Allanite Geochemical Response to Hydrothermal Alteration by Alkaline, Low-Temperature Fluids
Abstract
:1. Introduction
2. Geological Setting and Research Material
3. Analytical Methods
3.1. Electron Probe Microanalysis
3.2. LA-ICP-MS Analysis of Trace Elements
4. Results
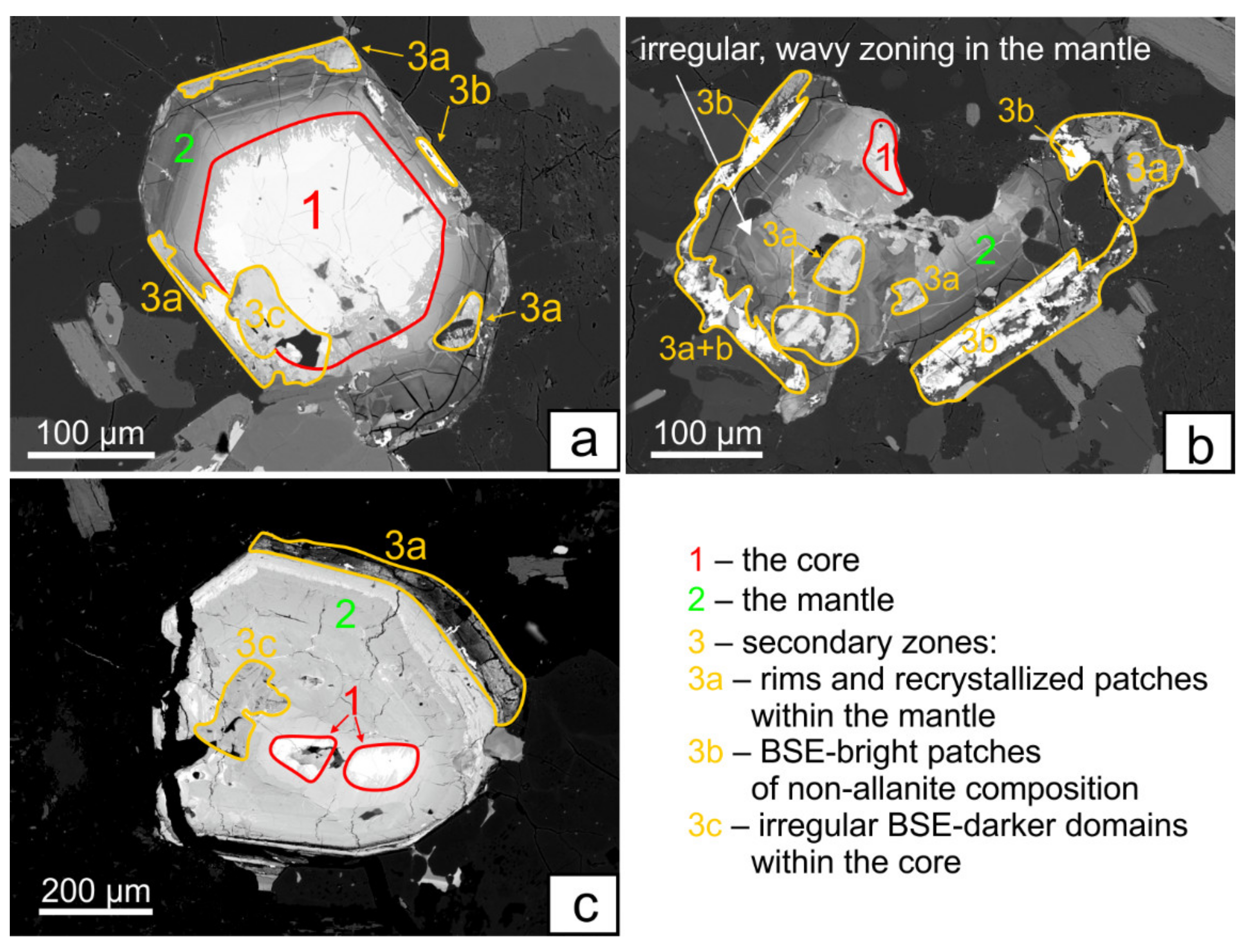
4.1. Textural Characterization
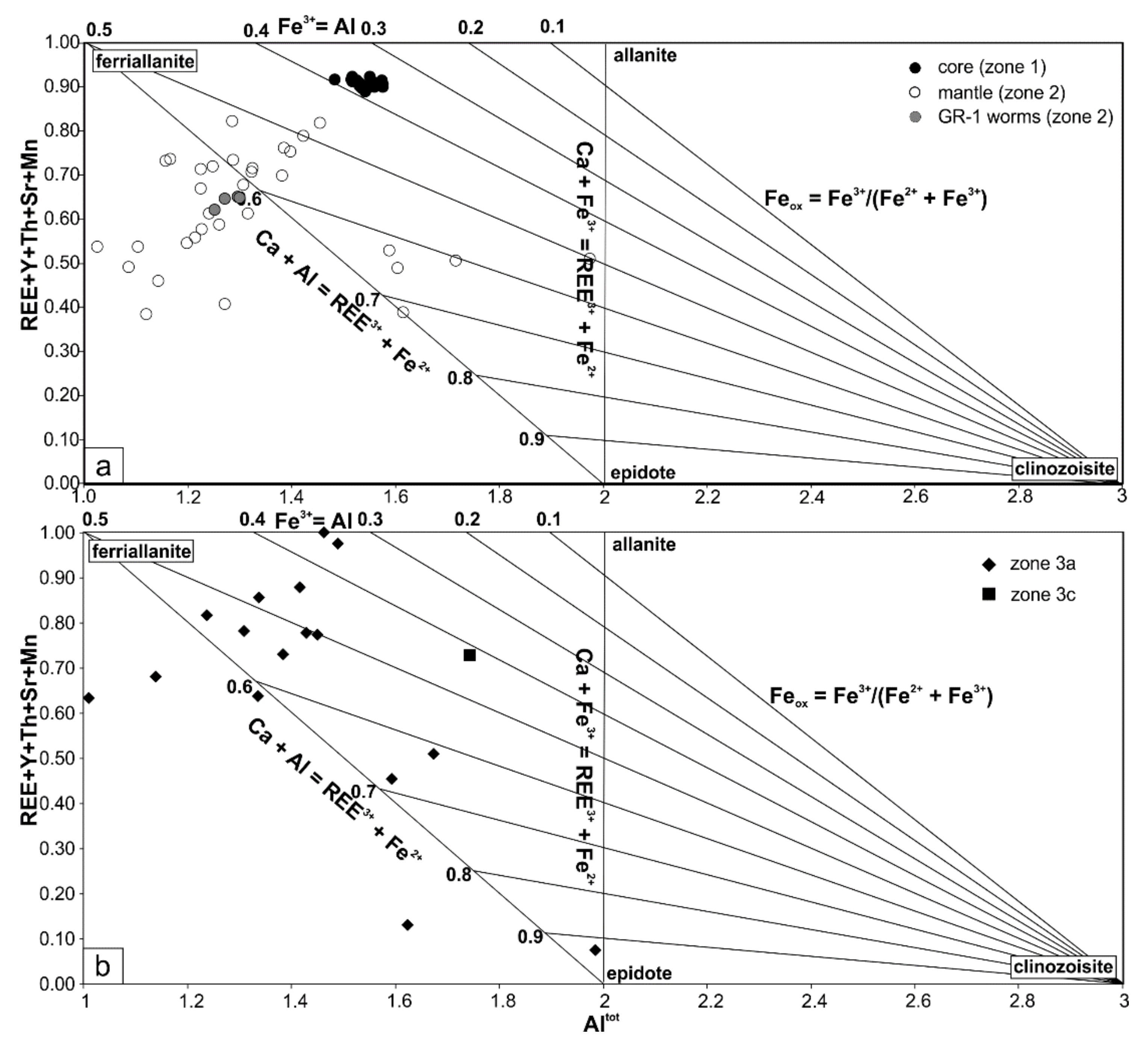
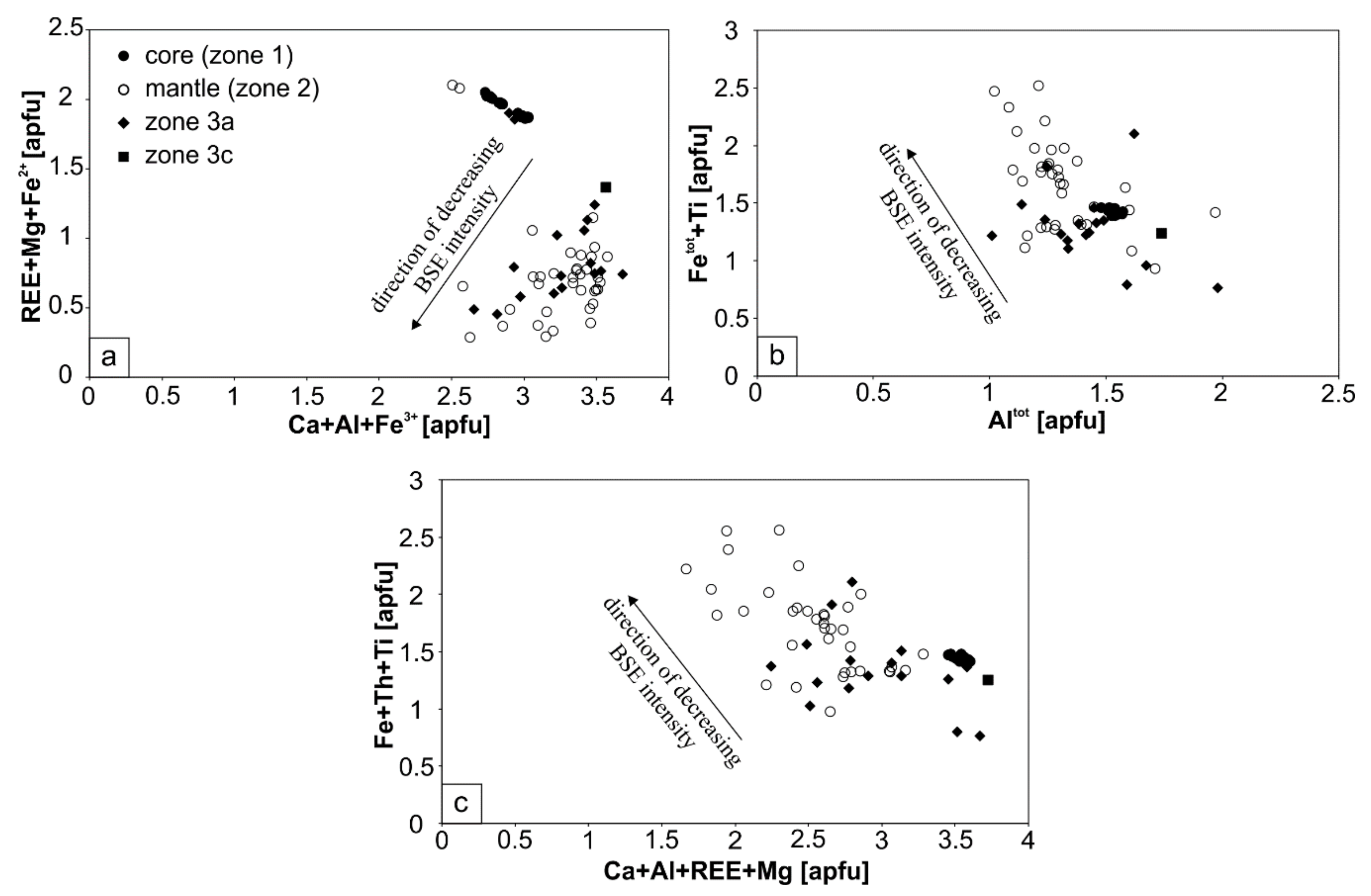
4.2. Major and Trace Element Composition
4.1.1. The Core—Zone 1
4.1.2. The Mantle—Zone 2
4.1.3. Secondary—Zones 3
5. Discussion
5.1. Crystallization Conditions of the Allanite Core
5.2. Late-Stage Infiltration by Fluids
5.2.1. First-Stage Alteration—Origin of the Mantles (Zone 2)
5.2.2. Second-Stage Interaction with Fluids—Formation of Zones 3
5.3. Composition of the Hydrothermal Fluids
5.4. Influence of Metamictization
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ward, C.D.; McArthur, J.M.; Walsh, J.N. Rare earth element behavior during evolution and alteration of the Dartmoor granite, SW England. J. Pet. 1992, 33, 785–815. [Google Scholar] [CrossRef]
- Petrík, I.; Broska, I.; Lipka, J.; Siman, P. Granitoid Allanite-(Ce) Substitution Relations, Redox Conditions and REE Distributions (on an Example of I-Type Granitoids, Western Carpathians, Slovakia). Geol. Carpathian 1995, 46, 79–94. [Google Scholar]
- Bea, F. Residence of REE, Y, Th and U in granites and crustal protoliths; implications for the chemistry of crustal melts. J. Pet. 1996, 37, 521–552. [Google Scholar] [CrossRef]
- Jiang, N.; Sun, S.; Chu, X.; Mizuta, T.; Ishiyama, D. Mobilization and enrichment of high-field strength elements during late- and post-magmatic processes in the Shuiquangou syenitic complex, Northern China. Chem. Geol. 2003, 200, 117–128. [Google Scholar] [CrossRef]
- Vlach, S.R.F.; Gualda, G.A.R. Allanite and chevkinite in A-type granites and syenites of the Graciosa Province, southern Brazil. Lithos 2007, 97, 98–121. [Google Scholar] [CrossRef]
- Gieré, R.; Sorensen, S.S. Allanite and other REE-rich epidote group minerals. Rev. Mineral. Geochem. 2004, 56, 431–493. [Google Scholar] [CrossRef]
- Wood, S.A.; Ricketts, A. Allanite–(Ce) from the Eocene Casto granite, Idaho: Response to hydrothermal alteration. Can. Mineral. 2000, 38, 81–100. [Google Scholar] [CrossRef]
- Chabiron, A.; Cuney, M. Altération de l’allanite dans les granites sous la caldeira de Streltsovka (Transbaïkalie, Russie). Une source possible d’uranium pour les gisements. Earth Planet. Sci. 2001, 332, 99–105. [Google Scholar]
- Poitrasson, F. In situ investigations of allanite hydrothermal alteration: Examples from calc-alkaline and anorogenic granites of Corsica (southeast France). Contrib. Mineral. Pet. 2002, 142, 485–500. [Google Scholar] [CrossRef]
- Meintzer, R.E.; Mitchell, R.S. The epigene alteration of allanite. Can. Mineral. 1988, 26, 945–955. [Google Scholar]
- Anenburg, M.; Katzir, Y.; Rhede, D.; Jöns, N.; Bach, W. Rare earth element evolution and migration in plagiogranites: A record preserved in epidote and allanite of the Troodos ophiolite. Contrib. Mineral. Pet. 2015, 169, 25. [Google Scholar] [CrossRef]
- Anenburg, M.; Mavrogenes, J.A.; Bennett, V.C. The fluorapatite P-REE-Th vein deposit at Nolans Bore: Genesis by carbonatite metasomatism. J. Pet. 2020. [Google Scholar] [CrossRef]
- Morin, J.A. Allanite in granitic rocks of the Kenora-Vermilion Bay area, Northwestern Ontario. Can. Mineral. 1977, 15, 297–302. [Google Scholar]
- Słaby, E.; Martin, H. Mafic and Felsic Magma Interaction in Granites: The Hercynian Karkonosze Pluton (Sudetes, Bohemian Massif). J. Pet. 2008, 49, 353–391. [Google Scholar] [CrossRef] [Green Version]
- Frost, B.R.; Barnes, C.G.; Collins, W.J.; Arculus, R.J.; Ellis, D.J.; Frost, C.D. A Geochemical Classification for Granitic Rocks. J. Pet. 2001, 42, 2033–2048. [Google Scholar] [CrossRef]
- Whalen, J.B.; Currie, K.L.; Chappell, B.W. A-type granites: Geochemical characteristics, discrimination and petrogenesis. Contrib. Mineral. Pet. 1987, 95, 407–419. [Google Scholar] [CrossRef]
- Słaby, E.; Michalik, M.; Mierzejewski, M. Zr-Y-HREE enriched granitic vein rock from Karkonosze (SW Poland)—Record of early fractionation of crustal melt? Pol. Mineral. Soc. Spec. Pap. 2005, 25, 204–207. [Google Scholar]
- Słaby, E. CHARAC- non-CHARAC behaviour of Y/Ho and Zr/ Hf in Karkonosze hybridic and granitic melts. Pol. Mineral. Soc. Spec. Pap. 2005, 25, 200–203. [Google Scholar]
- Kusiak, M.A.; Dunkley, D.J.; Słaby, E.; Martin, H.; Budzyń, B. Sensitive high-resolution ion microprobe analysis of zircon reequilibrated by late magmatic fluids in a hybridized pluton. Geology 2009, 37, 1063–1066. [Google Scholar] [CrossRef]
- Mazur, S.; Aleksandrowski, P. The Tepla(?)/Saxothuringian suture in the Karkonosz–Izera Massif, western Sudetes, Central European Variscides. Int. J. Earth Sci. 2001, 90, 341–360. [Google Scholar] [CrossRef]
- Žák, J.; Verner, K.; Sláma, J.; Kachlík, V.; Chlupáčová, M. Multistagemagma emplacement and progressive strain accumulation in the Krkonoše–Jizera plutonic complex, Bohemian Massif. Tectonics 2013, 32, 1493–1512. [Google Scholar] [CrossRef]
- Kryza, R.; Pin, C.; Oberc-Dziedzic, T.; Crowley, Q.G.; Larionov, A. Deciphering the geochronology of a large granitoid pluton (Karkonosze Granite, SW Poland): An assessment of U–Pb zircon SIMS and Rb–Sr whole-rock dates relative to U–Pb zircon CA-ID-TIMS. Int. Geol. Rev. 2014, 56, 756–782. [Google Scholar] [CrossRef]
- Barbarin, B. A review of the relationships between granitoid types, their origins and their geodynamic environments. Lithos 1999, 46, 605–626. [Google Scholar] [CrossRef]
- White, A.J.R.; Chappell, B.W. Granitoid types and their distribution in the Lachlan Fold Belt, southeastern Australia. In Circum-Pacific Plutonic Terranes; Roddick, J.A., Ed.; Geological Society of America: Memoir, CO, USA, 1983; Volume 159, pp. 21–34. [Google Scholar]
- Clarke, D.B. Granitoid Rocks; Chapman & Hall: London, UK, 1992. [Google Scholar]
- Chappell, B.W.; White, A.J.R. Two contrasting granite types: 25 years later. Aust. J. Earth Sci. 2001, 48, 489–499. [Google Scholar] [CrossRef]
- Słaby, E.; Galbarczyk-Gąsiorowska, L.; Baszkiewicz, A. Mantled alkali-feldspar megacrysts from the marginal part of the Karkonosze granitoid massif (SW Poland). Acta Geol. Pol. 2002, 52, 501–519. [Google Scholar]
- Słaby, E.; Götze, J. Feldspar crystallization under magma mixing conditions shown by cathodoluminescence and geochemical modelling: Case study from the Karkonosze pluton (SW Poland). Mineral. Mag. 2004, 68, 541–557. [Google Scholar] [CrossRef]
- Słaby, E.; Galbarczyk-Gąsiorowska, L.; Seltmann, R.; Müller, A. Alkali feldspar megacryst growth: Geochemical modelling. Mineral. Pet. 2007, 68, 1–29. [Google Scholar] [CrossRef]
- Słaby, E.; Seltmann, R.; Kober, B.; Müller, A.; Galbarczyk-Gąsiorowska, L.; Jeffries, T. LREE distribution patterns in zoned alkali feldspar megacrysts—Implication for parental melt composition. Mineral. Mag. 2007, 71, 193–217. [Google Scholar] [CrossRef]
- Lisowiec, K.; Słaby, E.; Förster, H.J. Polytopic vector analysis (PVA) modelling of whole-rock and apatite chemistry from the Karkonosze composite pluton (Poland, Czech Republic). Lithos 2015, 230, 105–120. [Google Scholar] [CrossRef] [Green Version]
- Słaby, E.; Martin, H. Mechanisms of differentiation of the Karkonosze granite. Pol. Mineral. Soc. Spec. Pap. 2005, 26, 264–267. [Google Scholar]
- Słaby, E.; Götze, J.; Wörner, G.; Simon, K.; Wrzalik, R.; Śmigielski, M. K-feldspar phenocrysts in microgranular magmatic enclaves: A cathodoluminescence and geochemical study of crystal growth as a marker of magma mingling dynamics. Lithos 2008, 105, 85–97. [Google Scholar] [CrossRef]
- Gros, K.; Słaby, E.; Birski, Ł.; Kozub-Budzyń, G.; Slama, J. Titanite major and trace element composition following the evolution path of a composite pluton. Mineral. Petrol. (under review).
- Stormer, J.C.J.; Pierson, M.L.; Tacker, R.C. Variation of F and Cl X-ray intensity due to anisotropic diffusion in apatite during electron microprobe analysis. Am. Mineral. 1993, 78, 641–648. [Google Scholar]
- Goldoff, B.; Webster, J.D.; Harlov, D.E. Characterization of fluor-chlorapatites by electron probe microanalysis with a focus on time-dependent intensity variation of halogens. Am. Mineral. 2012, 97, 1103–1115. [Google Scholar] [CrossRef]
- Stock, M.J.; Humphreys, M.C.S.; Smith, V.C.; Johnson, R.D.; Pyle, D.M. EIMF New constraints on electron-beam induced halogen migration in apatite. Am. Mineral. 2015, 100, 281–293. [Google Scholar] [CrossRef] [Green Version]
- Yavuz, F.; Yıldırım, D.K. A Windows program for calculation and classification of epidote–supergroup minerals. Period. Mineral. 2018, 87, 269–285. [Google Scholar]
- Armbruster, T.; Bonazzi, P.; Akasaka, M.; Bermanec, V.; Chopin, C.; Giere, R.; Heuss-Assbichler, S.; Liebscher, A.; Menchetti, S.; Pan, Y.; et al. Recommended nomenclature of epidote-group minerals. Eur. J. Mineral. 2006, 18, 551–567. [Google Scholar] [CrossRef] [Green Version]
- Tunheng, A.; Hirata, T. Development of signal smoothing device for precise elemental analysis using laser ablation-ICP-mass spectrometry. J. Anal. Atom. Spectrom. 2004, 19, 932–934. [Google Scholar] [CrossRef]
- Van Achterbergh, E.; Ryan, C.G.; Jackson, S.E.; Griffin, W.L. Data reduction software for LA-ICP-MS. In Laser Ablation-ICPMS in the Earth Sciences: Principles and Applications; Sylvester, P.J., Ed.; Mineralogical Association of Canada: Québec, QC, Canada, 2001; pp. 239–243. [Google Scholar]
- Sun, S.S.; McDonough, W.F. Chemical and isotopic systematics of oceanic basalts: Implications for mantle composition and processes. In Magmatism in the Ocean Basins; Special Publications; Saunders, A.D., Norry, M.J., Eds.; Geological Society: London, UK, 1989; Volume 42, pp. 313–345. [Google Scholar]
- Broska, I.; Petrík, I.; Williams, T.C. Coexisting monazite and allanite in peraluminous granitoids of the Tribeè Mountains, Western Carpathians. Am. Mineral. 2000, 85, 22–32. [Google Scholar] [CrossRef]
- Vazquez, J.A.; Manning, C.E.; Reid, M.R. Experimental Determination of Allanite Stability in High-Silica Rhyolite; Abstract V41C-1407; American Geophysical Union: Washington, DC, USA, 2004. [Google Scholar]
- Hermann, J. Allanite: Thorium and light rare earth element carrier in subducted crust. Chem. Geol. 2002, 192, 289–306. [Google Scholar] [CrossRef]
- Chesner, C.A.; Ettlinger, A.D. Composition of volcanic allanite from the Toba Tuffs, Sumatra, Indonésia. Am. Mineral. 1989, 74, 750–758. [Google Scholar]
- Anenburg, M.; Mavrogenes, J.A. Carbonatitic versus hydrothermal origin for fluorapatite REE-Th deposits: Experimental study of REE transport and crustal “antiskarn” metasomatism. Am. J. Sci. 2018, 318, 335–366. [Google Scholar] [CrossRef]
- Spruzeniece, L.; Piazolo, S.; Maynard-Casely, H. Deformation-resembling microstructure created by fluid-mediated dissolution–precipitation reactions. Nat. Commun. 2017, 8, 14032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Agudo, E.; Putnis, C.V.; Putnis, A. Coupled dissolution and precipitation at mineral–fluid interfaces. Chem. Geol. 2014, 383, 132–146. [Google Scholar] [CrossRef]
- Allegre, C.J.; Provost, A.; Jaupart, C. Oscillatory zoning: A pathological case of crystal growth. Nature 1981, 294, 223–228. [Google Scholar] [CrossRef]
- Brugger, J.; Lahaye, Y.; Costa, S.; Lambert, D.; Bateman, R. Inhomogeneous distribution of REE in scheelite and dynamics of Archaean hydrothermal systems (Mt. Charlotte and Drysdale gold deposits, Western Australia). Contrib. Mineral. Pet. 2000, 139, 251–264. [Google Scholar] [CrossRef]
- Shore, M.; Fowler, A.D. Oscillatory zoning in minerals; a common phenomenon. Can. Mineral. 1996, 34, 1111–1126. [Google Scholar]
- Barker, S.L.; Cox, S.F. Oscillatory zoning and trace element incorporation in hydrothermal minerals: Insights from calcite growth experiments. Geofluids 2011, 11, 48–56. [Google Scholar] [CrossRef]
- Mitropoulos, P. Primary allanite in andesitic rocks from the Poros Volcano, Greece. Mineral. Mag. 1987, 51, 601–604. [Google Scholar] [CrossRef]
- Pe-Piper, G.; Piper, D.J.W.; Papoutsa, A. Mid Carboniferous lamprophyres, Cobequid Fault Zone, eastern Canada, linked to sodic granites, voluminous gabbro, and albitization. Lithos 2018, 296–299, 316–331. [Google Scholar] [CrossRef]
- Smith, M.P.; Henderson, P.; Jeffries, T. The formation and alteration of allanite in skarn from the Beinn an Dubhaich granite aureole, Skye. Eur. J. Mineral. 2002, 14, 471–486. [Google Scholar] [CrossRef]
- Allen, D.E.; Seyfried, W.E., Jr. REE controls in ultramafic hosted MOR hydrothermal systems: An experimental study at elevated temperature and pressure. Geochim. Cosmochim. Acta 2005, 69, 675–683. [Google Scholar] [CrossRef]
- Migdisov, A.A.; Williams-Jones, A.E.; Wagner, T. An experimental study of the solubility and speciation of the Rare Earth Elements (III) in fluoride- and chloride-bearing aqueous solutions at temperatures up to 300C. Geochim. Cosmochim. Acta 2009, 73, 7087–7109. [Google Scholar] [CrossRef]
- Buda, G.; Nagy, G. Some REE-bearing accessory minerals in two rock types of Variscan granitoids, Hungary. Geol. Carpath. 1995, 46, 67–78. [Google Scholar]
- Anenburg, M.; Burnham, A.D.; Mavrogenes, J.A. REE redistribution textures in altered fluorapatite: Symplectites, veins and phosphate-silicate-carbonate assemblages from the Nolans Bore P–REE–Th deposit, NT, Australia. Can. Mineral. 2018, 56, 331–354. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Bühn, B.; Rankin, A.H. Composition of natural, volatile-rich Na–Ca–REE–Sr carbonatitic fluids trapped in fluid inclusions. Geochim. Cosmochim. Acta 1999, 63, 3781–3797. [Google Scholar] [CrossRef]
- Loges, A.; Migdisov, A.A.; Wagner, T.; Williams-Jones, A.E.; Markl, G. An experimental study of the aqueous solubility and speciation of Y(III) fluoride at temperatures up to 250C. Geochim. Cosmochim. Acta 2013, 123, 403–415. [Google Scholar] [CrossRef]
- Williams-Jones, A.E.; Migdisov, A.A.; Samson, I.M. Hydrothermal mobilization of the rare earth elements—A tale of “Ceria” and “Yttria”. Elements 2012, 8, 355–360. [Google Scholar] [CrossRef]
- Nisbet, H.; Migdisov, A.A.; Williams-Jones, A.E.; Xu, H.; van Hinsberg, V.J.; Roback, R. Challenging the thorium-immobility paradigm. Sci. Rep.-UK 2019, 9, 17035. [Google Scholar] [CrossRef]
- Keppler, H.; Wyllie, P.J. Partitioning of Cu, Sn, Mo, W, U, and Th between melt and aqueous fluid in the systems haplogranite-H2O−HCl and haplogranite-H2O−HF. Contr. Mineral. Pet. 1991, 109, 139–150. [Google Scholar] [CrossRef]
- Mochnacka, K.; Banaś, M. Occurrence and genetic relationships of uranium and thorium mineralization in the Karkonosze Izera Block (the Sudety Mts, SW Poland). Ann. Soc. Geol. Pol. 2000, 70, 137–150. [Google Scholar]
- Nisbet, H.; Migdisov, A.; Xu, H.; Guo, X.; van Hinsberg, V.; Williams-Jones, A.E.; Boukhalfa, H.; Roback, R. An experimental study of the solubility and speciation of thorium in chloride-bearing aqueous solutions at temperatures up to 250 °C. Geochim. Cosmochim. Acta 2018, 239, 363–373. [Google Scholar] [CrossRef]
- Oberli, F.; Meier, M.; Berger, A.; Rosenberg, C.L.; Gieré, R. U–Th–Pb and 230Th/238U disequilibrium isotope systematics: Precise accessory mineral chronology and melt evolution tracing in the Alpine Bergell intrusion. Geochim. Cosmochim. Acta 2004, 68, 2543–2560. [Google Scholar] [CrossRef]
- Giuli, G.; Alonso-Mori, R.; Cicconi, M.R.; Paris, E.; Glatzel, P.; Eeckhout, S.G.; Scaillet, B. Effect of alkalis on the Fe oxidation state and local environment in peralkaline rhyolitic glasses. Am. Mineral. 2012, 97, 468–475. [Google Scholar] [CrossRef] [Green Version]
- Anenburg, M.; O’Neill, H.S.C. Redox in Magmas: Comment on a Recent Treatment of the Kaiserstuhl Volcanics (Braunger et al., Journal of Petrology, 59, 1731–1762, 2018) and Some Other Misconceptions. J. Pet. 2019, 60, 1825–1832. [Google Scholar] [CrossRef]
- Volovetskii, M.V.; Lukanin, O.A.; Rusakov, V.S.; Kargal’tsev, A.A. Influence of oxygen fugacity and temperature on the redox state of iron in natural silicic aluminosilicate melts. Geochem. Int. 2012, 50, 330–343. [Google Scholar] [CrossRef]
- Uher, P.; Ondrejka, M.; Bačík, P.; Broska, I.; Konečný, P. Britholite, monazite, REE carbonates, and calcite: Products of hydrothermal alteration of allanite and apatite in A-type granite from Stupné, Western Carpathians, Slovakia. Lithos 2015, 236–237, 212–225. [Google Scholar] [CrossRef]
- Berger, A.; Gnos, E.; Janots, E.; Fernandez, A.; Giese, J. Formation and composition of rhabdophane, bastnäsite and hydrated thorium minerals during alteration: Implications for geochronology and low-temperature processes. Chem. Geol. 2008, 254, 238–248. [Google Scholar] [CrossRef]
- Migdisov, A.; Williams-Jones, A.E.; Brugger, J.; Caporuscio, F.A. Hydrothermal transport, deposition, and fractionation of the REE: Experimental data and thermodynamic calculations. Chem. Geol. 2016, 439, 13–42. [Google Scholar] [CrossRef] [Green Version]
- Migdisov, A.A.; Williams-Jones, A.E. Hydrothermal transport and deposition of the rare earth elements by fluorine-bearing aqueous liquids. Miner. Depos. 2014, 49, 987–997. [Google Scholar] [CrossRef]
- Migdisov, A.; Guo, X.; Nisbet, H.; Xu, H.; Williams-Jones, A.E. Fractionation of REE, U, and Th in natural ore-forming hydrothermal systems: Thermodynamic modelling. J. Chem. Thermodyn. 2019, 128, 305–319. [Google Scholar] [CrossRef] [Green Version]
- Kozłowski, A.; Marcinowska, A. Hydrothermal activity in the Karkonosze, Strzegom and Strzelin massifs—A fluid inclusion study. In Granitoids in Poland; Archivum Mineralogiae Monograph, no. 1; Kozłowski, A., Wiszniewska, J., Eds.; Faculty of Geology of The Warsaw University: Warsaw, Poland, 2007; pp. 243–252. [Google Scholar]
- Budzyń, B.; Harlov, D.E.; Williams, M.L.; Jercinovic, M.J. Experimental determination of stability relations between monazite, fluorapatite, allanite, and REE-epidote as a function of pressure, temperature, and fluid composition. Am. Mineral. 2011, 96, 1547–1567. [Google Scholar] [CrossRef]
- Krenn, E.; Harlov, D.E.; Finger, F.; Wunder, B. LREE-redistribution among fluorapatite, monazite, and allanite at high pressures and temperatures. Am. Mineral. 2012, 97, 1881–1890. [Google Scholar] [CrossRef]
- Wilamowski, A. Chloritization and polytypism of biotite in the Lomnica granite, Karkonosze Massif, Sudetes, Poland: Stable isotope evidence. Chem. Geol. 2002, 182, 529–547. [Google Scholar] [CrossRef]
- Mochnacka, K.; Oberc-Dziedzic, T.; Mayer, W.; Pieczka, A. Ore mineralization related to geological evolution of the Karkonosze- Izera Massif (the Sudetes, Poland)—Towards a model. Ore Geol. Rev. 2004, 64, 215–238. [Google Scholar] [CrossRef]
- Kozłowski, A. Pneumatolitic and hydrothermal activity in the Karkonosze Block. Acta Geol. Pol. 1978, 28, 171–222. [Google Scholar]
- Mikulski, S.Z.; Stein, H.J. Re–Os age for molybdenite from the West Sudetes, SW Poland. In Granitoids in Poland; Archivum Mineralogiae Monograph, no. 1; Kozłowski, A., Wiszniewska, J., Eds.; Faculty of Geology of The Warsaw University: Warsaw, Poland, 2007; Volume 1, pp. 203–216. [Google Scholar]
- Mikulski, S.Z.; Bagiński, B.; Dzierżanowski, P. The CHIME age calculations on monazite and xenotime in aplogranite from the Szklarska Poręba Huta. Pol. Mineral. Soc. Spec. Pap. 2004, 24, 287–290. [Google Scholar]
- Mitchell, R.S. Metamict minerals: A review. Mineral. Rec. 1973, 4, 177–182. [Google Scholar]







| Grain. | GR-1 | GR-1 | GR-1 | GR-1 | GR-1 | GR-2 | GR-2 | GR-3 | GR-3 | GR-3 | GR-3 | SOK-1 | SOK-1 | SOK-1 | SOK-1 | SOK-2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spot no. (EPMA/LA-ICP-MS) | 1/16 a | 1r | 1’/8 | 2’/10 | 4’/7 | 28/23 | 18’/22 | 7/40 | 8’/35 | 10’/43 | 11’/44 | 10/14 | 17/13 | 18/18 | 19/19 | 22/20 |
| Zone | zone 1 | zone 2 (worms) | zone 2 (bright) | zone 2 (dark) | zone 3c | zone 1 | zone 2 (dark) | zone 1 | zone 3a | zone 2 (dark) | zone 3b | zone 1 | zone 2 (medium) | zone 2 | zone 3a | zone 2 (bright) |
| ThO2 | 0.52 | 1.25 | 1.72 | 5.43 | 0.67 | 0.58 | 1.59 | 0.68 | 1.95 | 2.82 | 2.88 | 0.47 | 1.60 | 2.40 | 6.05 | 0.56 |
| Sm2O3 | 0.32 | 0.29 | 0.29 | 0.21 | 0.26 | 0.28 | 0.30 | 0.26 | 0.45 | 0.14 | 0.84 | 0.19 | 0.19 | 0.24 | 0.17 | 0.21 |
| Nd2O3 | 2.92 | 2.35 | 2.37 | 1.76 | 2.51 | 3.06 | 2.63 | 2.87 | 3.65 | 1.69 | 6.65 | 2.68 | 2.10 | 2.74 | 1.89 | 2.48 |
| Pr2O3 | 1.22 | 0.82 | 0.87 | 0.53 | 0.92 | 1.25 | 1.10 | 1.10 | 1.15 | 0.71 | 1.97 | 1.12 | 0.84 | 1.06 | 0.31 | 0.97 |
| Ce2O3 | 12.22 | 8.39 | 7.15 | 3.59 | 9.60 | 11.82 | 8.35 | 12.20 | 9.17 | 5.05 | 14.10 | 12.23 | 9.89 | 10.13 | 5.73 | 11.04 |
| La2O3 | 7.47 | 5.57 | 4.63 | 1.65 | 5.73 | 7.07 | 5.51 | 7.75 | 4.73 | 3.28 | 9.81 | 7.79 | 6.06 | 5.44 | 2.31 | 6.96 |
| Y2O3 | 0.15 | 0.33 | 0.39 | 0.58 | 0.24 | 0.13 | 0.37 | 0.14 | 0.27 | 0.19 | 0.92 | 0.10 | 0.10 | 0.09 | 0.15 | 0.12 |
| FeO | 15.83 | 20.95 | 21.66 | 15.91 | 15.49 | 15.61 | 19.38 | 16.34 | 16.75 | 14.95 | 4.13 | 16.00 | 13.83 | 13.03 | 9.79 | 16.59 |
| MnO | 0.32 | 0.22 | 0.22 | 0.13 | 0.50 | 0.29 | 0.25 | 0.31 | 0.41 | 0.20 | <DL | 0.30 | 0.54 | 0.50 | 0.41 | 0.35 |
| TiO2 | 1.49 | 2.74 | 2.95 | 4.56 | 0.50 | 1.48 | 2.58 | 1.84 | 1.84 | 3.79 | 1.99 | 1.63 | 3.64 | 2.70 | 3.92 | 1.78 |
| CaO | 10.22 | 7.07 | 6.28 | 3.47 | 13.07 | 10.24 | 6.80 | 10.35 | 9.14 | 4.77 | 2.10 | 10.46 | 8.66 | 9.68 | 6.37 | 9.54 |
| K2O | <DL | <DL | 0.04 | 0.31 | <DL | <DL | 0.15 | <DL | 0.07 | 0.22 | 0.13 | <DL | <DL | 0.02 | 0.31 | < DL |
| P2O5 | 0.11 | 0.10 | 0.07 | 0.34 | 0.14 | 0.13 | 0.13 | 0.14 | 0.11 | 0.12 | 0.12 | 0.12 | 0.48 | 0.31 | 0.98 | 0.16 |
| SiO2 | 30.30 | 32.99 | 33.72 | 29.80 | 32.02 | 30.54 | 32.76 | 30.36 | 31.53 | 28.24 | 6.62 | 30.87 | 33.40 | 30.65 | 27.38 | 31.23 |
| Al2O3 | 13.52 | 12.09 | 11.69 | 9.63 | 15.95 | 13.37 | 12.10 | 12.98 | 12.91 | 12.67 | 3.66 | 13.51 | 11.78 | 11.13 | 7.83 | 12.83 |
| MgO | 0.74 | 0.17 | 0.43 | 0.25 | 0.23 | 0.72 | 0.49 | 0.82 | 0.47 | 0.32 | <DL | 0.64 | 0.57 | 0.34 | 0.34 | 0.55 |
| Na2O | <DL | 0.06 | 0.35 | 0.25 | <DL | <DL | 0.30 | <DL | 0.37 | 0.25 | 0.07 | <DL | 0.56 | 0.25 | 0.37 | 0.13 |
| F b | 0.09 | 0.38 | 0.10 | 0.23 | <DL | <DL | 0.18 | <DL | 0.10 | 0.28 | 2.19 | <DL | 0.18 | 0.27 | 0.12 | 0.16 |
| Total | 97.42 | 95.77 | 94.91 | 78.62 | 97.82 | 96.56 | 94.95 | 98.14 | 95.08 | 79.68 | 58.16 | 98.11 | 94.40 | 91.00 | 74.40 | 95.66 |
| Sr | 30 | 238 | 199 | 46 | 35 | 231 | 31 | 201 | 170 | 163 | 181 | 412 | 911 | 656 | 271 | |
| Eu | 57 | 78 | 65 | 63 | 66 | 70 | 52 | 61 | 45 | 149 | 66 | 63 | 68 | 45 | 71 | |
| Gd | 1014 | 1955 c | 1353 | 1393 | 1021 | 1098 | 1286 | 886 | 1852 | 720 | 3048 | 825 | 713 | 753 | 524 | 837 |
| Tb | 98 | 199 | 232 | 108 | 107 | 185 | 91 | 204 | 100 | 368 | 79 | 72 | 70 | 58 | 81 | |
| Dy | 384 | 1107 | 1252 | 512 | 401 | 972 | 356 | 819 | 536 | 1754 | 310 | 289 | 268 | 244 | 321 | |
| Ho | 52 | 201 | 242 | 81 | 54 | 172 | 48 | 111 | 83 | 292 | 43 | 42 | 37 | 38 | 45 | |
| Er | 115 | 569 | 709 | 196 | 102 | 489 | 93 | 234 | 213 | 678 | 89 | 94 | 76 | 88 | 95 | |
| Tm | 11 | 81 | 103 | 27 | 11 | 73 | 10 | 29 | 34 | 76 | 10 | 11 | 9 | 11 | 11 | |
| Yb | 65 | 575 | 695 | 215 | 62 | 524 | 57 | 191 | 247 | 421 | 53 | 69 | 51 | 69 | 61 | |
| Lu | 7 | 85 | 105 | 31 | 7 | 77 | 7 | 25 | 34 | 55 | 7 | 10 | 7 | 11 | 9 | |
| Zr | 25 | 17 | 15 | 7 | 14 | 21 | 24 | 8 | 18 | 7 | 39 | 246 | 247 | 1456 | 25 | |
| U | 43 | 320 | 402 | 54 | 48 | 301 | 62 | 378 | 1038 | 759 | 126 | 369 | 209 | 1373 | 168 | |
| Nb | <DL | 1 | 1 | <DL | <DL | 1 | 1 | <DL | 1 | 1 | 1 | 5 | 3 | 12 | 1 | |
| Sn | 13 | 31 | 63 | 216 | 16 | 27 | 16 | 50 | 49 | 41 | 16 | 32 | 31 | 88 | 23 | |
| Ba | 3 | 515 | 317 | <DL | 26 | 509 | 7 | 247 | 291 | 295 | 46 | 84 | 82 | 129 | 72 | |
| Hf | 1 | 1 | 1 | 1 | 1 | 2 | 1 | <DL | 1 | <DL | 1 | 1 | 3 | 22 | 1 | |
| Ta | <DL | <DL | <DL | <DL | <DL | <DL | <DL | <DL | <DL | <DL | 0 | <DL | <DL | <DL | <DL | |
| ΣREE [wt %] | 20.80 | 17.61 | 13.50 | 7.09 | 16.47 | 20.24 | 15.66 | 20.81 | 16.71 | 9.49 | 29.20 | 20.65 | 16.42 | 16.88 | 9.00 | 18.66 |
| La/Sm | 23 | 19 | 16 | 8 | 22 | 25 | 18 | 29 | 10 | 23 | 12 | 41 | 31 | 23 | 13 | 32 |
| (La/Yb)N | 669 | - | 47 | 14 | 154 | 657 | 61 | 794 | 144 | 77 | 135 | 849 | 508 | 613 | 194 | 659 |
| Th/U | 106 | 16 | 47 | 119 | 108 | 106 | 46 | 97 | 45 | 24 | 33 | 33 | 38 | 101 | 39 | 29 |
| Cations, calculated based on the structure refinement of Armbruster et al. [39]: | ||||||||||||||||
| Grain | GR-1 | GR-1 | GR-1 | GR-1 | GR-1 | GR-2 | GR-2 | GR-3 | GR-3 | GR-3 | SOK-1 | SOK-1 | SOK-1 | SOK-1 | SOK-2 | |
| Spot no. (EPMA/LA-ICP-MS) | 1/16 | 1r | 1’/8 | 2’/10 | 4’/7 | 28/23 | 18’/22 | 7/40 | 8’/35 | 10’/43 | 10/14 | 17/13 | 18/18 | 19/19 | 22/20 | |
| Zone | zone 1 d | zone 2 (worms) | zone 2 (bright) | zone 2 (dark) | zone 3c | zone 1 | zone 2 (dark) | zone 1 | zone 3a | zone 2 (dark) | zone 1 | zone 2 (medium) | zone 2 (bright) | zone 3a | zone 2 (bright) | |
| Si e | 2.952 | 3.000 | 3.000 | 3.000 | 2.966 | 2.988 | 3.000 | 2.939 | 3.000 | 3.000 | 2.975 | 3.000 | 3.000 | 3.000 | 3.000 | |
| P | 0.009 | - | - | - | 0.011 | 0.010 | - | 0.012 | - | - | 0.010 | - | - | - | - | |
| Al | 0.039 | 0.000 | 0.000 | 0.000 | 0.023 | 0.002 | 0.000 | 0.049 | 0.000 | 0.000 | 0.015 | 0.000 | 0.000 | 0.000 | 0.000 | |
| Total T-site | 3.000 | 3.000 | 3.000 | 3.000 | 3.000 | 3.000 | 3.000 | 3.000 | 3.000 | 3.000 | 3.000 | 3.000 | 3.000 | 3.000 | 3.000 | |
| Ti | 0.109 | 0.188 | 0.197 | 0.346 | 0.035 | 0.109 | 0.177 | 0.134 | 0.132 | 0.303 | 0.118 | 0.246 | 0.199 | 0.323 | 0.129 | |
| Al | 1.512 | 1.295 | 1.226 | 1.143 | 1.718 | 1.540 | 1.306 | 1.432 | 1.448 | 1.586 | 1.519 | 1.247 | 1.284 | 1.011 | 1.453 | |
| Fe3+ | 0.404 | 1.494 | 1.521 | 1.339 | 0.533 | 0.352 | 1.396 | 0.439 | 1.033 | 1.061 | 0.372 | 1.039 | 1.067 | 0.897 | 1.044 | |
| Fe2+ | 0.868 | 0.000 | 0.000 | 0.000 | 0.656 | 0.895 | 0.054 | 0.872 | 0.299 | 0.000 | 0.900 | 0.000 | 0.000 | 0.000 | 0.289 | |
| Mg | 0.107 | 0.023 | 0.057 | 0.037 | 0.032 | 0.104 | 0.067 | 0.119 | 0.066 | 0.050 | 0.091 | 0.076 | 0.050 | 0.056 | 0.078 | |
| Mn2+ | 0.000 | 0.000 | 0.000 | 0.011 | 0.026 | 0.000 | 0.000 | 0.004 | 0.021 | 0.000 | 0.000 | 0.041 | 0.041 | 0.038 | 0.008 | |
| Total M-site | 3.000 | 3.000 | 3.000 | 2.876 | 3.000 | 3.000 | 3.000 | 3.000 | 3.000 | 3.000 | 3.000 | 2.648 | 2.641 | 2.324 | 3.000 | |
| Mn2+ | 0.026 | 0.017 | 0.016 | 0.000 | 0.014 | 0.024 | 0.019 | 0.021 | 0.012 | 0.018 | 0.025 | 0.000 | 0.000 | 0.000 | 0.021 | |
| Fe2+ | 0.016 | 0.048 | 0.091 | 0.000 | 0.010 | 0.031 | 0.034 | 0.011 | 0.000 | 0.000 | 0.018 | 0.000 | 0.000 | 0.000 | 0.000 | |
| Ca | 1.067 | 0.689 | 0.598 | 0.375 | 1.297 | 1.074 | 0.667 | 1.073 | 0.932 | 0.543 | 1.080 | 0.833 | 1.015 | 0.748 | 0.982 | |
| Na | 0.001 | 0.010 | 0.060 | 0.049 | - | - | 0.053 | - | 0.068 | 0.052 | - | 0.097 | 0.048 | 0.078 | 0.024 | |
| K | - | - | 0.004 | 0.039 | - | - | 0.018 | - | 0.009 | 0.030 | - | - | 0.003 | 0.043 | - | |
| P | - | 0.008 | 0.006 | 0.029 | - | - | 0.010 | - | 0.009 | 0.010 | - | 0.037 | 0.026 | 0.091 | 0.013 | |
| REE | 0.875 | 0.608 | 0.520 | 0.323 | 0.661 | 0.853 | 0.621 | 0.873 | 0.689 | 0.438 | 0.860 | 0.638 | 0.715 | 0.431 | 0.775 | |
| Th | 0.012 | 0.026 | 0.035 | 0.124 | 0.014 | 0.013 | 0.033 | 0.015 | 0.042 | 0.068 | 0.010 | 0.033 | 0.053 | 0.151 | 0.012 | |
| Total A-site | 2.000 | 1.407 | 1.335 | 0.942 | 2.000 | 2.000 | 1.465 | 2.000 | 1.764 | 1.165 | 2.000 | 1.644 | 1.873 | 1.552 | 1.832 | |
| Feox Armbruster et al. [39] | 0.31 | 0.97 | 0.94 | 1.00 | 0.44 | 0.28 | 0.94 | 0.33 | 0.78 | 1.00 | 0.29 | 1.00 | 1.00 | 1.00 | 0.78 | |
| Feox Petrik et al. [2] | 0.34 | 0.50 | 0.57 | 0.62 | 0.43 | 0.38 | 0.49 | 0.39 | 0.41 | 0.47 | 0.38 | 0.45 | 0.41 | 0.56 | 0.40 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gros, K.; Słaby, E.; Jokubauskas, P.; Sláma, J.; Kozub-Budzyń, G. Allanite Geochemical Response to Hydrothermal Alteration by Alkaline, Low-Temperature Fluids. Minerals 2020, 10, 392. https://doi.org/10.3390/min10050392
Gros K, Słaby E, Jokubauskas P, Sláma J, Kozub-Budzyń G. Allanite Geochemical Response to Hydrothermal Alteration by Alkaline, Low-Temperature Fluids. Minerals. 2020; 10(5):392. https://doi.org/10.3390/min10050392
Chicago/Turabian StyleGros, Katarzyna, Ewa Słaby, Petras Jokubauskas, Jiří Sláma, and Gabriela Kozub-Budzyń. 2020. "Allanite Geochemical Response to Hydrothermal Alteration by Alkaline, Low-Temperature Fluids" Minerals 10, no. 5: 392. https://doi.org/10.3390/min10050392






