β2GP1, Anti-β2GP1 Antibodies and Platelets: Key Players in the Antiphospholipid Syndrome
Abstract
:1. Introduction
2. β2GP1
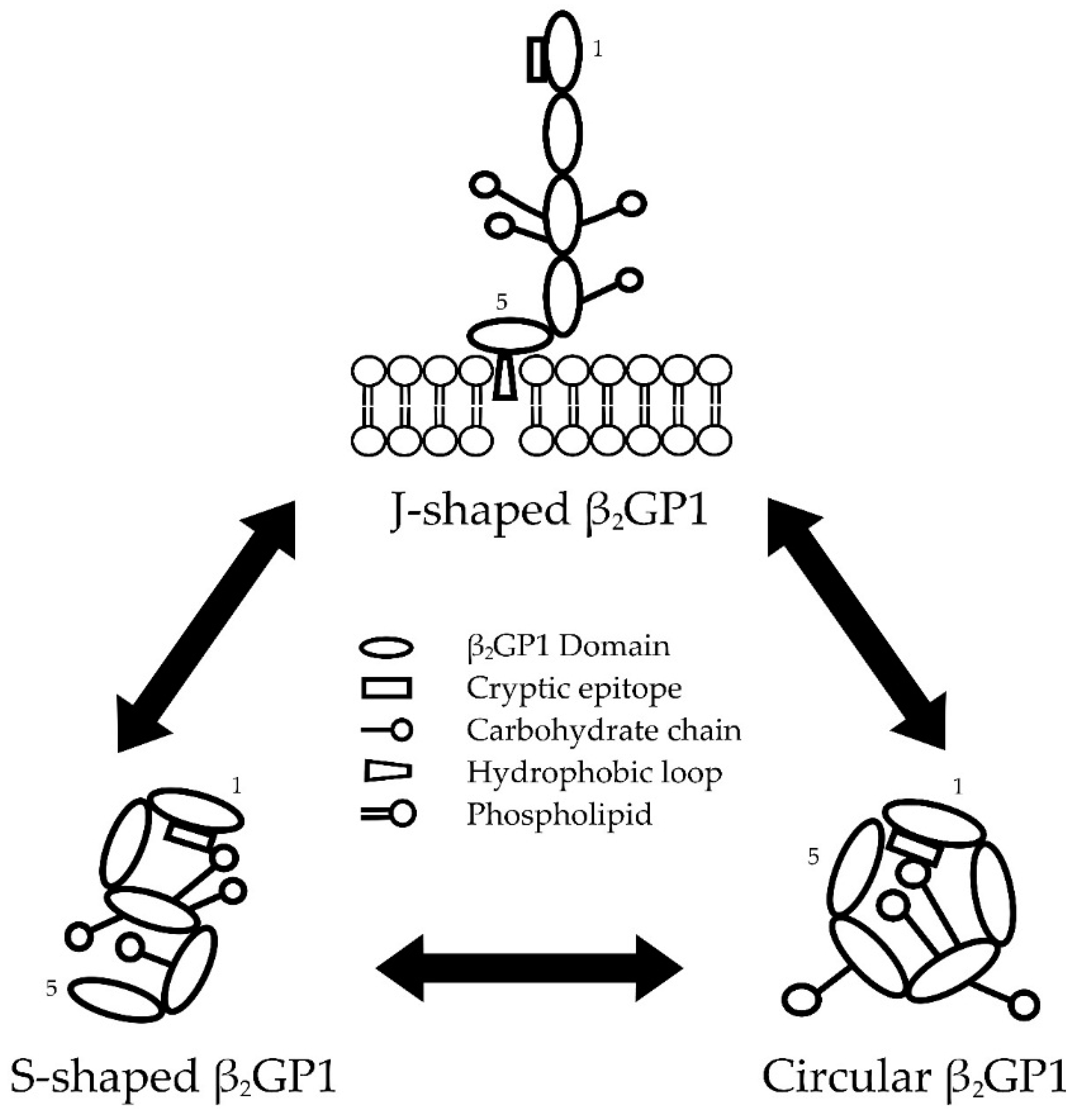
2.1. Conformations of β2GP1
2.1.1. Transformation between β2GP1 Conformations
2.1.2. Factors Affecting β2GP1 Conformation
2.2. Physiological Role(s) of β2GP1
3. Anti-β2GP1 Antibodies
3.1. Clinical Significance of Anti-β2GP1 Antibodies
3.2. Etiology of Anti-β2GP1 Antibodies
3.3. The Two Hit Hypothesis
3.4. Types of Anti-β2GP1 Antibodies
3.5. Anti-DI-β2GP1 Antibodies as a Diagnostic Tool
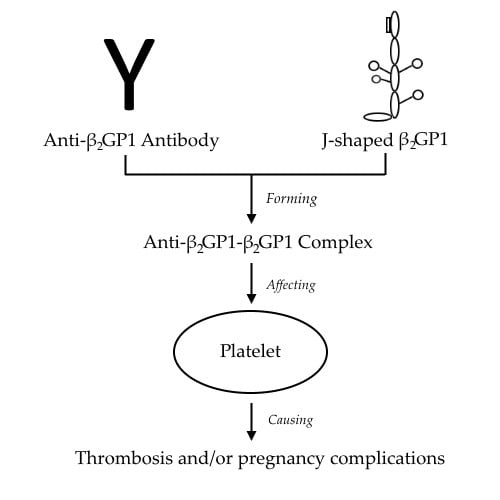
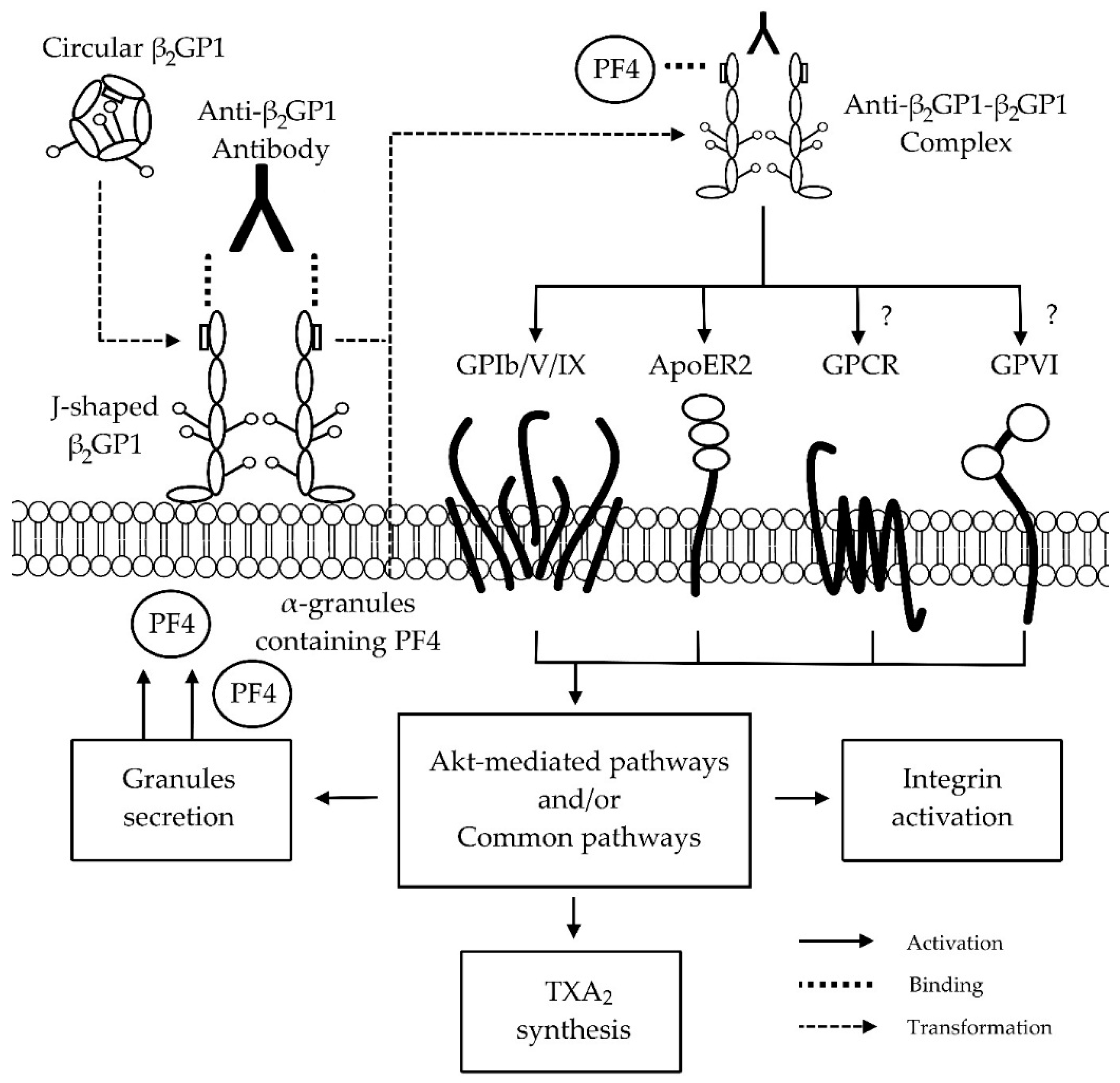
4. Anti-β2GP1-β2GP1 Complexes and Platelets
5. Conclusion and Further Research
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| APL | Anti-phospholipid |
| LAC | Lupus anti-coagulant |
| aCL | Anti-cardiolipin |
| Anti-β2GP1 | Anti-beta 2 glycoprotein 1 |
| APS | Antiphospholipid syndrome |
| SLE | Systemic lupus erythematosus |
| ELISA | Enzyme-linked immunosorbent assays |
| D | Domain |
| Anti-DI-β2GP1 | Anti-domain I-beta 2 glycoprotein 1 |
| EDTA | Ethylenediaminetetraacetic acid |
| ADP | Adenosine diphosphate |
| VWF | Von Willebrand factor |
| GP | Glycoprotein |
| ADAMTS13 | vWF protease |
| Ig | Immunoglobulin |
| ApoER2 | Apolipoprotein E receptor 2 |
| GPCR | Guanine nucleotide-binding protein-coupled receptors |
| PF4 | Platelet factor 4 |
References
- Keeling, D.; Mackie, I.; Moore, G.W.; Greer, I.A.; Greaves, M. Guidelines on the investigation and management of antiphospholipid syndrome. Br. J. Haematol. 2012, 157, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Biggioggero, M.; Meroni, P.L. The geoepidemiology of the antiphospholipid antibody syndrome. Autoimmun. Rev. 2010, 9, A299–A304. [Google Scholar] [CrossRef] [PubMed]
- De Groot, P.G.; Urbanus, R.T. The significance of autoantibodies against β2-glycoprotein I. Blood 2012, 120, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Fickentscher, C.; Magorivska, I.; Janko, C.; Biermann, M.; Bilyy, R.; Nalli, C.; Tincani, A.; Medeghini, V.; Meini, A.; Nimmerjahn, F. The pathogenicity of anti-β2GP1-IgG autoantibodies depends on Fc glycosylation. J. Immunol. Res. 2015, 2015, 1–15. [Google Scholar] [CrossRef]
- Miyakis, S.; Lockshin, M.; Atsumi, T.; Branch, D.; Brey, R.; Cervera, R.; Derksen, R.; De Groot, P.; Koike, T.; Meroni, P. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J. Thromb. Haemost. 2006, 4, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Urbanus, R.T.; Derksen, R.H.; De Groot, P.G. Current insight into diagnostics and pathophysiology of the antiphospolipid syndrome. Blood Rev. 2008, 22, 93–105. [Google Scholar] [CrossRef] [PubMed]
- De Groot, P.G.; Urbanus, R.T. The future of antiphospholipid antibody testing. Semin. Thromb. Hemost. 2012, 38, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Reber, G.; Boehlen, F.; De Moerloose, P. Technical aspects in laboratory testing for antiphospholipid antibodies: Is standardization an impossible dream? Semin. Thromb. Hemost. 2008, 34, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Du, V.X.; Kelchtermans, H.; De Groot, P.G.; De Laat, B. From antibody to clinical phenotype, the black box of the antiphospholipid syndrome: Pathogenic mechanisms of the antiphospholipid syndrome. Thromb. Res. 2013, 132, 319–326. [Google Scholar] [CrossRef] [PubMed]
- De Laat, B.; Derksen, R.H.; Urbanus, R.T.; De Groot, P.G. IgG antibodies that recognize epitope Gly40-Arg43 in domain I of β2–glycoprotein I cause LAC, and their presence correlates strongly with thrombosis. Blood 2005, 105, 1540–1545. [Google Scholar] [CrossRef] [PubMed]
- De Laat, B.; Derksen, R.; Reber, G.; Musial, J.; Swadzba, J.; Bozic, B.; Cucnik, S.; Regnault, V.; Forastiero, R.; Woodhams, B. An international multicentre-laboratory evaluation of a new assay to detect specifically lupus anticoagulants dependent on the presence of anti-beta2-glycoprotein I autoantibodies. J. Thromb. Haemost. 2011, 9, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Chamley, L.; McKay, E.; Pattison, N. Cofactor dependent and cofactor independent anticardiolipin antibodies. Thromb. Res. 1991, 61, 291–299. [Google Scholar] [CrossRef]
- Tripodi, A.; De Groot, P.G.; Pengo, V. Antiphospholipid syndrome: Laboratory detection, mechanisms of action and treatment. J. Intern. Med. 2011, 270, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Giannakopoulos, B.; Yan, X.; Yu, P.; Berndt, M.C.; Andrews, R.K.; Rivera, J.; Iverson, G.M.; Cockerill, K.A.; Linnik, M.D.; et al. Anti-β2-glycoprotein I antibodies in complex with β2-glycoprotein I can activate platelets in a dysregulated manner via glycoprotein Ib-IX-V. Arthritis Rheum. 2006, 54, 2558–2567. [Google Scholar] [CrossRef] [PubMed]
- Hulstein, J.J.; Lenting, P.J.; De Laat, B.; Derksen, R.H.; Fijnheer, R.; De Groot, P.G. β2-glycoprotein I inhibits von Willebrand factor–dependent platelet adhesion and aggregation. Blood 2007, 110, 1483–1491. [Google Scholar] [CrossRef] [PubMed]
- Korporaal, S.J.; Relou, I.A.; Van Eck, M.; Strasser, V.; Bezemer, M.; Gorter, G.; Van Berkel, T.J.; Nimpf, J.; Akkerman, J.-W.N.; Lenting, P.J. Binding of low density lipoprotein to platelet apolipoprotein E receptor 2' results in phosphorylation of p38MAPK. J. Biol. Chem. 2004, 279, 52526–52534. [Google Scholar] [CrossRef] [PubMed]
- Jurk, K.; Kehrel, B.E. Platelets: Physiology and biochemistry. Semin. Thromb. Hemost. 2005, 31, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Galli, M.; Luciani, D.; Bertolini, G.; Barbui, T. Lupus anticoagulants are stronger risk factors for thrombosis than anticardiolipin antibodies in the antiphospholipid syndrome: A systematic review of the literature. Blood 2003, 101, 1827–1832. [Google Scholar] [CrossRef] [PubMed]
- Galli, M.; Barbui, T. Antiphospholipid antibodies and pregnancy. Best Pract. Res. Clin. Haematol. 2003, 16, 211–225. [Google Scholar] [CrossRef]
- Favaloro, E.J.; Silvestrini, R. Assessing the usefulness of anticardiolipin antibody assays. Am. J. Clin. Pathol. 2002, 118, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Bahar, A.; Kwak, J.; Beer, A.; Kim, J.; Nelson, L.; Beaman, K.; Gilman-Sachs, A. Antibodies to phospholipids and nuclear antigens in non-pregnant women with unexplained spontaneous recurrent abortions. J. Reprod. Immunol. 1993, 24, 213–222. [Google Scholar] [CrossRef]
- Spadaro, A.; Riccieri, V.; Terracina, S.; Rinaldi, T.; Taccari, E.; Zoppinia, A. Class specific rheumatoid factors and antiphospholipid syndrome in systemic lupus erythematosus. Lupus 2000, 9, 56–60. [Google Scholar] [CrossRef]
- Reber, G.; de Moerloose, P. Anti-β2-glycoprotein I antibodies—When and how should they be measured? Thromb. Res. 2004, 114, 527–531. [Google Scholar] [CrossRef]
- Faden, D.; Tincani, A.; Tanzi, P.; Spatola, L.; Lojacono, A.; Tarantini, M.; Balestrieri, G. Anti-beta 2 glycoprotein I antibodies in a general obstetric population: Preliminary results on the prevalence and correlation with pregnancy outcome. Anti-beta2 glycoprotein I antibodies are associated with some obstetrical complications, mainly preeclampsia-eclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol. 1997, 73, 37–42. [Google Scholar] [PubMed]
- Atsumi, T.; Ieko, M.; Bertolaccini, M.L.; Ichikawa, K.; Tsutsumi, A.; Matsuura, E.; Koike, T. Association of autoantibodies against the phosphatidylserine-prothrombin complex with manifestations of the antiphospholipid syndrome and with the presence of lupus anticoagulant. Arthritis Rheum. 2000, 43, 1982–1993. [Google Scholar] [CrossRef]
- McIntyre, J.A.; Wagenknecht, D.R.; Sugi, T. Phospholipid binding plasma proteins required for antiphospholipid antibody detection—An overview. Am. J. Reprod. Immunol. 1997, 37, 101–110. [Google Scholar] [CrossRef]
- Matsuura, E.; Igarashi, Y.; Fujimoto, M.; Ichikawa, K.; Koike, T. Anticardiolipin cofactor(s) and differential diagnosis of autoimmune disease. Lancet 1990, 336, 177–178. [Google Scholar] [CrossRef]
- McNeil, H.P.; Simpson, R.J.; Chesterman, C.N.; Krilis, S.A. Anti-phospholipid antibodies are directed against a complex antigen that includes a lipid-binding inhibitor of coagulation: Beta 2-glycoprotein I (apolipoprotein H). Proc. Natl. Acad. Sci. USA. 1990, 87, 4120–4124. [Google Scholar] [CrossRef] [PubMed]
- Galli, M.; Comfurius, P.; Maassen, C.; Hemker, H.C.; de Baets, M.H.; van Breda-Vriesman, P.J.; Barbui, T.; Zwaal, R.F.; Bevers, E.M. Anticardiolipin antibodies (ACA) directed not to cardiolipin but to a plasma protein cofactor. Lancet 1990, 335, 1544–1547. [Google Scholar] [CrossRef]
- Schultze, H. Glycoproteins of human plasma. Bull. Schweiz. Akad. Med. Wiss. 1961, 17, 77–91. [Google Scholar] [PubMed]
- Lozier, J.; Takahashi, N.; Putnam, F.W. Complete amino acid sequence of human plasma beta 2-glycoprotein I. Proc. Natl. Acad. Sci. USA. 1984, 81, 3640–3644. [Google Scholar] [CrossRef] [PubMed]
- Polz, E.; Kostner, G.M. The binding of β2-glycoprotein I to human serum lipoproteins: Distribution among density fractions. FEBS Lett. 1979, 102, 183–186. [Google Scholar] [CrossRef]
- Vlachoyiannopoulos, P.; Krilis, S.; Hunt, J.; Manoussakis, M.; Moutsopoulos, H. Patients with anticardiolipin antibodies with and without antiphospholipid syndrome: Their clinical features and β2-glycoprotein I plasma levels. Eur. J. Clin. Invest. 1992, 22, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Miyakis, S.; Giannakopoulos, B.; Krilis, S.A. Beta 2 glycoprotein I-function in health and disease. Thromb. Res. 2004, 114, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, Y.; Rahman, A. Domain I of β2-glycoprotein I: Its role as an epitope and the potential to be developed as a specific target for the treatment of the antiphospholipid syndrome. Lupus 2010, 19, 400–405. [Google Scholar] [CrossRef]
- Agar, C.; De Groot, P.G.; Marquart, J.A.; Meijers, J. Evolutionary conservation of the lipopolysaccharide binding site of β2-glycoprotein I. Thromb. Haemost. 2011, 106, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Steinkasserer, A.; Estaller, C.; Weiss, E.H.; Sim, R.B.; Day, A.J. Complete nucleotide and deduced amino acid sequence of human beta 2-glycoprotein I. Biochem. J. 1991, 277, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Bouma, B.; De Groot, P.G.; Van Den Elsen, J.M.; Ravelli, R.B.; Schouten, A.; Simmelink, M.J.; Derksen, R.H.; Kroon, J.; Gros, P. Adhesion mechanism of human β2-glycoprotein I to phospholipids based on its crystal structure. EMBO J. 1999, 18, 5166–5174. [Google Scholar] [CrossRef]
- Pelkmans, L.; De Laat, B. Antibodies against domain I of β2-glycoprotein I: The one and only? Lupus 2012, 21, 769–772. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, T.; Schousboe, I.; Boel, E.; Mulvihill, E.M.; Hansen, R.R.; Moller, K.B.; Moller, N.P.H.; Sottrup-Jensen, L. Molecular cloning and mammalian expression of human β2-glycoprotein I cDNA. FEBS Lett. 1991, 289, 183–186. [Google Scholar] [CrossRef]
- Agar, C.; Van Os, G.M.; Morgelin, M.; Sprenger, R.R.; Marquart, J.A.; Urbanus, R.T.; Derksen, R.H.; Meijers, J.C.; De Groot, P.G. β2-Glycoprotein I can exist in two conformations: Implications for our understanding of the antiphospholipid syndrome. Blood 2010, 116, 1336–1343. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbacher, R.; Zeth, K.; Diederichs, K.; Gries, A.; Kostner, G.M.; Laggner, P.; Prassl, R. Crystal structure of human β2-glycoprotein I: Implications for phospholipid binding and the antiphospholipid syndrome. EMBO J. 1999, 18, 6228–6239. [Google Scholar] [CrossRef] [PubMed]
- Hammel, M.; Kriechbaum, M.; Gries, A.; Kostner, G.M.; Laggner, P.; Prassl, R. Solution structure of human and bovine β2-glycoprotein I revealed by small-angle X-ray scattering. J. Mol. Biol. 2002, 321, 85–97. [Google Scholar] [CrossRef]
- Koike, T.; Ichikawa, K.; Kasahara, H.; Atsumi, T.; Tsutsumi, A.; Matsuura, E. Epitopes on β2GPI recognized by anticardiolipin antibodies. Lupus 1998, 7, 14–17. [Google Scholar] [CrossRef]
- Ioannou, Y.; Pericleous, C.; Giles, I.; Latchman, D.S.; Isenberg, D.A.; Rahman, A. Binding of antiphospholipid antibodies to discontinuous epitopes on domain I of human β2-glycoprotein I: Mutation studies including residues R39 to R43. Arthritis Rheum. 2007, 56, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Chighizola, C.B.; Gerosa, M.; Meroni, P.L. New tests to detect antiphospholipid antibodies: Anti-domain I beta-2-glycoprotein-I antibodies. Curr. Rheumatol. Rep. 2014, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]
- De Laat, B.; De Groot, P.G. Autoantibodies directed against domain I of beta2-glycoprotein I. Curr. Rheumatol. Rep. 2011, 13, 70–76. [Google Scholar] [CrossRef] [PubMed]
- De Laat, B.; Derksen, R.H.W.M.; Van Lummel, M.; Pennings, M.T.T.; De Groot, P.G. Pathogenic anti-β2-glycoprotein I antibodies recognize domain I of β2-glycoprotein I only after a conformational change. Blood 2006, 107, 1916–1924. [Google Scholar] [CrossRef] [PubMed]
- Wurm, H. β2-Glycoprotein-I (apolipoprotein H) interactions with phospholipid vesicles. Int. J. Biochem. 1984, 16, 511–515. [Google Scholar] [CrossRef]
- Perutková, Š.; Frank-Bertoncelj, M.; Rozman, B.; Kralj-Iglič, V.; Iglič, A. Influence of ionic strength and beta2-glycoprotein I concentration on agglutination of like-charged phospholipid membranes. Coll. Surf. B 2013, 111, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, M.; Hagihara, Y.; Nishii, I.; Yamazaki, T.; Kato, H.; Goto, Y. Identification of the phospholipid-binding site of human β2-glycoprotein I domain V by heteronuclear magnetic resonance. J. Mol. Biol. 2000, 304, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, M.; Vreys, I.; Wittevrongel, C.; Boon, D.; Vermylen, J.; Hoylaerts, M.F.; Arnout, J. Thrombogenicity of β2-glycoprotein I–dependent antiphospholipid antibodies in a photochemically induced thrombosis model in the hamster. Blood 2003, 101, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Giannakopoulos, B.; Krilis, S.A. The pathogenesis of the antiphospholipid syndrome. N. Engl. J. Med. 2013, 368, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Dupuy d'Angeac, A.; Stefas, I.; Graafland, H.; de Lamotte, F.; Rucheton, M.; Palais, C.; Eriksson, A.-K.; Bosc, P.; Rosé, C.; Chicheportiche, R. Biotinylation of glycan chains in β2 glycoprotein I induces dimerization of the molecule and its detection by the human autoimmune anti-cardiolipin antibody EY2C9. Biochem. J. 2006, 393, 117–127. [Google Scholar] [CrossRef]
- Kondo, A.; Miyamoto, T.; Yonekawa, O.; Giessing, A.M.; Østerlund, E.C.; Jensen, O.N. Glycopeptide profiling of beta-2-glycoprotein I by mass spectrometry reveals attenuated sialylation in patients with antiphospholipid syndrome. J. Proteomics 2009, 73, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, A.-J.; Marcos, M.; Mirón-Canelo, J.-A.; Cervera, R.; Espinosa, G. Val247Leu beta2-glycoprotein-I allelic variant is associated with antiphospholipid syndrome: Systematic review and meta-analysis. Autoimmun. Rev. 2012, 11, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, S.; Atsumi, T.; Matsuura, E.; Kaihara, K.; Yamamoto, D.; Ichikawa, K.; Koike, T. Significance of valine/leucine247 polymorphism of β2-glycoprotein I in antiphospholipid syndrome: Increased reactivity of anti-β2-glycoprotein I autoantibodies to the valine247 β2-glycoprotein I variant. Arthritis Rheum. 2005, 52, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Haupt, H.; Schwick, H.G.; Storiko, K. On a hereditary beta-2-glycoprotein I deficiency. Humangenetik 1968, 5, 291–293. [Google Scholar] [PubMed]
- Nimpf, J.; Wurm, H.; Kostner, G. Interaction of beta 2-glycoprotein-I with human blood platelets: Influence upon the ADP-induced aggregation. Thromb. Haemost. 1985, 54, 397–401. [Google Scholar] [PubMed]
- Rahgozar, S. Revisiting Beta 2 glycoprotein I, the major autoantigen in the antiphospholipid syndrome. Iran. J. Immunol. 2012, 9, 73–85. [Google Scholar] [PubMed]
- Passam, F.; Rahgozar, S.; Qi, M.; Raftery, M.; Wong, J.; Tanaka, K.; Ioannou, Y.; Zhang, J.; Gemmell, R.; Qi, J. Redox control of β2-glycoprotein I–von Willebrand factor interaction by thioredoxin-1. J. Thromb. Haemost. 2010, 8, 1754–1762. [Google Scholar] [CrossRef] [PubMed]
- Maiti, S.N.; Balasubramanian, K.; Ramoth, J.A.; Schroit, A.J. Beta-2-glycoprotein 1-dependent macrophage uptake of apoptotic cells. Binding to lipoprotein receptor-related protein receptor family members. J. Biol. Chem. 2008, 283, 3761–3766. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Monem, H.; Dasgupta, S.K.; Le, A.; Prakasam, A.; Thiagarajan, P. Phagocytosis of platelet microvesicles and β2–glycoprotein I. Thromb. Haemost. 2010, 104, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M.; Wasylik, S.; Morgelin, M.; Olin, A.I.; Meijers, J.; Derksen, R.H.; De Groot, P.G.; Herwald, H. The antibacterial activity of peptides derived from human β2 glycoprotein I is inhibited by protein H and M1 protein from Streptococcus pyogenes. Mol. Microbiol. 2008, 67, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Agar, C.; De Groot, P.G.; Morgelin, M.; Monk, S.D.; Van Os, G.; Levels, J.H.; De Laat, B.; Urbanus, R.T.; Herwald, H.; Van der Poll, T.; et al. β2-glycoprotein I: A novel component of innate immunity. Blood 2011, 117, 6939–6947. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Reddel, S.W.; Herzog, H.; Wang, Y.X.; Brighton, T.; France, M.P.; Robertson, S.A.; Krilis, S.A. Impaired thrombin generation in β2-glycoprotein I null mice. J. Biol. Chem. 2001, 276, 13817–13821. [Google Scholar] [PubMed]
- De Groot, P.G.; Derksen, R. Pathophysiology of antiphospholipid antibodies. Neth. J. Med. 2004, 62, 267–272. [Google Scholar] [PubMed]
- Forastiero, R.; Martinuzzo, M.; Carreras, L.O.; Maclouf, J. Anti-β2 glycoprotein I antibodies and platelet activation in patients with antiphospholipid antibodies: Association with increased excretion of platelet-derived thromboxane urinary metabolites. Thromb. Haemost. 1998, 79, 42–45. [Google Scholar] [PubMed]
- Urbanus, R.T.; De Laat, H.B.; De Groot, P.G.; Derksen, R.H. Prolonged bleeding time and lupus anticoagulant: A second paradox in the antiphospholipid syndrome. Arthritis Rheum. 2004, 50, 3605–3609. [Google Scholar] [CrossRef] [PubMed]
- Abou-Nassar, K.; Carrier, M.; Ramsay, T.; Rodger, M.A. The association between antiphospholipid antibodies and placenta mediated complications: A systematic review and meta-analysis. Thromb. Res. 2011, 128, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Carroll, T.Y.; Mulla, M.J.; Han, C.S.; Brosens, J.J.; Chamley, L.W.; Giles, I.; Pericleous, C.; Rahman, A.; Sfakianaki, A.K.; Paidas, M.J. Modulation of trophoblast angiogenic factor secretion by antiphospholipid antibodies is not reversed by heparin. Am. J. Reprod. Immunol. 2011, 66, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Rand, J.; Wu, X.; Quinn, A.; Taatjes, D. The annexin A5-mediated pathogenic mechanism in the antiphospholipid syndrome: Role in pregnancy losses and thrombosis. Lupus 2010, 19, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Willis, R.; Shoenfeld, Y.; Pierangeli, S.S.; Blank, M. What is the origin of antiphospholipid antibodies? In Antiphospholipid Syndrome; Springer: New York, NY, USA, 2012; pp. 23–39. [Google Scholar]
- Hashimoto, Y.; Kawamura, M.; Ichikawa, K.; Suzuki, T.; Sumida, T.; Yoshida, S.; Matsuura, E.; Ikehara, S.; Koike, T. Anticardiolipin antibodies in NZW × BXSB F1 mice. A model of antiphospholipid syndrome. J. Immunol. 1992, 149, 1063–1068. [Google Scholar] [PubMed]
- Ida, A.; Hirose, S.; Hamano, Y.; Kodera, S.; Jiang, Y.; Abe, M.; Zhang, D.; Nishimura, H.; Shirai, T. Multigenic control of lupus-associated antiphospholipid syndrome in a model of (NZW × BXSB) F1 mice. Eur. J. Immunol. 1998, 28, 2694–2703. [Google Scholar] [CrossRef]
- Castro-Marrero, J.; Balada, E.; Vilardell-Tarres, M.; Ordi-Ros, J. Genetic risk factors of thrombosis in the antiphospholipid syndrome. Br. J. Haematol. 2009, 147, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Epstein, F.H.; Albert, L.J.; Inman, R.D. Molecular mimicry and autoimmunity. N. Engl. J. Med. 1999, 341, 2068–2074. [Google Scholar] [CrossRef] [PubMed]
- Sherer, Y.; Blank, M.; Shoenfeld, Y. Antiphospholipid syndrome (APS): Where does it come from? Best Pract. Res. Clin. Rheumatol. 2007, 21, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, M.; Matsuda, J. Induction of anticardiolipin antibody and/or lupus anticoagulant in rabbits by immunization with lipoteichoic acid, lipopolysaccharide and lipid A. Lupus 1996, 5, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Subang, R.; Levine, J.S.; Janoff, A.S.; Davidson, S.M.; Taraschi, T.F.; Koike, T.; Minchey, S.R.; Whiteside, M.; Tannenbaum, M.; Rauch, J. Phospholipid-bound β2-glycoprotein I induces the production of anti-phospholipid antibodies. J. Autoimmun. 2000, 15, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Merrill, J.T. Do antiphospholipid antibodies develop for a purpose? Curr. Rheumatol. Rep. 2006, 8, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Van Os, G.M.; Meijers, J.C.; Agar, C.; Seron, M.V.; Marquart, J.A.; Akesson, P.; Urbanus, R.T.; Derksen, R.H.; Herwald, H.; Morgelin, M.; et al. Induction of anti-β2-glycoprotein I autoantibodies in mice by protein H of Streptococcus pyogenes. J. Thromb. Haemost. 2011, 9, 2447–2456. [Google Scholar] [CrossRef] [PubMed]
- Biasiolo, A.; Rampazzo, P.; Brocco, T.; Barbero, F.; Rosato, A.; Pengo, V. [Anti-β2 Glycoprotein I—β2 Glycoprotein I] immune complexes in patients with antiphospholipid syndrome and other autoimmune diseases. Lupus 1999, 8, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Fischetti, F.; Durigutto, P.; Pellis, V.; Debeus, A.; Macor, P.; Bulla, R.; Bossi, F.; Ziller, F.; Sblattero, D.; Meroni, P. Thrombus formation induced by antibodies to β2-glycoprotein I is complement dependent and requires a priming factor. Blood 2005, 106, 2340–2346. [Google Scholar] [CrossRef] [PubMed]
- Arad, A.; Proulle, V.; Furie, R.A.; Furie, B.C.; Furie, B. β2-glycoprotein-1 autoantibodies from patients with antiphospholipid syndrome are sufficient to potentiate arterial thrombus formation in a mouse model. Blood 2011, 117, 3453–3459. [Google Scholar] [CrossRef] [PubMed]
- Asherson, R.A. The catastrophic antiphospholipid syndrome, 1998. A review of the clinical features, possible pathogenesis and treatment. Lupus 1998, 7, S55–S62. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, M.B. Antiphospholipid syndrome. East. J. Med. 2009, 14, 51–56. [Google Scholar]
- Shoenfeld, Y.; Krause, I.; Kvapil, F.; Sulkes, J.; Lev, S.; Von Landenberg, P.; Font, J.; Zaech, J.; Cervera, R.; Piette, J. Prevalence and clinical correlations of antibodies against six β2-glycoprotein-I-related peptides in the antiphospholipid syndrome. J. Clin. Immunol. 2003, 23, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Cucnik, S.; Kveder, T.; Artenjak, A.; Gallova, Z.U.; Swadzba, J.; Musial, J.; Iwaniec, T.; Stojanovich, L.; Alessandri, C.; Valesini, G. Avidity of anti-β2-glycoprotein I antibodies in patients with antiphospholipid syndrome. Lupus 2012, 21, 764–765. [Google Scholar] [CrossRef] [PubMed]
- Carter, A.M. Complement activation: An emerging player in the pathogenesis of cardiovascular disease. Scientifica 2012, 2012, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Jefferis, R.; Kumararatne, D. Selective IgG subclass deficiency: Quantification and clinical relevance. Clin. Exp. Immunol. 1990, 81, 357. [Google Scholar] [CrossRef] [PubMed]
- Palarasah, Y.; Skjodt, K.; Brandt, J.; Teisner, B.; Koch, C.; Vitved, L.; Skjoedt, M.O. Generation of a C3c specific monoclonal antibody and assessment of C3c as a putative inflammatory marker derived from complement factor C3. J. Immunol. Methods 2010, 362, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Banzato, A.; Pozzi, N.; Frasson, R.; De Filippis, V.; Ruffatti, A.; Bison, E.; Padayattil, S.; Denas, G.; Pengo, V. Antibodies to domain I of β2 glycoprotein I are in close relation to patients risk categories in antiphospholipid syndrome (APS). Thromb. Res. 2011, 128, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, N.; Banzato, A.; Bettin, S.; Bison, E.; Pengo, V.; De Filippis, V. Chemical synthesis and characterization of wild-type and biotinylated N-terminal domain 1–64 of beta2-glycoprotein I. Protein Sci. 2010, 19, 1065–1078. [Google Scholar] [CrossRef] [PubMed]
- Meneghel, L.; Ruffatti, A.; Gavasso, S.; Tonello, M.; Mattia, E.; Spiezia, L.; Tormene, D.; Hoxha, A.; Fedrigo, M.; Simioni, P. Detection of IgG anti-domain I beta2 glycoprotein I antibodies by chemiluminescence immunoassay in primary antiphospholipid syndrome. Clin. Chim. Acta 2015, 446, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Van Lummel, M.; Pennings, M.T.; Derksen, R.H.; Urbanus, R.T.; Lutters, B.C.; Kaldenhoven, N.; De Groot, P.G. The binding site in β2-glycoprotein I for ApoER2' on platelets is located in domain V. J. Biol. Chem. 2005, 280, 36729–36736. [Google Scholar] [CrossRef] [PubMed]
- Pennings, M.T.; Derksen, R.H.; Urbanus, R.T.; Tekelenburg, W.L.; Hemrika, W.; De Groot, P.G. Platelets express three different splice variants of ApoER2 that are all involved in signaling. J. Thromb. Haemost. 2007, 5, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- Kolyada, A.; Porter, A.; Beglova, N. Inhibition of thrombotic properties of persistent autoimmune anti-β2GPI antibodies in the mouse model of antiphospholipid syndrome. Blood 2014, 123, 1090–1097. [Google Scholar] [CrossRef] [PubMed]
- Palatinus, A.A.; Ahuja, K.D.; Adams, M.J. Effects of antiphospholipid antibodies on in vitro platelet aggregation. Clin. Appl. Thromb. Hemost. 2012, 18, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Betts, N.A.; Ahuja, K.D.; Adams, M.J. Anti-β2GP1 antibodies have variable effects on platelet aggregation. Pathol.-J. RCPA 2013, 45, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Sikara, M.P.; Routsias, J.G.; Samiotaki, M.; Panayotou, G.; Moutsopoulos, H.M.; Vlachoyiannopoulos, P.G. β2 Glycoprotein I (β2GPI) binds platelet factor 4 (PF4): Implications for the pathogenesis of antiphospholipid syndrome. Blood 2010, 115, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Ashby, B.; Daniel, J.L.; Smith, J.B. Mechanisms of platelet activation and inhibition. Hematol. Oncol. Clin. North. Am. 1990, 4, 1–26. [Google Scholar] [PubMed]
- Li, Z.; Delaney, M.K.; O’Brien, K.A.; Du, X. Signaling during platelet adhesion and activation. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2341–2349. [Google Scholar] [CrossRef] [PubMed]
- Pennings, M.; Derksen, R.; Van Lummel, M.; Adelmeijer, J.; Vanhoorelbeke, K.; Urbanus, R.; Lisman, T.; De Groot, P. Platelet adhesion to dimeric β2-glycoprotein I under conditions of flow is mediated by at least two receptors: Glycoprotein Ibα and apolipoprotein E receptor 2'. J. Thromb. Haemost. 2007, 5, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Lutters, B.C.; Derksen, R.H.; Tekelenburg, W.L.; Lenting, P.J.; Arnout, J.; De Groot, P.G. Dimers of β2-glycoprotein I increase platelet deposition to collagen via interaction with phospholipids and the apolipoprotein E receptor 2'. J. Biol. Chem. 2003, 278, 33831–33838. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, H.L.D.; Carvalho, G.R.D.; Aarestrup, F.M.; Correa, J.O.D.A.; Azevedo, M.R.A. Evaluation of platelet aggregation in the presence of antiphospholipid antibodies: Anti-β2GP1 and anticardiolipin. Rev. Bras. Reumatol. 2013, 53, 400–404. [Google Scholar] [CrossRef]
- Ho, Y.C.; Ahuja, K.D.; Adams, M.J. Effects of anti-β2GP1 antibodies on collagen induced platelet aggregation. 2016; in preparation. [Google Scholar]
| Assays | Principle of Detection | Antibodies Detected | Clinical Significance [5] |
|---|---|---|---|
| LAC | Clotting assay | LAC (mainly against β2GP1 and prothrombin) | |
| aCL antibody | Immunological assay | aCL antibody (IgG, IgM, IgA) |
|
| Anti-β2GP1 antibody | Immunological assay | Anti-β2GP1 antibody (IgG, IgM, IgA) |
|
| Anti-prothrombin antibody | Immunological assay | Anti-prothrombin and anti-phosphatidylserine-prothrombin complex |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, Y.C.; Ahuja, K.D.K.; Körner, H.; Adams, M.J. β2GP1, Anti-β2GP1 Antibodies and Platelets: Key Players in the Antiphospholipid Syndrome. Antibodies 2016, 5, 12. https://doi.org/10.3390/antib5020012
Ho YC, Ahuja KDK, Körner H, Adams MJ. β2GP1, Anti-β2GP1 Antibodies and Platelets: Key Players in the Antiphospholipid Syndrome. Antibodies. 2016; 5(2):12. https://doi.org/10.3390/antib5020012
Chicago/Turabian StyleHo, Yik C., Kiran D. K. Ahuja, Heinrich Körner, and Murray J. Adams. 2016. "β2GP1, Anti-β2GP1 Antibodies and Platelets: Key Players in the Antiphospholipid Syndrome" Antibodies 5, no. 2: 12. https://doi.org/10.3390/antib5020012