Through a Glass Darkly—Some Thoughts on Symmetry and Chemistry
Abstract
1. Introduction
Methods
2. Minerals Bend Light…
Plane and Circular Polarized Light
3. …and so to Biomaterials
4. The Atom Arrives!
4.1. The New Laws
4.1.1. The Law of Definite Proportion
4.1.2. The Law of Multiple Proportions
4.1.3. The Law of Reciprocal Proportion
4.2. The Atomic Theory of Dalton
4.3. From Atoms to Molecules
4.4. And So to Valency and Bonds
4.5. The Depiction of Molecules
4.6. The Importance of Models
4.7. Do Molecules Have Shape?
5. Introducing the Tetrahedral Carbon Atom
5.1. Back to Optical Activity
5.2. The Year Was 1874
5.3. The Cahn-Ingold-Prelog System
6. Other Asymmetric Atoms
6.1. Nitrogen
6.2. Phosphorus, Antimony and Arsenic
6.3. Sulfur
6.4. Other Atoms
7. Inorganic Chemistry Gets into the Act
8. Chirality and Other Parts of the Vocabulary
8.1. An Aside on Right- and Left-Handedness
8.2. Words in and Out of Context
8.3. Enter Chirality
8.4. When the Stereochemical World Came (Slowly) Tumbling Down
9. Chiral Molecules without Asymmetric Atoms
9.1. Axial Chirality
9.2. Helical Chirality—An Aside?
9.3. Planar Chirality
9.4. Topological Chirality
10. Order out of Chaos—Or Chaos out of Order?
11. Polemic or Iconoclasm? The Case for More Unity in Stereochemical Notation
11.1. Phenomenological Descriptors
11.2. The Case of Classical “Asymmetric” Carbon Atoms
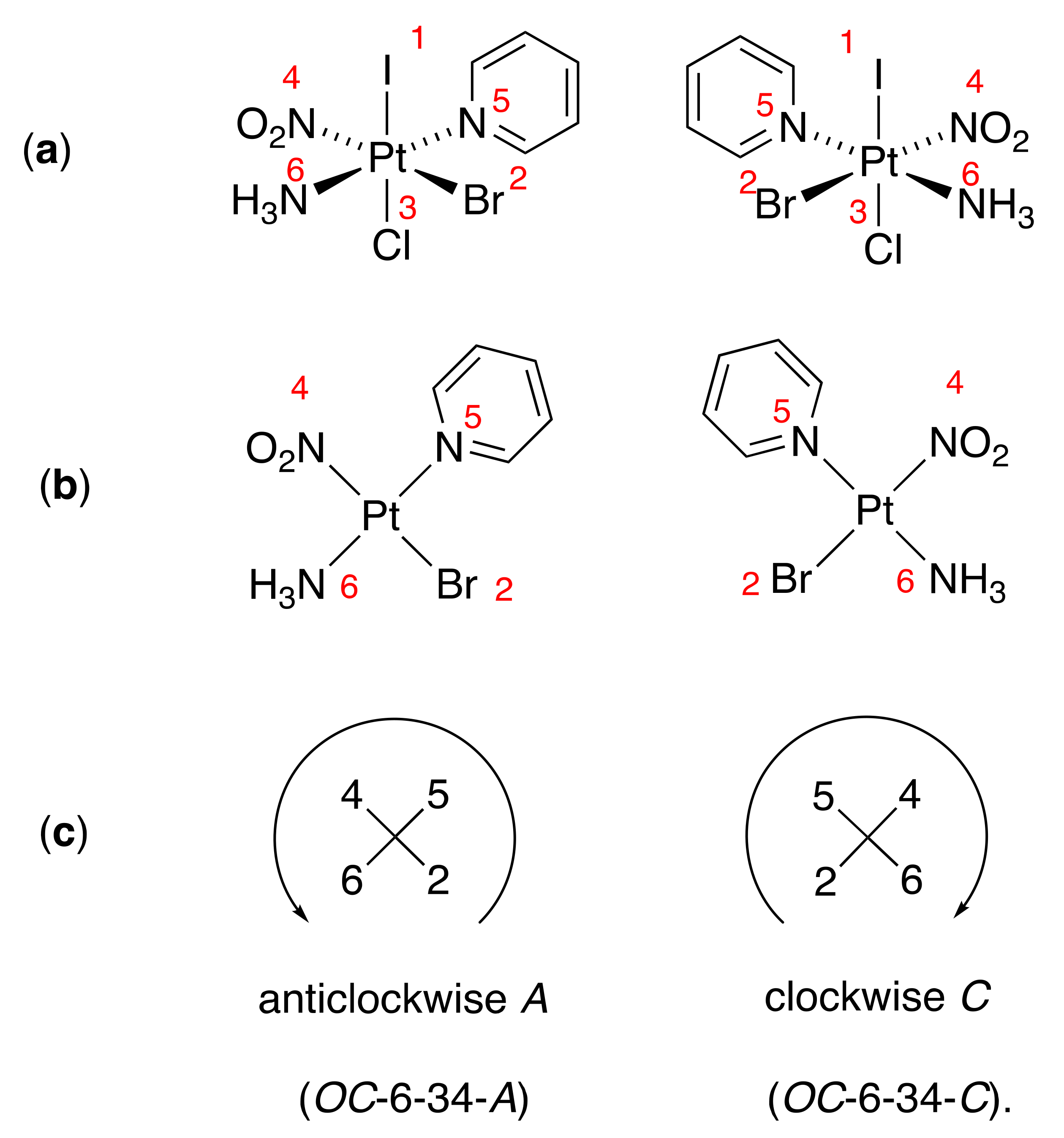
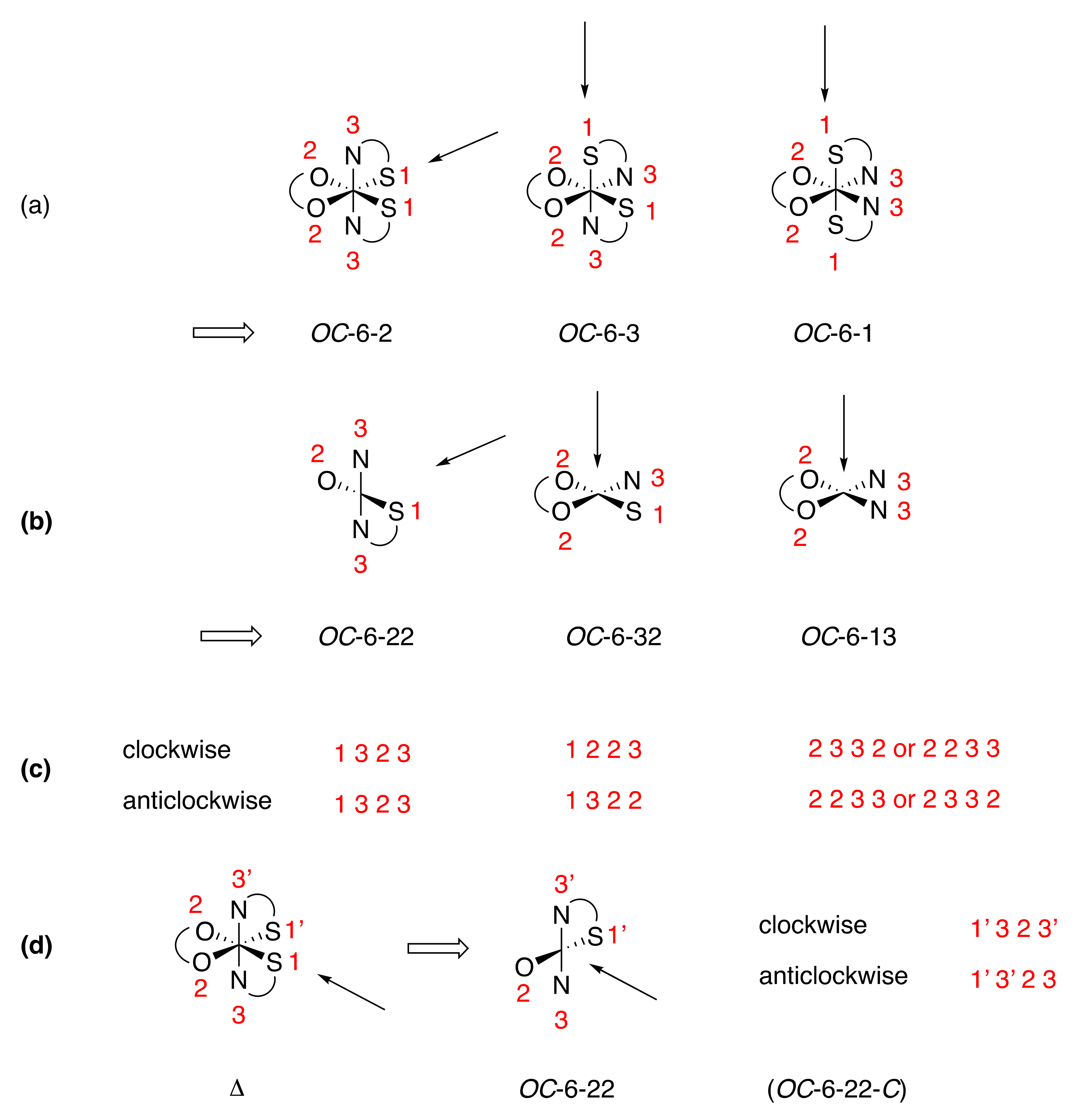
11.3. Lumpy Potatoes and Stereogenic Metal Centers
12. Conclusions and Closing Thoughts
Funding
Acknowledgments
Conflicts of Interest
References
- Aubert, M.; Brumm, A.; Ramli, M.; Sutikna, T.; Saptomo, E.W.; Hakim, B.; Morwood, M.J.; van den Bergh, G.D.; Kinsley, L.; Dosseto, A. Pleistocene Cave Art From Sulawesi, Indonesia. Nature 2014, 514, 223–227. [Google Scholar] [CrossRef]
- Pike, A.W.; Hoffmann, D.L.; García-Diez, M.; Pettitt, P.B.; Alcolea, J.; De Balbín, R.; González-Sainz, C.; de las Heras, C.; Lasheras, J.A.; Montes, R.; et al. U-Series Dating of Paleolithic Art in 11 Caves in Spain. Science 2012, 336, 1409–1413. [Google Scholar] [CrossRef]
- Graves, R. The Greek Myths; Penguin: London, UK, 2011; ISBN 9780241952740. [Google Scholar]
- Carroll, L. Through the Looking Glass, and What Alice Found There; Macmillan and Co.: London, UK, 1871. [Google Scholar]
- Mason, S.F. The Foundations of Classical Stereochemistry. Top. Stereochem. 1976, 9, 1–34. [Google Scholar] [CrossRef]
- Ramberg, P.J. Chemical Structure, Spatial Arrangement: The Early History of Stereochemistry, 1874–1914; Ashgate: Aldershot, UK, 2003; ISBN 0754603970. [Google Scholar]
- Bartholini, E. Experimenta Crystalli Islandici Disdiaclastici Quibus Mira and Insolita Refractio Detegitur; Daniel Paulli: Copenhagen, Denmark, 1669. [Google Scholar]
- Bartholin, E. An Account of Sundry Experiments Made and Communicated By That Learn’d Mathematician, Dr. Erasmus Bartholin, Upon a Chrystal-Like Body, Sent to Him Out of Island. Philos. Trans. R. Soc. Lond. 1670, 5, 2139–2148. [Google Scholar] [CrossRef]
- Bartholin, E. Experiments With the Double Refracting Iceland Crystal Which Led to the Discovery of a Marvelous and Strange Refraction; Brandt: Westtown, PA, USA, 1959. [Google Scholar]
- Buchwald, J.Z. Experimental Investigations of Double Refraction From Huygens to Malus. Arch. Hist. Exact Sci. 1980, 21, 311–373. [Google Scholar] [CrossRef]
- Huyghens, C. Traité de la Lumière. où Sont Expliquées les Causes de ce Qui Luy Arrive Dans la Reflexion, & Dans la Refraction. et Particulierement dans L’etrange Refraction du Criystal D’islande; Pierre Vander AA: Leiden, The Netherlands, 1690. [Google Scholar]
- Huygens, C. Treatise on Light. In Which Are Explained the Causes of that Which Occurs in Reflexion, & in Refraction. And Particularly in the Strange Refraction of Iceland Crystal; MacMillan and Co. Ltd.: London, UK, 1912. [Google Scholar]
- Huyghens, C. Traité de la Lumière; Gauthiers-Villars et Cie: Paris, France, 1920. [Google Scholar]
- Ward, R. The Development of the Polarimeter in Relation to Problems in Pure and Applied Chemistry: An Aspect of Nineteenth Century Scientific Instrumentation. Ph.D. Dissertation, University of London, London, UK, 1980. [Google Scholar]
- Landolt, H.; Robb, D.C.; Veley, V.H. The Handbook of the Polariscope; MacMillan and Co.: London, UK, 1882. [Google Scholar]
- Landolt, H. Das Optische Drehungsvermögen Organische Substanzen und die Praktische Anwendungen Desselben für Chemiker, Physiker und Zuckertechniker; Friedrich Vieweg und Sohn: Braunschweig, Germany, 1879. [Google Scholar]
- Landolt, H.; Schönrock, O.; Lindner, P.; Schütt, F.; Berndt, L.; Posner, T. Das Optische Drehungsvermögen Organische Substanzen und Dessen Praktische Anwendungen, 2nd ed.; Friedrich Vieweg und Sohn: Braunschweig, Germany, 1898. [Google Scholar]
- Landolt, H.; Schönrock, O.; Lindner, P.; Schütt, F.; Berndt, L.; Posner, T.; Long, J.H. The Optical Rotating Power of Organic Substances and Its Practical Applications; The Chemical Publishing Co.: Washington, USA, 1902. [Google Scholar]
- Newton, I. Opticks: Or, a Treatise of the Reflexions, Refractions, Inflexions and Colours of Light. Also Two Treatises of the Species and Magnitude of Curvilinear Figures; Sam Smith and Benj. Walford, Printers to the Royal Society: London, UK, 1704. [Google Scholar]
- Newton, I. Opticks: Or, a Treatise of the Reflexions, Refractions, Inflexions and Colours of Light. The Fourth Edition, Corrected; William Innys: London, UK, 1730. [Google Scholar]
- Hecht, E. Optics. Global Edition, 5th ed.; Pearson Education Limited: Harlow, UK, 2017. [Google Scholar]
- Frankel, E. The Search for a Corpuscular Theory of Double Refraction: Malus, Laplace and the Prize Competition of 1808. Centaurus 1974, 18, 223–244. [Google Scholar] [CrossRef] [PubMed]
- Frankel, E. Corpuscular Optics and the Wave Theory of Light: The Science and Politics of a Revolution in Physics. Soc. Stud. Sci. 1976, 6, 141–184. [Google Scholar] [CrossRef]
- Young, T. The Bakerian Lecture: Experiments and Calculations Relative to Physical Optics. Phil. Trans. R. Soc. Lond. 1804, 94, 1–16. [Google Scholar]
- Young, T. A Course of Lectures on Natural Philosophy and the Mechanical Arts; J. Johnson: London, UK, 1807. [Google Scholar]
- Young, T. An Account of Some Cases of the Production of Colours, Not Hitherto Described. Phil. Trans. R. Soc. Lond. 1802, 92, 387–397. [Google Scholar] [CrossRef]
- Malus, E.L. Sur Une Proprieé De La Lumière Réfléchie Par Des Corps Diaphanes. Mém. Soc. D’arcueil 1809, 2, 143. [Google Scholar]
- Fresnel, A.; de Senarmont, H.; Verdet, E.; Fresnel, L. Oeuvres Compleèes D’Augustin Fresnel, Henri De Senaramont, Emile Verdet and Leonor Fresnel Vol. 1; Imprimerie Impériale: Paris, France, 1870. [Google Scholar]
- Fresnel, A.; de Senarmont, H.; Verdet, E.; Fresnel, L. Oeuvres Compleèes D’Augustin Fresnel, Henri de Senaramont, Emile Verdet and Leonor Fresnel Vol. 2; Imprimerie Impériale: Paris, France, 1868. [Google Scholar]
- Fresnel, A.; de Senarmont, H.; Verdet, E.; Fresnel, L. Oeuvres Compleèes D’Augustin Fresnel, Henri de Senaramont, Emile Verdet and Leonor Fresnel Vol. 3; Imprimerie Impériale: Paris, France, 1866. [Google Scholar]
- Arago, F. Oevres De François Arago; Gide and T.O. Weigel: Paris, France; Leipzig, Germany, 1858. [Google Scholar]
- Buchwald, J.Z. The Battle between Arago and Biot Over Fresnel. J. Opt. 1989, 20, 109–117. [Google Scholar] [CrossRef]
- Leclercq, F. Biot, la Polarisation Chromatique et la Théorie Des Accès. Rev. D’histoire Sci. 2011, 64, 121–156. [Google Scholar] [CrossRef]
- Biot, J. Phénomènes De Polarisation Succesive, Observés Dans Des Fluides Homogènes. Bull. Soc. Philomath. 1815, 4, 190–193. [Google Scholar]
- Biot, J. Phénomènes De Polarisation Succesive, Observés Dans Des Fluides Homogènes. J. Mines 1815, 38, 205–210. [Google Scholar]
- Biot, J.-B. Traité de Physique Experimentale et Mathematique; Deterville: Paris, France, 1816. [Google Scholar]
- Herschel, J.F.W. On the Rotation Impressed by Plates of Rock Crystal on the Planes of Polarization of the Rays of Light, as Connected with Certain Peculiarities in Its Crystallization. Trans. Camb. Phil. Soc. 1820, 1, 43–53. [Google Scholar]
- Haüy, R.-J. Traité de Miéralogie, 2nd ed.; Bachelier: Paris, France, 1822; Volume 4. [Google Scholar]
- Haüy, R.-J. Traité de Miéralogie; Louis: Paris, France, 1801; Volume 4. [Google Scholar]
- Haüy, R.-J. Essai D’Une Théorie sur la Structure des Crystaux. Appliquée À Plusieurs Genres de Substances Crystallisées; Gogué & Néé de la Rochelle: Paris, France, 1784. [Google Scholar]
- Haüy, R.-J. Tableau Comparatif des Résultats de la Cristallographie et de L’Analyse Chimique, Relativement À la Classification des Minéraux; Courcier: Paris, France, 1809. [Google Scholar]
- Pullman, B. The Atom in the History of Human Thought. Transl. By Axel Reisinger; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- Meinel, C. Early Seventeenth-Century Atomism: Theory, Epistemology, and the Insufficiency of Experiment. ISIS 1988, 79, 68–103. [Google Scholar] [CrossRef]
- Chalmers, A. The Scientist’s Atom and the Philosopher’s Stone: How Science Succeeded and Philosophy Failed to Gain Knowledge of Atoms; Springer: Dordrecht, The Netherlands, 2009; ISBN 9789048123629. [Google Scholar]
- Hudson, R. What Was Perrin Really Doing in His Proof of the Reality of Atoms. HOPOS J. Int. Soc. Hist. Phil. Sci. 2020, 10, 194–218. [Google Scholar] [CrossRef]
- Banchetti-Robino, M.P. The Chemical Philosophy of Robert Boyle; Oxford University Press: New York, NY, USA, 2020; ISBN 9780197502501. [Google Scholar]
- Newman, W. The Significance of “chymical Atomism”. Early Sci. Med. 2009, 14, 248–264. [Google Scholar] [CrossRef]
- Proust, J.L. Extrait D’un Mémoire Intitulé: Recherches Fuste Bleu De Prusse, Par Proust. J. Phys. Chim. Hist. Nat. Arts 1794, 2, 334–341. [Google Scholar]
- Proust, J.L. Extrait D’un Mémoire Intitulé: Recherches Fuste Bleu De Prusse, Par Proust. Ann. Chim. 1797, 23, 85–101. [Google Scholar]
- Higgins, W. A Comparative View of the Phlogistic and Antiphlogistic Theories. With Inductions. To Which is Annexed an Analysis of the Human Calculus With Observations on Its Origin Etc; J. Murray: London, UK, 1789. [Google Scholar]
- Higgins, W. A Comparative View of the Phlogistic and Antiphlogistic Theories. With Inductions. To Which is Annexed an Analysis of the Human Calculus With Observations on Its Origin Etc, 2nd ed.; J. Murray: London, UK, 1791. [Google Scholar]
- Higgins, W. Experiments and Observations on the Atomic Theory and Electrical Phenomena; Graisberry and Campbell: Dublin, Ireland, 1814. [Google Scholar]
- Higgins, W. On the Origin of the Atomic Theory. Philos. Mag. 1816, 48, 363–371. [Google Scholar] [CrossRef]
- Higgins, W. On the Origin of the Atomic Theory. Philos. Mag. 1816, 48, 408–417. [Google Scholar] [CrossRef]
- Higgins, W. Remarks on a Paper By Mr. Dalton on the Chemical Compounds of Azote and Oxygen, &c. Philos. Mag. 1817, 49, 241–250. [Google Scholar] [CrossRef][Green Version]
- Higgins, W. On the Atomic Theory. Philos. Mag. 1818, 51, 161–172. [Google Scholar] [CrossRef][Green Version]
- Higgins, W. On the Atomic Theory. Philos. Mag. 1818, 51, 81–91. [Google Scholar] [CrossRef]
- Higgins, W. On Dr. Murray’s Statement Respecting the Origin of the Doctrine of Definite Proportions, and the Arrangement of the Elementary Principles of Chemical Compounds. Philos. Mag. 1819, 53, 401–410. [Google Scholar] [CrossRef]
- Nash, J. The Discovery of the Atomic Theory Claimed for Mr. Higgins. Philos. Mag. 1814, 43, 54–57. [Google Scholar] [CrossRef][Green Version]
- Meldrum, A.N. Two Great Irish Chemists. Bryan Higgins (1737–1820) and William Higgins (D 1825). New Irel. Rev. 1909, 32, 350–364. [Google Scholar]
- Meldrum, A.N. Two Great Irish Chemists. Bryan Higgins (1737–1820) and William Higgins (D 1825). New Irel. Rev. 1910, 32, 275–286. [Google Scholar]
- Meldrum, A.N. The Development of the Atomic Theory: (7) the Rival Claims of William Higgins and John Dalton. Mem. Manch. Phil. Soc. 1911, 55, 1–11. [Google Scholar]
- White, J.H. William Higgins and the Atomic Hypothesis. Sci. Prog. Twent. Century (1919–1933) 1929, 24, 300–306. [Google Scholar] [CrossRef]
- Reilly, J.; MacSweeney, D.T. William Higgins, a Pioneer of the Atomic Theory. Sci. Proc. R. Dublin. Soc. 1929, 19, 139–157. [Google Scholar]
- Partington, J.R. William Higgins and John Dalton. Nature 1951, 167, 120–121. [Google Scholar] [CrossRef]
- Soddy, F. William Higgins and John Dalton. Nature 1951, 167, 734–735. [Google Scholar] [CrossRef] [PubMed]
- Partington, J.R. William Higgins and John Dalton. Nature 1951, 167, 735–736. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, T.S. William Higgins, Chemist (1763–1825). Endeavour 1952, 11, 47–52. [Google Scholar] [CrossRef]
- Wheeler, T.S. William Higgins, Chemist (1763–1825). Part 1. Studies 1954, 43, 78–91. [Google Scholar] [CrossRef]
- Wheeler, T.S. William Higgins, Chemist (1763–1825). Part 2. Studies 1954, 43, 207–218. [Google Scholar] [CrossRef]
- Wheeler, T.S. William Higgins, Chemist (1763–1825). Part 3. Studies 1954, 43, 327–338. [Google Scholar] [CrossRef]
- Partington, J.R. William Higgins, Chemist (1763–1825). Nature 1955, 176, 8–9. [Google Scholar] [CrossRef]
- Wheeler, T.S.; Partington, J.R. The Life and Work of William Higgins, Chemist (1763–1825); Pergamon: Oxford, UK, 1960. [Google Scholar]
- Higgins, W.; Wheeler, T.S.; Partington, J.R. The Life and Work of William Higgins, Chemist, 1763-1825 ncluding Reprints of “a Comparative View of the Phlogistic and Antiphlogistic Theories” and “Observations on the Atomic Theory and Electrical Phenomena”; Pergamon Press: New York, NY, USA, 1960. [Google Scholar]
- Grossman, M.I. William Higgins At the Dublin Society, 1810–20: The Loss of a Professorship and a Claim to the Atomic Theory. Notes Rec. R. Soc. Lond. 2010, 64, 417–434. [Google Scholar] [CrossRef]
- Grossman, M.I. John Dalton and the London Atomists: William and Bryan Higgins, William Austin, and New Daltonian Doubts About the Origin of the Atomic Theory. Notes Rec. R. Soc. Lond. 2014, 68, 339–356. [Google Scholar] [CrossRef][Green Version]
- Grossman, M.I. John Dalton and the Origin of the Atomic Theory: Reassessing the Influence of Bryan Higgins. Br. J. Hist. Sci. 2017, 50, 657–676. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, W.K. Memoir of Bryan Higgins, M.d., and of William Higgins, Professor of Chemistry to the Royal Dublin Society; With a Short Notice of Irish Chemists and the State of Chemistry in Ireland Before the Year 1800; Hodges and Smith: Dublin, Ireland, 1849. [Google Scholar]
- Dalton, J. Foundations of the Atomic Theory Comprising Papers & Extracts By Dalton, Wollaston and Thomson; W. Clay: Edinburgh, UK, 1899. [Google Scholar]
- Dalton, J. A New System of Chemical Philosophy Vol. 1, Part 1; R. Bickerstaff: London, UK, 1808. [Google Scholar]
- Dalton, J. A New System of Chemical Philosophy Vol. 1, Part 2; R. Bickerstaff: London, UK, 1810. [Google Scholar]
- Dalton, J. A New System of Chemical Philosophy Vol. 2; R. Bickerstaff: London, UK, 1827. [Google Scholar]
- Wenzel, C.F. Lehre von der Verwandtschaft der Körper; Gotthelf August Gerlach: Dresden, Germany, 1777. [Google Scholar]
- Partington, J.R. Jeremias Benjamin Richter and the Law of Reciprocal Proportions.—I. Ann. Sci. 1951, 7, 173–198. [Google Scholar] [CrossRef]
- Partington, J.R. Jeremias Benjamin Richter and the Law of Reciprocal Proportions.—II. Ann. Sci. 1953, 9, 289–314. [Google Scholar] [CrossRef]
- Richter, J.B. Anfangsgründe der Stöchyometrie Oder Messkunst Chymischer Elemente, 3 Parts; Johann Friedrich Korn dem Eltern: Breslau, Germany; Hirschberg, Germany, 1792. [Google Scholar]
- Richter, J.B. Ueber Die Neuern Gegenstände der Chymie; Johann Friedrich Korn: Bresslau, Germany; Hirschberg, Germany, 1792; Volume 11. [Google Scholar]
- Hess, M. On the Scientific Labours of Jeremias Benjamin Richter. Addressed to the Imperial Academy of Sciences of St. Petersburg, At the Public Sitting of Dec. 29, 1840. Philos. Mag. 1842, 21, 81–96. [Google Scholar] [CrossRef][Green Version]
- Hess, M. Sur Les Travaux De Jérémie-Banjamin Richter. Recl Act. Acad. Sci. St. Petersb. 1841, 1840, 51–73. [Google Scholar] [CrossRef][Green Version]
- Rocke, A.J. Chemical Atomism in the Nineteenth Century. From Dalton to Cannizzaro; Ohio State University Press: Columbus, OH, USA, 1984. [Google Scholar]
- Rocke, A.J. Chemical Atomism the Evolution of Chemical Theory in the Nineteenth Century. In Tools and Modes of Representation in the Laboratory Sciences; Klein, U., Ed.; Springer: Dordrecht, Germany, 2001; Volume 222, pp. 1–12. ISBN 978-90-481-5859-1. [Google Scholar]
- Fleck, G. Atomism in Late Nineteenth-Century Physical Chemistry. J. Hist. Ideas 1963, 24, 106–114. [Google Scholar] [CrossRef]
- Thackray, A.W. The Origin of Dalton’s Chemical Atomic Theory: Daltonian Doubts Resolved. Isis 1966, 57, 35–55. [Google Scholar] [CrossRef]
- Dalton, J. On the Absorption of Gases By Water and Other Liquids. In Memoirs of the Literary and Philosophical Society of Manchester, Second Series. 1805, Volume 1, pp. 271–287. Available online: https://web.lemoyne.edu/~giunta/dalton52.html (accessed on 11 August 2021).
- Grossman, M.I. John Dalton’s “Aha” Moment: The Origin of the Chemical Atomic Theory. Ambix 2021, 68, 49–71. [Google Scholar] [CrossRef]
- Pratt, H.T. A Letter Signed: The Very Beginnings of Dalton’s Atomic Theory. Ambix 2010, 57, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Yourgrau, P. A World Without Time: The Forgotten Legacy of Gödel and Einstein; Basic Books: New York, NY, USA, 2004; ISBN 0465092934. [Google Scholar]
- Cercignani, C. Ludwig Boltzmann—The Man Who Trusted Atoms; Oxford University Press: Oxford, UK, 1998; ISBN 9780198501541. [Google Scholar]
- Duhem, P. La Science Allemande; A. Hermann & Fils: Paris, France, 1915. [Google Scholar]
- The Atomic Debates: Brodie and the Rejection of the Atomic Theory: Three Studies; Brock, W.H., Ed.; Leicester University Press: Leicester, UK, 1967. [Google Scholar]
- Philosophy of Chemistry: Synthesis of a New Discipline. Boston Studies in the Philosophy and History of Science; Scerri, E., McIntyre, L., Eds.; Springer: Dordrecht, The Netherlands, 2015; Volume 306, ISBN 978-94-017-9364-3. [Google Scholar]
- Rocke, A.J. The Reception of Chemical Atomism in Germany. Isis 1979, 70, 519–536. [Google Scholar] [CrossRef]
- Nye, M.J. Molecular Reality: A Perspective on the Scientific Work of Jean Perrin; Elsevier: New York, NY, USA, 1972. [Google Scholar]
- Chalmers, A.F. Atom and Aether in Nineteenth-Century Physical Science. Found. Chem. 2008, 10, 157–166. [Google Scholar] [CrossRef]
- Wurtz, A. The Atomic Theory. Transl. E. Cleminshaw; D. Appleton and Co.: New York, NY, USA, 1881. [Google Scholar]
- Perrin, J. Les Atomes; Libraire Félix Alcan: Paris, France, 1913. [Google Scholar]
- Patterson, G.D. Les Atomes: A Landmark Book in Chemistry. Found. Chem. 2010, 12, 223–233. [Google Scholar] [CrossRef]
- Needham, P. Resisting Chemical Atomism: Duhem’s Argument. Philos. Sci. 2008, 75, 921–931. [Google Scholar] [CrossRef]
- Needham, P. What is Water? Analysis 2000, 60, 13–21. [Google Scholar] [CrossRef]
- Zwier, K.R. Dalton’s Chemical Atoms Versus Duhem’s Chemical Equivalents. Philos. Sci. 2011, 78, 842–853. [Google Scholar] [CrossRef]
- Needham, P. Has Daltonian Atomism Provided Chemistry with Any Explanations. Philos. Sci. 2004, 71, 1038–1047. [Google Scholar] [CrossRef]
- Needham, P. When Did Atoms Begin to Do Any Explanatory Work in Chemistry. Int. Stud. Philos. Sci. 2004, 18, 199–219. [Google Scholar] [CrossRef]
- Needham, P. Was Duhem Justified in Not Distinguishing Between Physical and Chemical Atomism. Transversal Int. J. Hist. Sci. 2017, 2, 108. [Google Scholar] [CrossRef]
- Constable, E.C.; Housecroft, C.E. Before Radicals Were Free—The Radical Particulier of De Morveau. Chemistry 2020, 2, 19. [Google Scholar] [CrossRef]
- Constable, E.C.; Housecroft, C.E. Chemical Bonding: The Journey From Miniature Hooks to Density Functional Theory. Molecules 2020, 25, 2623. [Google Scholar] [CrossRef]
- Windler, S.C.H. Ueber Das Substitutionsgesetz Und Die Theorie Der Typen. Ann. Chem. Pharm. 1840, 33, 308–310. [Google Scholar] [CrossRef]
- Boyle, R. The Correspondence of Robert Boyle; Pickering & Chatto: London, UK, 2001; Volume 3. [Google Scholar]
- Berzelius, J.J. Essay on the Cause of Chemical Proportions, and on Some Circumstances Relating to Them; Together with a Short and Easy Method of Expressing Them. Ann. Philos. 1813, 2, 443–454. [Google Scholar]
- Berzelius, J.J. Essay on the Cause of Chemical Proportions, and on Some Circumstances Relating to Them; Together with a Short and Easy Method of Expressing Them. Ann. Philos. 1814, 3, 244–255. [Google Scholar]
- Berzelius, J.J. Essay on the Cause of Chemical Proportions, and on Some Circumstances Relating to Them; Together with a Short and Easy Method of Expressing Them. Ann. Philos. 1814, 3, 93–106. [Google Scholar]
- Berzelius, J.J. Essay on the Cause of Chemical Proportions, and on Some Circumstances Relating to Them; Together with a Short and Easy Method of Expressing Them. Ann. Philos. 1814, 3, 353–364. [Google Scholar]
- Berzelius, J.J. Essay on the Cause of Chemical Proportions, and on Some Circumstances Relating to Them; Together with a Short and Easy Method of Expressing Them. Ann. Philos. 1814, 3, 51–62. [Google Scholar]
- Goodman, D.C. Wollaston and the Atomic Theory of Dalton. Hist. Stud. Nat. Sci. 1969, 1, 37–59. [Google Scholar] [CrossRef]
- Thomson, T. A System of Chemistry, 3rd ed; Bell & Bradfute and E. Balfour: Edinburgh, UK, 1807; Volume 4. [Google Scholar]
- Wollaston, W.H. On Super-Acid and Sub-Acid Salts. Philos. Mag. 1808, 31, 276–281. [Google Scholar] [CrossRef]
- Wollaston, W.H. Xii. The Bakerian Lecture. On the Elementary Particles of Certain Crystals. Philos. Mag. 1813, 42, 61–69. [Google Scholar] [CrossRef][Green Version]
- Gerhardt, C.; Chancel, G.C.B. Ueber Die Constitution Der Organischen Verbindungen. J. Prakt. Chem. 1851, 53, 257–279. [Google Scholar] [CrossRef]
- Frankland, E. On a New Series of Organic Bodies Containing Metals. Phil. Trans. 1852, 142, 417–444. [Google Scholar]
- Couper, A.S. On a New Chemical Theory. Philos. Mag. (1798–1977) 1858, 16, 104–116. [Google Scholar] [CrossRef]
- Couper, A.S. Sur Une Nouvelle Théorie Chimique. Ann. Chim. Phys. 1858, 53, 469–489. [Google Scholar]
- Couper, A.S. Sur Une Nouvelle Théorie Chimique. C. R. Hebd. Seances Acad. Sci. 1858, 46, 1157–1160. [Google Scholar]
- Anschütz, R. Life and Chemical Work of Archibald Scott Couper. Proc. R. Soc. Edinburgh. 1909, 29, 193–273. [Google Scholar] [CrossRef]
- Kekulé, A. Ueber Die, S.G. Gepaarten Verbindungen Und Die Theorie Der Mehratomigen Radicale. Ann. Chem. Pharm 1857, 104, 129–150. [Google Scholar] [CrossRef]
- Kekulé, A. Ueber Die Constitution Und Die Metamorphosen Der Chemischen Verbindungen Und Über Die Chemische Natur Des Kohlenstoffs. Ann. Chem. Pharm 1858, 106, 129–159. [Google Scholar] [CrossRef]
- Rocke, A.J. Kekulé, Butlerov, and the Historiography of the Theory of Chemical Structure. Br. J. Hist. Sci. 1981, 14, 27–57. [Google Scholar] [CrossRef]
- Rocke, A.J. Subatomic Speculations and the Origin of Structure Theory. Ambix 1983, 30, 1–18. [Google Scholar] [CrossRef]
- Rocke, A.J. Image and Reality. Kekulé, Kopp, and the Scientific Imagination; University of Chicago Press: Chicago, IL, USA, 2010; ISBN 9780226723358. [Google Scholar]
- Russell, C.A. The History of Valency; Leicester University Press: Leicester, UK, 1971; ISBN 978-0391000339. [Google Scholar]
- Palmer, W.G. A History of the Concept of Valency; Cambridge University Press: Cambridge, UK, 1965. [Google Scholar]
- Butlerov, A. Einiges Über Die Chemische Structur der Körper. Z. Chem. 1861, 4, 549–560. [Google Scholar]
- Butlerov, A. Lehrbuch der Organischen Chemie: Zur Einführung in das Specielle Studium Derselben; Quandt & Händel: Leipzig, Germany, 1867. [Google Scholar]
- Montaudo, G. The Discovery of Tetrahedral Carbon: Contributions of Paternò and Cannizzaro. Gazz. Chim. Ital. 1997, 127, 837–842. [Google Scholar]
- Wislicenus, J. Ueber Die Isomeren Milchsäuren. Zweite Abhandlung. Ueber Die Optisch-Active Milchsäure der Fleischflüssigkeit, Die Paramilchsäure. Justus Liebigs Ann. Chem. 1873, 167, 302–346. [Google Scholar] [CrossRef]
- Crosland, M.P. Historical Studies in the Language of Chemistry; Heinemann: London, UK, 1963. [Google Scholar]
- Kekulé, M.A. Observations on Mr. Couper’s New Chemical Theory. Philos. Mag. 1858, 16, 478–479. [Google Scholar] [CrossRef]
- Leicester, H.M. Alexander Mikhaĭlovich Butlerov. J. Chem. Educ. 1940, 17, 203. [Google Scholar] [CrossRef]
- Loschmidt, J.J. Chemische Studien. I. A. Constitutions-Formeln der Organischen Chemie in Geographischer Darstellung. B. Das Mariott’sche Gesetz; Carl Ferold’s Sohn: Vienna, Austria, 1861. [Google Scholar]
- Meyer, L. Die Modernen Theorien der Chemie und Ihre Bedeutung für Die Chemische Statik; Maruschke & Berendt: Breslau, Germany, 1864. [Google Scholar]
- Meyer, L. Modern Theories of Chemistry. Translated From the German, 5th ed.; Bedson, P.P., Williams, W.C., Eds.; Longmans, Green, and Co: London, UK; New York, NY, USA, 1888. [Google Scholar]
- Brown, A.C. On the Theory of Isomeric Compounds. Trans. R. Soc. Edinb. 1864, 707–719. [Google Scholar] [CrossRef]
- Ramsay, O.B. Molecules in Three Dimensions I. Chemistry 1974, 47(1), 6–9. [Google Scholar]
- Ramsay, O.B. Molecules in Three Dimensions II. Chemistry 1974, 47(2), 6–11. [Google Scholar]
- Greenaway, F. John Dalton and the Atom; Heinemann: London, UK, 1966. [Google Scholar]
- Ramsay, O.B. Stereochemistry; Heyden: London, UK; Philadelphia, PA, USA; Rheine, Germany, 1981. [Google Scholar]
- Mauskopf, S.H. The Atomic Structural Theories of Ampère and Gaudin: Molecular Speculation and Avogadro’s Hypothesis. Isis 1969, 60, 61–74. [Google Scholar] [CrossRef]
- Ampère, A.M.; De Lettre, M.; Le Ampeèr, a.M. Comte Berthollet, Sur La Détermination Des Proportions Dans Lesquelles Les Corps Se Combinent D’Après Le Nombre Et La Disposition Respective Des Molécules Dont Leurs Particules Intégrantes Sont Composées. Ann. Chim. 1814, 90, 43–86. [Google Scholar]
- Gaudin, M.-A. L’Architecture du Monde des Atomes; Gauthier-Villars: Paris, France, 1873. [Google Scholar]
- Paternò, E. Intorno All’azione del Percloruro di Fosforo sul Clorale. G. Sci. Nat. Econ. 1869, 5, 117–122. [Google Scholar]
- Meyer, V. Ergebnisse Und Ziele Der Stereo-Chemischen Forschung. Ber. Dtsch. Chem. Ges. 1890, 23, 567–619. [Google Scholar] [CrossRef]
- Biot, J. Mémoire Sur La Polarization Circulaire Et Sur Ses Applications À La Chimie Organique. Mem. Acad.Sci. Inst. Fr. 1835, 13, 39–175. [Google Scholar]
- Biot, J. Pour Discerner Les Mélanges Et Les Combinaisons Chimiques Définies Ou Non Définies, Qui Agissent Sur La Lumière Polarisée; Suivies D’Applications Aux Combinaisons De L’Acide Tartarique Avec L’Eau, L’Alcool Et L’Esprit De Bois”. Mem. Acad.Sci. Inst. Fr. 1838, 15, 93–279. [Google Scholar]
- Findlay, A. Use of the Name ‘Racemic Acid. Nature 1937, 140, 22. [Google Scholar] [CrossRef]
- Gay-Lussac, J.L. Cours de Chimie Par M. Gay-Lussac Comprenant L’histoire des Sels, ia Chimie Végétale et Animale; Pichon et Didier: Paris, France, 1828. [Google Scholar]
- Flack, H.D. Louis Pasteur’s Discovery of Molecular Chirality and Spontaneous Resolution in 1848, Together With a Complete Review of His Crystallographic and Chemical Work. Acta Cryst. A 2009, 65, 371–389. [Google Scholar] [CrossRef] [PubMed]
- Pasteur, L. Researches on Molecular Asymmetry of Natural Organic Products. Alembic Club Reprints No. 14; The Alembic Club: Edinburgh, UK, 1897. [Google Scholar]
- Pasteur, L. Researches on the Molecular Asymmetry of Natural Organic Products. Of Natural Organic Products; The Alembic Club: Edinburgh, UK, 1905. [Google Scholar]
- Pasteur, L.; Cahours, A.; Wurtz, A.; Berthelot, M.; Barral, J.A.; Deville, S.-C.H.; Dumas. Leçons de Chimie Professés en 1860; L. Hachette et Cie: Paris, France, 1861. [Google Scholar]
- van’t Hoff, J.H. Sur Les Formules De Structure Dans L’espace. Arch. Neerl. Sci. Exactes Nat. 1874, 9, 445. [Google Scholar]
- van’t Hoff, J.H. Voorstel Tot Uitbreiding der Tegenwoordige in de Scheikunde Gebruikte Structuurformules in de Ruimte, Benevens Een Daarmee Samenhangende Opmerking Omtrent Het Verband Tusschen Optisch Actief Vermogen En Chemische Constitutie Van Organische Verbindi; Greven: Utrecht, The Netherlands, 1874. [Google Scholar]
- Le Bel, J.A. Sur Les Relations Qui Existent Entre Les Formules Atomiques Des Corps Organiques Et Le Pouvoir Rotatoire De Leurs Dissolutions. Bull. Soc. Chim. Fr. 1874, 22, 337–347. [Google Scholar]
- Riddell, F.G.; Robinson, M.J.T.; Van’t Hoff, J.H.; Le, J.A. Bel—their Historical Context. Tetrahedron 1974, 30, 2001–2007. [Google Scholar] [CrossRef]
- Gal, J. Louis Pasteur, Chemical Linguist: Founding the Language of Stereochemistry. Helv. Chim. Acta 2019, 102, e1900098. [Google Scholar] [CrossRef]
- Ramsay, O.B. Van’t Hoff-Le Bel Centennial; American Chemical Society: Washington, DC, USA, 1975. [Google Scholar]
- van’t Hoff, J.H. Die Lagerung der Atome Im Raume, 2nd ed.; Vieweg: Braunschweig, Germany, 1894. [Google Scholar]
- van’t Hoff, J.H. La Chimie Dans L’Espace; P.M. Bazendijk: Rotterdam, The Netherlands, 1875. [Google Scholar]
- van’t Hoff, J.H. Dix Années Dans L’histoire D’une Théorie; P.M. Bazendijk: Rotterdam, The Netherlands, 1887. [Google Scholar]
- van’t Hoff, J.H. The Arrangement of Atoms in Space, 2nd ed.; Eiloart, A., Ed.; Longmans, Green and Co.: London, UK, 1898. [Google Scholar]
- van’t Hoff, J.H. Die Lagerung Der Atome Im Raume; Herrmann, F., Ed.; Vieweg: Braunschweig, Germany, 1877. [Google Scholar]
- Maitland, P.; Mills, W.H. Experimental Demonstration of the Allene Asymmetry. Nature 1935, 135, 994. [Google Scholar] [CrossRef]
- Kolbe, H. Zeichen Der Zett. J. Prakt. Chem. 1877, 15, 473–477. [Google Scholar]
- Ramberg, P.J. Arthur Michael’s Critique of Stereochemistry, 1887–1899. Hist. Stud. Phys. Biol. Sci. 1995, 26, 89–138. [Google Scholar] [CrossRef]
- Snelders, H.A.M. The Reception of J. H. Van’t Hoff’s Theory of the Asymmetric Carbon Atom. J. Chem. Educ. 1974, 51, 2. [Google Scholar] [CrossRef]
- Van’t Hoff, J.H.; Springer, G.F. J. H. Van’t Hoff’s Inaugural Lecture: Imagination in Science. In Imagination in Science; Springer: Berlin, Heidelberg, 1967; pp. 6–18. ISBN 9783662377475. [Google Scholar]
- Rocke, A.J. The Quiet Revolution; University of California Press: Berkeley, CA, USA, 1993; ISBN 9780520081109. [Google Scholar]
- Rocke, A.J. Kolbe Versus the “Transcendental Chemists”: The Emergence of Classical Organic Chemistry. Ambix 1987, 34, 156–168. [Google Scholar] [CrossRef]
- Root-Bernstein, R.S. Visual Thinking: The Art of Imagining Reality. Trans. Am. Phil. Soc. 1985, 75, 50. [Google Scholar] [CrossRef]
- Barthelme, D.; Maxwell, W.; Borges, J.L.; Bernstein, J.; Stewart, D. The New Yorker, 1969, 9th August; New Yorker Magazine: Manhattan, NY, USA, 1969. [Google Scholar]
- Cahn, R.S.; Ingold, C.K. Specification of Configuration About Quadricovalent Asymmetric Atoms. J. Chem. Soc. 1951, 612. [Google Scholar] [CrossRef]
- Cahn, R.S.; Ingold, C.K.; Prelog, V. The Specification of Asymmetric Configuration in Organic Chemistry. Experientia 1956, 12, 81–94. [Google Scholar] [CrossRef]
- Cahn, R.S.; Ingold, C.; Prelog, V. Specification of Molecular Chirality. Angew. Chem. Int. Ed. Engl. 1966, 5, 385–415. [Google Scholar] [CrossRef]
- Moss, G.P. Basic Terminology of Stereochemistry (Iupac Recommendations 1996). Pure Appl. Chem. 1996, 68, 2193–2222. [Google Scholar] [CrossRef]
- IUPAC. Nomenclature of Organic Chemistry; Royal Society of Chemistry: Cambridge, UK, 2013; ISBN 9780854041824. [Google Scholar]
- Prelog, V.; Helmchen, G. Basic Principles of the Cip-System and Proposals for a Revision. Angew. Chem. Int. Ed. 1982, 21, 567–583. [Google Scholar] [CrossRef]
- Hantzsch, A.; Werner, A. Ueber Räumliche Anordnung Der Atome in Stickstoffhaltigen Molekülen. Ber. Dtsch. Chem. Ges. 1890, 23, 11–30. [Google Scholar] [CrossRef]
- Wedekind, E.; Klatte, K.A. Über Die Aktivierung Einer Asymmetrischen Tertiärbase in Gestalt Von Salzen Mit Optisch-Aktiven Säuren. (54. Mitteilung Über Das Asymmetrische Stickstoffatom). Ber. Dtsch. Chem. Ges. 1927, 60, 2325–2334. [Google Scholar] [CrossRef]
- Prelog, V.; Wieland, P. Über Die Spaltung Dertröger’Schen Base in Optische Antipoden, Ein Beitrag Zur Stereochemie Des Dreiwertigen Stickstoffs. Helv. Chim. Acta 1944, 27, 1127–1134. [Google Scholar] [CrossRef]
- Werner, A. Beitrag Zur Konstitution Anorganischer Verbindungen. Z. Anorg. Allg. Chem. 1893, 3, 267–330. [Google Scholar] [CrossRef]
- Pope, W.J.; Peachey, S.J. Asymmetric Optically Active Nitrogen Compounds. Dextro- and Lævo-A-benzylphenylallylmethylammonium Iodides and Bromides. J. Chem. Soc. Trans. 1899, 75, 1127–1131. [Google Scholar] [CrossRef]
- Meisenheimer, J. Eine Neue Art Von Asymmetrie Beim Stickstoffatom. Ber. Dtsch. Chem. Ges. 1908, 41, 3966–3976. [Google Scholar] [CrossRef]
- Meisenheimer, J.; Lichtenstadt, L. Über Optisch-Aktive Verbindungen Des Phosphors. Ber. Dtsch. Chem. Ges. 1911, 44, 356–359. [Google Scholar] [CrossRef]
- Holliman, F.G.; Mann, F.G. The Sterochemistry of Organic Derivatives of Phosphorus. Part Ii. The Synthesis of 2: 2-Disubstituted 1:2:3:4-Tetrahydroisophosphinolinium Salts and the Optical Resolution of 2-Phenyl-2-p-hydroxyphenyl-1:2:3:4-Tetrahydroisophosphinolinium Bromide. J. Chem. Soc. 1947, 1634. [Google Scholar] [CrossRef]
- Kumli, K.F.; McEwen, W.E.; Vander Werf, C.A. Resolution of a Non-Heterocyclic Quaternary Phosphonium Iodide. J. Am. Chem. Soc. 1959, 81, 248–249. [Google Scholar] [CrossRef]
- Horner, L.; Winkler, H.; Rapp, A.; Mentrup, A.; Hoffmann, H.; Beck, P. Phosphororganische Verbindungen Optisch Aktive Tertiäre Phosphine Aus Optisch Aktiven Quartären Phosphoniumsalzen. Tetrahedron Lett. 1961, 2, 161–166. [Google Scholar] [CrossRef]
- Campbell, I.G.M. The Configuration of Heterocyclic Compounds. Part Xi. Preparation of Phenoxstibines and Resolution of 10-P-carboxyphenyl-2-methylphenoxstibine. J. Chem. Soc. 1947, 4–10. [Google Scholar] [CrossRef]
- Campbell, I.G.M. The Configuration of Heterocyclic Antimony Compounds. Part I. Preparation of 9-Stibiafluorenes and Optical Resolution of 2-Carboxy-9-p-tolyl-9-stibiafluorene. J. Chem. Soc. 1950, 3109. [Google Scholar] [CrossRef]
- Campbell, I.G.M. The Configuration of Heterocyclic Antimony Compounds. Part Ii. Symmetric and Enantiomorphic 9-Stibiafluorenes. J. Chem. Soc. 1952, 4448. [Google Scholar] [CrossRef]
- Campbell, I.G.M.; Morrill, D.J. The Configuration of Heterocyclic Antimony Compounds. Part Iii. Resolution and Racemisation of New Members of the 9-Stibiafluorene Series. J. Chem. Soc. 1955, 1662. [Google Scholar] [CrossRef]
- Campbell, I.G.M. The Stereochemistry of Triarylstibines. Synthesis and Optical Resolution of P-Carboxyphenyl-2-diphenylylphenylstibine. J. Chem. Soc. 1955, 3116. [Google Scholar] [CrossRef]
- Campbell, I.G.M.; White, A.W. The Stereochemistry of Triarylstibines. Part Ii. Synthesis of Unsymmetrically Substituted Triarylstibines and Optical Resolution of P-Carboxyphenyl-1-naphthylphenylstibine. J. Chem. Soc. 1958, 1184. [Google Scholar] [CrossRef]
- Burrows, G.J.; Turner, E.E. Experiments on the Production of Compounds Containing Arsenic as a Centre of Optical Activity. J. Chem. Soc., Trans. 1921, 119, 426–437. [Google Scholar] [CrossRef]
- Mills, W.H.; Raper, R. The Resolution of an Asymmetric Arsenic Compound Into Its Optically Active Forms. J. Chem. Soc. Trans. 1925, 127, 2479–2483. [Google Scholar] [CrossRef]
- Horner, L.; Fuchs, H. Optisch Aktive Tertiäre Arsine Aus Optisch Aktiven Quartären Arsoniumsalzen. Tetrahedron Lett. 1962, 3, 203–204. [Google Scholar] [CrossRef]
- Phillips, H. Investigations on the Dependence of Rotatory Power on Chemical Constitution. Part Xxvii. The Optical Properties of N-Alkyl P-Toluenesulphinates. J. Chem. Soc. Trans. 1925, 127, 2552–2587. [Google Scholar] [CrossRef]
- Harrison, P.W.B.; Kenyon, J.; Phillips, H. The Dependence of Rotatory Power on Chemical Constitution. Part Xxix. The Resolution of Sulphoxides Into Their Optically Active Forms. J. Chem. Soc. 1926, 129, 2079–2090. [Google Scholar] [CrossRef]
- Constable, E.C.; Housecroft, C.E. Coordination Chemistry: The Scientific Legacy of Alfred Werner. Chem. Soc. Rev. 2013, 42, 1429–1439. [Google Scholar] [CrossRef]
- Constable, E.C. Stereogenic Metal Centres—From Werner to Supramolecular Chemistry. Chem. Soc. Rev. 2013, 42, 1637–1651. [Google Scholar] [CrossRef] [PubMed]
- Werner, A.; Vilmos, A. Beitrag Zur Konstitution Anorganischer Verbindungen. Xvii. Mitteilung. Über Oxalatodiäthylendiaminkobaltisalze. Z. Anorg. Allg. Chem. 1899, 21, 145–158. [Google Scholar] [CrossRef]
- Werner, A. Zur Kenntnis Des Asymmetrischen Kobaltatoms. I. Ber. Dtsch. Chem. Ges. 1911, 44, 1887–1898. [Google Scholar] [CrossRef]
- Werner, A. Zur Kenntnis Des Asymmetrischen Kobaltatoms. II. Ber. Dtsch. Chem. Ges. 1911, 44, 2445–2455. [Google Scholar] [CrossRef]
- Werner, A. Zur Kenntnis Des Asymmetrischen Kobaltatoms. III. Ber. Dtsch. Chem. Ges. 1911, 44, 3272–3278. [Google Scholar] [CrossRef]
- Werner, A. Zur Kenntnis Des Asymmetrischen Kobaltatoms. IV. Ber. Dtsch. Chem. Ges. 1911, 44, 3279–3284. [Google Scholar] [CrossRef]
- Werner, A. Zur Kenntnis Des Asymmetrischen Kobaltatoms. V. Ber. Dtsch. Chem. Ges. 1912, 45, 121–130. [Google Scholar] [CrossRef]
- Werner, A.; McCutcheon, T.P. Zur Kenntnis Des Asymmetrischen Kobaltatoms. VI. Ber. Dtsch. Chem. Ges. 1912, 45, 3281–3287. [Google Scholar] [CrossRef]
- Werner, A.; Shibata, Y. Zur Kenntnis Des Asymmetrischen Kobaltatoms. VII. Ber. Dtsch. Chem. Ges. 1912, 45, 3287–3293. [Google Scholar] [CrossRef]
- Werner, A.; Tschernoff, G. Zur Kenntnis Des Asymmetrischen Kobaltatoms. VIII. Ber. Dtsch. Chem. Ges. 1912, 45, 3294–3301. [Google Scholar] [CrossRef]
- Werner, A. Über Spiegelbild-Isomerie Bei Eisenverbindungen. Ber. Dtsch. Chem. Ges. 1912, 45, 433–436. [Google Scholar] [CrossRef]
- Werner, A. Über Spiegelbildisomerie Bei Rhodium-Verbindungen. I. Ber. Dtsch. Chem. Ges. 1912, 45, 1228–1236. [Google Scholar] [CrossRef]
- Werner, A. Zur Kenntnis Des Asymmetrischen Kobaltatoms IX. Ber. Dtsch. Chem. Ges. 1913, 46, 3674–3683. [Google Scholar] [CrossRef]
- Werner, A. Zur Kenntnis Des Asymmetrischen Kobaltatoms X. Ber. Dtsch. Chem. Ges. 1914, 47, 1961–1979. [Google Scholar] [CrossRef]
- Werner, A. Zur Kenntnis Des Asymmetrischen Kobaltatoms XI. Über Oxalo-Diäthylendiamin-kobaltisalze Und Eine Neue Spaltungsmethode Für Racemische Anorganische Verbindungen. Ber. Dtsch. Chem. Ges. 1914, 47, 2171–2182. [Google Scholar] [CrossRef]
- Werner, A. Zur Kenntnis Des Asymmetrischen Kobaltatoms XII. Über Optische Aktivität Bei Kohlenstofffreien Verbindungen. Ber. Dtsch. Chem. Ges. 1914, 47, 3087–3094. [Google Scholar] [CrossRef]
- Kauffman, G.B. The Discovery of Optically Active Coordination Compounds: A Milestone in Stereochemistry. Isis 1975, 66, 38–62. [Google Scholar] [CrossRef]
- Kauffman, G.B. A Stereochemical Achievement of the First Order: Alfred Werner’s Resolution of Cobalt Complexes, 85 Years Later. Bull. Hist. Chem. 1997, 20, 50–59. [Google Scholar]
- Ernst, K.H.; Berke, H. Optical Activity and Alfred Werner’s Coordination Chemistry. Chirality 2011, 23, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Essen, L.N.; Gel’man, A.D. Preparation of Complexes of Quadrivalent Platinum With Six Different Substituents. Zh. Neorg. Khim. 1956, 1, 2475–2487. [Google Scholar]
- Essen, L.N.; Gel’man, A.D. The Preparation of Quadrivalent Platinum Complex Compounds With Five and Six Different Substituents in the Inner Spheres of the Trans and Cis Configurations. Proc. Acad. Sci. USSR 1956, 108, 309–312. [Google Scholar]
- Essen, L.N.; Gel’man, A.D. The Preparation of Quadrivalent Platinum Complex Compounds With Five and Six Different Substituents in the Inner Spheres of the Trans and Cis Configurations. Dokl. Akad. Nauk SSSR 1956, 108, 651–654. [Google Scholar]
- Essen, L.N.; Zakharova, F.A.; Gel’man, A.D. Synthesis of Isomers With Six Different Substituents. Zh. Neorg. Khim. 1958, 3, 2654–2661. [Google Scholar]
- Essen, L.N.; Zakharova, F.A.; Gel’man, A.D. Preparation of new geometric isomers of [PtBrClINO2NH3py]. Zh. Neorg. Khim. 1967, 12, 1405–1406. [Google Scholar]
- Spitsyn, V.I. Fifty years of development of inorganic chemistry in the Soviet Union. Russ. Chem. Rev. 1967, 36, 808–817. [Google Scholar] [CrossRef]
- Essen, L.N.; Bukhtiyarova, T.N. Preparation of New Geometric Isomers of [PtBrClino2NH3py]. Zh. Neorg. Khim. 1967, 12, 1405–1406. [Google Scholar]
- Gel’dman, A.D.; Essen, L.N. Complex Compounds of Quadrivalent Platinum With Six Different Substituents in the Inner Sphere. Dokl. Akad. Nauk SSSR 1950, 75, 693–695. [Google Scholar]
- Drayer, D.E. The Early History of Stereochemistry: From the Discovery of Molecular Asymmetry and the First Resolution of a Racemate By Pasteur to the Asymmetrical Chiral Carbon of Van’t Hoff and Le Bel. In Drug Stereochemistry: Analytical Methods and Pharmacology, 2nd ed.; Wainer, I.W., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1993; pp. 1–24. [Google Scholar]
- Drayer, D.E. The Early History of Stereochemistry: From the Discovery of Molecular Asymmetry and the First Resolution of a Racemate By Pasteur to the Asymmetrical Chiral Carbon of Van’t Hoff and Le Bel. Clin. Res. Regul. Aff. 2001, 18, 181–203. [Google Scholar] [CrossRef]
- Meijer, E.W. Jacobus Henricus Van ‘t Hoff; Hundred Years of Impact on Stereochemistry in the Netherlands. Angew. Chem. Int. Ed. 2001, 40, 3783–3789. [Google Scholar] [CrossRef]
- Cintas, P. On the Origin of Tetrahedral Carbon: A Case for Philosophy of Chemistry? Found. Chem. 2002, 4, 149–161. [Google Scholar] [CrossRef]
- Ochiai, H. Philosophical Foundations of Stereochemistry. HYLE 2015, 21, 1–18. [Google Scholar]
- Ruch, E. Homochiralität Als Klassifizierungsprinzip Von Molekülen Spezieller Molekülklassen. Theor. Chim. Acta 1968, 11, 183–192. [Google Scholar] [CrossRef]
- Ruch, E. Algebraic Aspects of the Chirality Phenomenon in Chemistry. Acc. Chem. Res. 1972, 5, 49–56. [Google Scholar] [CrossRef]
- Ruch, E. Chiral Derivatives of Achiral Molecules: Standard Classes and the Problem of a Right-Left Classification. Angew. Chem. Int. Ed. 1977, 16, 65–72. [Google Scholar] [CrossRef]
- Ruch, E.; Schönhofer, A. Näherungsformeln Für Spiegelungsantimetrische Moleküleigenschaften. Theor. Chim. Acta 1968, 10, 91–110. [Google Scholar] [CrossRef]
- Cintas, P. Tracing the Origins and Evolution of Chirality and Handedness in Chemical Language. Angew. Chem. Int. Ed. 2007, 46, 4016–4024. [Google Scholar] [CrossRef] [PubMed]
- King, R.B. Chirality and Handedness: The Ruch “shoe-Potato” Dichotomy in the Right-Left Classification Problem. Ann. N. Y. Acad. Sci. 2003, 988, 158–170. [Google Scholar] [CrossRef]
- King, R.B. Chirality Algebra and the Right–Left Classification Problem. In Advances in BioChirality; Pályi, G., Zucchi, C., Caglioti, L., Eds.; Elsevier: Amsterdam, The Netherlands, 1999; pp. 13–34. ISBN 9780080434049. [Google Scholar]
- King, R.B. Nonhanded Chirality in Octahedral Metal Complexes. Chirality 2001, 13, 465–473. [Google Scholar] [CrossRef]
- Connelly, N.G.; Damhus, T. Nomenclature of Inorganic Chemistry: Iupac Recommendations 2005; RSC Publishing: Cambridge, UK, 2005. [Google Scholar]
- Cotton, A. Absorption inégale Des Rayons Circulaires Droit Et Gauche Dans Certains Corps Actifs. C. R. Hebd. Seances Acad. Sci. 1895, 120, 989–991. [Google Scholar]
- Cotton, A. Dispersion Rotatoire Anomale Des Corps Absorbants. C. R. Hebd. Seances Acad. Sci. 1895, 120, 1044–1046. [Google Scholar]
- Cotton, A. Recherches Sur L’absorption Et La Dispersion De La Lumière Par Les Milieux Doués Du Pouvoir Rotatoire. Ann. Chim. Phys. Sér. 7 1896, 8, 347–432. [Google Scholar] [CrossRef]
- Constable, E.C.; Housecroft, C.E. When Stereochemistry Raised Its Ugly Head in Coordination Chemistry—An Appreciation of Howard Flack. Chemistry 2020, 2, 49. [Google Scholar] [CrossRef]
- Mathieu, J.-P. Activité Optique Et Solubilité De Quelques Cobaltammines. C. R. Hebd. Seances Acad. Sci. 1934, 199, 278–280. [Google Scholar]
- Mathieu, J.-P. Configuration De Quelques Complexes Hexacoordinés Optiquement Actifs. C. R. Hebd. Seances Acad. Sci. 1934, 198, 1598–1600. [Google Scholar]
- Mathieu, J.-P. Absorption, Activité Optique Et Configuration De Complexes Minéraux. C. R. Hebd. Seances Acad. Sci. 1935, 201, 1183–1184. [Google Scholar]
- Mathieu, J.-P. Recherches Expérimentales Sur Le Dichroisme Circulaire Et Sur Quelques Applications Physico-Chimiques De Ce Phénomène. Ann. Phys. 1935, 11, 371–460. [Google Scholar] [CrossRef]
- Mathieu, J.-P. Recherches Sur Les Complexes De Werner Activité Optique Et Configuration Des Ions Du Type Mea3. J. Chim. Phys. 1936, 3, 476–498. [Google Scholar] [CrossRef]
- Mathieu, J.-P. The Werner Complexes. Absorption of the Hexacoördinates of Cobalt and Chromium in Aqueous Solution. Bull. Soc. Chim. Fr. Mem. 1936, 3, 463–475. [Google Scholar]
- Mathieu, J.-P. The Werner Complexes. Optical Activity and Configuration of the Ions Containing the Groups M En2 and M Ox2. Bull. Soc. Chim. Fr. Mem. 1936, 3, 476–498. [Google Scholar]
- Mathieu, J.-P. Werner Complexes; Optical Activity and the Configuration of Ions of the Type Ma3. J. Chim. Phys. 1936, 33, 78–96. [Google Scholar] [CrossRef]
- Mathieu, J.-P. Experiments on the Complexes of Werner. Substitution in the Optically Active Complex Chlorides. Bull. Soc. Chim. Fr. Mem. 1937, 4, 687–700. [Google Scholar]
- Mathieu, J.-P. Recent Views on the Stereochemistry of Complex Inorganic Compounds. Bull. Soc. Chim. Fr.; Mem. 1938, 5, 725–805. [Google Scholar]
- Mathieu, J.-P. The Werner Complexes-absorption and Optical Activity of Cobalt Compounds With a Double Nucleus. Bull. Soc. Chim. Fr. Mem. 1938, 5, 105–113. [Google Scholar]
- Mathieu, J.-P. Researches on Werner Complexes. Cobaltammines Containing Optically Active Amino Acids. Bull. Soc. Chim. Fr. Mem. 1939, 6, 873–882. [Google Scholar]
- Mathieu, J.-P. Researches on Werner Complexes. Optical Activity and Configuration of Platinum. Iv. Triethylenediamine Ion. Bull. Soc. Chim. Fr. Mem. 1939, 6, 1258–1259. [Google Scholar]
- Mathieu, J.-P. Activité Optique Naturelle. In Handbuch der Physik/Encyclopedia of Physics: Spektroskopie II/Spectroscopy II; Springer: Berlin/Heidelberg, Germany, 1957; pp. 333–432. ISBN 9783642458620. [Google Scholar]
- Kuhn, W.; Bein, K. Konfiguration und Optische Drehung Bei Anorganischen Komplexverbindungen. Z. Anorg. Allgem. Chem. 1934, 21, 321–348. [Google Scholar] [CrossRef]
- Kuhn, W. Das Problem Der Absoluten Konfiguration Optisch Aktiver Stoffe. Sci. Nat. 1938, 26, 289–296. [Google Scholar] [CrossRef]
- Jensen, K.A. Tentative Proposals for Nomenclature of Absolute Configurations Concerned with Six-Coordinated Complexes Based on the Octahedron. Inorg. Chem. 1970, 9, 1–5. [Google Scholar] [CrossRef]
- IUPAC. Compendium of Chemical Terminology, 2nd ed.; the “gold Book”; Online Version Created by Chalk, S.j. Available online: https://doi.org/10.1351/goldbook (accessed on 4 August 2021).
- Crosland, M.P. Historical Studies in the Language of Chemistry; Dover: New York, NY, USA, 1978. [Google Scholar]
- Senning, A. Elsevier’s Dictionary of Chemoetymology: The Whies and Whences of Chemical Nomenclature and Terminology; Elsevier: Amsterdam, The Netherlands, 2007; ISBN 9780444522399. [Google Scholar]
- Senning, A. The Etymology of Chemical Names: Tradition and Convenience Vs. Rationality in Chemical Nomenclature; de Gruyter: Berlin, Germany, 2019; ISBN 9783110612714. [Google Scholar]
- Gal, J. Louis Pasteur, In defense of Louis Pasteur: Critique of Gerald Geison’s deconstruction of Pasteur’s discovery of molecular chirality. Chirality 2019, 31, 261–282. [Google Scholar] [CrossRef]
- Gal, J. Louis Pasteur, Language, and Molecular Chirality. I. Background and Dissymmetry. Chirality 2011, 23, 1–16. [Google Scholar] [CrossRef]
- Gal, J. Stereochemical Vocabulary for Structures That Are Chiral But Not Asymmetric: History, Analysis, and Proposal for a Rational Terminology. Chirality 2011, 23, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Gal, J. Molecular Chirality in Chemistry and Biology: Historical Milestones. Helv. Chim. Acta 2013, 96, 1617–1657. [Google Scholar] [CrossRef]
- Gal, J. Molecular Chirality: Language, History, and Significance. In Differentiation of Enantiomers I; Schurig, V., Ed.; Springer International Publishing: Cham, Switzerland, 2013; pp. 1–20. ISBN 9783319032382. [Google Scholar]
- Schmitz-Emans, M. Science in Wonderland. In Literature and Science; Schmitz-Emans, M., Ed.; Königshausen und Neumann: Würzburg, Germany, 2008. [Google Scholar]
- Kelvin, W.T. The Molecular Tactics of a Crystal; Clarendon Press: Oxford, UK, 1894. [Google Scholar]
- Larmor, J. On Electro-Crystalline Properties as Conditioned By Atomic Lattices. Proc. R. Soc. Lond. Ser. A 1921, 99, 1–10. [Google Scholar] [CrossRef]
- Larmor, J. The Structural Significance of Optical Rotatory Quality. Rep. Br. Ass. Advmt. Sci. 1922, 351–352. [Google Scholar]
- Raman, C.V. Crystals of Quartz With Iridescent Faces. Proc. Indian Acad. Sci. Sect. A 1950, 31, 275–279. [Google Scholar] [CrossRef]
- Whyte, L.L. Chirality. Nature 1957, 180, 513. [Google Scholar] [CrossRef]
- Whyte, L.L. Chirality. Nature 1958, 182, 198. [Google Scholar] [CrossRef]
- Purdie, T. Resolution of Lactic Acid Into Its Optically Active Components. J. Chem. Soc. Trans. 1893, 63, 1143–1157. [Google Scholar] [CrossRef]
- Kipping, F.S.; Pope, W.J. Studies of the Terpenes and Allied Compounds. The Sulphonic Derivatives of Camphor. Part I. J. Chem. Soc., Trans. 1893, 63, 548–604. [Google Scholar] [CrossRef]
- Kipping, F.S.; Pope, W.J. Enantiomorphism. J. Chem. Soc., Trans. 1898, 73, 606–617. [Google Scholar] [CrossRef]
- Kipping, F.S.; Pope, W.J. Characterisation of Racemic Compounds. J. Chem. Soc., Trans. 1899, 75, 36–46. [Google Scholar] [CrossRef]
- Einhorn, A. Ueber Die Beziehungen Des Cocaïns Zum Atropin. Ber. Dtsch. Chem. Ges. 1890, 23, 1338–1344. [Google Scholar] [CrossRef]
- Zelinsky, N. Ueber Die Stereoisomerie Der Dimethyldioxyglutarsäuren. Ber. Dtsch. Chem. Ges. 1891, 24, 4006–4017. [Google Scholar] [CrossRef]
- Gal, J. Carl Friedrich Naumann and the Introduction of Enantio Terminology: A Review and Analysis on the 150th Anniversary. Chirality 2007, 19, 89–98. [Google Scholar] [CrossRef]
- Schoenflies, A. Krystallsysteme Und Krystallstructur; Teubner: Leipzig, Germany, 1891. [Google Scholar]
- Naumann, C.F. Elemente Der Theoretischen Krystallographie; W. Engelmann: Leipzig, Germany, 1856. [Google Scholar]
- Ostwald, W. Outlines of General Chemistry; Walker, J., Ed.; MacMillan and Co: London, UK, 1890. [Google Scholar]
- Attfield, J. Chemistry: General, Medical, and Pharmaceutical, Including the Chemistry of the U. S. Pharmacopia. A Manual on the General Principles of the Science, and Their Applications in Medicine and Pharmacy; Lea Brothers & Co.: Philadelphia, PA, USA, 1894. [Google Scholar]
- Hinrichs, G.D. Introduction to General Chemistry. A Graded Course of One Hundred Lectures; Lemcke and Buechner: New York, NY, USA; Leipzig, Germany, 1897. [Google Scholar]
- Kuhn, R. Molekulare Asymmetrie. In Stereochemie: Eine zusammenfassung der Ergebnisse, Grundlagen und Probleme; Freudenberg, K., Ed.; Franz Deutike: Vienna, Austria, 1933; pp. 803–824. [Google Scholar]
- Christie, G.H.; Kenner, J. The Molecular Configurations of Polynuclear Aromatic Compounds. Part 1. The Resolution of Γ-6:6′-Dinitro- and 4:6:4′:6′-Tetranitro-diphenic Acids Into Optically Active Components. J. Chem. Soc., Trans. 1922, 121, 614–620. [Google Scholar] [CrossRef]
- Cain, J.C.; Coulthard, A.; Micklethwait, F.M.G. Studies in the Diphenyl Series. Part 2. The Dinitrobenzidines: A New Form of Isomerism. J. Chem. Soc. Trans. 1912, 101, 2298–2304. [Google Scholar] [CrossRef]
- King, H. The Possibility of a New Instance of Optical Activity Without an Asymmetric Carbon Atom. Proc. Chem. Soc. 1914, 30, 250. [Google Scholar] [CrossRef]
- Thorpe, J.F. John Cannell Cain. J. Chem. Soc. Trans. 1921, 119, 533–537. [Google Scholar]
- Berthod, M.; Mignani, G.; Woodward, G.; Lemaire, M. Modified Binap: The How and the Why. Chem. Rev. 2005, 105, 1801–1836. [Google Scholar] [CrossRef]
- Miyashita, A.; Yasuda, A.; Takaya, H.; Toriumi, K.; Ito, T.; Souchi, T.; Noyori, R. Synthesis of 2,2’-Bis(diphenylphosphino)-1,1’-binaphthyl (Binap), an Atropisomeric Chiral Bis(triaryl)phosphine, and Its Use in the Rhodium(I)-Catalyzed Asymmetric Hydrogenation of.alpha.-(acylamino)acrylic Acids. J. Am. Chem. Soc. 1980, 102, 7932–7934. [Google Scholar] [CrossRef]
- Flanders, M.; Swann, D. At the Drop of a Hat. 1960. Available online: https://www.youtube.com/watch?v=AYr0eNtpDHs (accessed on 11 August 2021).
- Chen, C.-F.; Shen, Y. Helicene Chemistry; Springer: Berlin, Germany, 2016; ISBN 9783662531662. [Google Scholar]
- Lemiere, G.L.; Alderweireldt, F.C. Proposition for a New Definition of the Chiral Plane and Its Consequences for the Specification of Planar Chirality. J. Org. Chem. 1980, 45, 4175–4179. [Google Scholar] [CrossRef]
- Hirschmann, H.; Hanson, K.R. Elements of Stereoisomerism and Prostereoismerism. J. Org. Chem. 1971, 36, 3293–3306. [Google Scholar] [CrossRef]
- Simon, J. Topological Chirality of Certain Molecules. Topology 1986, 25, 229–235. [Google Scholar] [CrossRef]
- Chambron, J.-C.; Dietrich-Buchecker, C.O.; Sauvage, J.-P. From Classical Chirality to Topologically Chiral Catenands and Knots. Top. Curr. Chem. 1993, 165, 131–162. [Google Scholar] [CrossRef]
- Segawa, Y.; Kuwayama, M.; Hijikata, Y.; Fushimi, M.; Nishihara, T.; Pirillo, J.; Shirasaki, J.; Kubota, N.; Itami, K. Topological Molecular Nanocarbons: All-Benzene Catenane and Trefoil Knot. Science 2019, 365, 272–276. [Google Scholar] [CrossRef]
- Mitchell, D.K.; Sauvage, J.-P. A Topologically Chiral [2]catenand. Angew. Chem. Int. Ed. 1988, 27, 930–931. [Google Scholar] [CrossRef]
- Constable, E.C.; Hartshorn, R.M.; Housecroft, C.E. 1,1’-Biisoquinolines-neglected Ligands in the Heterocyclic Diimine Family That Provoke Stereochemical Reflections. Molecules 2021, 26, 1584. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson. Scene–Bishop’s Breakfast Table. Judy Lond. Serio-Comic J. 1895. [Google Scholar]
- Kramer, S. There Was a Little Girl: Its First Printings · Its Authorship · Its Variants. Pap. Bibl. Soc. Am. 1946, 40, 287–310. [Google Scholar] [CrossRef]
- Castiglioni, E.; Abbate, S.; Longhi, G. Experimental Methods for Measuring Optical Rotatory Dispersion: Survey and Outlook. Chirality 2011, 23, 711–716. [Google Scholar] [CrossRef] [PubMed]
























Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Constable, E.C. Through a Glass Darkly—Some Thoughts on Symmetry and Chemistry. Symmetry 2021, 13, 1891. https://doi.org/10.3390/sym13101891
Constable EC. Through a Glass Darkly—Some Thoughts on Symmetry and Chemistry. Symmetry. 2021; 13(10):1891. https://doi.org/10.3390/sym13101891
Chicago/Turabian StyleConstable, Edwin C. 2021. "Through a Glass Darkly—Some Thoughts on Symmetry and Chemistry" Symmetry 13, no. 10: 1891. https://doi.org/10.3390/sym13101891
APA StyleConstable, E. C. (2021). Through a Glass Darkly—Some Thoughts on Symmetry and Chemistry. Symmetry, 13(10), 1891. https://doi.org/10.3390/sym13101891





