Photosynthetic Efficiency is Higher in Asymmetric Leaves than in Symmetric Leaves of the Same Plant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Species and Study Sites
2.2. Measurements of Chlorophyll Fluorescence and Leaf Traits
2.3. Measurements of Asymmetry in Leaf Shape
2.4. Statistical Analysis
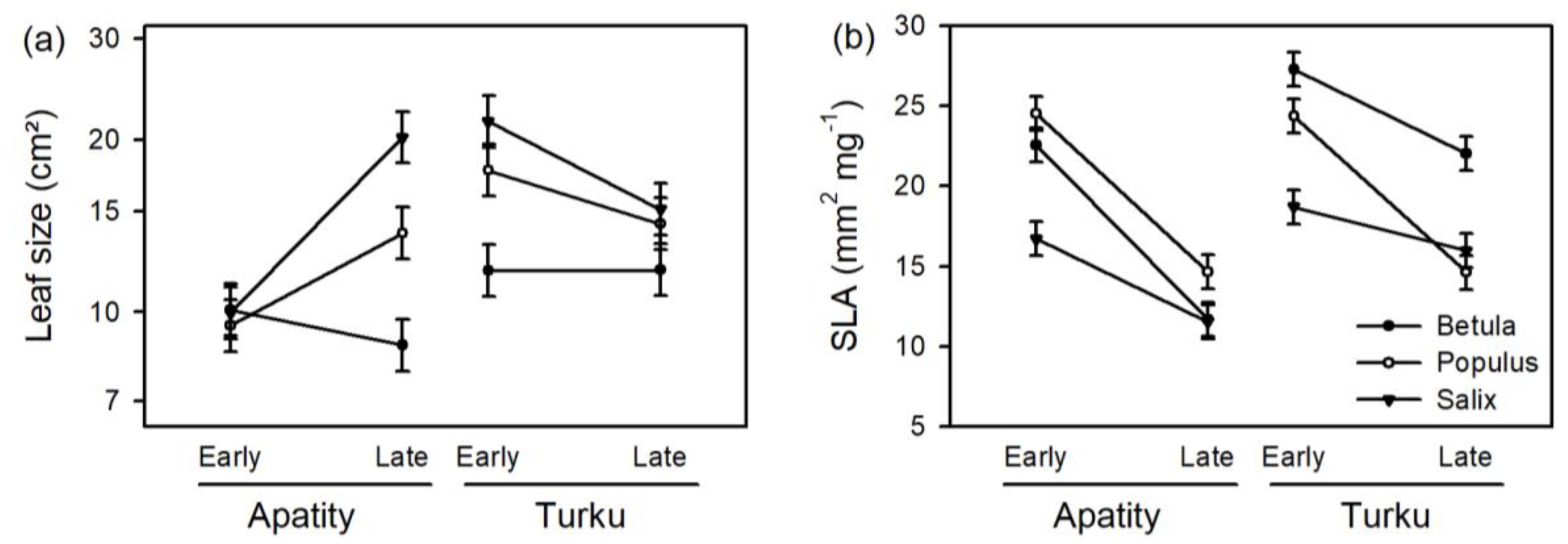
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tuomi, J.; Vuorisalo, T. Hierarchical selection in modular organisms. Trends Ecol. Evol. 1989, 4, 209–213. [Google Scholar] [CrossRef]
- Silvertown, J.; Gordon, D.M. A framework for plant behavior. Annu. Rev. Ecol. Syst. 1989, 20, 349–366. [Google Scholar] [CrossRef]
- Bazzaz, F.A. Habitat selection in plants. Am. Nat. 1991, 137, S116–S130. [Google Scholar] [CrossRef]
- Augspurger, C.K.; Bartlett, E.A. Differences in leaf phenology between juvenile and adult trees in a temperate deciduous forest. Tree Physiol. 2003, 23, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Stuefer, J.F. Two types of division of labour in clonal plants: benefits, costs and constraints. Perspect. Plant Ecol. Evol. Syst. 1998, 1, 47–60. [Google Scholar] [CrossRef]
- Granado-Yela, C.; García-Verdugo, C.; Carrillo, K.; Rubio de Casas, R.; Kleczkowski, L.A.; Balaguer, L. Temporal matching among diurnal photosynthetic patterns within the crown of the evergreen sclerophyll Olea europaea L. Plant Cell Environ. 2011, 34, 800–810. [Google Scholar] [CrossRef] [PubMed]
- Roslin, T.; Gripenberg, S.; Salminen, J.P.; Karonen, M.; O’Hara, R.B.; Pihlaja, K. Seeing the trees for the leaves—oaks as mosaics for a host-specific moth. Oikos 2006, 113, 106–120. [Google Scholar] [CrossRef]
- Herrera, C.M. Multiplicity in Unity: Plant Subindividual Variation and Interactions with Animals (Interspecific Interactions); Univ. Chicago Press: Chicago, IL, USA, 2009. [Google Scholar]
- Hoglund, S. Timing of growth determines fitness and performance of a galling insect on willow. Ecol. Entomol. 2014, 39, 159–167. [Google Scholar] [CrossRef]
- Kozlov, M.V.; Zverev, V.; Zvereva, E.L. Do defoliating insects distinguish between symmetric and asymmetric leaves within a plant? Ecol. Entomol. 2018, 43, 656–664. [Google Scholar] [CrossRef]
- Møller, A.P.; Swaddle, J.P. Asymmetry, Developmental Stability, and Evolution; Oxford Univ. Press: Oxford, UK, 1997. [Google Scholar]
- Polak, M. Developmental Instability: Causes and Consequences; Oxford Univ. Press: Oxford, UK, 2003. [Google Scholar]
- Freeman, D.C.; Graham, J.H.; Emlen, J.M. Developmental stability in plants: symmetries, stress and epigenesis. Genetica 1993, 89, 97–119. [Google Scholar] [CrossRef]
- Kozlov, M.V.; Wilsey, B.J.; Koricheva, J.; Haukioja, E. Fluctuating asymmetry of birch leaves increases under pollution impact. J. Appl. Ecol. 1996, 33, 1489–1495. [Google Scholar] [CrossRef]
- Kozlov, M.V.; Zvereva, E.L.; Zverev, V.E. Impacts of Point Polluters on Terrestrial Biota: Comparative Analysis of 18 Contaminated Areas; Springer: Dordrecht, Netherlands, 2009. [Google Scholar]
- Hagen, S.B.; Ims, R.A.; Yoccoz, N.G.; Sørlibråten, O. Fluctuating asymmetry as an indicator of elevation stress and distribution limits in mountain birch (Betula pubescens). Plant Ecol. 2008, 195, 157–163. [Google Scholar] [CrossRef]
- Zvereva, E.L.; Kozlov, M.V.; Haukioja, E. Stress responses of Salix borealis to pollution and defoliation. J. Appl. Ecol. 1997, 34, 1387–1396. [Google Scholar] [CrossRef]
- Graham, J.H.; Raz, S.; Hel-Or, H.; Eviatar, N. Fluctuating asymmetry: methods, theory, and applications. Symmetry 2010, 2, 466–540. [Google Scholar] [CrossRef]
- Kozlov, M.V. Plant studies on fluctuating asymmetry in Russia: mythology and methodology. Russ. J. Ecol. 2017, 48, 1–9. [Google Scholar] [CrossRef]
- Sandner, T.M.; Matthies, D. Fluctuating asymmetry of leaves is a poor indicator of environmental stress and genetic stress by inbreeding. Silene vulgaris. Ecol. Indic. 2017, 79, 247–253. [Google Scholar] [CrossRef]
- Sherry, R.A.; Lord, E.M. Developmental stability in leaves of Clarkia tembloriensis (Onagraceae) as related to population outcrossing rates and heterozygosity. Evolution 1996, 50, 80–91. [Google Scholar] [CrossRef]
- Cowart, N.M.; Graham, J.H. Within- and among-individual variation in fluctuating asymmetry of leaves in the fig (Ficus carica L.). Int. J. Plant Sci. 1999, 160, 116–121. [Google Scholar] [CrossRef]
- De Sibio, P.R.; Rossi, M.N. Oviposition of a leaf-miner on Erythroxylum tortuosum (Erythroxylaceae) leaves: hierarchical variation of physical leaf traits. Aust. J. Bot. 2012, 60, 136–142. [Google Scholar] [CrossRef]
- Sandner, T.; Zverev, V.; Kozlov, M.V. Can the use of landmarks improve the suitability of fluctuating asymmetry in plant leaves as an indicator of stress? Ecol. Indic. 2019, 97, 457–465. [Google Scholar] [CrossRef]
- Cornelissen, T.; Stiling, P. Perfect is best: low leaf fluctuating asymmetry reduces herbivory by leaf miners. Oecologia 2005, 142, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Rinderle, U. Chlorophyll Fluorescence Signatures as Vitality Indicators in Forest Decline Research. In Applications of chlorophyll fluorescence; Lichtenthaler, K., Rinderle, U., Eds.; Kluwer Academic Publishers: Dordrecht, Netherlands, 1988; pp. 143–149. [Google Scholar]
- Öqwist, G.; Wass, R. A portable, microprocessor operated instrument for measuring chlorophyll fluorescence kinetics in stress physiology. Physiol. Plantarum 1988, 73, 211–217. [Google Scholar] [CrossRef]
- Otronen, M.; Rosenlund, H.M. Morphological asymmetry and chlorophyll fluorescence in Scots pine (Pinus sylvestris): responses to variation in soil moisture, nutrients and defoliation. Ann. Bot. Fenn. 2001, 38, 285–294. [Google Scholar]
- Biber, P.D. Determining salinity-tolerance of giant Salvinia using chlorophyll fluorescence. Gulf Caribbean Res. 2009, 21, 31–36. [Google Scholar] [CrossRef]
- Zvereva, E.L.; Kozlov, M.V. Growth and reproduction of dwarf shrubs, Vaccinium myrtillus and V. vitis-idaea, in a severely polluted area. Basic Appl. Ecol. 2005, 6, 261–274. [Google Scholar] [CrossRef]
- Nikiforou, C.; Manetas, Y. Ecological stress memory: Evidence in two out of seven species through the examination of the relationship between leaf fluctuating asymmetry and photosynthesis. Ecol. Indic. 2017, 74, 530–534. [Google Scholar] [CrossRef]
- Pantin, F.; Simonneau, T.; Muller, B. Coming of leaf age: control of growth by hydraulics and metabolics during leaf ontogeny. New Phytologist 2012, 196, 349–366. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, M.V.; Gavrikov, D.E.; Zverev, V.; Zvereva, E.L. Local insect damage reduces fluctuating asymmetry in next-year’s leaves of downy birch. Insects 2018, 9, 56. [Google Scholar] [CrossRef] [PubMed]
- Wilsey, B.J.; Haukioja, E.; Koricheva, J.; Sulkinoja, M. Leaf fluctuating asymmetry increases with hybridization and elevation in tree-line birches. Ecology 1998, 79, 2092–2099. [Google Scholar] [CrossRef]
- Zvereva, E.L.; Kozlov, M.V. Effects of pollution induced habitat disturbance on willow response to simulated herbivory. J. Ecol. 2001, 89, 21–30. [Google Scholar] [CrossRef]
- Rasband, W.S. ImageJ. U.S. National Institutes of Health: Bethesda, USA. 2017. Available online: http://imagej.nih.gov/ij/ (accessed on 07 May 2019).
- Klingenberg, C.P.; McIntyre, G.S. Geometric morphometrics of developmental instability: analyzing patterns of fluctuating asymmetry with Procrustes methods. Evolution 1998, 52, 1363–1375. [Google Scholar] [CrossRef] [PubMed]
- Klingenberg, C.P. MorphoJ: an integrated software package for geometric morphometrics. Mol. Ecol. Resour. 2011, 11, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Harper, J.L. The value of a leaf. Oecologia 1989, 80, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Ruohomäki, K.; Haukioja, E.; Repka, S.; Lehtila, K. Leaf value: effects of damage to individual leaves on growth and reproduction of mountain birch shoots. Ecology 1997, 78, 2105–2117. [Google Scholar] [CrossRef]
- Zangerl, A.R. Leaf value and optimal defense in Pastinaca sativa L. (Umbelliferae). Amer. Midl. Nat. 1986, 116, 432–436. [Google Scholar] [CrossRef]
- Hu, H.; Wang, L.; Wang, Q.; Jiao, L.; Hua, W.; Zhou, Q.; Huang, H. Photosynthesis, chlorophyll fluorescence characteristics, and chlorophyll content of soybean seedlings under combined stress of bisphenol a and cadmium. Environ. Toxicol. Chem. 2014, 33, 2455–2462. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, G.C.; Govindjee. Chlorophyll a Fluorescence—a Signature of Photosynthesis. Advances in Photosynthesis and Respiration; Kluwer Academic Publishers: Dordrecht, Netherlands, 2004; Volume 19. [Google Scholar]
- Bucher, S.F.; Bernhardt–Römermann, M.; Römermann, C. Chlorophyll fluorescence and gas exchange measurements in field research: an ecological case study. Photosynthetica 2018, 56, 1161–1170. [Google Scholar] [CrossRef]
- Gallé, A.; Flexas, J. Gas-exchange and Chlorophyll Fluorescence Measurements in Grapevine Leaves in the Field. In Methodologies and Results in Grapevine Research; Delrot, S., Medrano, H., Or, E., Bavaresco, L., Grando, S., Eds.; Springer Science + Business Media B.V.: Berlin/Heidelberg, Germany, 2010; pp. 107–121. [Google Scholar]
- Jumrani, K.; Bhatia, V.S.; Pandey, G.P. Impact of elevated temperatures on specific leaf weight, stomatal density, photosynthesis and chlorophyll fluorescence in soybean. Photosynt. Res. 2017, 131, 333–350. [Google Scholar] [CrossRef]
- Bokhorst, S.; Berg, M.P.; Edvinsen, G.K.; Ellers, J.; Heitman, A.; Jaakola, L. Impact of multiple ecological stressors on a sub-arctic ecosystem: no interaction between extreme winter warming events, nitrogen addition and grazing. Frontiers Plant Sci. 2018, 9, 1787. [Google Scholar] [CrossRef]
- Evans, J.R. Phorosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 1989, 78, 9–19. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Yang, G.; Tan, C.; Zhao, C. Effects of nitrogen stress on the photosynthetic CO2 assimilation, chlorophyll fluorescence, and sugar-nitrogen ratio in corn. Sci. Reports 2014, 5, 9311. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, M.V.; Niemelä, P. Difference in needle length—a new and objective indicator of pollution impact on Scots pine (Pinus sylvestris). Water Air Soil Pollut. 1999, 116, 365–370. [Google Scholar] [CrossRef]
- Chitwood, D.H.; Headland, L.R.; Ranjan, A.; Martinez, C.C.; Braybrook, S.A.; Koenig, D.P. Leaf asymmetry as a developmental constraint imposed by auxin-dependent phyllotactic patterning. Plant Cell 2012, 24, 2318–2327. [Google Scholar] [CrossRef] [PubMed]
- Alves-Silva, E.; Del-Claro, K. Herbivory-induced stress: leaf developmental instability is caused by herbivore damage in early stages of leaf development. Ecol. Indic. 2016, 61, 359–365. [Google Scholar] [CrossRef]
- Sack, L.; Melcher, P.J.; Liu, W.H.; Middleton, E.; Pardee, T. How strong is intracanopy leaf plasticity in temperate deciduous trees? Am. J. Bot. 2006, 93, 829–839. [Google Scholar] [CrossRef] [Green Version]
- Tholen, D.; Boom, C.; Zhu, X.G. Prospects for improving photosynthesis by altering leaf anatomy. Plant Sci. 2012, 197, 92–101. [Google Scholar] [CrossRef]
- Jurik, T.W.; Chabot, J.F.; Chabot, B.F. Ontogeny of photosynthetic performance in Fragaria virginiana under changing light regimes. Plant Physiol. 1979, 63, 542–547. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C.; Döll, M.; Fietz, H.-J.; Bach, T.; Kozel, U.; Meier, D.; Rahmsdorf, U. Photosynthetic activity, chloroplast ultrastructure, and leaf characteristics of high-light and low-light plants and of sun and shade leaves. Photosynth. Res. 1981, 2, 115–141. [Google Scholar] [CrossRef]
- Valladares, F.; Niinemets, Ü. The Architecture of Plant Crowns: form Design Rules to Light Capture and Performance. In Functional plant ecology; Pugnaire, F., Valladares, F., Eds.; Taylor and Francis: New York, NY, USA, 2007; pp. 101–149. [Google Scholar]
- Freeman, D.C.; Brown, M.L.; Dobson, M.; Jordan, Y.; Kizy, A.; Micallef, C. Developmental instability: measures of resistance and resilience using pumpkin (Cucurbita pepo L.). Biol. J. Linn. Soc. 2003, 78, 27–41. [Google Scholar] [CrossRef]
- Kusi, J. Variations in phenotypic plasticity and fluctuating asymmetry of leaf morphology of three Quercus (oak) species in response to environmental factors. 2013. Available online: http://dc.etsu.edu/etd/1160 (accessed on 07 May 2019).
- Sandner, T.M.; Matthies, D. Inbreeding limits responses to environmental stress in Silene vulgaris. Environ. Exptl Bot. 2018, 147, 86–94. [Google Scholar] [CrossRef]
- Kozlov, M.V. Contrasting response of mountain birch to damage by Eriocrania leafminers in polluted and unpolluted habitats. Can. J. Bot. 2005, 83, 73–79. [Google Scholar] [CrossRef]
- Nykänen, H.; Koricheva, J. Damage-induced changes in woody plants and their effects on insect herbivore performance: a metaanalysis. Oikos 2004, 104, 247–268. [Google Scholar] [CrossRef]
- Delaney, K.J.; Haile, F.J.; Peterson, R.K.; Higley, L.G. Impairment of leaf photosynthesis after insect herbivory or mechanical injury on common milkweed, Asclepias syriaca. Environ. Entomol. 2008, 37, 1332–1343. [Google Scholar] [CrossRef]
- Roy, B.A.; Stanton, M.L. Asymmetry of wild mustard, Sinapis arvensis (Brassicaceae), in response to severe physiological stresses. J. Evol. Biol. 1999, 12, 440–449. [Google Scholar] [CrossRef]
- Wan, S.; Xia, J.; Liu, W.; Niu, S. Photosynthetic overcompensation under nocturnal warming enhances grassland carbon sequestration. Ecology 2009, 90, 2700–2710. [Google Scholar] [CrossRef] [Green Version]
- Luo, H.H.; Zhang, Y.L.; Zhang, W.F. Effects of water stress and rewatering on photosynthesis, root activity, and yield of cotton with drip irrigation under mulch. Photosynthetica 2016, 54, 65–73. [Google Scholar] [CrossRef]


| Plant Species | Explanatory Variable | Degrees of Freedom | Mean Sum of Squares | F | P |
|---|---|---|---|---|---|
| Betula pubescens | Individual | 237 | 9.91×10-3 | 6.74 | <0.001 |
| Side | 3 | 4.38×10-3 | 2.98 | 0.032 | |
| Individual × Side | 237 | 1.47×10-3 | 44.05 | <0.001 | |
| Error | 480 | 3.34×10-5 | |||
| Salix caprea | Individual | 237 | 4.56×10-3 | 1.82 | <0.001 |
| Side | 3 | 5.16×10-3 | 2.06 | 0.106 | |
| Individual × Side | 237 | 2.50×10-3 | 55.56 | <0.001 | |
| Error | 480 | 4.51×10-5 | |||
| Populus tremula | Individual | 395 | 8.78×10-3 | 3.17 | <0.001 |
| Side | 5 | 1.27×10-3 | 0.46 | 0.807 | |
| Individual × Side | 395 | 2.77×10-3 | 6.24 | <0.001 | |
| Error | 800 | 4.44×10-4 |
| Explanatory Variable | Degrees of Freedom | Leaf Size | Specific Leaf Area | ||
|---|---|---|---|---|---|
| F | P | F | P | ||
| Site | 1, 48 | 19.67 | <0.0001 | 33.83 | <0.0001 |
| Season | 1, 48 | 1.04 | 0.3134 | 142.20 | <0.0001 |
| Species | 2, 48 | 16.12 | <0.0001 | 25.51 | <0.0001 |
| Site × Season | 1, 48 | 17.23 | 0.0001 | 5.03 | 0.0296 |
| Species × Site | 2, 48 | 0.28 | 0.7585 | 13.11 | <0.0001 |
| Species × Season | 2, 48 | 1.42 | 0.2513 | 8.09 | 0.0009 |
| Species × Site × Season | 2, 48 | 8.46 | 0.0007 | 1.67 | 0.1994 |
| Explanatory Variable | Degrees of Freedom | F | P |
|---|---|---|---|
| Site | 1, 48.1 | 232.56 | <0.0001 |
| Season | 1, 49.1 | 333.74 | <0.0001 |
| Species | 2, 50.5 | 4.69 | 0.0135 |
| Site × Season | 1, 48.5 | 288.86 | <0.0001 |
| Species × Site | 2, 48.1 | 0.76 | 0.4753 |
| Species × Season | 2, 48.1 | 8.36 | 0.0008 |
| Species × Site × Season | 2, 49.0 | 6.56 | 0.0030 |
| Leaf FA | 1, 201.4 | 6.06 | 0.0146 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozlov, M.V.; Zverev, V.; Sandner, T.M. Photosynthetic Efficiency is Higher in Asymmetric Leaves than in Symmetric Leaves of the Same Plant. Symmetry 2019, 11, 834. https://doi.org/10.3390/sym11060834
Kozlov MV, Zverev V, Sandner TM. Photosynthetic Efficiency is Higher in Asymmetric Leaves than in Symmetric Leaves of the Same Plant. Symmetry. 2019; 11(6):834. https://doi.org/10.3390/sym11060834
Chicago/Turabian StyleKozlov, Mikhail V., Vitali Zverev, and Tobias M. Sandner. 2019. "Photosynthetic Efficiency is Higher in Asymmetric Leaves than in Symmetric Leaves of the Same Plant" Symmetry 11, no. 6: 834. https://doi.org/10.3390/sym11060834
APA StyleKozlov, M. V., Zverev, V., & Sandner, T. M. (2019). Photosynthetic Efficiency is Higher in Asymmetric Leaves than in Symmetric Leaves of the Same Plant. Symmetry, 11(6), 834. https://doi.org/10.3390/sym11060834






