Development of a Smart Metered-Dose Inhaler for Asthma Based on Computational Fluid Dynamics
Abstract
:1. Introduction
2. Materials and Methods
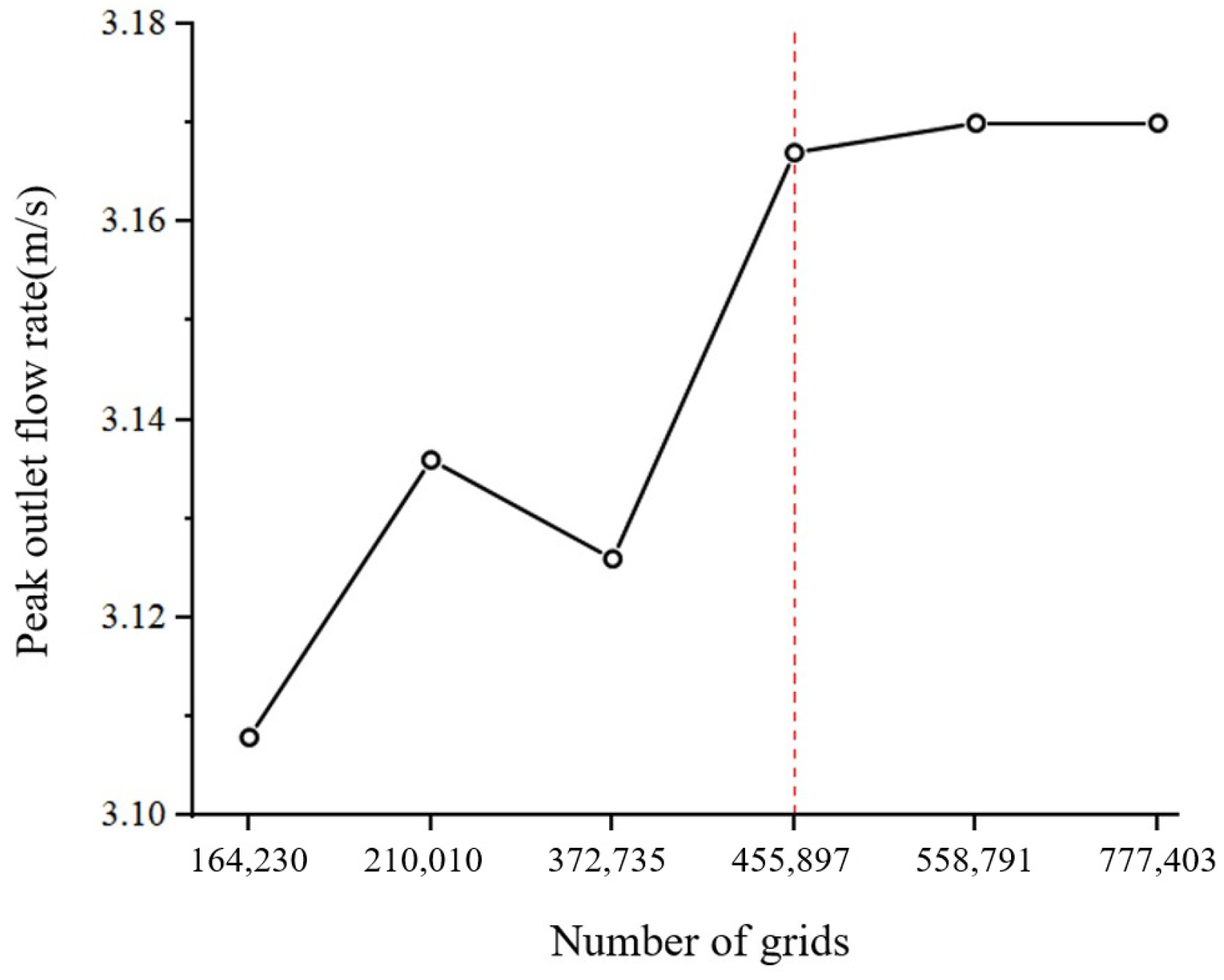
2.1. Modeling and Meshing of the Human Mouth and Throat Regions
2.2. Numerical Simulation Methods for Computational Fluid Dynamics Analysis
- Particle force equation:
- 2.
- Random walk model
- 3.
- Particle deposition model
3. Quantitative Study of Drug Particle Deposition in the Mouth and Throat Regions
3.1. Influence of Peak Inspiratory Flow Rate on the Drug Deposition Fraction in the Mouth and Throat Regions
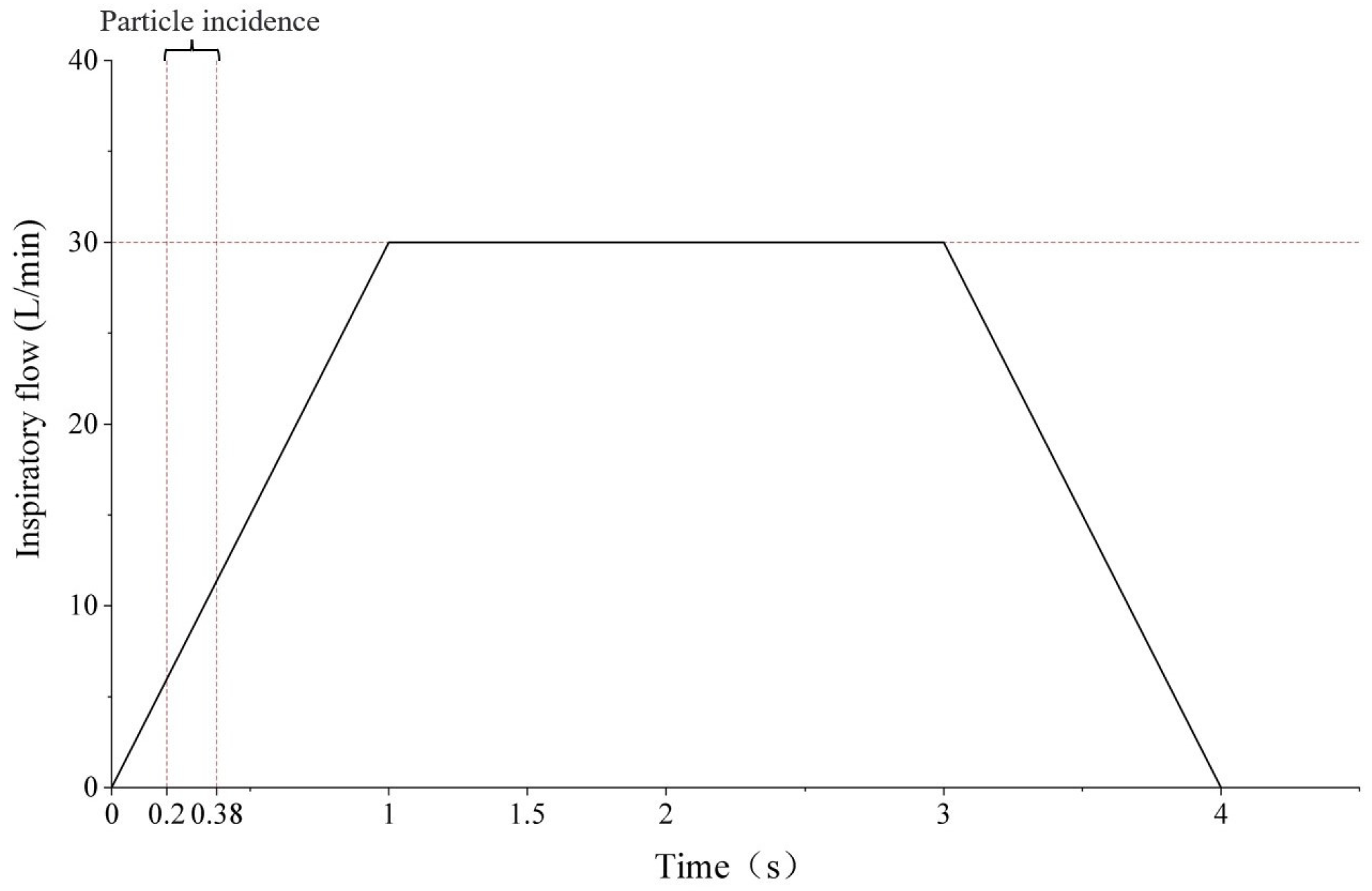
3.1.1. Boundary Conditions
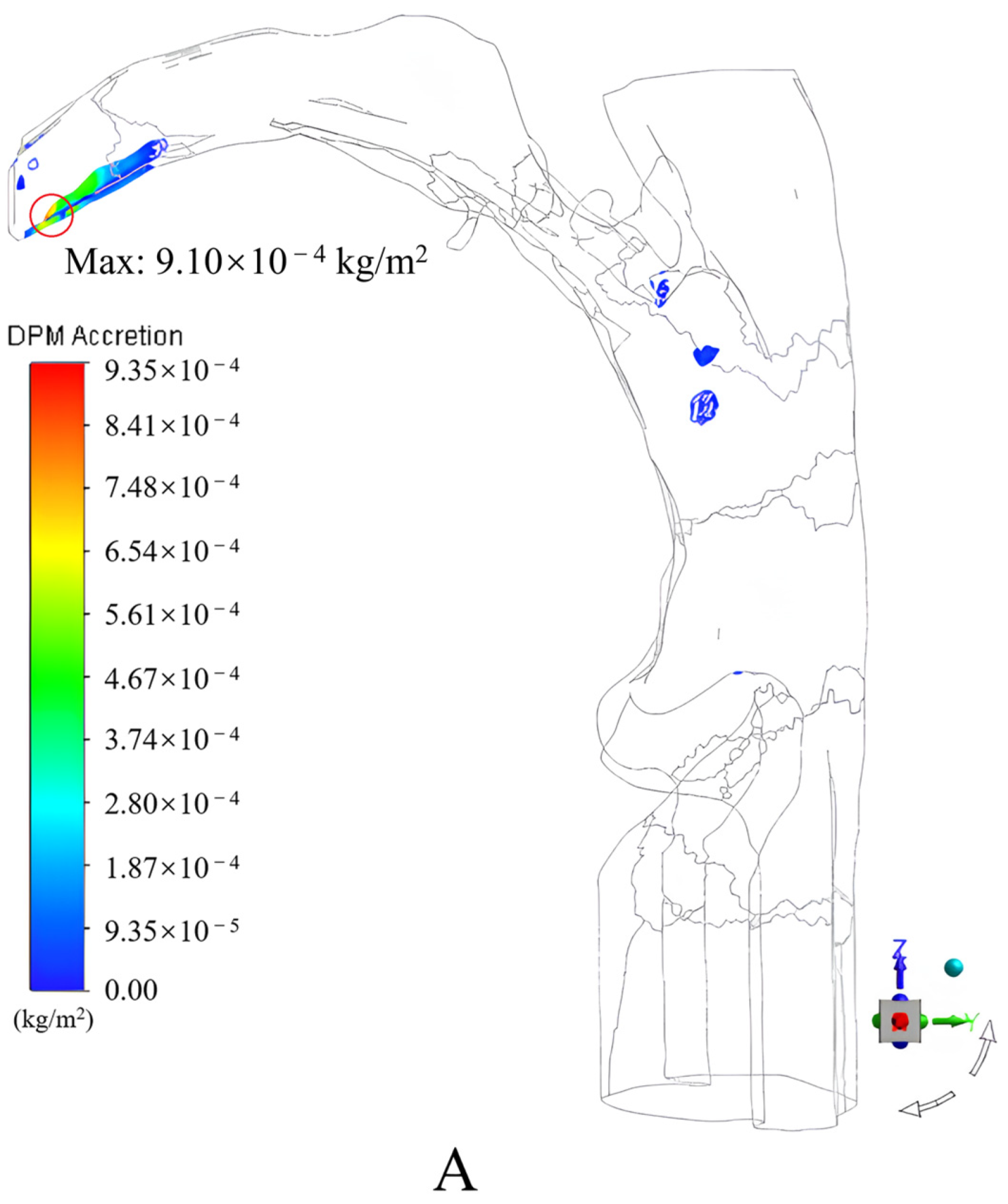
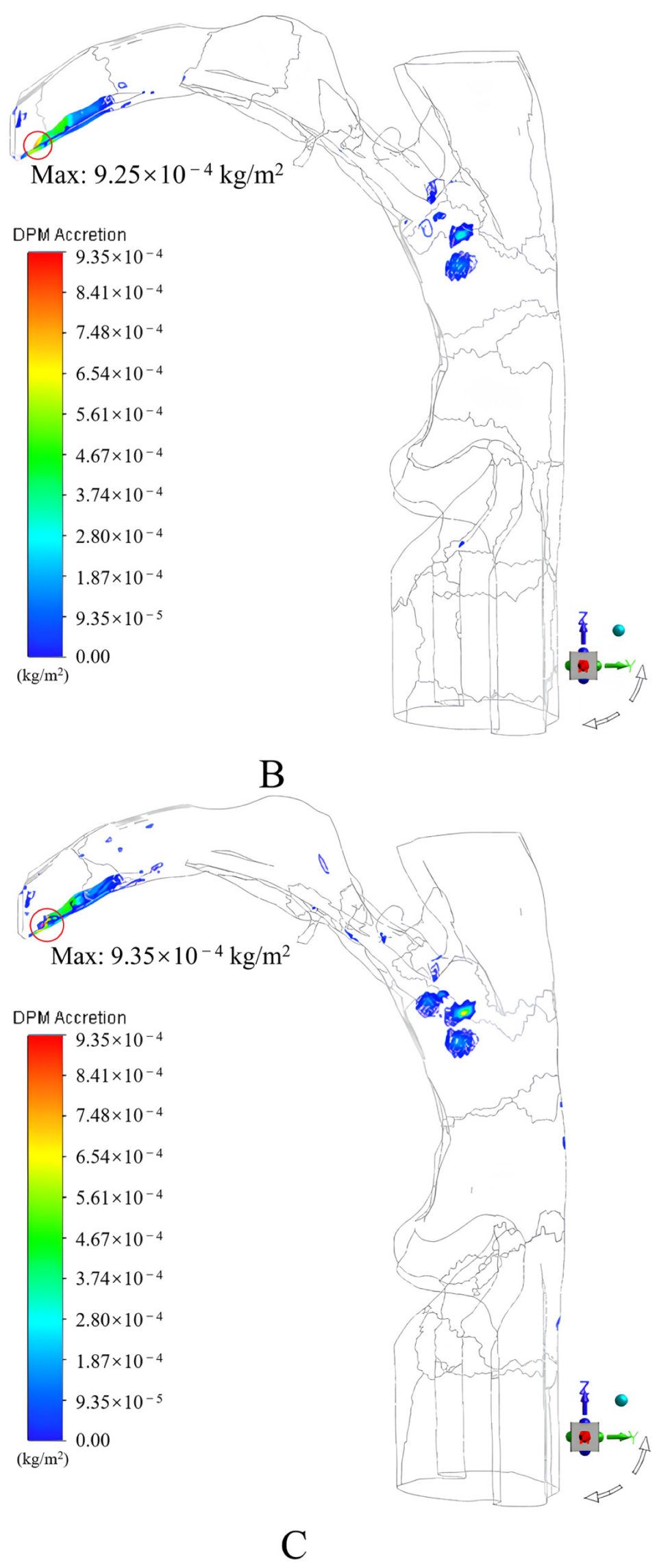
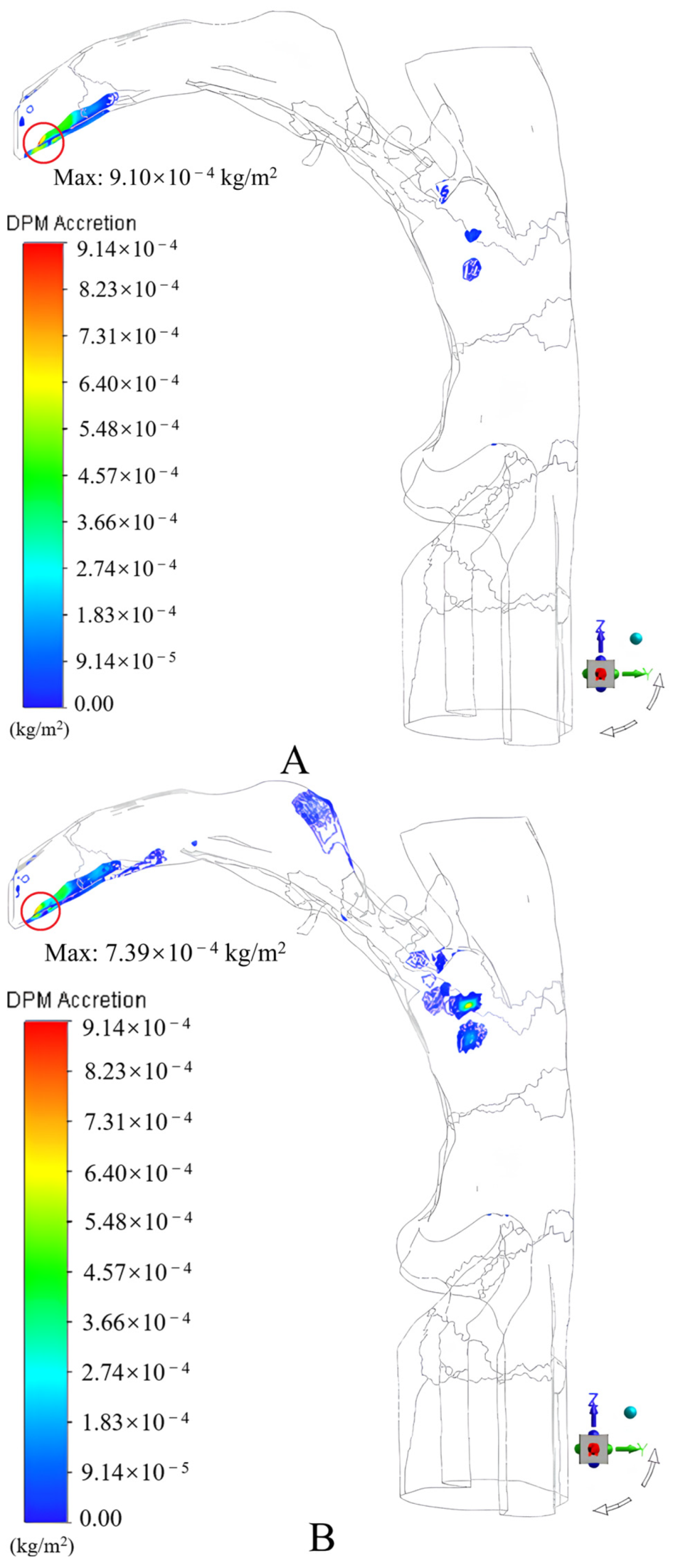
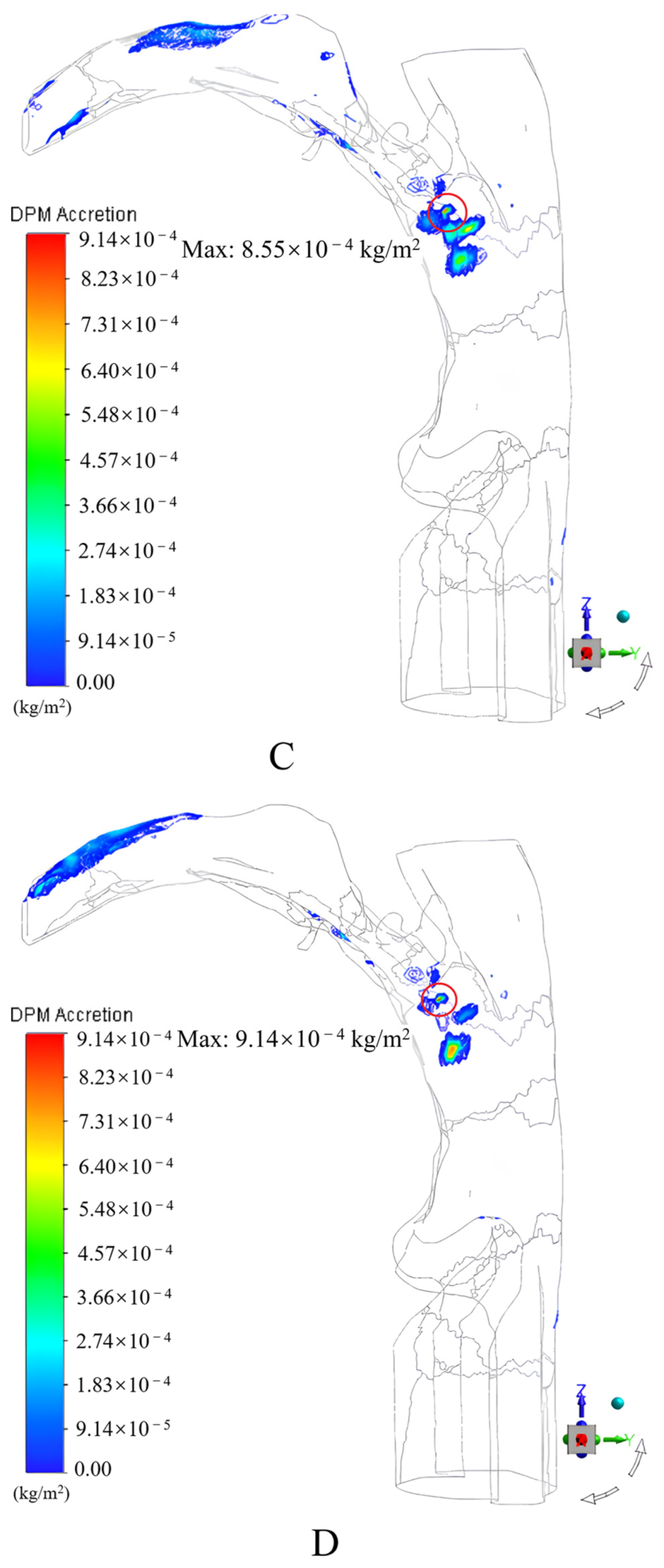
3.1.2. Results and Discussion
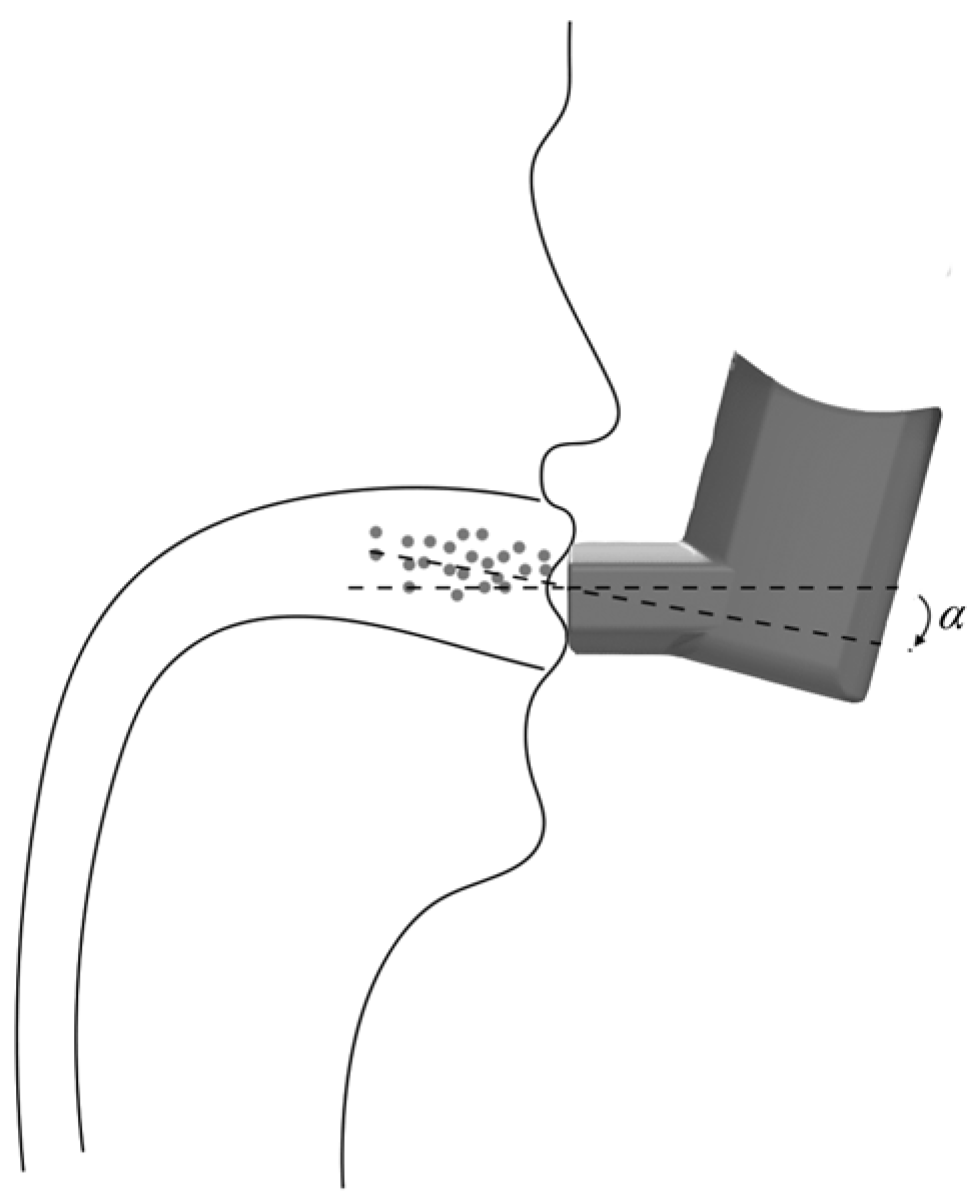
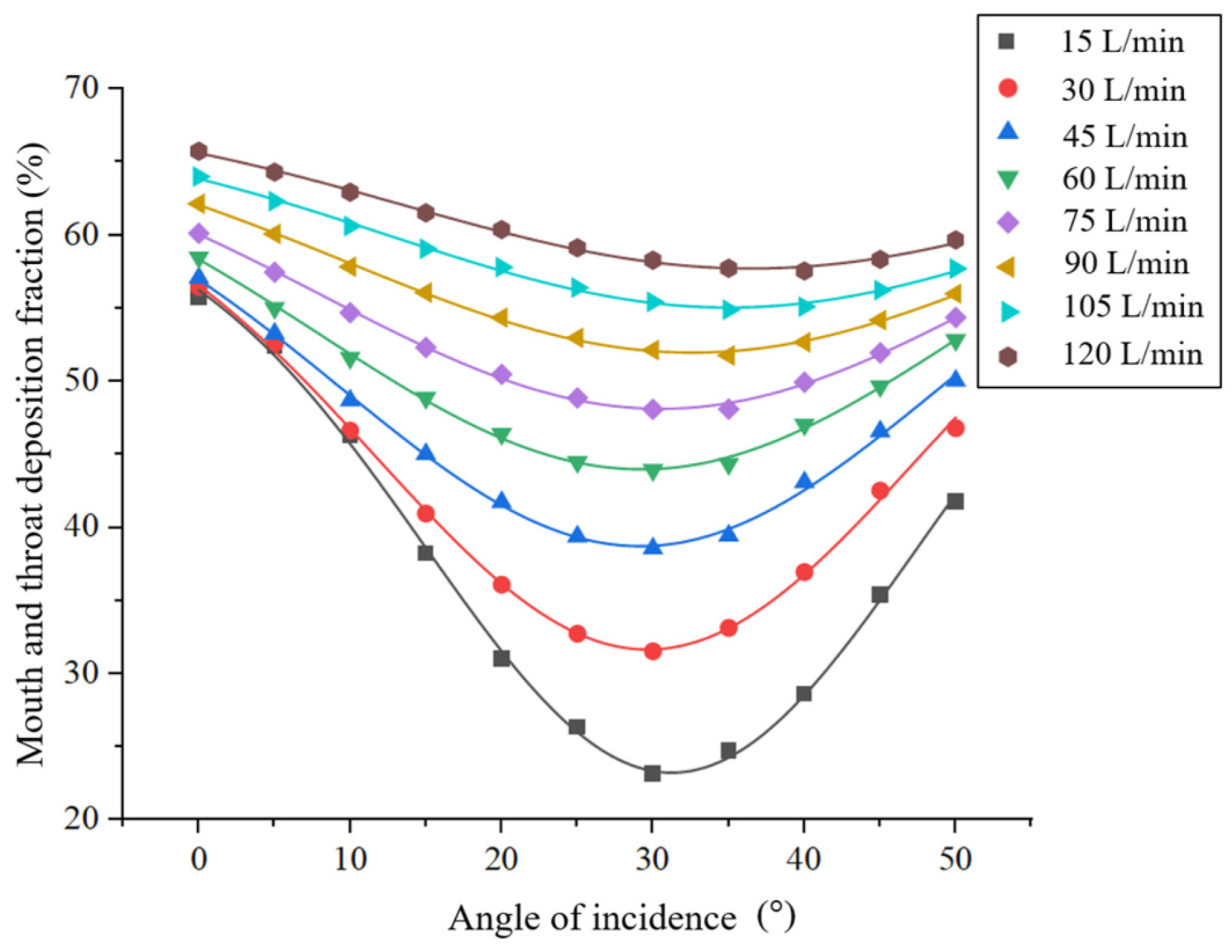
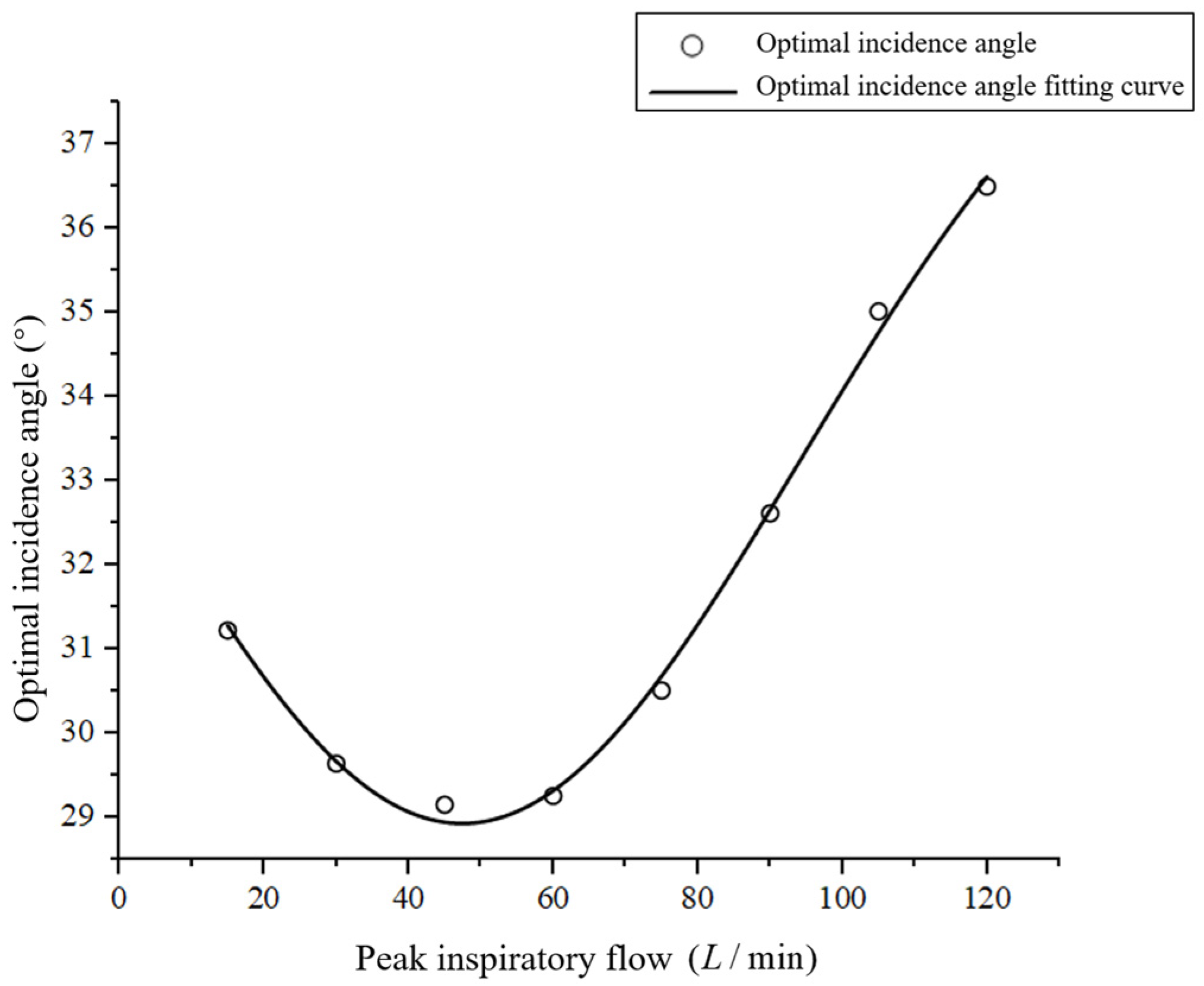
3.2. Effect of the Angle of Incidence on the Drug Deposition Fraction in the Mouth and Throat Regions
3.2.1. Boundary Conditions
3.2.2. Results and Discussion
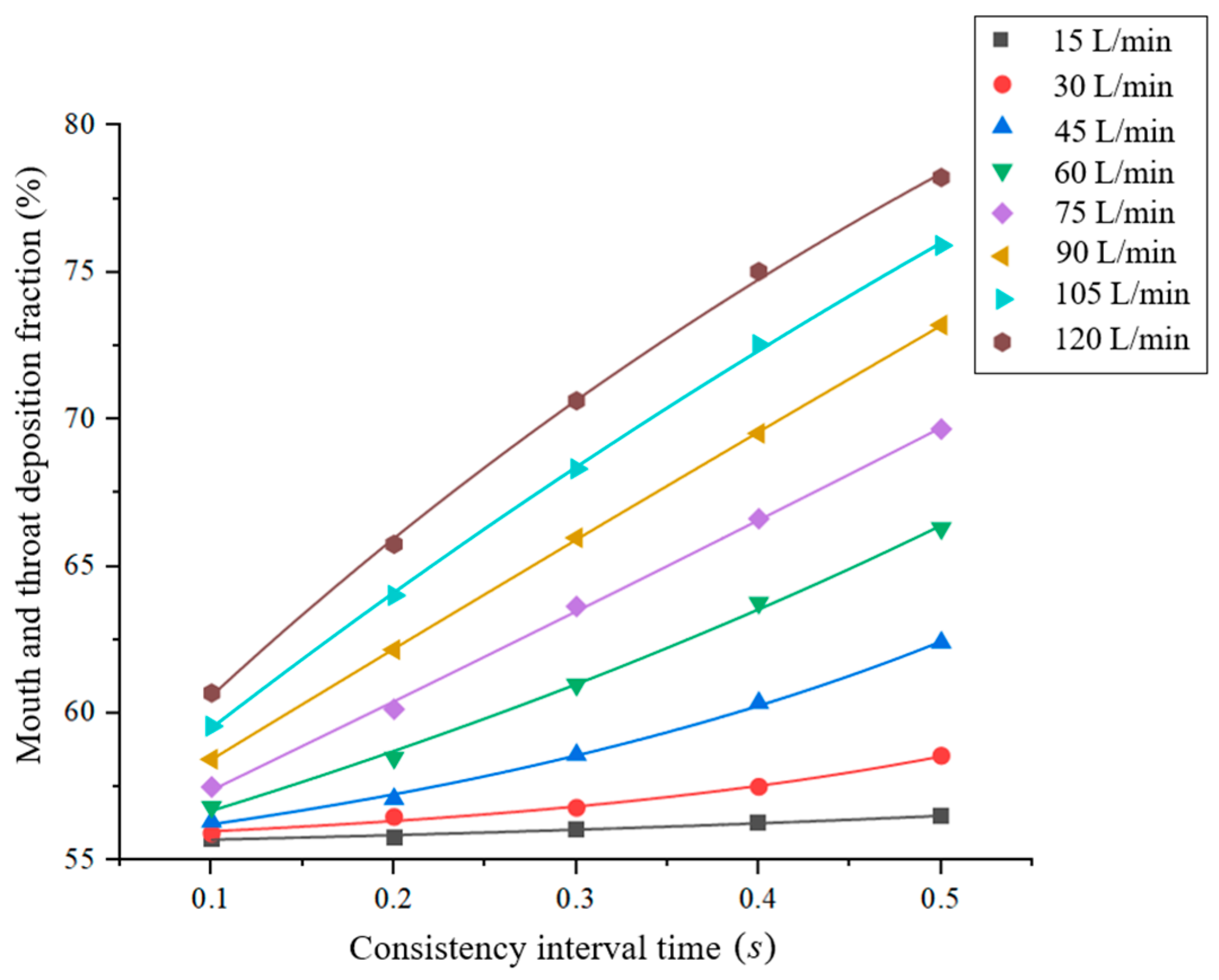
3.3. Effect of Inspiration–Press Interval Time on the Deposition Fraction in the Mouth and Throat Regions
3.3.1. Boundary Conditions
3.3.2. Results and Discussion
4. Design of an Intelligent Dosing Inhaler Based on the Obtained Simulation Results
- The intelligent MDI must include a peak suction flow monitoring mechanism and an angle adjustment mechanism for detecting the patient’s peak inspiratory flow and adjusting the angle of incidence to the optimal angle of incidence;
- The intelligent MDI must include an automatic pressing mechanism that enables rapid detection of the inspiratory action and provides an immediate drive for pressing the aerosol canister to achieve inspiration–press synchronization.
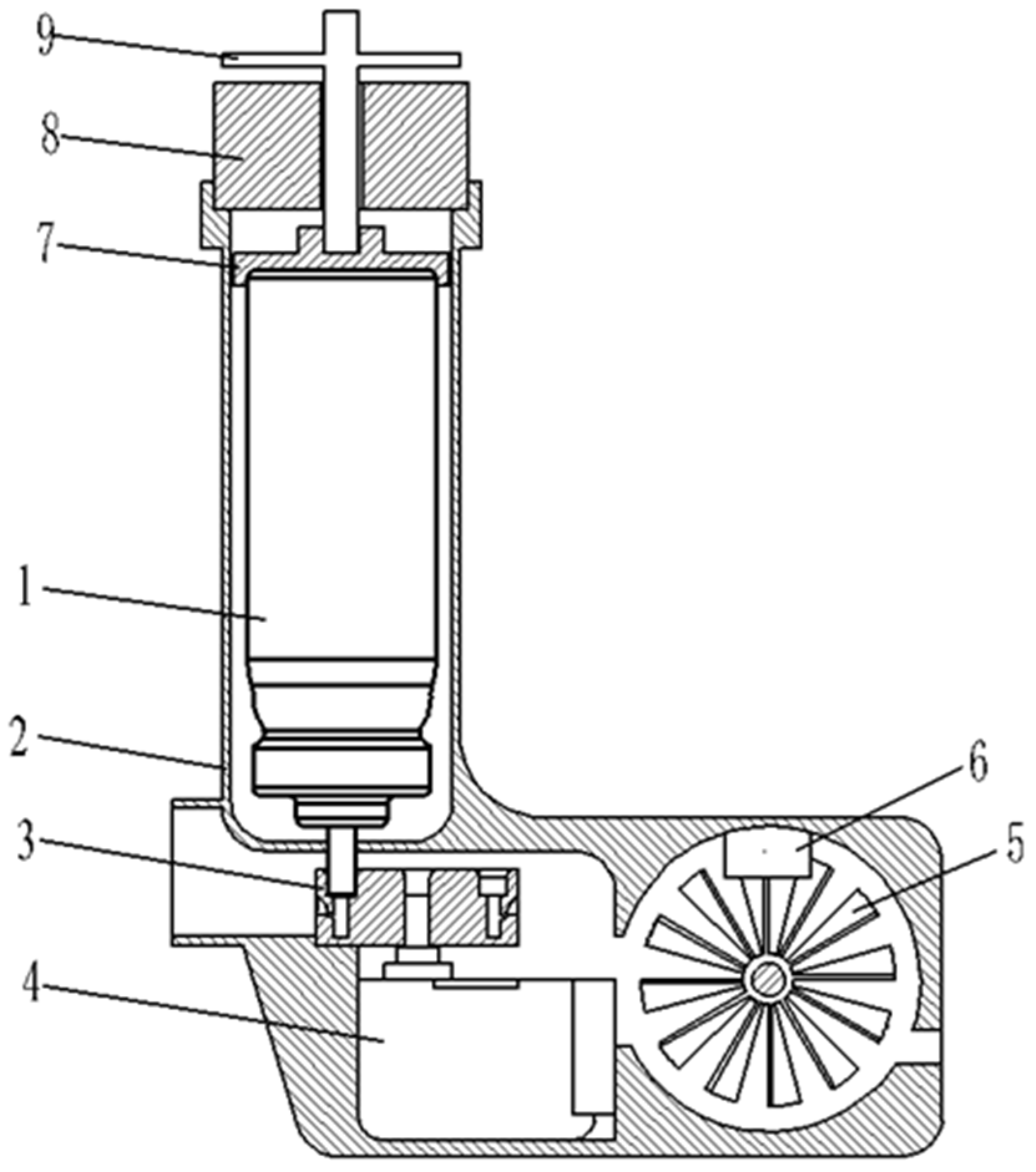
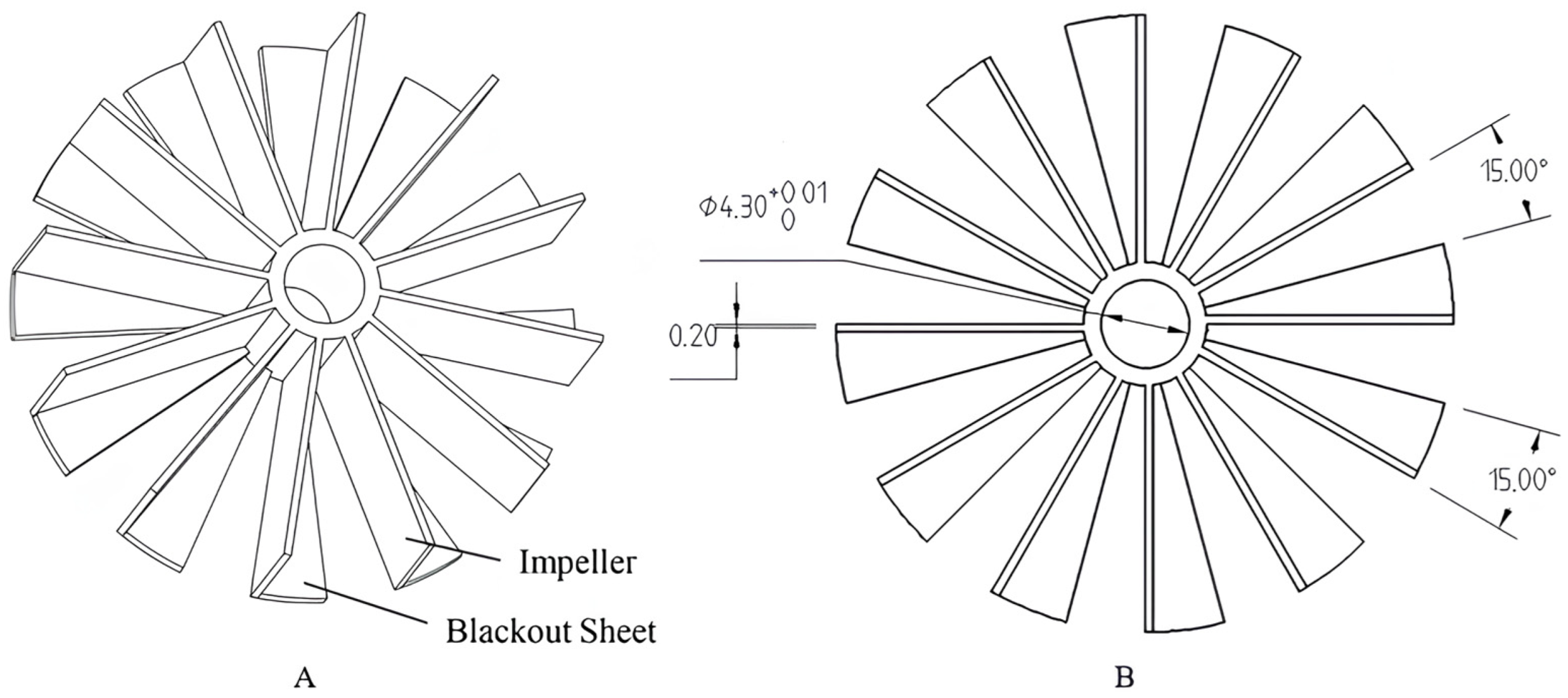
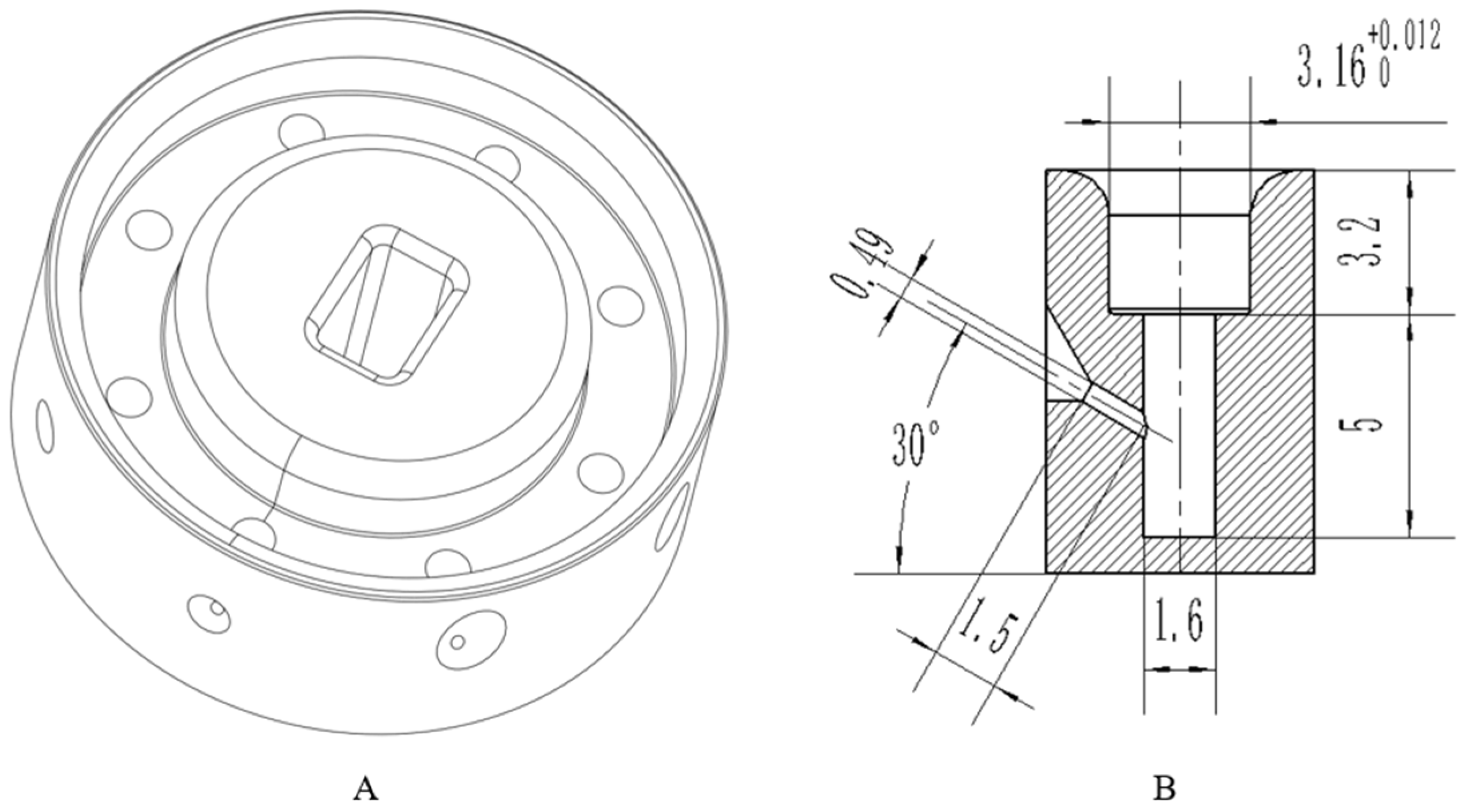
4.1. Structural Design
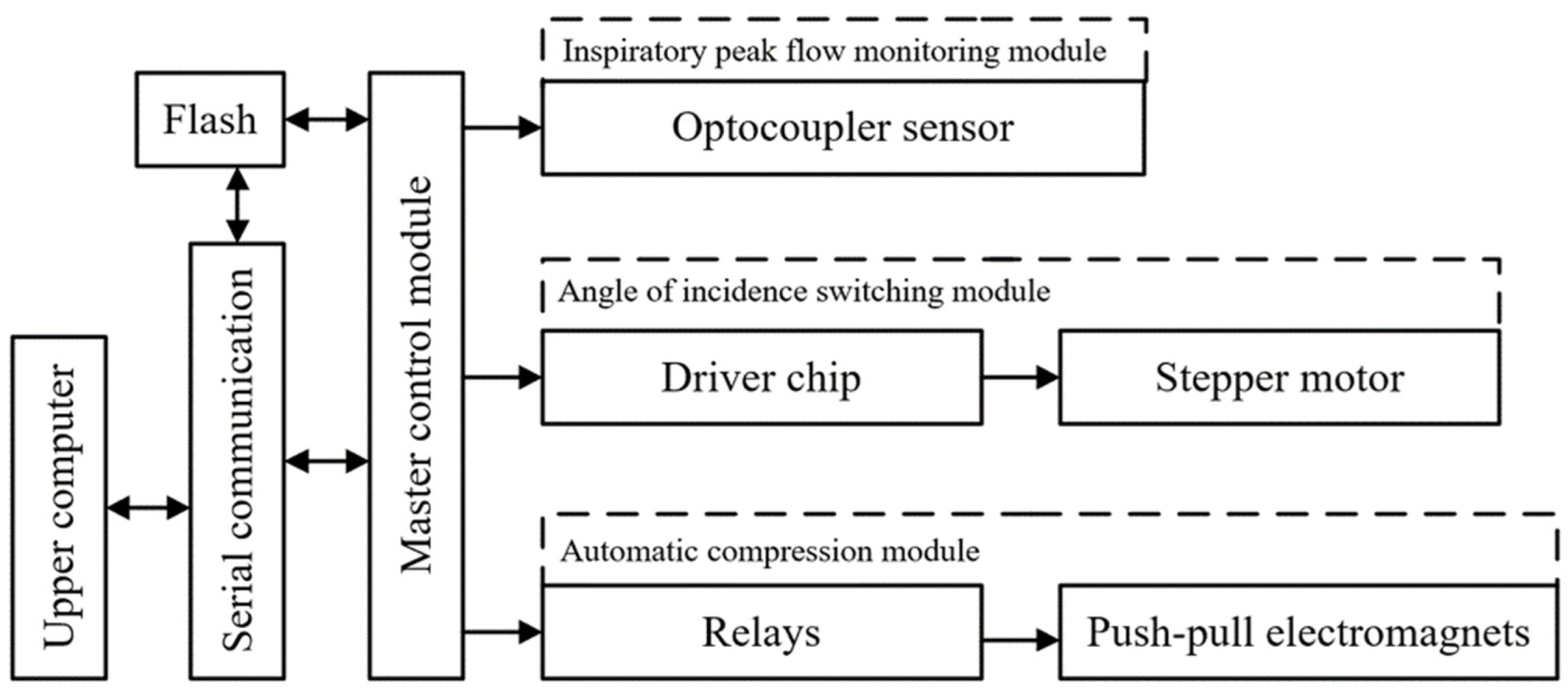
4.2. Design of the Control System
- The speed of the disc impeller is monitored by the microcontroller and the slotted optocoupler, and the peak inspiratory flow rate of the patient is obtained through microcontroller processing;
- A small stepper motor is driven by the driver chip to rotate the angle switching nozzle to switch to the nozzle with the optimal incidence angle corresponding to the patient’s current peak inspiratory flow rate;
- Using a relay with a push–pull solenoid, the aerosol canister is quickly pressed to optimally shorten the drug injection interval when the patient inhales again;
- The medication data of the patient are then uploaded to a PC via serial communication and to a database via the upper computer software, which includes device ID, medication time, peak inspiratory flow, and interval time.
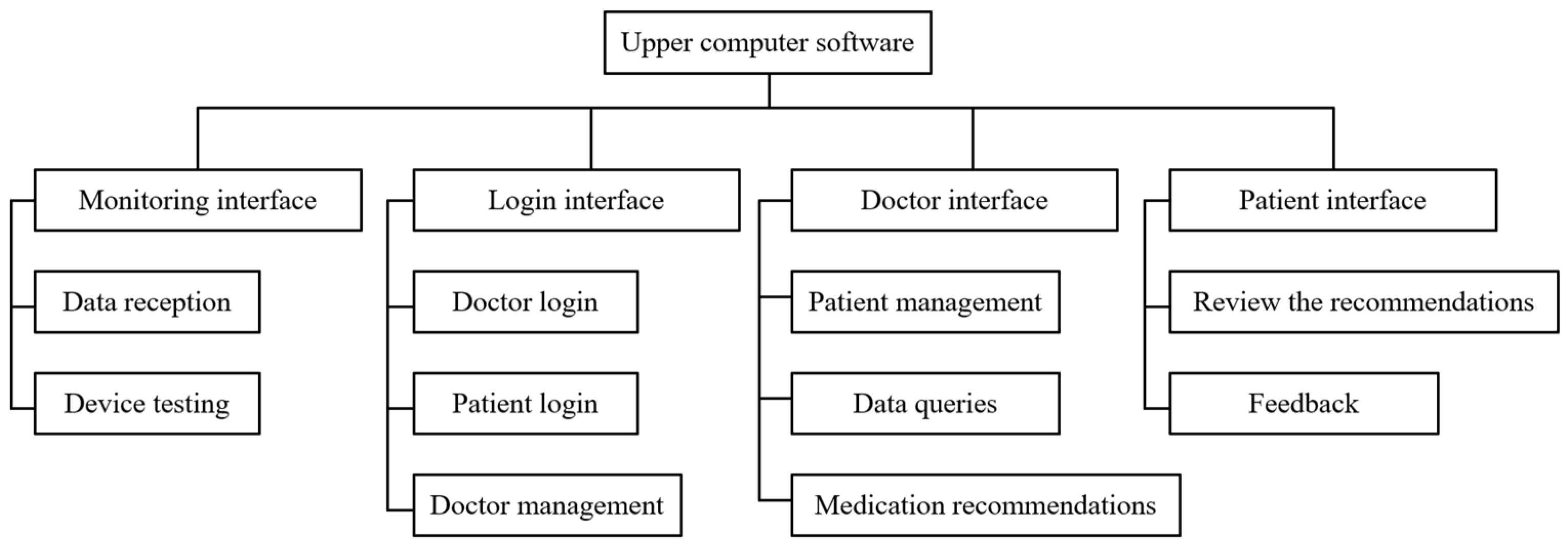
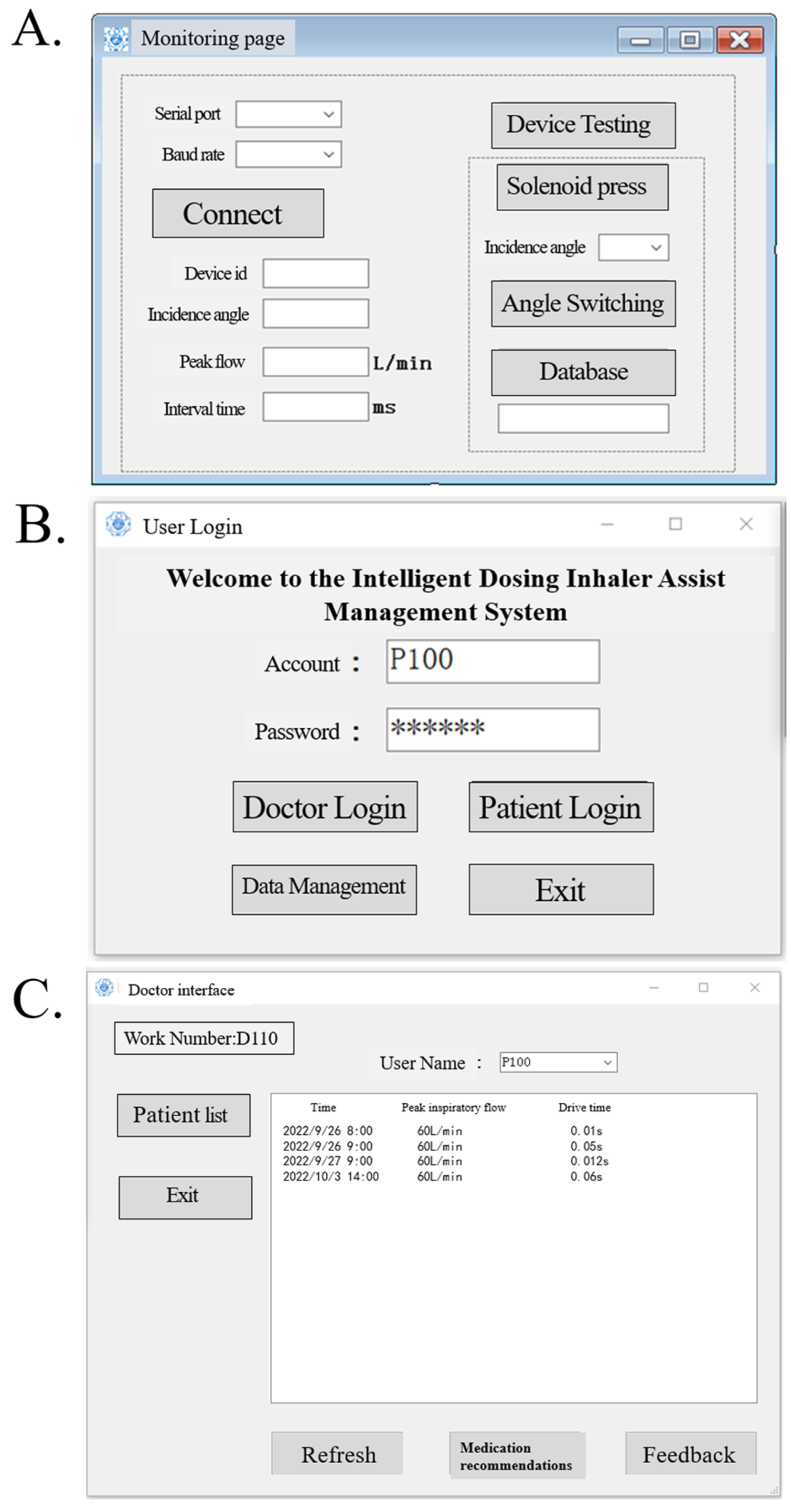
4.3. Upper Computer Design
- The upper computer software reads the data transmitted by the device in real time and saves the data to the database while also testing whether certain parts of the device are functioning properly;
- The upper computer software registers and deletes the accounts of physicians and patients (i.e., device IDs) and saves them to the database;
- Physicians and patients are able to login to the upper computer software, and the physicians can access the medication data of patients from the database and provide recommendations, and patients can consult physicians or provide feedback on the recommendations through the software.
5. Prototype Preparation and Experimental Test
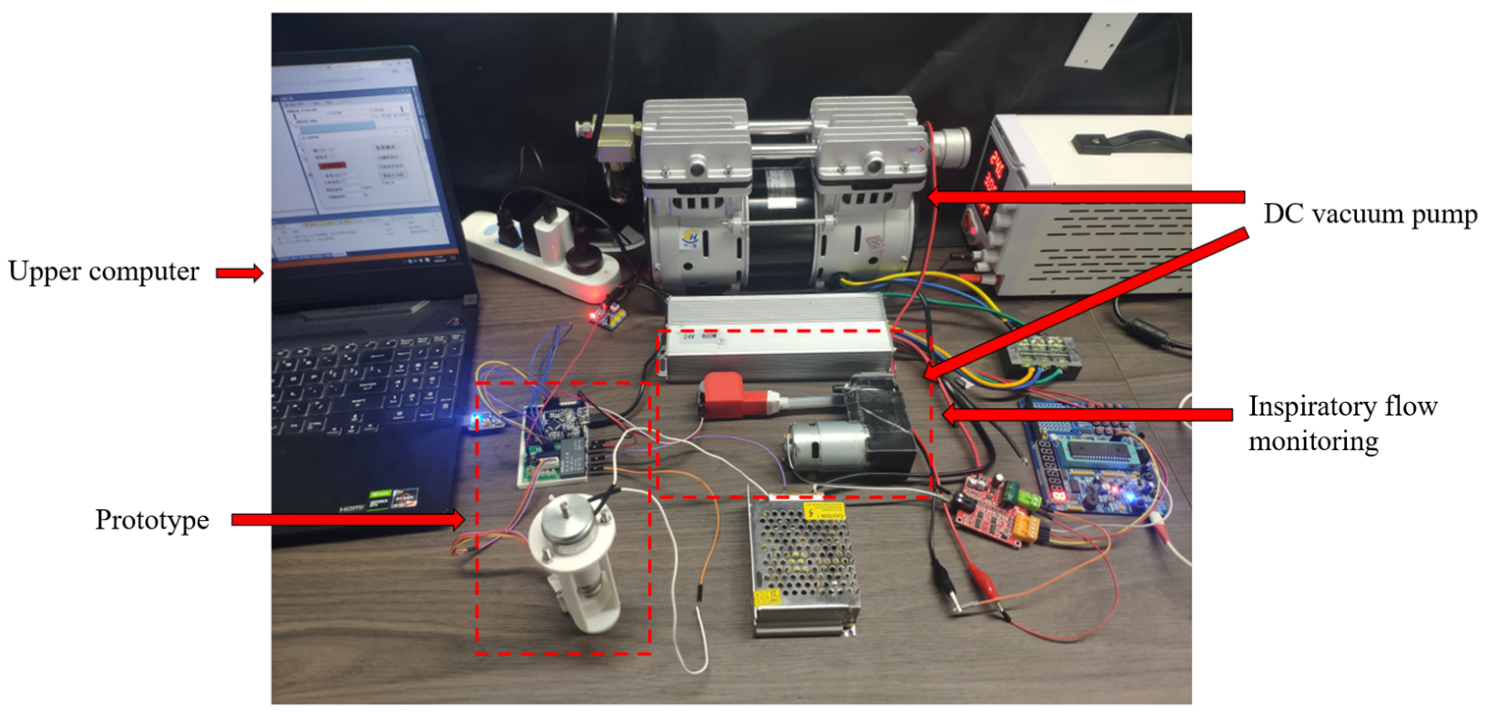
5.1. Prototype Preparation
5.2. Inspiratory Flow and Incidence Angle Test
5.2.1. Accuracy Test of Inspiratory Flow Monitoring and Incidence Angle Adjustments
- The DC vacuum pump was connected to the inspiratory flow monitoring device in the test platform and the constant flow rate was then tested from 15 to 120 L/min at 15 L/min intervals. Each flow rate was tested thrice, and the average of the peak inspiratory flow rates obtained from the final statistics was adopted as the displayed flow rate;
- We tested whether the upper computer software was able to receive the monitoring results in real time, and whether the receiving function worked normally, the test results were analyzed and are summarized in Table 5.
5.2.2. Inspiration–Press Synchronization Test
- the DC vacuum pump was connected to the inspiratory flow monitoring device in the test platform; the constant flow rate was then tested from 15 to 120 L/min at 15 L/min intervals. Each flow rate was tested thrice, and the average of the inspiration–press intervals obtained from the final tally was used as the interval time;
- we observed whether the solenoid pressed the driver, the upper computer software could adequately receive the monitoring results in real time, and whether the receiving function worked normally; the test results were analyzed and are summarized in Table 6.
5.3. Comprehensive Performance Analysis of the Prototype
6. Discussion
7. Conclusions
- An incidence angle of 0°, and an increase in the peak inspiratory flow rate caused the drug particles to easily contact and deposit themselves on the convex tip of the tongue and body surface of the tongue under the inertial impact of the inspiratory airflow, the deposition fraction of the drug particles in the mouth and throat gradually increased from 55.78% to 65.75%;
- When the peak inspiratory flow remained constant, the deposition fraction of drug particles in the mouth and throat first decreased and then increased as the incidence angle increased. Thus, there existed certain optimal incidence angles corresponding to the lowest deposition fractions at different peak inspiratory flows;
- When the angle of incidence and peak inspiratory flow remained constant, the deposition fraction positively correlated with the inspiration–press interval; therefore, inspiration–press synchronization was identified to be a critical factor;
- According to the test results corresponding to each component of the prototype designed based on the simulation results and the test results of the ideal prototype performance, the smart MDI for asthma developed in this study can reduce the deposition fraction of drug particles in the mouth and throat to a certain extent, thereby improving the efficacy of the drug.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, K.; Yang, T.; Xu, J.; Yang, L.; Zhao, J.; Zhang, X.; Bai, C.; Kang, J.; Ran, P.; Shen, H.; et al. Prevalence, risk factors, and management of asthma in China: A national cross-sectional study. Lancet 2019, 394, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Zhang, Q.; Li, C.; Lin, J. Prevalence of and risk factors for asthma among people aged 45 and older in China: A cross-sectional study. BMC Pulm. Med. 2021, 21, 311. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Deng, L.; Lu, C.; Li, Y.; Norbäck, D. Parental stress and air pollution increase childhood asthma in China. Environ. Res. 2018, 165, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Song, P.; Zhu, Y.; Lei, H.; Chan, K.Y.; Campbell, H.; Theodoratou, E.; Rudan, I.; Global Health Epidemiology Research Group. The disease burden of childhood asthma in China: A systematic review and meta-analysis. J. Glob. Health 2020, 10, 010801. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Xu, Y. Internal Medicine, 8th ed.; People’s Medical Publishing House: Beijing, China, 2013. [Google Scholar]
- Ebmeier, S.; Thayabaran, D.; Braithwaite, I.; Bénamara, C.; Weatherall, M.; Beasley, R. Trends in international asthma mortality: Analysis of data from the WHO Mortality Database from 46 countries (1993–2012). Lancet 2017, 390, 935–945. [Google Scholar] [CrossRef]
- Lundbäck, B.; Backman, H.; Lötvall, J.; Rönmark, E. Is asthma prevalence still increasing? Expert Rev. Respir. Med. 2016, 10, 39–51. [Google Scholar] [CrossRef]
- Feng, X. Epidemiological Investigation of Bronchial Asthma Prevalence and Related Risk Factors in China; Peking Union Medical College: Beijing, China, 2014. [Google Scholar]
- Zhou, X.; Shen, H.; Zhong, N. Guidelines for bronchial asthma prevent and management (2020 edition). Chin. J. Tuberc. Respir. Dis. 2020, 43, 26. [Google Scholar]
- Cheng, Y. Inhalation Formulation 10 Billion Yuan Market Pattern Is Changing. Medical Report. 2021, 22, 349. [Google Scholar]
- Kim, Y.; Li, D.; Park, S.; Yi, D.; Yeoh, G.; Abbas, A. Computational investigation of particle penetration and deposition pattern in a realistic respiratory tract model from different types of dry powder inhalers. Int. J. Pharm. 2021, 612, 121293. [Google Scholar] [CrossRef]
- De Boer, A.H.; Thalberg, K. Metered dose inhalers (MDIs). In Inhaled Medicines; Academic Press: Cambridge, MA, USA, 2021; pp. 65–97. [Google Scholar]
- Yousefi, M.; Inthavong, K.; Tu, J. Effect of Pressurized Metered Dose Inhaler Spray Characteristics and Particle Size Distribution on Drug Delivery Efficiency. J. Aerosol Med. Pulm. Drug Deliv. 2017, 30, 359–372. [Google Scholar] [CrossRef]
- Lim, S.H.; Park, S.; Lee, C.C.; Ho, P.C.L.; Kang, L. A 3D Printed Human Upper Respiratory Tract Model for Particulate Deposition Profiling. Int. J. Pharm. 2021, 597 (Suppl. S2), 120307. [Google Scholar] [CrossRef] [PubMed]
- Stein, S.W.; Sheth, P.; Hodson, P.D.; Myrdal, P.B. Advances in Metered Dose Inhaler Technology: Hardware Development. AAPS PharmSciTech 2014, 15, 326–338. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, K.W.; Guentsch, E.; Hoskinson, M.; Finlay, W.H. On the suitability of k–ε turbulence modeling for aerosol deposition in the mouth and throat: A comparison with experiment. J. Aerosol Sci. 2000, 31, 739–749. [Google Scholar] [CrossRef]
- Zhang, Y.; Finlay, W.H.; Matida, E. Particle deposition measurements and numerical simulation in a highly idealized mouth–throat. J. Aerosol Sci. 2004, 35, 789–803. [Google Scholar] [CrossRef]
- Cui, X.; Ge, H.; Song, W.; Wang, L.; Feng, Y.; Wang, J. Large eddy simulation of gas flow characteristics in the whole respiratory tract. J. Med. Biomech. 2021, 36 (Suppl. S1), 97. [Google Scholar]
- Shen, Y.; Ji Zhe, W. Numerical modeling of particulate matter deposition in the respiratory tract. Med. Biomech. 2016, 31, 193–198. [Google Scholar]
- Cunningham, E. On the velocity of steady fall of spherical particles through fluid medium. Proc. R. Soc. Lond. Ser. A Contain. Pap. A Math. Phys. Character 1910, 83, 357–365. [Google Scholar]
- Wu, X.; Zhang, W.; Chen, F.; Li, M.; Ding, Y. Effect of Improved-Seven-step Method for Inhalation. Pharm. Clin. Res. 2020, 28, 286–288. [Google Scholar] [CrossRef]
- Deeks, E.D.; Lyseng-Williamson, K.A. K-haler® breath-triggered inhaler: A profile of the properties of the device. Drugs Ther. Perspect. 2019, 35, 315–320. [Google Scholar] [CrossRef]
- Zou, W.; Zhao, C. Performance comparison of flexible pressure sensors in breathing airflow detection. Electron. Meas. Technol. 2020, 43, 153–158. [Google Scholar] [CrossRef]
- Prime, D.; De Backer, W.; Hamilton, M.; Cahn, A.; Preece, A.; Kelleher, D.; Baines, A.; Moore, A.; Brealey, N.; Moynihan, J. Effect of disease severity in asthma and chronic obstructive pulmonary disease on inhaler-specific inhalation profiles through the ELLIPTA® dry powder inhaler. J. Aerosol Med. Pulm. Drug Deliv. 2015, 28, 486–497. [Google Scholar] [CrossRef] [PubMed]
- Farkas, Á.; Horváth, A.; Kerekes, A.; Nagy, A.; Kugler, S.; Tamási, L.; Tomisa, G. Effect of delayed pMDI actuation on the lung deposition of a fixed-dose combination aerosol drug. Int. J. Pharm. 2018, 547, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Alatrash, A.; Matida, E. Characterization of medication velocity and size distribution from pressurized metered-dose inhalers by phase Doppler anemometry. J. Aerosol Med. Pulm. Drug Deliv. 2016, 29, 501–513. [Google Scholar] [CrossRef]
- Liu, X.; Doub, W.H.; Guo, C. Evaluation of metered dose inhaler spray velocities using Phase Doppler Anemometry (PDA). Int. J. Pharm. 2012, 423, 235–239. [Google Scholar] [CrossRef]
- Oliveira, R.F.; Teixeira, S.; Silva, L.F.; Teixeira, J.C.; Antunes, H. (Eds.) Study of a pressurized metered-dose inhaler spray parameters in fluent. In Proceedings of the World Congress on Engineering (WCE 2010), London, UK, 30 June–2 July 2010. [Google Scholar]
- Yaqoubi, S.; Chan, H.K.; Nokhodchi, A.; Dastmalchi, S.; Alizadeh, A.A.; Barzegar-Jalali, M.; Adibkia, K.; Hamishehkar, H. A quantitative approach to predicting lung deposition profiles of pharmaceutical powder aerosols. Int. J. Pharm. 2021, 602, 120568. [Google Scholar] [CrossRef] [PubMed]
- Kwok, P.C.L.; Salama, R.O.; Chan, H.K. Inhalation Drug Delivery: Techniques and Products; Colombo, P., Traini, D., Buttini, F., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Huang, F.; Zhou, X.; Dai, W.; Yu, J.; Zhou, Z.; Tong, Z.; Yu, A. In Vitro and In Silico Investigations on Drug Delivery in the Mouth-Throat Models with Handihaler. Pharm. Res. 2022, 39, 3005–3019. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.S.; Fu, C.S.; Yazzie, D.; Zhou, Y. Respiratory deposition patterns of salbutamol pMDI with CFC and HFA-134a formulations in a human airway replica. J. Aerosol Med. 2001, 14, 255–266. [Google Scholar] [CrossRef]
- Tao, X.; Zhao, J.; Duan, Y.; Liu, X.; Tang, Y. Current status of learning to use inhalation devices in children with asthma. Chin. J. Med. Guide 2021, 23, 603–605. [Google Scholar]






| Inspiratory Flow Q (L/min) | Mass Flow Rate q (kg/s) | Inspiratory Flow Q (L/min) | Mass Flow Rate q (kg/s) |
|---|---|---|---|
| 15 | 2.85 × 10−4 | 75 | 1.42 × 10−3 |
| 30 | 5.70 × 10−4 | 90 | 1.71 × 10−3 |
| 45 | 8.54 × 10−4 | 105 | 1.99 × 10−3 |
| 60 | 1.14 × 10−3 | 120 | 2.28 × 10−3 |
| Parameter Type | Numerical Value |
|---|---|
| Particle size distribution mode | Rosin–Rammler function |
| Minimum particle size (μm) | 1.22 |
| Maximum particle size (μm) | 49.50 |
| Mean particle size (μm) | 12.82 |
| Inspiratory Flow Qmax (L/min) | Deposition Fraction DF (%) | Inspiratory Flow Qmax (L/min) | Deposition Fraction DF (%) |
|---|---|---|---|
| 15 | 55.78 | 75 | 60.14 |
| 30 | 56.47 | 90 | 62.16 |
| 45 | 57.08 | 105 | 64.01 |
| 60 | 58.49 | 120 | 65.75 |
| Angle of incidence α (°) | 0 | 15 | 30 |
| Deposition fraction DF (%) | 56.47 | 40.96 | 31.53 |
| Test Flow (L/min) | Displayed Flow (L/min) | Relative Error (%) | Actual Angle of Incidence (°) | Displayed Angle of Incidence (°) |
|---|---|---|---|---|
| 120 | 116.52 | 2.90 | 36.62 | 36 |
| 105 | 102.16 | 2.70 | 34.77 | 34 |
| 90 | 87.41 | 2.88 | 32.65 | 32 |
| 75 | 72.82 | 2.91 | 30.69 | 30 |
| 60 | 58.83 | 1.95 | 29.33 | 29 |
| 45 | 43.97 | 2.29 | 28.95 | 29 |
| 30 | 29.03 | 3.23 | 29.68 | 30 |
| 15 | 14.32 | 4.53 | 31.29 | 31 |
| Peak Inspiratory Flow (L/Min) | Interval Time (s) | Press aerosol Canister? (Yes/No) |
|---|---|---|
| 120 | 0.1276 | Yes |
| 105 | 0.1340 | Yes |
| 90 | 0.1423 | Yes |
| 75 | 0.1542 | Yes |
| 60 | 0.1713 | Yes |
| 45 | 0.1998 | Yes |
| 30 | 0.2537 | Yes |
| 15 | 0.4219 | Yes |
| Peak Inspiratory Flow (L/min) | MDI-Administered Drug Deposition Fraction in the Mouth and Throat (%) | Difference (%) | |
|---|---|---|---|
| No Drug Delivery Device Was Used | Use of a Drug Delivery Device | ||
| 120 | 65.75 | 52.62 | 13.13 |
| 105 | 64.01 | 49.97 | 14.04 |
| 90 | 62.16 | 48.06 | 14.10 |
| 75 | 60.14 | 45.14 | 15.00 |
| 60 | 58.49 | 42.41 | 16.08 |
| 45 | 57.08 | 39.57 | 17.51 |
| 30 | 56.47 | 34.74 | 21.73 |
| 15 | 55.78 | 29.47 | 26.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Wei, M. Development of a Smart Metered-Dose Inhaler for Asthma Based on Computational Fluid Dynamics. Symmetry 2023, 15, 1712. https://doi.org/10.3390/sym15091712
Zhang Z, Wei M. Development of a Smart Metered-Dose Inhaler for Asthma Based on Computational Fluid Dynamics. Symmetry. 2023; 15(9):1712. https://doi.org/10.3390/sym15091712
Chicago/Turabian StyleZhang, Zhiguo, and Maoning Wei. 2023. "Development of a Smart Metered-Dose Inhaler for Asthma Based on Computational Fluid Dynamics" Symmetry 15, no. 9: 1712. https://doi.org/10.3390/sym15091712




