Fluctuating Asymmetry in Two Common Freshwater Fishes as a Biological Indicator of Urbanization and Environmental Stress within the Middle Chattahoochee Watershed
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study System and Defining Urbanization
2.2. Land-Use Analyses
2.3. Fish Collection
2.4. Metric and Meristic Analyses
2.5. Fluctuating Asymmetry Analysis
3. Results
3.1. Land-Use Analyses and Chemical Factors of Urbanization
3.2. Fluctuating Asymmetry
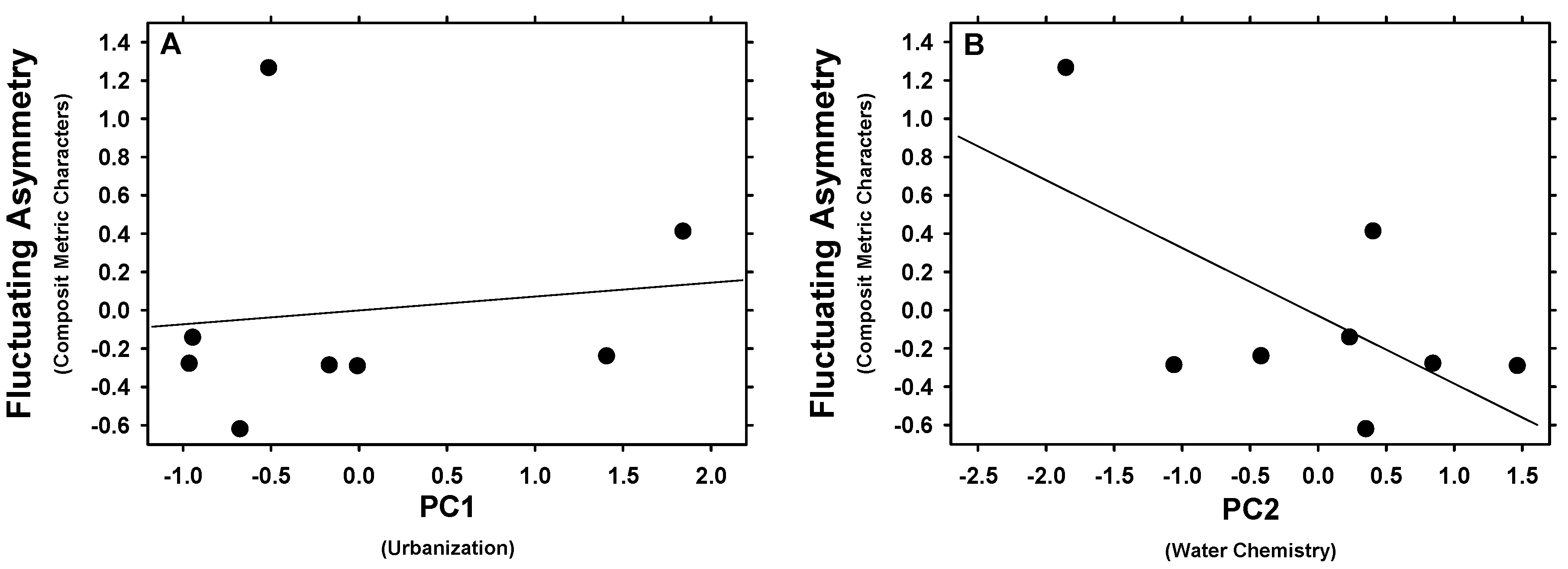
3.3. Fluctuating Asymmetry Related to Land-Use and Chemical Factors of Urbanization
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Moller, A.P.; Swaddle, J.P. Asymmetry, Developmental Stability, and Evolution; Oxford University Press: New York, NY, USA, 1997. [Google Scholar]
- Moller, A.P. Developmental stability and fitness: A review. Am. Nat. 1997, 149, 916–932. [Google Scholar]
- Zakharov, L.A. A measure of fluctuating asymmetry for a set of characters. Acta Zool. Fenn. 1992, 191, 37–77. [Google Scholar]
- Palmer, R.A. Fluctuating asymmetry analyses: A primer. In Developmental Stability: Its Origins and Evolutionary Implications; Markow, T.A., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1994; pp. 335–364. [Google Scholar]
- Lucentini, L.; Carosi, A.; Erra, R.; Giovinazzo, G.; Lorenzoni, M.; Mearelli, M. Fluctuating asymmetry in perch, Perca fluviatilia (Percidae) from three lakes of the Region Umbria (Italy) as a tool to demonstrate the impact of man-made lakes on developmental stability. Ital. J. Zool. 1998, 65, 445–447. [Google Scholar]
- Van Valen, L. A study of fluctuating asymmetry. Evolution 1962, 16, 125–142. [Google Scholar]
- Palmer, R.A. Waltzing with asymmetry. BioScience 1996, 46, 518–532. [Google Scholar] [CrossRef]
- Graham, J.H.; Shimizu, K.; Emlen, J.M.; Freeman, D.C.; Merkel, J. Growth models and the expected distribution of fluctuating asymmetry. Biol. J. Linn. Soc. 2003, 80, 57–65. [Google Scholar] [CrossRef]
- Hochwender, C.G.; Fritz, R.S. Fluctuating asymmetry in a Salix hybrid system: The importance of genetic versus environmental causes. Evolution 1999, 53, 408–416. [Google Scholar] [CrossRef]
- Palmer, R.A.; Strobeck, C. Fluctuating asymmetry: Measurement, analysis, and patterns. Annu. Rev. Ecol. Evol. Syst. 1986, 17, 391–421. [Google Scholar] [CrossRef]
- Palmer, R.A.; Strobeck, C. Fluctuating asymmetry as a measure of developmental stability: Implications of non-normal distributions and power of statistical tests. Acta Zool. Fennica 1992, 191, 57–72. [Google Scholar]
- Siegel, P.; Siegel, M.I.; Krimmer, E.C.; Doyle, W.J.; Barry, H. Fluctuating asymmetry as an indicator of the stressful prenatal effects of D9-tetrahydrocannabinol in the laboratory rat. Toxicol. Appl. Pharm. 1977, 42, 339–344. [Google Scholar] [CrossRef]
- Hardersen, S. The role of behavioural ecology of damselflies in the use of fluctuating asymmetry as a bioindicator of water pollution. Ecol. Entomol. 2000, 25, 45–53. [Google Scholar] [CrossRef]
- Bonada, N.; Williams, D.D. Exploration of the utility of fluctuating asymmetry as an indicator of river condition using larvae of the caddisfly Hydropsyche morose (Trichoptera: Hydropsychidae). Hydrobiologia 2002, 481, 147–156. [Google Scholar] [CrossRef]
- Seixas, L.B.; Neves Dos Santos, A.F.G.; Neves Dos Santos, L. Fluctuating asymmetry: A tool for impact assessment on fish populations in a tropical polluted bay, Brazil. Ecol. Indic. 2016, 71, 522–532. [Google Scholar] [CrossRef]
- Tull, J.C.; Brussard, P.F. Fluctuating asymmetry as an indicator of environmental stress from off-highway vehicles. J. Wildl. Manag. 2006, 71, 1944–1948. [Google Scholar] [CrossRef]
- Lajus, D.; Rainer, K.; Brix, O. Fluctuating asymmetry and other parameters of morphological variation of eelpout Zoarces viviparus (Zoarcidae, Teleostei) from different parts of its distributional range. Sarsia 2003, 88, 247–260. [Google Scholar] [CrossRef]
- Estes, E.C.; Katholi, C.R.; Angus, R.A. Elevated fluctuating asymmetry in eastern mosquitofish (Gambusia holbrooki) from a river receiving paper mill effluent. Environ. Toxicol. Chem. 2006, 25, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Pickett, S.T.A.; Cadenasso, M.L.; Grove, J.M.; Nilon, C.H.; Poutay, R.V.; Zipperer, W.C.; Constanza, R. Urban ecological systems: Linking terrestrial ecology, physical, and socioeconomic components of metropolitan areas. Annu. Rev. Ecol. Syst. 2001, 32, 127–157. [Google Scholar] [CrossRef]
- Weller, B.; Ganzhorn, J.U. Carabid beetle community composition, body size, and fluctuating asymmetry along an urban-rural gradient. Basic Appl. Ecol. 2004, 5, 193–201. [Google Scholar] [CrossRef]
- Hahs, A.K.; McDonnell, M.J. Selecting independent measures to quantify Melbourne’s urban-rural gradient. Landsc. Urban Plan. 2006, 78, 435–448. [Google Scholar] [CrossRef]
- Duh, J.; Shandas, V.; Chang, H.; George, L.A. Rates of urbanization and the resiliency of air and water quality. Sci. Total Environ. 2008, 400, 238–256. [Google Scholar] [CrossRef] [PubMed]
- Poff, N.L.; Allan, J.D.; Bain, M.B.; Karr, J.R.; Prestegaard, K.L.; Richter, B.D.; Sparks, R.E.; Stromberg, J.C. The natural flow regime. BioScience 1997, 47, 769–784. [Google Scholar] [CrossRef]
- Graham, J.H.; Emlen, J.M.; Freeman, C.D. Developmental stability and its applications in ecotoxicology. Ecotoxicology 1993, 2, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Valentine, D.W.; Soule, M. Effect of p,p’-DDT on developmental stability of pectoral fin rays in the grunion, Leuresthes tenius. Fish. Bull. 1973, 71, 921–926. [Google Scholar]
- Ames, L.J.; Felley, J.D.; Smith, M.H. Amounts of asymmetry in Centrarchid fish inhabiting heated and nonheated reservoirs. Trans. Am. Fish. Soc. 1979, 108, 489–495. [Google Scholar] [CrossRef]
- Allenbach, D.M. Fluctuating asymmetry and exogenous stress in fishes: A review. Rev. Fish Biol. Fish. 2011, 21, 355–376. [Google Scholar] [CrossRef]
- Martin, S.M.; Lutterschmidt, W.I. A checklist to the common Cyprinid and Centrachid fishes of the Bull and Upatoi Creeks Watershed of Georgia with a brief glimpse of correlative urban influences and land use. Southeast. Nat. 2013, 12, 769–780. [Google Scholar] [CrossRef]
- Anderson, S.M.; Fiorillo, R.A.; Cook, T.J.; Lutterschmidt, W.I. Helminth parasites of two species of Lepomis (Osteichthyes: Centrarchidae) from an urban watershed and their potential use in environmental monitoring. Ga. J. Sci. 2015, 73, 123–135. [Google Scholar]
- Buck, J.C.; Lutterschmidt, W.I. Parasite abundance decrease with host density: Evidence of the encounter-dilution effect for a parasite with a complex life cycle. Hydrobiology 2017, 784, 201–210. [Google Scholar] [CrossRef]
- Deal, B. Ecological urban dynamics: The convergence of spatial modelling and sustainability. Build. Res. Inf. 2001, 29, 381–393. [Google Scholar] [CrossRef]
- Wang, Y.; Choi, W.; Deal, B.M. Long-term impacts of land-use change on non-point source pollutant loads for the St. Louis metropolitan area, USA. Environ. Manag. 2005, 35, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Callender, E.; Rice, K.C. The urban environmental gradient: Anthropogenic influences on the spatial and temporal distributions of lead and zinc in sediments. Environ. Sci. Technol. 2000, 34, 232–238. [Google Scholar] [CrossRef]
- Helms, B.S.; Feminella, J.W. Detection of biotic responses to urbanization using fish assemblages from small streams of western Georgia, USA. Urban Ecosyst. 2005, 8, 39–57. [Google Scholar] [CrossRef]
- Styers, D.M.; Chappelka, A.H. Urbanization and atmospheric deposition: Use of bioindicators in determining patterns of land-use change in western Georgia. Water Air Soil Pollut. 2009, 200, 371–386. [Google Scholar] [CrossRef]
- Griffith, G.E.; Omernik, J.M.; Comstock, J.A.; Lawrence, S.; Martin, G.; Goddard, A.; Hulcher, V.J.; Foster, T. Ecoregions of Alabama and Georgia; US Geological Survey: Reston, VA, USA, 2001.
- Crim, J.F. Water Quality Changes Across an Urban-Rural Land Use Gradient in Streams of the West Georgia Piedmont. Master’s Thesis, Auburn University, Auburn, AL, USA, December 2007. [Google Scholar]
- Clesceri, L.S.; Greenberg, A.E.; Eaton, A.D. (Eds.) Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1998.
- Paul, M.J.; Meyer, J.L. Streams in the urban landscape. Annu. Rev. Ecol. Syst. 2001, 32, 333–365. [Google Scholar] [CrossRef]
- Schoonover, J.E.; Lockaby, B.G. Land cover impacts on stream nutrients and fecal coliform in the lower Piedmont of west Georgia. J. Hydrol. 2006, 331, 371–382. [Google Scholar] [CrossRef]
- Camargo, J.A.; Alonso, A. Ecological and toxicological effects of inorganic nitrogen pollution in aquatic ecosystems: A global assessment. Environ. Int. 2006, 32, 831–849. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, R.K.; Sheridan, J.M. Streamflow water quality in the Georgia coastal plain. In Proceedings of the 1989 Georgia Water Resources Conference, Athens, GA, USA, 16–17 May 1989.
- Dickerson, B.R.; Vinyard, G.L. Effects of high levels of the total dissolved solids in Walker lake, Nevada, on survival and growth of Lahontan cutthroat trout. Trans. Am. Fish. Soc. 1999, 128, 507–515. [Google Scholar] [CrossRef]
- Biggs, T.W.; Dunne, T.; Martinelli, L.A. Natural controls and human impacts on stream nutrient concentrations in a deforested region of the Brazilian Amazon basin. Biogeochemistry 2004, 68, 227–257. [Google Scholar] [CrossRef]
- Theobald, D.M. Placing exurban land-use change in a human modification framework. Front. Ecol. Environ. 2004, 2, 139–144. [Google Scholar] [CrossRef]
- Jones, J.A.; Swanson, F.J.; Wemple, B.C.; Snyder, K.U. Effects of roads on hydrology, geomorphology, and disturbance patches in stream networks. Conserv. Biol. 2000, 14, 76–85. [Google Scholar] [CrossRef]
- Klein, R.D. Urbanization and stream quality impairment. Water Resour. Bull. 1979, 15, 948–963. [Google Scholar] [CrossRef]
- Merila, J.; Bjorklund, M. Fluctuating asymmetry and measurement error. Syst. Biol. 1995, 44, 97–101. [Google Scholar] [CrossRef]
- Roe, K.J.; Harris, P.M.; Mayden, R.L. Phyolgenetic relationships of the genera of North American sunfishes and basses (Percoidei: Centrarchidae) as evidenced by the mitochondrial cytochrome b gene. Copeia 2002, 2002, 897–905. [Google Scholar] [CrossRef]
- Collar, D.C.; O’Meara, B.C.; Wainright, P.C.; Near, T.J. Piscivory limits diversification of feeding morphology in Centrarchid fishes. Evolution 2009, 63, 1557–1573. [Google Scholar] [CrossRef] [PubMed]
- Yule, G.U. Notes on the theory of association of attributes in statistics. Biometrika 1903, 2, 121–134. [Google Scholar] [CrossRef]
- Pertoldi, C.; Kristensen, T.N. A new fluctuating asymmetry index, or the solution for the scaling effect? Symmetry 2015, 7, 327–335. [Google Scholar] [CrossRef]
- Allan, J.D. Landscapes and riverscapes: The influence of land use on stream ecosystems. Annu. Rev. Ecol. Evolut. Syst. 2004, 35, 257–284. [Google Scholar] [CrossRef]
- Richards, C.; Johnson, L.B.; Host, G.E. Landscape-scale influences on stream habitats and biota. Can. J. Fish. Aquat. Sci. 1996, 53, 295–311. [Google Scholar] [CrossRef]
- Roy, A.H.; Rosemond, A.D.; Paul, M.J.; Leigh, D.S.; Wallace, J.B. Stream macroinvertebrate response to catchment urbanization (Georgia, USA). Freshw. Biol. 2003, 48, 329–346. [Google Scholar] [CrossRef]
- Matthews, W.J. Physiochemical tolerance and selectivity of stream fishes as related to their geographic ranges and local distributions. In Community and Evolutionary Ecology of North American Stream Fishes; University of Oklahoma Press: Norman, OK, USA, 1987; pp. 111–120. [Google Scholar]
- Wang, L.; Lyons, J.; Kanehl, P.; Bannerman, R. Impacts of urbanization on stream habitat and fish across multiple spatial scales. Environ. Manag. 2001, 28, 255–266. [Google Scholar] [CrossRef]
- Bjorksten, T.; David, P.; Pomiankowski, A.; Fowler, K. Fluctuating asymmetry of sexual and nonsexual traits in stalk-eyed flies: A poor indicator of developmental and genetic quality. J. Evol. Biol. 2001, 13, 89–97. [Google Scholar] [CrossRef]
- Karr, J.R. Assessment of biotic integrity using fish communities. Fisheries 1981, 6, 21–27. [Google Scholar] [CrossRef]
- Fausch, K.D.; Lyons, J.; Karr, J.R.; Angermeier, P.L. Fish Communities as Indicators of Environmental Degradation. Am. Fish. Soc. Symp. 1990, 8, 123–144. [Google Scholar]
- Cookson, N.; Schorr, M.S. Correlation of watershed housing density with environmental conditions and fish assemblages in a Tennessee ridge and valley stream. J. Freshw. Ecol. 2009, 24, 553–561. [Google Scholar] [CrossRef]
- Yoder, C.O.; Miltner, R.J.; White, D. Assessing the status of aquatic life designated uses in urban and suburban watersheds. In National Conference on Retrofit Opportunities for Water Resource Protection in Urban Environment, Chicago, IL, USA, 9–12 February 1998; pp. 16–28.
- Adams, S.M. Status and use of biological indicators for evaluating the effects of stress on fish. J. Parasitol. 1990, 70, 466–474. [Google Scholar]
- Marcogliese, D.J. Parasites of the superorganism: Are they indicators of ecosystem health? Int. J. Parasitol. 2005, 35, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Lafferty, K.D. Environmental parasitology: What can parasites tell us about human impacts on the environment? Parasitol. Today 1997, 13, 251–256. [Google Scholar] [CrossRef]
- Kennedy, C.R. Freshwater fish parasites and environmental quality: An overview and caution. Parassitologia 1997, 39, 249–254. [Google Scholar] [PubMed]
- Sures, B. Environmental parasitology: Relevancy of parasites in monitoring environmental pollution. Trends Parasitol. 2004, 20, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.A.; Billiard, S.M. Parasites of winter flounder (Pleuronectes americanus) as an additional bioindicator of stress-related exposure to untreated pulp and paper mill effluent: A 5-year field study. Arch. Environ. Contam. Toxicol. 2007, 52, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Karr, J.R.; Yant, P.R.; Faush, K.D.; Schlosser, I.J. Spatial and temporal variability of the index of biotic integrity in three Midwestern streams. Trans. Am. Fish. Soc. 1987, 116, 1–11. [Google Scholar] [CrossRef]
- Georgia Spatial Data Infrastructure. Georgia GIS Clearinghouse: Map Data and Aerial Photography. Georgia Spatial Web, 2011. Available online: www.georgiaspatial.org (accessed on 23 July 2013).
- United States Census Bureau TIGER/Line Shapefiles and TIGER/Line Files. United States Census Bureau Web. Available online: http://www.census.gov (accessed on 23 July 2013).




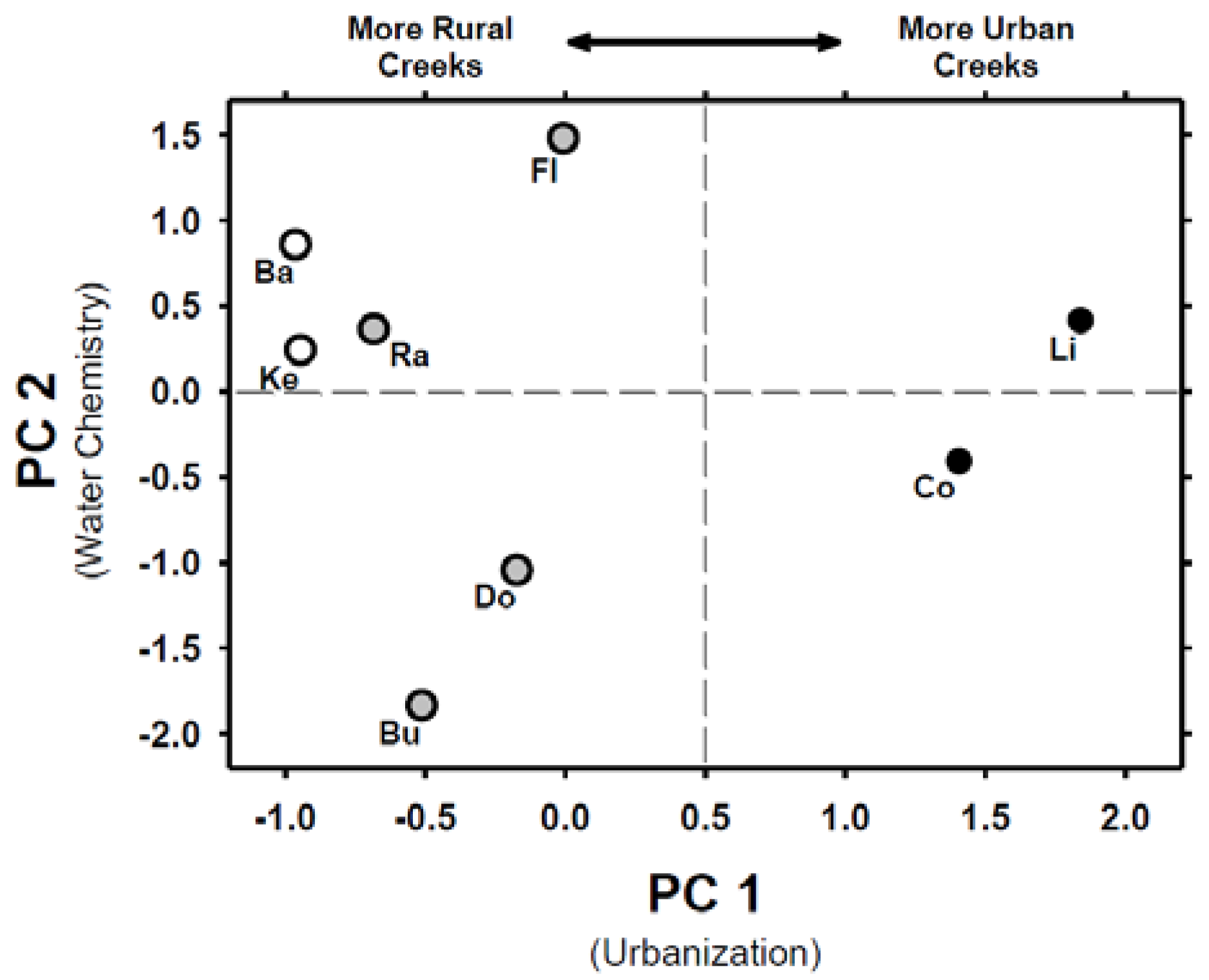


| Chemical Factor | Cause and Effects | Citations |
| Orthophosphates, fluoride, chlorine and sulfates | Fertilizers, cleaning agents, human and food wastes, wastewater treatments. Acidification, increased primary production, eutrophication and changes in nutrient cycling. | [38,39,40,41] |
| Total suspended solids | Increased erosion, decreased water quality. Fish growth and survival, nutrient cycling. | [39,42,43] |
| Land-Use Factor | Potential Effects | Citations |
| Urban land use change | Changes in fish assemblage, water quality, stream landscapes, nutrient cycling etc. | [21,34,44,45] |
| Impervious surfaces | Influence water quality, water quantity, hydrology, nutrient cycling, flood pulse index, increased down cutting, changes in riparian zone, etc. | [19,34,39] |
| Road density | Changes in riparian areas (increased erosion) and stream geomorphology, flow, flooding, discharge, increased disturbance, etc. | [21,39,45,46] |
| Population density | Changes in water quality, water quantity, nutrient cycling, etc. | [19,44,45] |
| Creek | Percent Urban Land Use | Percent Urban Impervious Surface | Total Length (km) of Road | Population Density (People/km2) |
|---|---|---|---|---|
| Lindsey | 90.05 | 81.11 | 87.1 | 9,922.6 |
| Cooper | 84.21 | 67.91 | 154.9 | 14,832.5 |
| Flatrock | 46.19 | 23.59 | 19.2 | 2,488.2 |
| Bull | 30.82 | 8.13 | 57.6 | 1,048.5 |
| Dozier | 47.42 | 22.50 | 52.6 | 265.1 |
| Randall | 29.40 | 7.45 | 67.1 | 158.6 |
| Kendall | 27.54 | 2.85 | 34.9 | 145.1 |
| Baker | 14.34 | 1.30 | 26.7 | 121.1 |
| Creek | TSS (total suspended solids) (mg/L) | Fluoride (ppm) | Chlorine (ppm) | Sulfate (ppm) | Orthophosphate (mg/L) |
|---|---|---|---|---|---|
| Lindsey | 0 | 18.8452 | 11.2172 | 5.7066 | 0.0470 |
| Cooper | 2 | 0.1382 | 9.2143 | 3.9300 | 0.0120 |
| Flatrock | 2 | 0.1952 | 9.9335 | 6.1606 | 0.0030 |
| Bull | 4 | 0.1208 | 2.6962 | 2.8727 | 0.0650 |
| Dozier | 5 | 19.1136 | 6.8703 | 2.2944 | 0.0090 |
| Randall | 1 | 0.0889 | 5.2048 | 3.3805 | 0.0038 |
| Kendall | 0 | 0.4775 | 4.2805 | 2.0065 | 0.0045 |
| Baker | 0 | 0.2275 | 3.8476 | 4.4178 | 0.0130 |
| Creek | L. auritus | L. macrochirus | Total |
|---|---|---|---|
| Lindsey | 86 | 33 | 119 |
| Cooper | 17 | 36 | 53 |
| Flatrock | 36 | 23 | 59 |
| Bull | 19 | 5 | 24 |
| Dozier | 18 | 4 | 22 |
| Randall | 28 | 1 | 29 |
| Kendall | 43 | 29 | 72 |
| Baker | 36 | 14 | 50 |
| Total | 283 | 145 | 428 |
| Metric Character | MS (SxI) | MS (Within) | FA | ME | F | P |
|---|---|---|---|---|---|---|
| Eye-Peduncle Distance (3) | 0.27778 | 0.08539 | 0.09617 | 0.08539 | 1.20 | 0.299 |
| Eye Diameter (4) | 0.04332 | 0.01283 | 0.01524 | 0.01283 | 2.569 | 0.013 |
| Eye-Snout Distance (5) | 0.04657 | 0.01376 | 0.01641 | 0.01376 | 2.597 | 0.012 |
| PC1 Urbanization | PC2 Water Chemistry | |||||
|---|---|---|---|---|---|---|
| FA Metric Character | F | P | r2 | F | P | r2 |
| Eye-Peduncle Distance | 2.592 | 0.159 | 0.302 | 2.604 | 0.158 | 0.303 |
| Eye Diameter | 1.738 | 0.235 | 0.225 | 0.592 | 0.471 | 0.090 |
| Eye-Snout Distance | 0.446 | 0.529 | 0.069 | 4.223 | 0.086 | 0.413 |
| Composite FA | 0.130 | 0.731 | 0.021 | 3.501 | 0.111 | 0.368 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lutterschmidt, W.I.; Martin, S.L.; Schaefer, J.F. Fluctuating Asymmetry in Two Common Freshwater Fishes as a Biological Indicator of Urbanization and Environmental Stress within the Middle Chattahoochee Watershed. Symmetry 2016, 8, 124. https://doi.org/10.3390/sym8110124
Lutterschmidt WI, Martin SL, Schaefer JF. Fluctuating Asymmetry in Two Common Freshwater Fishes as a Biological Indicator of Urbanization and Environmental Stress within the Middle Chattahoochee Watershed. Symmetry. 2016; 8(11):124. https://doi.org/10.3390/sym8110124
Chicago/Turabian StyleLutterschmidt, William I., Samantha L. Martin, and Jacob F. Schaefer. 2016. "Fluctuating Asymmetry in Two Common Freshwater Fishes as a Biological Indicator of Urbanization and Environmental Stress within the Middle Chattahoochee Watershed" Symmetry 8, no. 11: 124. https://doi.org/10.3390/sym8110124





