrossRef]
- Louden, J.D. Raman Microscopy. In Practical Raman Spectroscopy; Gardiner, D.J., Graves, P.R., Eds.; Springer: Berlin/Heidelberg, Germany, 1989; pp. 119–151. ISBN 978-3-642-74040-4. [Google Scholar]
- Newbury, D.E. Microbeam Analysis of Samples of Unusual Shape. J. Phys. Colloq. 1984, 45, 775–780. [Google Scholar] [CrossRef]
- Potts, P.J.; Webb, P.C.; Williams-Thorpe, O. Investigation of a Correction Procedure for Surface Irregularity Effects Based on Scatter Peak Intensities in the Field Analysis of Geological and Archaeological Rock Samples by Portable X-Ray Fluorescence Spectrometry. J. Anal. At. Spectrom. 1997, 12, 769–776. [Google Scholar] [CrossRef]
- Potts, P.J.; West, M. Portable X-Ray Fluorescence Spectrometry; Royal Society of Chemistry: Cambridge, UK, 2008; ISBN 978-0-85404-552-5. [Google Scholar]
- Pouchou, J.-L.; Pichoir, F. Quantitative Analysis of Homogeneous or Stratified Microvolumes Applying the Model “PAP.”. In Electron Probe Quantitation; Heinrich, K.F.J., Newbury, D.E., Eds.; Springer: Boston, MA, USA, 1991; pp. 31–75. ISBN 978-1-4899-2617-3. [Google Scholar]
- Pouchou, J.-L. Les méthodes de quantification en microanalyse X. In Microscopie Electronique à Balayage et Microanalyses; Brisset, F., Ed.; EDP Sciences: Les Ulis, France, 2008. [Google Scholar]
- Calligaro, T.; Dran, J.-C.; Salomon, J. Chapter 5 Ion beam microanalysis. In Comprehensive Analytical Chemistry; Non-Destructive Microanalysis of Cultural Heritage Materials; Elsevier: Amsterdam, the Netherlands, 2004; Volume 42, pp. 227–276. [Google Scholar]
- Brisset, F.; Boivin, D. L’analyse EDS. In Microscopie Electronique à Balayage et Microanalyses; Brisset, F., Ed.; EDP Sciences: Les Ulis, France, 2008. [Google Scholar]
- Beck, L.; Lebon, M.; Pichon, L.; Menu, M.; Chiotti, L.; Nespoulet, R.; Paillet, P. PIXE Characterisation of Prehistoric Pigments from Abri Pataud (Dordogne, France). X-Ray Spectrom. 2011, 40, 219–223. [Google Scholar] [CrossRef]
- Pollard, A.M.; Batt, C.M.; Stern, B.; Young, S.M.M. Analytical Chemistry in Archaeology; Cambridge University Press: Cambridge, UK, 2007; ISBN 978-0-521-65572-9. [Google Scholar]
- Pollard, A.M.; Heron, C. Archaeological Chemistry; Royal Society of Chemistry: London, UK, 2015; ISBN 978-1-78262-611-4. [Google Scholar]
- Kanngießer, B.; Haschke, M.; Simionovici, A.; Chevallier, P.; Streli, C.; Wobrauschek, P.; Fabry, L.; Pahlke, S.; Comin, F.; Barrett, R.; et al. Methodological Developments and Applications. In Handbook of Practical X-Ray Fluorescence Analysis; Beckhoff, B., Kanngießer, B., Langhoff, N., Wedell, R., Wolff, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 433–833. ISBN 978-3-540-36722-2. [Google Scholar]
- Potts, P.J.; Williams-Thorpe, O.; Webb, P.C. The Bulk Analysis of Silicate Rocks by Portable X-Ray Fluorescence: Effect of Sample Mineralogy in Relation to the Size of the Excited Volume. Geostand. Newsl. 1997, 21, 29–41. [Google Scholar] [CrossRef]
- Mantler, M.; Willis, J.; Lachance, G.; Vrebos, B.A.R.; Mauser, K.-E.; Kawahara, N.; Rousseau, R.M.; Brouwer, P.N. Quantitative Analysis. In Handbook of Practical X-Ray Fluorescence Analysis; Beckhoff, B., Kanngießer, B., Langhoff, N., Wedell, R., Wolff, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 309–410. ISBN 978-3-540-36722-2. [Google Scholar]
- Speakman, R.J.; Shackley, M.S. Silo Science and Portable XRF in Archaeology: A Response to Frahm. J. Archeol. Sci. 2013, 40, 1435–1443. [Google Scholar] [CrossRef]
- Flemming, R.L. Micro X-Ray Diffraction (ΜXRD): A Versatile Technique for Characterization of Earth and Planetary Materials. Can. J. Earth Sci. 2007, 44, 1333–1346. [Google Scholar] [CrossRef]
- Waseda, Y.; Matsubara, E.; Shinoda, K. Diffraction from Polycrystalline Samples and Determination of Crystal Structure. In X-ray Diffraction Crystallography: Introduction, Examples and Solved Problems; Waseda, Y., Matsubara, E., Shinoda, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 107–167. ISBN 978-3-642-16635-8. [Google Scholar]
- Moore, D.M.; Reynolds, J.R.C. X-Ray Diffraction and the Identification and Analysis of Clay Minerals; Oxford University Press: New York, NY, USA, 1989. [Google Scholar]
- Meunier, A. Les Argiles; Collection Géosciences; Scientifiques GB; Contemporary Publishing International-GB Science Publisher, Impr.: Paris, France, 2002. [Google Scholar]
- Krishnan, K.; Hill, S.L. 3—FT-IR Microsampling Techniques. In Practical Fourier Transform Infrared Spectroscopy; Ferraro, J.R., Krishnan, K., Eds.; Academic Press: Cambridge, MA, USA, 1990; pp. 103–165. ISBN 978-0-12-254125-4. [Google Scholar]
- Bruno, T.J. Sampling Accessories for Infrared Spectrometry. Appl. Spectrosc. Rev. 1999, 34, 91–120. [Google Scholar] [CrossRef]
- Miliani, C.; Rosi, F.; Daveri, A.; Brunetti, B.G. Reflection Infrared Spectroscopy for the Non-Invasive in Situ Study of Artists’ Pigments. Appl. Phys. A 2012, 106, 295–307. [Google Scholar] [CrossRef]
- Fabbri, M.; Picollo, M.; Porcinai, S.; Bacci, M. Mid-Infrared Fiber-Optics Reflectance Spectroscopy: A Noninvasive Technique for Remote Analysis of Painted Layers. Part I: Technical Setup. Appl. Spectrosc. 2001, 55, 420–427. [Google Scholar] [CrossRef]
- Velliky, E.C.; Porr, M.; Conard, N.J. Ochre and Pigment Use at Hohle Fels Cave: Results of the First Systematic Review of Ochre and Ochre-Related Artefacts from the Upper Palaeolithic in Germany. PLoS ONE 2018, 13, e0209874. [Google Scholar] [CrossRef]
- Hodgskiss, T.; Wadley, L. How People Used Ochre at Rose Cottage Cave, South Africa: Sixty Thousand Years of Evidence from the Middle Stone Age. PLoS ONE 2017, 12, e0176317. [Google Scholar] [CrossRef]
- Beauvais, A.; Colin, F. Formation and Transformation Processes of Iron Duricrust Systems in Tropical Humid Environment. Chem. Geol. 1993, 106, 77–101. [Google Scholar] [CrossRef]
- Putzolu, F.; Abad, I.; Balassone, G.; Boni, M.; Cappelletti, P.; Graziano, S.F.; Maczurad, M.; Mondillo, N.; Najorka, J.; Santoro, L. Parent Rock and Climatic Evolution Control on the Genesis of Ni-Bearing Clays in Ni-Co Laterites: New Inferences from the Wingellina Deposit (Western Australia). Ore Geol. Rev. 2020, 120, 103431. [Google Scholar] [CrossRef]
- Kiehn, A.V.; Brook, G.A.; Glascock, M.D.; Dake, J.Z.; Robbins, L.H.; Campbell, A.C.; Murphy, M.L. Fingerprinting Specular Hematite from Mines in Botswana, Southern Africa. In Archaeological Chemistry; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2007; Volume 968, pp. 460–479. ISBN 978-0-8412-7413-6. [Google Scholar]
- Popelka-Filcoff, R.S.; Miksa, E.J.; Robertson, J.D.; Glascock, M.D.; Wallace, H. Elemental Analysis and Characterization of Ochre Sources from Southern Arizona. J. Archaeol. Sci. 2008, 35, 752–762. [Google Scholar] [CrossRef]
- Iriarte, E.; Foyo, A.; Sánchez, M.A.; Tomillo, C.; Setién, J. The Origin and Geochemical Characterization of Red Ochres from the Tito Bustillo and Monte Castillo Caves (Northern Spain). Archaeometry 2009, 2, 231–251. [Google Scholar] [CrossRef]
- Kingery-Schwartz, A.; Popelka-Filcoff, R.S.; Lopez, D.A.; Pottier, F.; Hill, P.; Glascock, M. Analysis of Geological Ochre: Its Geochemistry, Use, and Exchange in the US Northern Great Plains. Open J. Archaeom. 2013, 1, e15. [Google Scholar] [CrossRef]
- Dimuccio, L.A.; Rodrigues, N.; Larocca, F.; Pratas, J.; Amado, A.M.; de Carvalho, L.A.E.B. Geochemical and Mineralogical Fingerprints to Distinguish the Exploited Ferruginous Mineralisations of Grotta Della Monaca (Calabria, Italy). Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2017, 173, 704–720. [Google Scholar] [CrossRef]
- Zipkin, A.M.; Ambrose, S.H.; Hanchar, J.M.; Piccoli, P.M.; Brooks, A.S.; Anthony, E.Y. Elemental Fingerprinting of Kenya Rift Valley Ochre Deposits for Provenance Studies of Rock Art and Archaeological Pigments. Quat. Int. 2017, 430, 42–59. [Google Scholar] [CrossRef] [Green Version]
- Mauran, G.; Caron, B.; Détroit, F.; Nankela, A.; Bahain, J.-J.; Pleurdeau, D.; Lebon, M. Data Pretreatment and Multivariate Analyses for Ochre Sourcing: Application to Leopard Cave (Erongo, Namibia). J. Archaeol. Sci. Rep. 2021, 35, 102757. [Google Scholar] [CrossRef]
- Aitchison, J. The Statistical Analysis of Compositional Data. In Monographs on Statistics and Applied Probability; Chapman & Hall: London, UK, 1986; p. 416. [Google Scholar]
- Comas-Cufí, M.; Thió-Henestrosa, S. CoDaPack 2.0: A Stand-Alone, Multi-Platform Compositional Software. In Proceedings of the CoDaWork’11: 4th International Workshop on Compositional Data Analysis, Girona, Spain, 9–13 May 2011. [Google Scholar]
- How, M.W. The Mountain Bushmen of Basutoland; van Schaik, J.L., Ed.; Cambridge University Press: Cambridge, UK, 1962. [Google Scholar]
- Pomiès, M.; Menu, M.; Vignaud, C. Tem Observations of Goethite Dehydration: Application to Archaeological Samples. J. Eur. Ceram. Soc. 1999, 19, 1605–1614. [Google Scholar] [CrossRef]
- Lima de Faria, J. Dehydration of Goethite and Diaspore. Z. Für Krist. 1963, 119, 176–203. [Google Scholar] [CrossRef]
- Watari, F.; van Landuyt, J.; Delavignette, P.; Amelinckx, S.; Igata, N. X-Ray Peak Broadening as a Result of Twin Formation in Some Oxides Derived by Dehydration. Phys. Status Solidi A 1982, 73, 215–224. [Google Scholar] [CrossRef]
- Pomiès, M.-P.; Morin, G.; Vignaud, C. XRD Study of the Goethite-Hematite Transformation: Application to the Identification of Heated Prehistoric Pigments. Eur. J. Solid State Inorg. Chem. 1998, 35, 9–25. [Google Scholar] [CrossRef]
- Gualteri, A.F.; Venturelli, P. In Situ Study of the Goethite-Hematite Phase Transformation by Real Time Synchrotron. Am Miner. 1999, 84, 895–904. [Google Scholar] [CrossRef]
- Löffler, L.; Mäder, W. Anisotropic X-Ray Peak Broadening and Twin Formation in Hematite Derived from Natural and Synthetic Goethite. J. Eur. Ceram. Soc. 2006, 26, 131–139. [Google Scholar] [CrossRef]
- De Faria, D.L.A.; Lopes, F.N. Heated Goethite and Natural Hematite: Can Raman Spectroscopy Be Used to Differentiate Them? Vib. Spectrosc. 2007, 45, 117–121. [Google Scholar] [CrossRef]
- Zoppi, A.; Lofrumento, C.; Castellucci, E.M.; Sciau, P. Al-for-Fe Substitution in Hematite: The Effect of Low Al Concentrations in the Raman Spectrum of Fe2O3. J. Raman Spectrosc. 2008, 39, 40–46. [Google Scholar] [CrossRef]
- Onoratini, G.; Perinet, G. Données Minéralogiques Sur Les Colorants Rouges Préhistoriques de Provence: Démonstration Que Certains d’entre Eux Ont Été Obtenus Par Calcination de Goethite. Comptes Rendus Académie Sci. 1985, 301, 119–124. [Google Scholar]
- Baffier, D.; Girard, M.; Menu, M.; Vignaud, C. La Couleur à La Grande Grotte d’Arcysur-Cure (Yonne). L’Anthropologie 1999, 1999, 103–121. [Google Scholar]
- Pomiès, M.-P.; Menu, M.; Vignaud, C.; Goupy, J.; Mohen, J.-P. Lascaux, pigments préhistoriques à base d’oxydes de fer: Hématite naturelle collectée ou goethite chauffée? In Proceedings of the Art et Chimie, la Couleur: Actes du Congrès, Paris, France, 16–18 September 1998. [Google Scholar]
- Lahaye, C.; Godfrey-Smith, D.I.; Guibert, P.; Bechtel, F. Equivalent Thermal History (HE) of Ferruginous Sandstones Based on the Thermal Activation Characteristics of Quartz. Radiat. Meas. 2006, 41, 995–1000. [Google Scholar] [CrossRef]
- Aitken, M.J. Thermoluminescence Dating; Academic Press: London, UK, 1985. [Google Scholar]
- Dayet, L. Matériaux, Transformations et Fonctions de L’ocre Au Middle Stone Age: Le Cas de Diepkloof Rock Shelter Dans Le Contexte de l’Afrique Australe. Ph.D. Thesis, Université Bordeaux, Bordeaux, France, 2012. [Google Scholar]
- Parkington, J.E.; Rigaud, J.-P.H.; Poggenpoel, C.; Porraz, G.; Texier, P.-J. Introduction to the Project and Excavation of Diepkloof Rock Shelter (Western Cape, South Africa): A View on the Middle Stone Age. J. Archaeol. Sci. 2013, 40, 3369–3375. [Google Scholar] [CrossRef]
- Porraz, G.; Parkington, J.E.; Rigaud, J.-P.; Miller, C.E.; Poggenpoel, C.; Tribolo, C.; Archer, W.; Cartwright, C.R.; Charrié-Duhaut, A.; Dayet, L.; et al. The MSA Sequence of Diepkloof and the History of Southern African Late Pleistocene Populations. J. Archaeol. Sci. 2013, 40, 3542–3552. [Google Scholar] [CrossRef]
- Tribolo, C.; Mercier, N.; Douville, E.; Joron, J.-L.; Reyss, J.-L.; Rufer, D.; Cantin, N.; Lefrais, Y.; Miller, C.E.; Porraz, G.; et al. OSL and TL Dating of the Middle Stone Age Sequence at Diepkloof Rock Shelter (South Africa): A Clarification. J. Archaeol. Sci. 2013, 40, 3401–3411. [Google Scholar] [CrossRef]
- Calligaro, T.; MacArthur, J.D.; Salomon, J. An Improved Experimental Setup Analysis of Heavy and Light Elements Beam for the Simultaneous PIXE with a 3 MeV Proton External Beam. Nucl. Instrum. Methods Phys. B 1996, 109–110, 125–128. [Google Scholar] [CrossRef]
- Swann, C.P.; Fleming, S.J. Selective Filtering in PIXE Spectrometry. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 1990, 49, 65–69. [Google Scholar] [CrossRef]
- Campbell, J.L.; Boyd, N.I.; Grassi, N.; Bonnick, P.; Maxwell, J.A. The Guelph PIXE Software Package IV. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2010, 268, 3356–3363. [Google Scholar] [CrossRef]
- Miller, C.E.; Goldberg, P.; Berna, F. Geoarchaeological Investigations at Diepkloof Rock Shelter, Western Cape, South Africa. J. Archaeol. Sci. 2013, 40, 3432–3452. [Google Scholar] [CrossRef]
- Schmidt, P.; Porraz, G.; Slodczyk, A.; Bellot-gurlet, L.; Archer, W.; Miller, C.E. Heat Treatment in the South African Middle Stone Age: Temperature Induced Transformations of Silcrete and Their Technological Implications. J. Archaeol. Sci. 2013, 40, 3519–3531. [Google Scholar] [CrossRef] [Green Version]
- Porraz, G.; Texier, P.-J.; Archer, W.; Piboule, M.; Rigaud, J.-P.; Tribolo, C. Technological Successions in the Middle Stone Age Sequence of Diepkloof Rock Shelter, Western Cape, South Africa. J. Archaeol. Sci. 2013, 40, 3376–3400. [Google Scholar] [CrossRef]





| Reference | Archaeolgical Issue | Type of Remains | Sampling Limits | Method | Procedure | Sample Preparation |
|---|---|---|---|---|---|---|
| Attard Montalto et al. [60] | Provenance (provenience) | Ochre powders and pieces | None | ICP-AES | Invasive | Grinding, dissolution |
| Barham [2] (Young [66]) | Evidencing human exploitation | Ochre pieces | None | XRD | Invasive? | ? |
| ICP-MS | Invasive | Dissolution | ||||
| SEM-EDXS | Invasive | Polished blocks | ||||
| Beck et al. [67] | Classification, provenance (provenience) | Ochre pieces | Use-wear traces on the pieces | PIXE | Non-invasive | None |
| Belli et al. [68]; Gialanela et al. [43] | Heat treatment | Ochre lumps | None | XRD | Invasive | Removing of the external part, gentle grinding |
| SEM-EDXS | Invasive | Same | ||||
| TEM | Invasive | Same, suspension, one drop | ||||
| Raman spectrometry | Invasive | Same | ||||
| Bernatchez [69] | Classification | Ochre pieces | None | XRPD | Invasive | Grinding, powder |
| PIXE | Invasive | Grinding, pellets | ||||
| Brooks et al. [1] | Evidencing human exploitation, provenance | Ochre pieces | Use-wear traces on the pieces | SEM-EDXS | Non-invasive | None |
| LA-ICP-MS | Minimally invasive | None | ||||
| Cavallo et al. [16] | Provenance (provenience) | Ochre pieces | None | Petrography | Invasive | Polished thin-sections |
| SEM-EDXS | Invasive | Polished thin-sections | ||||
| Cavallo et al. [70,71] | Provenance (provenience), processing (heat treatment) | Ochre pieces | None | Powder XRD | Invasive | Grinding, powder |
| Cavallo et al. [32] | Heat treatment | Ochre pieces | None | Powder XRD | Invasive | Grinding, powder |
| Petrography | Invasive | Polished thin-sections | ||||
| SEM-EDXS | Invasive | Polished thin-sections | ||||
| TEM | Invasive | Grinding, suspension, one drop | ||||
| d’Errico et al. [41]; Salomon et al. [72] | Evidencing human exploitation, heat treatment, provenance (geological origin) | Ochre lumps | Very small collection | SEM-EDXS | Minimally Invasive | Micro-samples, no preparation |
| TEM | Minimally Invasive | Micro-samples, suspension, one drop | ||||
| PIXE-PIGE | Minimally Invasive | Micro-samples, enrobed in eopxy resin | ||||
| µXRD | Minimally Invasive | Micro-samples | ||||
| d’Errico et al. [73] | Raw material classification and selection | Ochre piece | Engraved piece | Visible spectroscopy | Non-invasive | None |
| XRF | Non-invasive | None | ||||
| Dayet et al. [28,74] | Raw material classification and selection, heat treatment, provenance (geological origin), changes over time | Ochre pieces | Use-wear traces on the pieces | SEM-EDXS | Non-invasive | None |
| XRD | Non-invasive/invasive | None/grinding | ||||
| Raman spectroscopy | Non-invasive | None | ||||
| Dayet et al. [75] | Raw material classification and selection | Pigment pieces | Use-wear traces on the pieces | SEM-EDXS | Non-invasive | None |
| µ-XRD | Non-invasive | None | ||||
| Raman spectroscopy | Non-invasive | None | ||||
| pXRF | Non-invasive | None | ||||
| Dayet et al. [17] | Provenance (provenience), group mobility | Ochre pieces/ferruginous materials | None | XRD | Invasive | Grinding, oriented powder |
| ICP-MS | Invasive | Grinding, dissolution | ||||
| ICP-OES | Invasive | Grinding, dissolution | ||||
| Dayet Bouillot et al. [27] | Raw material classification and selection, heat treatment, provenance (geological origin) | Ochre pieces | Use-wear traces on the pieces | SEM-EDXS | Non-invasive | None |
| XRD | Non-invasive | None | ||||
| Visible spectroscopy | - | - | ||||
| Dayet et al. [29] | Raw material classification, provenance (geological origin), changes over time | Pigment pieces | Museum collection | SEM-EDXS | Non-invasive | None |
| XRD | Non-invasive | None | ||||
| pXRF | Non-invasive | None | ||||
| Domingo et al. [61] | Identification of red pigments, processing and preparation | Pigment pieces and powders | None | SEM-EDXS | ? | ? |
| pXRF | ? | ? | ||||
| XRD | ? | ? | ||||
| FTIR | ? | ? | ||||
| GC (organic part only) | Likely invasive | ? | ||||
| Eiselt et al. [59] | Provenance (provenience) | Ochre pieces and powders | None | NAA | Invasive | Grinding/settling, drying and grinding |
| Fiore et al. [34] | Technology of paint production | Ochre lumps and sediments | None | XRD | ? | ? |
| FTIR | Invasive | KBr disks | ||||
| SEM-EDXS | ? | ? | ||||
| GC (organic part only) | - | Crushing, dissolution | ||||
| Garilli et al. [45] | Provenance (geological origin), heat treatment | Ochre sediments | None | SEM-EDXS | Partialy invasive | Dried and “homogenized” (method not given) |
| XRD | Partialy invasive | Drying, “homogenized” (method not given) | ||||
| ATR-FTIR | Partialy invasive | Drying, “homogenized” (method not given) | ||||
| Godfrey-Smith and Ilani [76] | Heat treatment | Hematite fragments | None | Thermoluminescence | Invasive | Grinding, extraction of quartz grains |
| XRD | Invasive | Grinding | ||||
| Goemare et al. [47] | Provenance (geological origin) | Pieces of hematite | Use-wear traces on the pieces | HH-XRF | Non-invasive | None |
| LA-ICP-MS | Minimally invasive | None | ||||
| PIXE | Non-invasive | None | ||||
| XRPD | Invasive | Grinding, extraction of the clay fraction | ||||
| Goemare et al. [77] | Provenance (geological origin) | Pieces of hematite | Use-wear traces on the pieces | HH-XRF | Non-invasive | None |
| PIXE | Non-invasive | None | ||||
| XRPD | Invasive | Grinding, extraction of the clay fraction | ||||
| Petrography | Invasive | Polished thin-sections | ||||
| Henshilwood et al. [42] | Evidencing human exploitation, provenance | Lumps and micro-fragments of ochre | None | PIXE | Invasive | Polished thin-sections |
| SEM-EDXS | Non-invasive/invasive | None/polished thin sections | ||||
| Petrography | Invasive | Polished thin-sections | ||||
| µXRD | Invasive | Polished thin-sections | ||||
| Hodgskiss, 2012 [26] | Raw material classification, properties and selection | Ochre pieces | Use-wear traces on the pieces | SEM-EDXS | Non-invasive | None |
| FTIR | Non-invasive | None | ||||
| Raman spectroscopy | Non-invasive | None | ||||
| Hovers et al., 2003 [24] | Provenance (geological origin) | Ochre lumps | None | Petrography | Invasive | Polished thin-sections |
| XRD | Invasive? | ? | ||||
| ICP-AES | Invasive | ? | ||||
| Hughes and Salomon, 2000 [78] | Classification, site comparison | Ochre pieces | None | Dosimetry | Invasive | ? |
| XRF | ? | ? | ||||
| SEM-EDXS | ? | ? | ||||
| TEM | ? | ? | ||||
| XRD | Invasive | Grinding | ||||
| Lebon et al., 2019 [52] | Raw material selection, links between different human activities | Ochre pieces, powders and residues | None | pXRF | Non-invasive | None |
| SEM-EDXS | Non-invasive | None | ||||
| µXRD | Non-invasive | None | ||||
| Powder XRD | Invasive | Grinding | ||||
| MacDonald et al., 2011 (2013) [18,79] | Site comparison, provenance (geological origin) | Ochre samples | None | NAA | Invasive | Heat-sealed in high-purity polyethylene vials |
| MacDonald et al., 2018 [19] | Provenance (provenience) | Ochre pieces | Use-wear traces on the pieces | XRD | Non-invasive | None |
| NAA | None-invasive/invasive | None/grinding | ||||
| MacDonald et al., 2020 [46] | Raw material properties | Sampling in an ochre mine | None | SEM-EDXS | Invasive | ? |
| XRD | Invasive | Levigated | ||||
| NAA | Invasive | ? | ||||
| Matarrese et al., 2011 (Di Prado et al., 2007) [49,80] | Site comparison, provenance (geological origin) | Pigment pieces | None | Petrography | Invasive | Polished thin-sections |
| XRD | Invasive | Grinding, clay separation | ||||
| Mathis et al., 2014 [36] | Provenance (geological origin), raw material selection | Ferruginous rocks | None | PIXE | Non-invasive? | None? |
| Mooney et al. [81] | Provenance (geological origin) | Archaeological ochre | None | Magnetic measurements | Non-invasive | None |
| Moyo et al. [44] | Raw material characterization, changes over time | Ochre pieces | Samples must not be damaged | XRF | Non-invasive/invasive | None/grinding |
| XRD | Invasive | Grinding | ||||
| FTIR | Invasive | Grinding, KBr pellets | ||||
| ICP-OES | Invasive | Grinding, digestion | ||||
| Pierce et al. [64] | Provenance (provenience), changes over time | Hematite pieces | None | NAA | Invasive | Grinding |
| Pradeau et al. [37] | Provenance (provenience), changes over time | Pieces of coloring materials | None/pieces with use-wear traces not analyzed | Petrography | Invasive | Polished thin-sections |
| SEM-EDXS | Invasive | Polished thin-sections | ||||
| XRD | Invasive | Grinding | ||||
| Pomiès et al. [81] | Heat treatment | Ochre pieces | None | XRD | Invasive | Grinding |
| TEM | Invasive | Grinding, suspension | ||||
| Popelka-Filcoff et al. [57] | Raw material classification | Ochre lumps and powders | None | NAA | Invasive | Grinding |
| Roebroeks et al. [4] | Evidencing human exploitation | Ochre sediments | None | XRD | Invasive | Grinding |
| SEM-EDXS | Invasive | Grinding | ||||
| Salomon et al. [35] (Salomon [25]) | Raw material characterization, heat treatment | Pieces of coloring materials | None/pieces with use-wear traces not analyzed | Petrography | Invasive | Polished thin-sections |
| SEM-EDXS | Invasive | Grinding | ||||
| XRD | Invasive | Grinding | ||||
| FTIR | Invasive | Grinding | ||||
| TEM | Invasive | Grinding, suspension | ||||
| Salomon et al. [82] | Heat treatment | Pieces of ferruginous rocks | None | µ-XRD | Non-invasive/minimally invasive | None/micro-samples |
| SEM-FEG | Non-invasive/minimally invasive | None/micro-samples | ||||
| TEM-FEG | Minimally Invasive | Micro-samples? | ||||
| San Juan-Foucher [83]; Pomiès and Vignaud [84] | Raw materail selection, heat treatment | Pieces of coloring materials | None | XRD | Invasive | Grinding |
| TEM | Invasive | Grinding, suspension | ||||
| Scadding et al. [62] | Provenance (provenience), raw material selection | Ochre manuports | None | LA-ICP-MS | Minimally invasive | None |
| Tortosa et al. [48] | Provenance (provenience), changes over time | Ochre fragments | None | XRD | Invasive | Grinding |
| ICP-MS | Invasive | Grinding | ||||
| Petrography | Invasive | Polished thin sections | ||||
| SEM-EDXS | Invasive | Polished thin sections | ||||
| XRF | Invasive | Grinding | ||||
| Trabska et al. [65] | Provenance (provenience) | Ferruginous artifacts | None | PIXE | ? | ? |
| XRF | ? | ? | ||||
| Velliky et al. [85] (Velliky et al. [86]) | Provenance (geological origin), changes over time | Pigment pieces | None | NAA | Invasive | ? |
| XRD | Invasive | Sub-sampling, grinding | ||||
| SEM-EDXS | Non-invasive? | None? | ||||
| Wadley [87] | Taphonomic analysis, accidental heating | Ochre powders | None | XRD | Invasive | Grinding |
| XRF | Invasive | Grinding | ||||
| ICP-MS | Invasive | Grinding | ||||
| Micro-morphology | Invasive | Polished thin sections | ||||
| Zarzycka et al. [63] | Provenance (provenience), group mobility | Ochre nodules | None | ICP-OES | Invasive | Grinding, dissolution |
| Zilhao et al. [50] | Evidencing human exploitation | Pigment lumps and residues | None | Powder XRD | Invasive | Grinding? |
| SEM-EDXS | Minimally invasive | Micro-sampling | ||||
| Raman spectroscopy | Minimally invasive | Micro-sampling |
| Reference | Context | Methods | Mode | Main Goal | Arch. Samples | Geol. Samples | Data Treatment |
|---|---|---|---|---|---|---|---|
| Barham [2] (Young [66]) | Twin Rivers, Zambia | XRF, ICP-MS, SEM-EDXS | Invasive | Seriation, geol. origin | 7 | - | Qualitative |
| Hovers et al. [24] | Qafzey, Israël | Pétrography, XRD, ICP-AES | Invasive | Seriation, geol. origin | 71 | 7 | Qualitative |
| Mooney et al. [81] | Australia | Magnetic parameters | Non-invasive | Provenience | 2 | 8 | Qualitative |
| Kiehn et al. [116] | Botswana | NAA | Invasive | Source discrimination | - | 72 | Multivariate statistical analyses |
| Popelka-Filcoff et al. [56] | Missouri, USA | NAA | Invasive | Source discrimination | - | 69 | Multivariate statistical analyses |
| Popelka-Filcoff et al. [57] | Jiskairumoko, Perou | NAA | Invasive | Seriation | 65 | - | Multivariate statistical analyses |
| Trabska et al. [65] | Dzierżysław 35, Poland | PIXE, TXRF | ? | Geol. origin | 19 | 11 | Multivariate statistical analyses |
| Bernatchez [69] | Nelson Bay Cave, South Africa | XRD, PIXE | Invasive | Seriation | 54 | - | Qualitative |
| Popelka-Filcoff et al. [117] | Arizona, USA | NAA | Invasive | Source discrimination | - | 110 | Multivariate statistical analyses |
| Iriarte et al. [118] | Tito Bustillo and Monte Castillo, Spain | Petrography, XRD, SEM-EDS, ICP-MS | Invasive | Source discrimination | - | 24 et 24 | Qualitative |
| Salomon [25]; Salomon et al. [35] | Arcy-sur-Cure, France | Macroscopic examination, SEM-EDS, XRD, petrography | Minimally invasive | Seriation, geol. origin | 100 | - | Qualitative |
| d’Errico et al. [41]; Salomon et al. [72] | Es Skhul, Israël | XRD, SEM-EDS, PIXE | Minimally invasive | Geol. origin | 4 | - | Qualitative |
| Eiselt et al. [59] | Arizona, USA | NAA | Invasive | Provenience | 25 | 54 | Multivariate statistical analyses |
| MacDonald et al. [18] | Canada | NAA | Invasive | Seriation, geol. origin | 3 | 61 | Multivariate statistical analyses |
| Attard Montalto et al. [60] | Malta | Petrography, ICP-AES | Invasive | Provenience | 21 | 58 | Multivariate statistical analyses |
| Beck et al. [67] | Arcy-sur-Cure, France | PIXE | Non-invasive | Seriation, geol. origin | 27 | - | Qualitative |
| Popelka-Filcoff et al. [58] | Austalia | k0-NAA | Invasive | Source discrimination, comparison between methods | - | 100 | Multivariate statistical analyses |
| Kingery-Schwartz et al. [119] | North America, USA | XRD, pXRF, NAA | Non-invasive, invasive | Source discrimination | - | ? | Qualitative, multivariate statistical analyses |
| Mathis et al. [36] | Ormesson, France | PIXE | Non-invasive | Geol. origin | ? | 29 | Bivariate analysis |
| Zipkin et al. [38] | Northern Malawi | NAA, LA-ICP-MS | Invasive | Source discrimination, comparison between methods | - | 22 | Multivariate statistical analyses |
| Dayet et al. [17] | Diepkloof rock shelter, South Africa | XRD, ICP-OES, ICP-MS | Invasive | Geol. origin, provenience | 28 | 80 | Qualitative, multivariate statistical analyses |
| Pradeau et al. [37] | Pendimoun and Giribaldi, France | SEM-EDS, XRD | Invasive | Geol. origin | 56 | ? | Qualitative |
| Cavallo et al. [16] | Fumane cave and Tagliente Rockshelter Italia | Petrography, SEM-EDXS | Invasive | Geol. origin | ? | 66 | Qualitative |
| Cavallo et al. [70] | Fumane cave and Tagliente Rockshelter Italia | Powder XRD | Invasive | Geol. origin | ? | - | Semi-quantitative |
| Dimuccio et al. [120] | Grotta della Monica, Italia | pXRF, XRD, Raman, FTIR | Invasive | Source characterization | - | 81 | Qualitative, multivariate statistical analyses |
| Zipkin et al. [121] | Central Kenya | LA-ICP-MS | Invasive | Source discrimination | - | 43 | Multivariate statistical analyses |
| MacDonald et al. [19] | Haney Cook and Ball villages, Northern America | XRD, NAA | Invasive | Provenience | 23 | - | Qualitative, multivariate statistical analyses |
| Velliky et al. [86] | Southwestern Germany | NAA | Invasive | Source discrimination | - | 139 | Multivariate statistical analyses |
| Zarzycka et al. [63] | La Prele Mamoth, USA | ICP-OES | Invasive | Provenience | 7 | 24 | Multivariate statistical analyses |
| Peirce et al. [64] | Central Missouri, 4 archaelogical sites, USA | NAA | Invasive | Provenience | 38 | 69 | Multivariate statistical analyses |
| Velliky et al. [85] | Hohle Fels, Geißenklösterle and Vogelherd, Germany | NAA, XRD, SEM-EDS | Invasive | Provenience, geol. origin | 210 | 139 | Qualitative, multivariate statistical analyses |
| Zipkin et al. [39] | Central Kenya | LA-ICP-MS | Invasive | Source discrimination | - | 36 | Multivariate statistical analyses |
| Mauran et al. [122] | Leopard cave, Namibia | ICP-OES, ICP-MS | Invasive | Geol. origin | 41 | 94 | Multivariate statistical analyses |
| Reference | Context | Arch. Samples | Methods | Experimental Samples | Conditions of Heating Experiments |
|---|---|---|---|---|---|
| Onoratini and Perinet [134] | 13 Paleolithic sites from south-east France | 60 | XRPD | 11 nat. goethite | |
| Pomiès et al. [126,129] | 5 Paleolithic sites from France | 30 + 15 | XRPD, TEM | Syn. goethite, 1 nat. goethite | Oven in air 250 to 1000 °C |
| Baffier et al. [135] | Arcy-sur-Cure, France | 3 | XRPD, TEM | - | - |
| Pomiès et al. [136] | Lacaux, France | 4 | XRPD, TEM | - | - |
| Godfrey-Smith et Ilani [76] | Qafzeh Cave, Israël | 4 | TL | - | - |
| Pomiès and Vignaud [84]; San Juan-Foucher [83] | Bois-Ragot, France | 14 | XRPD, TEM | - | - |
| Lahaye [137] | La Honteyre, France | 4 | TL | - | - |
| Salomon [25] | Arcy-sur-Cure, France | 70 | µXRD, TEM | ? | ? |
| Salomon [25]; Salomon et al. [82] | Combe-Saunière 1, France | 13 | µXRD, SEM-FEG, TEM | ? | ? |
| Salomon [25]; Salomon et al. [82] | Les Maîtreaux, France | 24 | µXRD, SEM-FEG, TEM | ? | ? |
| Salomon [25]; d’Errico et al. [41]; Salomon et al. [72] | Es-Skhul, Israël | 4 | µ-XRD, TEM | ? | ? |
| Gialanella et al. [43] | Riparo Delmari, Italia | 6 | XRPD, TEM, Raman | 3 nat. goethite | Furnace in air 1000 °C |
| Salomon et al. [82] | Grotte Blanchard | 6 | µXRD, SEM-FEG, TEM | ? | ? |
| Dayet et al. [27] | Klasies river main site | 39 | XRD on surfaces | - | - |
| Cavallo et al. [32] | Fumane cave, Italia | - | XRPD, TEM | - | - |
| Cavallo et al. [32] | Tagliente rockshelter, Italia | - | XRPD, TEM | - | - |
| Source | Rock Type | Geol. Formation | GPS Coordinates | Nearest Town | Ref. |
|---|---|---|---|---|---|
| Shale 1 | Shale | Table Mountain | 32°23′13″ S 18°27′10″ E | Elands Bay | 14040 to 14055 |
| Shale 2 | Shale | Klipheuwel | 32°18′57″ S 18°21′18″ E | Elands Bay | 14691 |
| Shale 3 | Shale | Klipheuwel | 32°27′30″ S 18°30′55″ E | Redelinghuys | 14693 |
| Shale 4 | Shale | Klipheuwel | 32°29′32″ S 18°33′47″ E | Redelinghuys | 14694 |
| Shale 5 | Shale | Table Mountain | 32°34′23″ S 18°43′50″ E | Het Kruis | 14692 |
| Shale 6 | Phyllite | Malmesbury | 32°31′21″ S 18°37′56″ E | Redelinghuys | 14696 |
| Shale 7 | Phyllite | Malmesbury | 32°52′36″ S 18°46′02″ E | Piketberg | 14700 |
| Ferr 1 | Ferricrete | Tertiary/quaternary | 32°31′21″ S 18°37′56″ E | Redelinghuys | 14697 |
| Ferr 2 | Indurated shale | Tertiary/quaternary | 32°42′08″ S 18°49′46″ E | Eendekuil | 14698 |
| Ferricrete | Tertiary/quaternary | 32°42′08″ S 18°49′46″ E | Eendekuil | 14699 | |
| Ferr 3 | Ferricrete | Tertiary/quaternary | 32°52′25″ S 18°47′14″ E | Piketberg | 14701 |
| Ferr 4 | Ferricrete | Tertiary/quaternary | 32°07′16″ S 18°26′30″ E | Lamberts Bay | 14337 |
| Rock Type | Ref. | Mode | H | G | Q | I/M | K | Ch | Pa | Und. CM | PF | C | A |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Shale | 14042d | O. powder | + | - | +++ | ++ | ++ | nd | nd | nd | - | - | - |
| Surface | X | ? | X | X | X | nd | nd | nd | nd | / | nd | ||
| 14043a | O. powder | + | - | +++ | ++ | ++ | nd | nd | nd | - | - | - | |
| Surface | X | ? | X | X | X | nd | nd | nd | nd | X | nd | ||
| 14045a | O. powder | + | - | +++ | ++ | ++ | nd | nd | nd | - | - | - | |
| Surface | X | ? | X | X | X | nd | nd | nd | nd | nd | nd | ||
| 14048a | O. powder | ++ | - | +++ | ++ | ++ | nd | nd | nd | - | nd | - | |
| Surface | X | ? | X | X | X | nd | nd | nd | nd | nd | nd | ||
| 14050b | O. powder | ++ | ? | + | +++ | + | nd | nd | nd | - | nd | - | |
| Surface | X | nd | / | X | X | nd | nd | nd | nd | nd | |||
| 14052a | O. powder | ++ | ? | + | +++ | + | nd | nd | nd | - | nd | - | |
| Surface | X | nd | / | X | X | nd | nd | nd | nd | nd | nd | ||
| 14691b | O. powder | + | nd | +++ | ++ | + | nd | nd | ++ | - | - | ||
| Surface | X | nd | X | X | nd | nd | nd | nd | X | nd | nd | ||
| 14691g | O. powder | + | nd | +++ | ++ | + | nd | nd | + | - | - | ||
| Surface | X | nd | X | / | / | nd | / | ||||||
| 14691j | O. powder | + | nd | +++ | ++ | + | nd | nd | nd | + | - | - | |
| Surface | X | nd | X | X | X | nd | nd | nd | X | nd | nd | ||
| 14691k | O. powder | + | nd | +++ | ++ | + | nd | nd | nd | + | - | - | |
| Surface | X | nd | X | X | nd | nd | nd | nd | nd | nd | nd | ||
| 14693d | O. powder | + | nd | +++ | ++ | - | nd | nd | + | - | - | nd | |
| Surface | + | nd | +++ | + | nd | nd | nd | nd | nd | nd | nd | ||
| 14693e | O. powder | + | nd | +++ | ++ | - | nd | nd | + | - | nd | nd | |
| Surface | X | nd | X | X | nd | nd | nd | nd | nd | nd | nd | ||
| 14693g | O. powder | + | nd | +++ | ++ | - | nd | nd | + | - | nd | - | |
| Surface | - | nd | X | X | nd | nd | nd | nd | nd | nd | nd | ||
| 14693j | O. powder | + | nd | +++ | ++ | - | nd | nd | + | - | - | nd | |
| Surface | X | nd | X | X | nd | nd | nd | nd | nd | nd | nd | ||
| 14694e | O. powder | + | nd | +++ | ++ | ? | + | nd | nd | - | nd | nd | |
| Surface | X | nd | X | X | ? | ? | nd | nd | nd | nd | nd | ||
| 14692b | O. powder | + | nd | +++ | ++ | ++ | nd | nd | nd | nd | - | nd | |
| Surface | X | nd | X | X | X | nd | nd | nd | nd | nd | nd | ||
| Phyllite (shale group) | 14696c | O. powder | nd | nd | + | +++ | ++ | nd | nd | nd | - | nd | nd |
| Surface | nd | nd | X | X | X | nd | nd | nd | - | nd | nd | ||
| 14700b | O. powder | nd | nd | + | +++ | + | nd | ? | nd | - | nd | nd | |
| Surface | nd | nd | X | X | X | nd | ? | nd | nd | nd | nd | ||
| Indurated shale (ferricrete group) | 14698f | O. powder | + | ++ | +++ | ++ | + | nd | nd | nd | + | nd | nd |
| Surface | X | X | X | X | X | nd | nd | nd | X | nd | nd | ||
| 14698a | O. powder | +++ | nd | ? | + | ++ | nd | nd | nd | - | nd | nd | |
| (cortex) | Surface | X | nd | ? | nd | nd | nd | nd | nd | nd | nd | nd | |
| Ferricrete | 14697a | O. powder | nd | +++ | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| (cortex) | Surface | nd | X | nd | nd | nd | nd | nd | nd | nd | nd | nd | |
| 14697b | O. powder | nd | +++ | + | nd | nd | nd | nd | nd | nd | nd | nd | |
| (cortex) | Surface | nd | X | nd | nd | nd | nd | nd | nd | nd | nd | nd | |
| 14699a | O. powder | nd | +++ | - | nd | + | nd | nd | nd | nd | nd | nd | |
| (cortex) | Surface | nd | X | nd | nd | nd | nd | nd | nd | nd | nd | nd | |
| 14701a | O. powder | nd | +++ | ++ | - | nd | nd | nd | nd | nd | nd | nd | |
| (cortex) | Surface | nd | X | + | nd | nd | nd | nd | nd | nd | nd | nd | |
| 14701b | O. powder | nd | +++ | nd | - | nd | nd | nd | nd | nd | nd | nd | |
| (cortex) | Surface | nd | X | nd | nd | nd | nd | nd | nd | nd | nd | nd | |
| 14337a | O. powder | +++ | nd | + | nd | - | nd | nd | nd | nd | nd | nd | |
| (cortex) | Surface | X | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Rock Type | Ref. | Oriented Powder | Surface | Difference of Composition |
|---|---|---|---|---|
| Shale/sandstone | 13716 | Quartz, I/M, maghemite, hematite | Quartz, maghemite | Heat, sensitivity |
| Shale | 13681 | Quartz, I/M, kaolinite, hematite | Identical | - |
| Shale | 13711 | I/M, quartz, hematite, kaolinite | Identical | - |
| Shale | 13715 | Quartz, I/M, kaolinite, hematite | Identical | - |
| Phyllite | 13718 | I/M, hematite | Identical | - |
| Shale | 13723 | Hematite, I/M, pyrophyllite * | Identical * | - |
| Shale | 13752 | I/M, quartz, hematite, goethite, kaolinite, anatase | Quartz, I/M, hematite, goethite | Sensitivity |
| Shale | 13757 | I/M, kaolinite, quartz, hematite, anatase | Kaolinite, I/M, hematite | Sensitivity, patina? |
| Shale | 13758 | Quartz, kaolinite, I/M, hematite | Identical | - |
| Shale | 13761 | I/M, quartz, hematite, kaolinite | Identical | - |
| Shale | 13771 | I/M, quartz, hematite | I/M, hematite | Patina? |
| Shale | 13773 | Quartz, hematite, I/M, kaolinite | Identical | - |
| Shale | 13777 | Quartz, kaolinite, I/M, hematite | I/M, maghemite | Heat, sensitivity, patina? |
| Shale | 13783 | I/M, quartz, hematite, kaolinite | Identical | - |
| Shale | 13804 | Quartz, I/M, hematite, kaolinite | Identical | - |
| Ferr/shale | 13725 | Hematite | Hematite, quartz (maghemite?) | Cortex |
| Ferr/shale | 13727 | Maghemite, hematite, quartz | Identical | - |
| Ferr/shale | 13750 | Hematite, I/M, kaolinite | Hematite, quartz | Sensitivity, cortex |
| Ferricrete | 13712 | Hematite | Identical | - |
| Ferricrete | 13690 | Maghemite, hematite, quartz | Identical | - |
| Ferricrete | 13722 | Goethite, kaolinite, hematite, quartz | Hematite | Heat, sensitivity, cortex |
| Ferricrete | 13724 | Hematite, quartz | Maghemite, hematite, quartz | Heat |
| Ferricrete | 13741 | Hematite, quartz | Identical | - |
| Ferricrete | 13743 | Hematite, maghemite | Hematite | Heat |
| Ferricrete | 13749 | Hematite, maghemite | Identical | - |
| Ferricrete | 13762 | Hematite, quartz, kaolinite | Quartz, hematite | Sensitivity, cortex |
| Ferricrete | 13793 | Hematite, maghemite, quartz | Maghemite, hematite | Cortex |
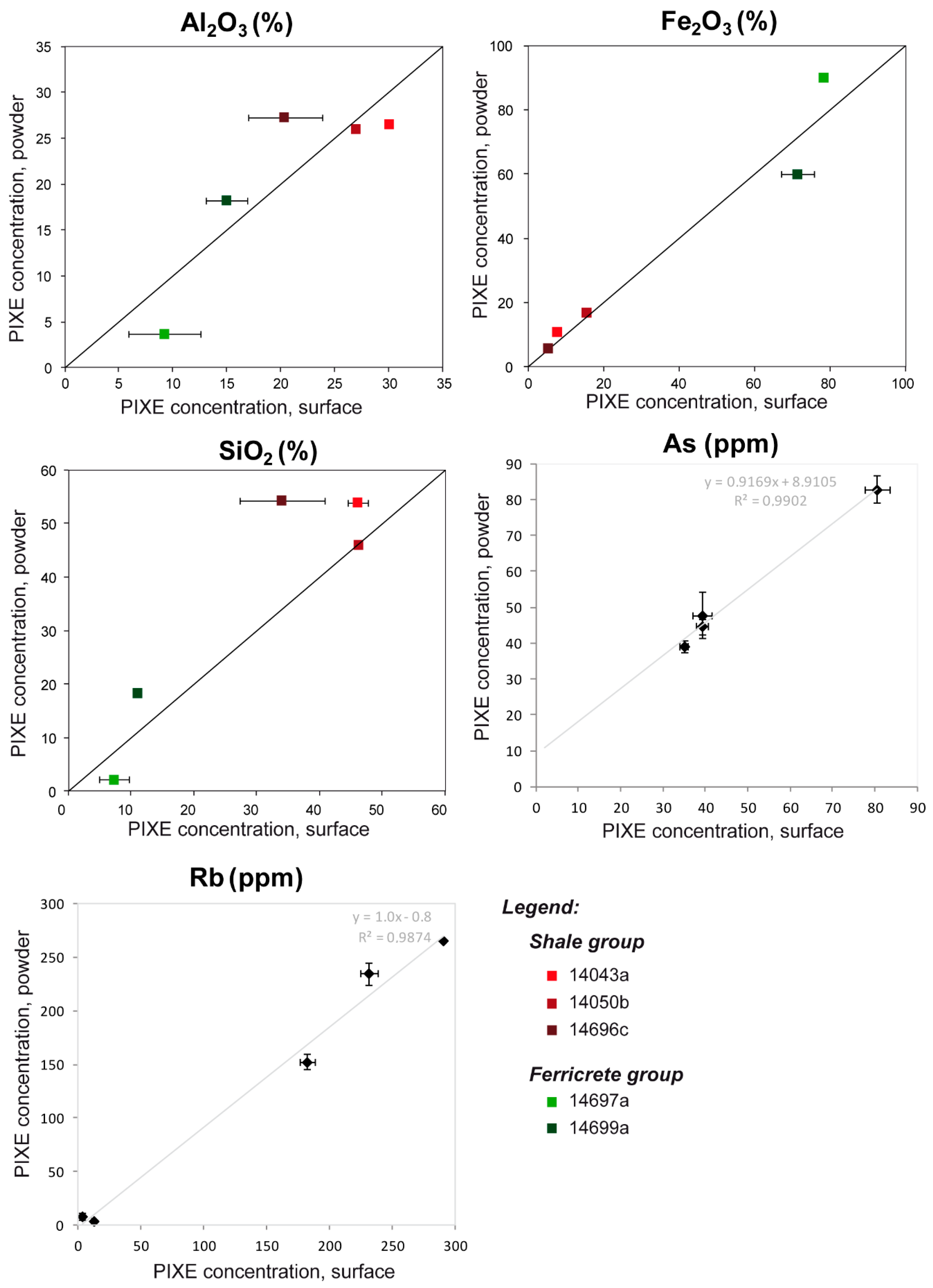
| Sample | Mode | Measurement | Na2O | MgO | Al2O3 | SiO2 | P2O5 | K2O | CaO | TiO2 | MnO | Fe2O3 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 14043a | Surface | AVERAGE | 0.5 | 0.7 | 24.2 | 56.5 | 0.7 | 5.1 | nd | 1.2 | nd | 11.0 | 100.0 |
| SD | 0.0 | 0.1 | 0.5 | 1.2 | 0.1 | 0.2 | - | 0.1 | - | 1.1 | - | ||
| Powder | AVERAGE | 0.5 | 0.9 | 25.0 | 55.9 | 0.4 | 5.0 | 0.1 | 1.1 | 0.1 | 11.0 | 100.0 | |
| SD | 0.1 | 0.0 | 0.2 | 0.6 | 0.1 | 0.0 | 0.0 | 0.1 | 0.1 | 0.3 | - | ||
| 14050b | Surface | AVERAGE | 0.3 | 1.0 | 25.2 | 47.9 | 0.7 | 6.4 | 0.1 | 1.4 | 0.1 | 16.9 | 100.0 |
| SD | 0.0 | 0.1 | 0.9 | 1.3 | 0.2 | 0.3 | 0.1 | 0.2 | 0.1 | 1.5 | - | ||
| Powder | AVERAGE | 0.8 | 1.2 | 26.2 | 46.5 | 0.5 | 6.3 | 0.3 | 1.3 | 0.1 | 16.7 | 100.0 | |
| SD | 0.0 | 0.0 | 0.1 | 0.1 | 0.1 | 0.0 | 0.0 | 0.1 | 0.0 | 0.1 | - | ||
| 14696c | Surface | AVERAGE | 7.2 | 2.5 | 24.0 | 52.4 | nd | 4.8 | nd | 1.1 | nd | 8.0 | 100.0 |
| SD | 0.8 | 0.1 | 1.9 | 1.0 | - | 0.4 | - | 0.1 | - | 0.4 | - | ||
| Powder | AVERAGE | 2.4 | 2.1 | 27.0 | 55.8 | nd | 5.7 | 0.1 | 1.0 | nd | 6.0 | 100.0 | |
| SD | 0.3 | 0.2 | 0.3 | 0.3 | - | 0.1 | 0.1 | 0.1 | - | 0.2 | - | ||
| 14697a | Surface | AVERAGE | 1.4 | 0.4 | 3.9 | 2.9 | 1.9 | 0.1 | 0.1 | 0.1 | nd | 89.3 | 100.0 |
| SD | 1.3 | 0.1 | 1.1 | 0.6 | 0.2 | 0.1 | 0.1 | 0.2 | - | 3.3 | - | ||
| Powder | AVERAGE | 0.4 | 0.5 | 4.0 | 2.5 | 2.5 | 0.1 | 0.2 | nd | nd | 89.7 | 100.0 | |
| SD | 0.0 | 0.1 | 0.4 | 0.1 | 0.1 | 0.0 | 0.0 | - | - | 0.4 | - | ||
| 14699a | Surface | AVERAGE | 0.1 | 0.1 | 8.2 | 6.3 | 3.9 | 0.2 | 0.1 | nd | nd | 81.0 | 100.0 |
| SD | 0.1 | 0.1 | 5.3 | 8.0 | 1.0 | 0.2 | 0.1 | - | - | 12.7 | - | ||
| Powder | AVERAGE | 0.2 | 0.2 | 17.7 | 17.7 | 3.3 | 0.4 | nd | 0.1 | nd | 60.4 | 100.0 | |
| SD | 0.0 | 0.0 | 0.2 | 0.2 | 0.1 | 0.0 | - | 0.1 | - | 0.4 | - |
| Sample | Mode | Maesurement | Na2O % | MgO % | Al2O3 % | SiO2 % | P2O5 % | SO3 % | Cl % | K2O % | CaO % | TiO2 % | MnO % | Fe2O3 % | Cr ppm | Ga ppm | As ppm | Rb ppm | Sr ppm | Y ppm | Zr ppm | Nb ppm | Ba ppm | Pb ppm | Th ppm |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 14043a | Surface | AVERAGE | 1.6 | 0.5 | 30.1 | 46.0 | 4.5 | 1.3 | 2.4 | 4.0 | 0.2 | 1.0 | 0.05 | 7.7 | 64 | 26 | 48 | 152 | 290 | 48 | 394 | 22 | 443 | 58 | 23 |
| SD | 0.3 | 0.0 | 0.3 | 1.6 | 0.6 | 0.1 | 0.1 | 0.1 | 0.0 | 0.2 | 0.00 | 1.1 | 5 | 4 | 6 | 26 | 39 | 7 | 28 | 8 | 215 | 13 | 3 | ||
| Powder | AVERAGE | 0.4 | 0.7 | 26.5 | 53.9 | 0.3 | 0.1 | 0.3 | 4.9 | 0.1 | 1.4 | 0.06 | 10.4 | 71 | 32 | 39 | 183 | 147 | 50 | 439 | 25 | 660 | 50 | 22 | |
| SD | 0.0 | 0.0 | 0.2 | 0.2 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.01 | 0.4 | 19 | 2 | 2 | 3 | 7 | 6 | 107 | 3 | 155 | 4 | 2 | ||
| 14050b | Surface | AVERAGE | 0.2 | 0.8 | 27.0 | 46.2 | 0.4 | 0.9 | 0.1 | 6.1 | 0.1 | 1.8 | 0.09 | 15.5 | 155 | 38 | 83 | 234 | 204 | 76 | 351 | 33 | 669 | 68 | 32 |
| SD | 0.0 | 0.0 | 0.3 | 0.5 | 0.0 | 0.3 | 0.0 | 0.2 | 0.0 | 0.1 | 0.01 | 0.2 | 6 | 0 | 4 | 9 | 13 | 11 | 36 | 3 | 106 | 2 | 2 | ||
| Powder | AVERAGE | 0.5 | 0.9 | 26.0 | 46.0 | 0.3 | 0.3 | 0.5 | 6.0 | 0.3 | 1.7 | 0.09 | 16.5 | 130 | 37 | 81 | 232 | 201 | 74 | 334 | 34 | 608 | 67 | 27 | |
| SD | 0.1 | 0.0 | 0.1 | 0.3 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.00 | 0.2 | 32 | 2 | 3 | 3 | 3 | 7 | 75 | 4 | 246 | 5 | 4 | ||
| 14696c | Surface | AVERAGE | 13.5 | 2.4 | 20.3 | 33.8 | 0.2 | 3.2 | 18.1 | 4.1 | 0.1 | 1.0 | nd | 4.7 | 67 | 24 | nd | 265 | 75 | 31 | 327 | 19 | 592 | 9 | 30 |
| SD | 6.7 | 0.5 | 3.4 | 6.7 | 0.0 | 0.0 | 6.2 | 0.5 | 0.0 | 0.2 | - | 0.2 | 33 | 1 | - | 15 | 9 | 1 | 301 | 4 | 118 | 3 | 7 | ||
| Powder | AVERAGE | 1.9 | 1.5 | 27.3 | 54.3 | 0.0 | 0.2 | 3.1 | 5.3 | 0.1 | 1.0 | 0.01 | 4.8 | 109 | 27 | 2 | 291 | 70 | 37 | 204 | 17 | 599 | 9 | 25 | |
| SD | 0.1 | 0.1 | 0.2 | 0.1 | 0.0 | 0.0 | 0.1 | 0.2 | 0.0 | 0.0 | 0.00 | 0.2 | 53 | 1 | - | - | 7 | 13 | 60 | 2 | 174 | 1 | 6 | ||
| 14697a | Surface | AVERAGE | 0.8 | 0.5 | 9.2 | 7.2 | 1.6 | 0.7 | 0.4 | 0.3 | 0.1 | 0.1 | nd | 78.3 | nd | 5 | 39 | 8 | 378 | 4 | 23 | 2 | 3068 | 12 | 11 |
| SD | 0.1 | 0.1 | 1.9 | 2.2 | 0.2 | 0.1 | 0.1 | 0.1 | 0.0 | 0.0 | - | 4.3 | - | 1 | 2 | 3 | 8 | 3 | 6 | 0 | 456 | 1 | 4 | ||
| Powder | AVERAGE | 0.3 | 0.3 | 3.7 | 2.2 | 1.8 | 0.5 | 0.5 | 0.1 | 0.2 | nd | nd | 90.1 | 59 | 5 | 35 | 4 | 55 | 5 | 18 | 3 | 441 | nd | 8 | |
| SD | 0.0 | 0.0 | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | - | - | 0.6 | 37 | - | 1 | 2 | 4 | 2 | 1 | 1 | 140 | - | 1 | ||
| 14699a | Surface | AVERAGE | 0.2 | 0.3 | 15.0 | 11.0 | 1.4 | 0.2 | 0.0 | 0.2 | 0.1 | 0.2 | nd | 71.4 | 117 | 15 | 45 | 4 | 13 | 9 | 32 | 2 | 86 | 30 | 6 |
| SD | 0.0 | 0.0 | 0.1 | 0.4 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | - | 0.4 | 47 | 1 | 2 | 1 | 1 | 1 | 3 | 40 | 1 | - | |||
| Powder | AVERAGE | 0.1 | 0.2 | 18.2 | 18.3 | 2.4 | 0.1 | 0.0 | 0.4 | 0.0 | 0.1 | nd | 59.9 | 98 | 5 | 39 | 13 | 8 | 6 | 16 | 2 | 79 | 25 | nd | |
| SD | 0.0 | 0.0 | 0.2 | 0.3 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | - | 0.5 | 31 | 1 | 2 | 2 | 2 | 2 | 4 | - | - | 4 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dayet, L. Invasive and Non-Invasive Analyses of Ochre and Iron-Based Pigment Raw Materials: A Methodological Perspective. Minerals 2021, 11, 210. https://doi.org/10.3390/min11020210
Dayet L. Invasive and Non-Invasive Analyses of Ochre and Iron-Based Pigment Raw Materials: A Methodological Perspective. Minerals. 2021; 11(2):210. https://doi.org/10.3390/min11020210
Chicago/Turabian StyleDayet, Laure. 2021. "Invasive and Non-Invasive Analyses of Ochre and Iron-Based Pigment Raw Materials: A Methodological Perspective" Minerals 11, no. 2: 210. https://doi.org/10.3390/min11020210






