Predicting the Potential Impact of Climate Change on Carbon Stock in Semi-Arid West African Savannas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling Design and Data Collection
2.2.1. Satellite Imagery and Data Pre-Processing
2.2.2. Vegetation Data
2.2.3. Soil Sampling, Processing, and Carbon Stock Calculation
- SOCstock: soil organic carbon (Mg C ha−1);
- C: carbon concentration in the finer fraction;
- Da: soil bulk density (Mg·cm−3);
- P: thickness of soil layer (cm);
- Frag: % of volume of coarse fragments/100; 100 will be used to convert the unit to Mg C ha−1.
2.2.4. Above and Belowground Biomass and Carbon Stock
2.2.5. Geographical Distribution of Carbon Stock under Changing Climate
2.2.6. Statistical Analyses
3. Results
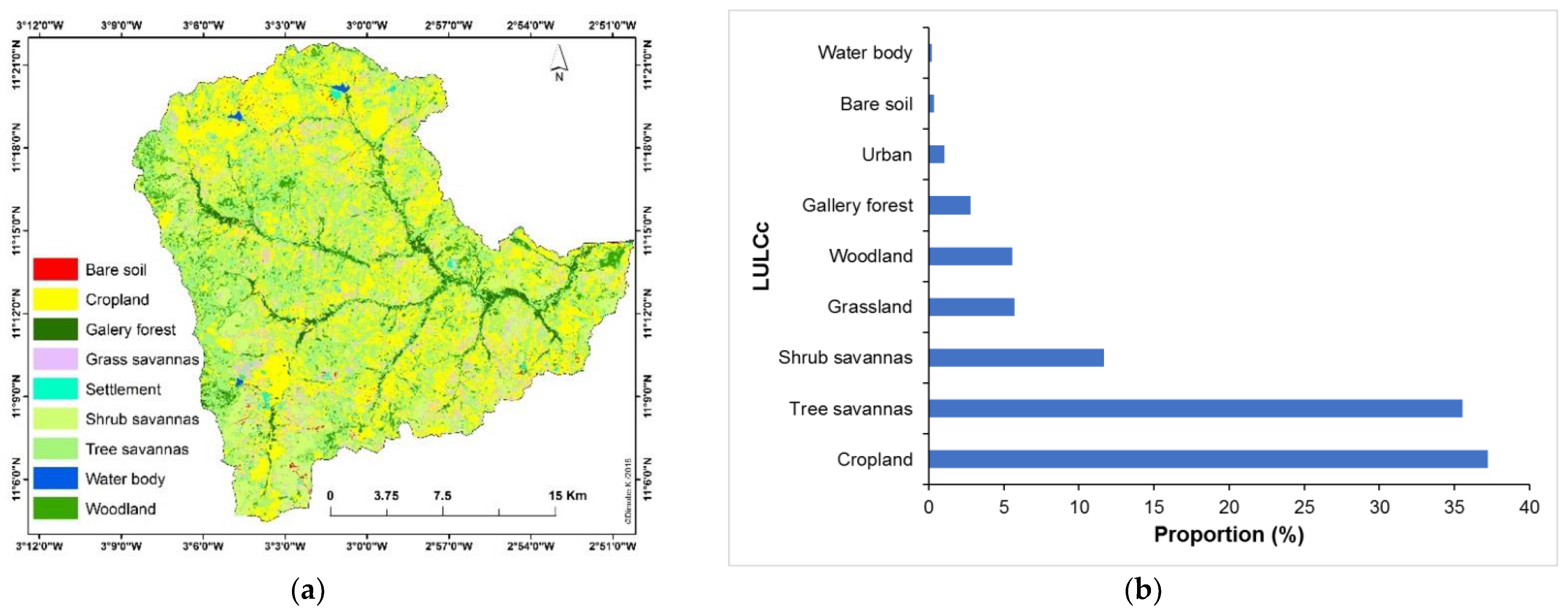
3.1. Main Land Use and Land Cover Categories
3.2. Vegetation Structure
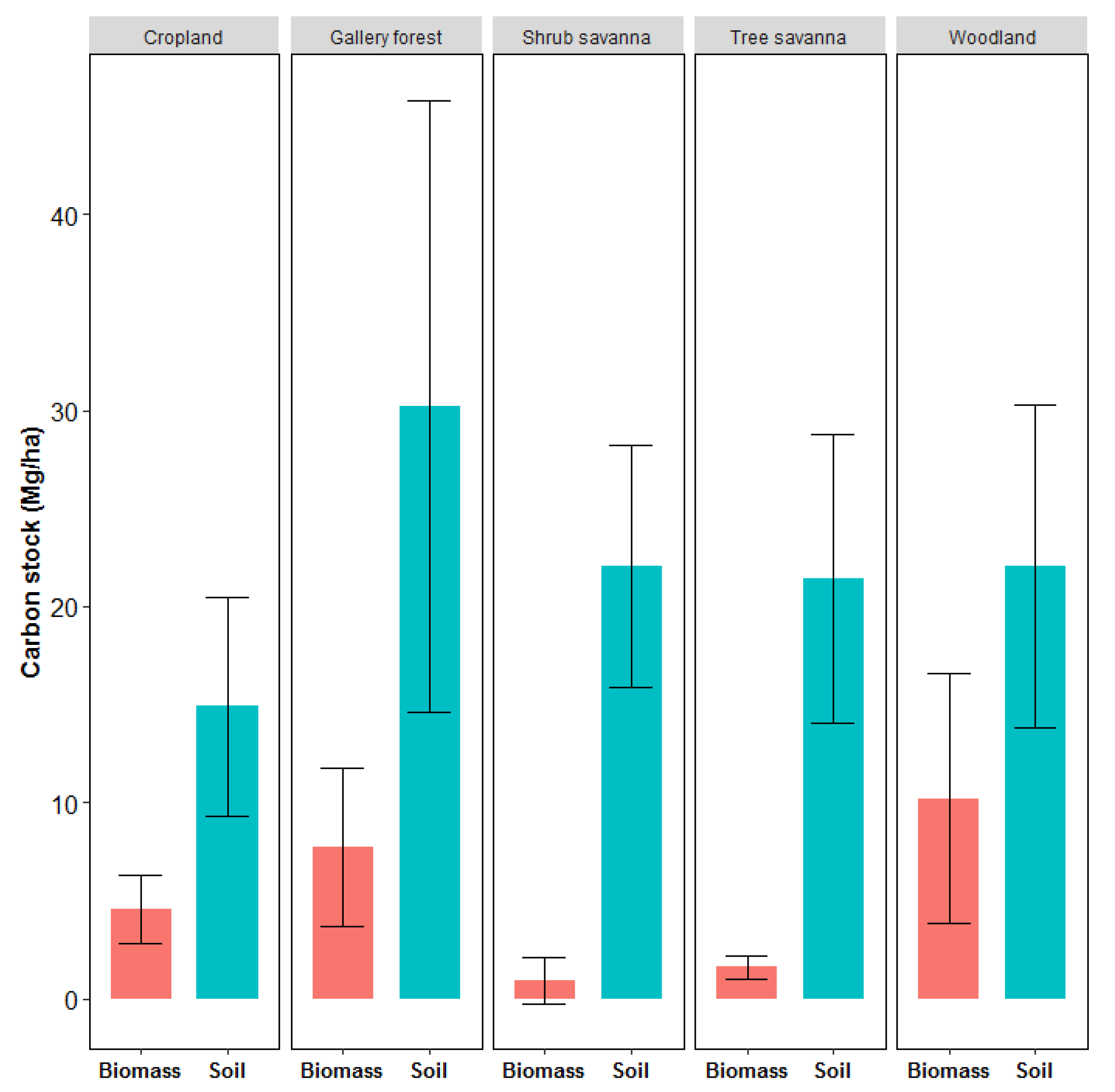
3.3. Carbon Stock in the Sudanese Savanna Landscape
3.3.1. Carbon Stock in Plant Biomass
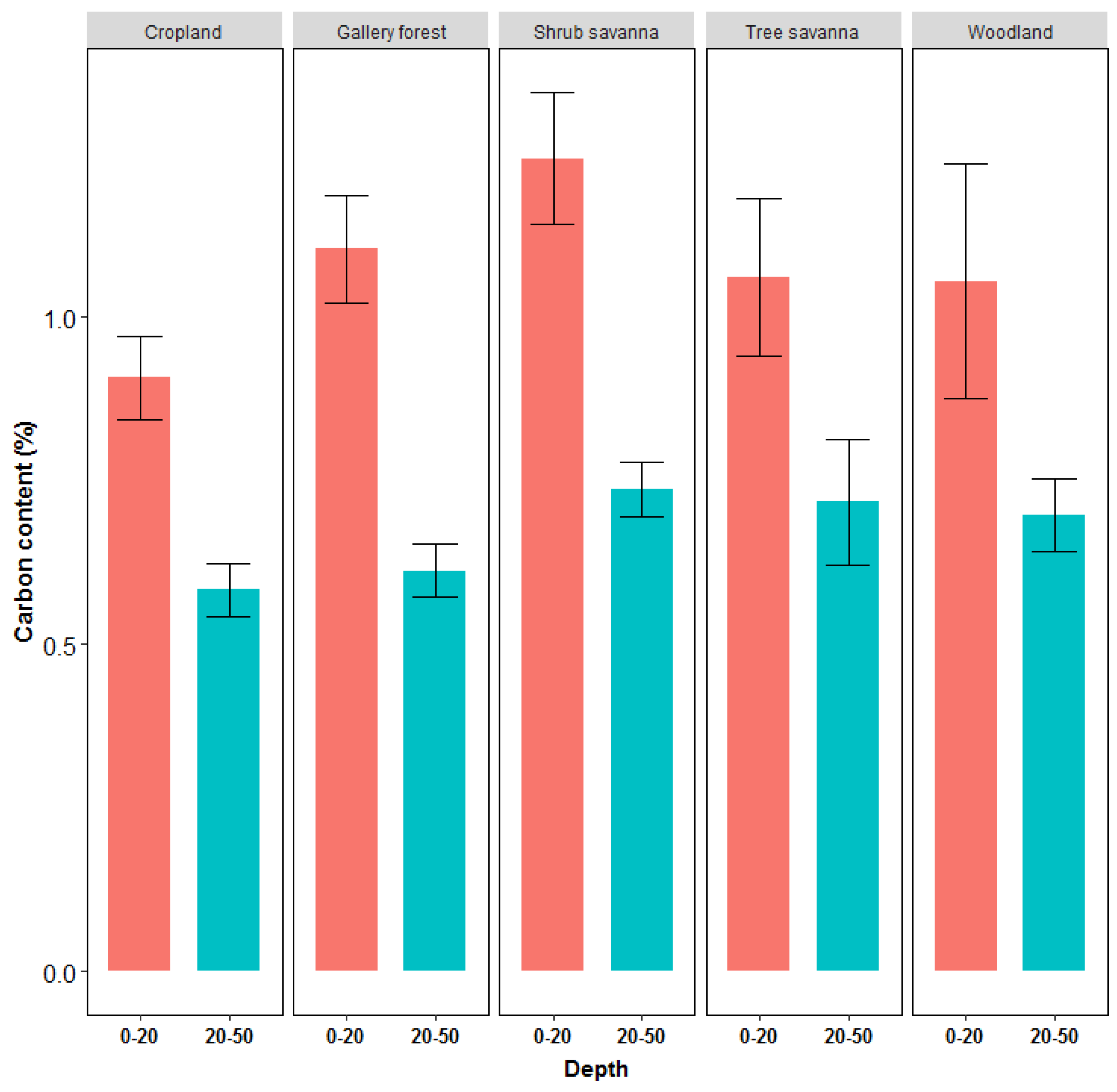
3.3.2. Soil Organic Carbon Stock
3.3.3. Total Carbon Stock in the Main LULCc
3.4. Geographical Distribution of Carbon Stock under Changing Climate
4. Discussion
4.1. Main Land Use and Land Cover Categories
4.2. Vegetation Structure
4.3. Carbon Stock in the Sudanese Savanna Landscape
4.3.1. Carbon in Plant Biomass
4.3.2. Soil Organic Carbon
4.4. Geographical Distribution of Carbon Stock under Changing Climate
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sylla, M.B.; Faye, A.; Giorgi, F.; Diedhiou, A.; Kunstmann, H. Projected Heat Stress Under 1.5 °C and 2 °C Global Warming Scenarios Creates Unprecedented Discomfort for Humans in West Africa. Earths Future 2018, 6, 1029–1044. [Google Scholar] [CrossRef]
- Sultan, B.; Roudier, P.; Quirion, P.; Alhassane, A.; Muller, B.; Dingkuhn, M.; Ciais, P.; Guimberteau, M.; Traore, S.; Baron, C.; et al. Assessing climate change impacts on sorghum and millet yields in the Sudanian and Sahelian savannas of West Africa. Environ. Res. Lett. 2013, 8, 014040. [Google Scholar] [CrossRef] [Green Version]
- Gockowski, J.; van Asten, P. Agricultural Intensification as a Climate Change and Food Security Strategy for Sub-Saharan Africa. In E. Wollenberg, A. Nihart, M.L. Tapio-Bostro and M. Grieg-Gran, Climate Change Mitigation and Agriculture. International Center for Research in Agroforestry-International Center for Tropical Agriculture: London-New York, 2012; pp. 382–390. Available online: http://hdl.handle.net/10568/80518 (accessed on 20 August 2018).
- Lott, J.E.; Ong, C.K.; Black, C.R. Understorey microclimate and crop performance in a Grevillea robusta-based agroforestry system in semi-arid Kenya. Agric. For. Meteorol. 2009, 149, 1140–1151. [Google Scholar] [CrossRef]
- Law, E.A.; Bryan, B.A.; Torabi, N.; Bekessy, S.A.; McAlpine, C.A.; Wilson, K.A. Measurement matters in managing landscape carbon. Ecosyst. Serv. 2015, 13, 6–15. [Google Scholar] [CrossRef]
- Thomson, A.M.; Calvin, K.V.; Chini, L.P.; Hurtt, G.; Edmonds, J.A.; Bond-Lamberty, B.; Frolking, S.; Wise, M.A.; Janetos, A.C. Climate mitigation and the future of tropical landscapes. Proc. Natl. Acad. Sci. USA 2010, 107, 19633–19638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumanski, J. Carbon sequestration, soil conservation, and the Kyoto protocol: Summary of implications. Clim. Chang. 2004, 65, 255–261. [Google Scholar] [CrossRef]
- Bali. Report of the Conference of Parties on Its Thirtheenth Session. Bali, Indonesia, 3–15 December 2007. Available online: https://unfccc.int/resource/docs/2007/cop13/eng/06.pdf (accessed on 14 July 2018).
- Angelsen, A.; Brockhaus, M.; Sunderlin, W.D.; Verchot, L.V. Analyse de la REDD+ Les Enjeux et les Choix; CIFOR: Bogor, Indonesia, 2013. [Google Scholar]
- Gibbs, H.K.; Brown, S.; Niles, J.O.; Foley, J.A. Monitoring and estimating tropical forest carbon stocks: Making REDD a reality. Environ. Res. Lett. 2007, 2, 045023. [Google Scholar] [CrossRef]
- Saatchi, S.S.; Harris, N.L.; Brown, S.; Lefsky, M.; Mitchard, E.T.; Salas, W.; Zutta, B.R.; Buermann, W.; Lewis, S.L.; Hagen, S. Benchmark map of forest carbon stocks in tropical regions across three continents. Proc. Natl. Acad. Sci. USA 2011, 108, 9899–9904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baccini, A.; Goetz, S.J.; Walker, W.S.; Laporte, N.T.; Sun, M.; Sulla-Menashe, D.; Hackler, J.; Beck, P.S.A.; Dubayah, R.; Friedl, M.A. Estimated carbon dioxide emissions from tropical deforestation improved by carbon-density maps. Nat. Clim. Chang. 2012, 2, 182. [Google Scholar] [CrossRef]
- Clark, D.B.; Kellner, J.R. Tropical forest biomass estimation and the fallacy of misplaced concreteness. J. Veg. Sci. 2012, 23, 1191–1196. [Google Scholar] [CrossRef] [Green Version]
- Basuki, T.M.; Van Laake, P.E.; Skidmore, A.K.; Hussin, Y.A. Allometric equations for estimating the above-ground biomass in tropical lowland Dipterocarp forests. For. Ecol. Manag. 2009, 257, 1684–1694. [Google Scholar] [CrossRef]
- Mbow, C.; Van Noordwijk, M.; Luedeling, E.; Neufeldt, H.; Minang, P.A.; Kowero, G. Agroforestry solutions to address food security and climate change challenges in Africa. Curr. Opin. Environ. Sustain. 2014, 6, 61–67. [Google Scholar] [CrossRef] [Green Version]
- Dimobe, K.; Goetze, D.; Ouédraogo, A.; Mensah, S.; Akpagana, K.; Porembski, S.; Thiombiano, A. Aboveground biomass allometric equations and carbon content of the shea butter tree (Vitellaria paradoxa C.F. Gaertn., Sapotaceae) components in Sudanian savannas (West Africa). Agrofor. Syst. 2018. [Google Scholar] [CrossRef]
- Dimobe, K.; Mensah, S.; Goetze, D.; Ouédraogo, A.; Kuyah, S.; Porembski, S.; Thiombiano, A. Aboveground biomass partitioning and additive models for Combretum glutinosum and Terminalia laxiflora in West Africa. Biomass Bioenergy 2018, 115, 151–159. [Google Scholar] [CrossRef]
- Brown, S. Estimating Biomass and Biomass Change of Tropical Forests: A Primer; Food & Agriculture Organization: Rome, Italy, 1997; ISBN 92-5-103955-0. [Google Scholar]
- Chave, J.; Andalo, C.; Brown, S.; Cairns, M.A.; Chambers, J.Q.; Eamus, D.; Fölster, H.; Fromard, F.; Higuchi, N.; Kira, T. Tree allometry and improved estimation of carbon stocks and balance in tropical forests. Oecologia 2005, 145, 87–99. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change: Mitigation. Contribution of Working Group III to the Fourth Assessment Report; IPCC: Geneva, Switzerland, 2007. [Google Scholar]
- Fearnside, P.M. Global warming and tropical land-use change: Greenhouse gas emissions from biomass burning, decomposition and soils in forest conversion, shifting cultivation and secondary vegetation. Clim. Chang. 2000, 46, 115–158. [Google Scholar] [CrossRef]
- Manlay, R.J.; Kairé, M.; Masse, D.; Chotte, J.-L.; Ciornei, G.; Floret, C. Carbon, nitrogen and phosphorus allocation in agro-ecosystems of a West African savanna: I. The plant component under semi-permanent cultivation. Agric. Ecosyst. Environ. 2002, 88, 215–232. [Google Scholar] [CrossRef]
- Ardö, J.; Olsson, L. Assessment of soil organic carbon in semi-arid Sudan using GIS and the CENTURY model. J. Arid Environ. 2003, 54, 633–651. [Google Scholar] [CrossRef]
- Feral, C.J.W.; Epstein, H.E.; Otter, L.; Aranibar, J.N.; Shugart, H.H.; Macko, S.A.; Ramontsho, J. Carbon and nitrogen in the soil–plant system along rainfall and land-use gradients in southern Africa. J. Arid Environ. 2003, 54, 327–343. [Google Scholar] [CrossRef]
- Archer, S.; Boutton, T.W.; McMurtry, C. Carbon And Nitrogen Accumulation in a Savanna Landscape: Field And Modeling Perspectives. In Global Environmental Change in the Ocean and on Land. Available online: https://pdfs.semanticscholar.org/d88c/3335ffe001ccee0d5076b8c8b4aa39ceb2e9.pdf (accessed on 21 October 2018).
- Hounkpatin, O.K.L.; de Hipt, F.O.; Bossa, A.Y.; Welp, G.; Amelung, W. Soil organic carbon stocks and their determining factors in the Dano catchment (Southwest Burkina Faso). Catena 2018, 166, 298–309. [Google Scholar] [CrossRef]
- Tiessen, H.; Feller, C.; Sampaio, E.; Garin, P. Carbon sequestration and turnover in semiarid savannas and dry forest. Clim. Chang. 1998, 40, 105–117. [Google Scholar] [CrossRef]
- Gifford, R.M.; Howden, M. Vegetation thickening in an ecological perspective: Significance to national greenhouse gas inventories. Environ. Sci. Policy 2001, 4, 59–72. [Google Scholar] [CrossRef]
- San José, J.J.; Montes, R.A. Management effects on carbon stocks and fluxes across the Orinoco savannas. For. Ecol. Manag. 2001, 150, 293–311. [Google Scholar] [CrossRef]
- Woomer, P.L.; Tieszen, L.L.; Tschakert, P.; Parton, W.J.; Touré, A. Landscape Carbon Sampling and Biogeochimical Modelling. A Two-Week Skills Development Workshop Conducted in Senegal. USGS, CSE, 2001. Available online: https://www.researchgate.net/profile/Larry_Tieszen/publication/229037894_Landscape_Carbon_Sampling_and_Biogeochemical_Modeling/links/0fcfd50a2991c39b06000000/Landscape-Carbon-Sampling-and-Biogeochemical-Modeling.pdf (accessed on 14 July 2018).
- Sawadogo, L.; Savadogo, P.; Tiveau, D.; Dayamba, S.D.; Zida, D.; Nouvellet, Y.; Oden, P.C.; Guinko, S. Allometric prediction of above-ground biomass of eleven woody tree species in the Sudanian savanna-woodland of West Africa. J. For. Res. 2010, 21, 475–481. [Google Scholar] [CrossRef]
- Mbow, C.; Verstraete, M.M.; Sambou, B.; Diaw, A.T.; Neufeldt, H. Allometric models for aboveground biomass in dry savanna trees of the Sudan and Sudan-Guinean ecosystems of Southern Senegal. J. For. Res. 2013, 19, 340–347. [Google Scholar] [CrossRef]
- Forkuor, G. Agricultural Land Use Mapping in West Africa Using Multi-sensor Satellite Imagery. Ph.D. Thesis, University of Würzburg, Würzburg, Germany, 2014. [Google Scholar]
- Waongo, M. Optimizing Planting Dates for Agricultural Decision-Making under Climate Change over Burkina Faso/West Africa. Ph.D. Thesis, 2015. Available online: https://d-nb.info/1077705913/34 (accessed on 15 July 2018).
- Sissoko, K.; van Keulen, H.; Verhagen, J.; Tekken, V.; Battaglini, A. Agriculture, livelihoods and climate change in the West African Sahel. Reg. Environ. Chang. 2011, 11, 119–125. [Google Scholar] [CrossRef]
- Sanfo, S. Politiques Publiques Agricoles et Lutte Contre la Pauvreté au Burkina Faso: Le cas de la Région du Plateau Central. Ph.D. Thesis, Paris 1. 2010. Available online: http://agritrop.cirad.fr/558233/1/document_558233.pdf (accessed on 30 August 2018).
- Thiombiano, A.; Glèlè-Kakai, R.; Bayen, P.; Boussim, J.I.; Mahamane, A. Méthodes et dispositifs d’inventaires forestiers en Afrique de l’Ouest: État des lieux et propositions pour une harmonisation. Ann. Sci. Agron. 2015, 19, 15–31. [Google Scholar]
- Sambaré, O.; Bognounou, F.; Wittig, R.; Thiombiano, A. Woody species composition, diversity and structure of riparian forests of four watercourses types in Burkina Fas. J. For. Res. 2011, 22, 145–158. [Google Scholar] [CrossRef]
- Aleza, K.; Wala, K.; Bayala, J.; Villamor, G.B.; Dourma, M.; Atakpama, W.; Akpagana, K. Population structure and regeneration status of Vitellaria Paradoxa (C. F. Gaertner) under different land management regimes in Atacora department, Benin. Agrofor. Syst. 2015, 89, 511–523. [Google Scholar] [CrossRef]
- Thiombiano, A.; Schmidt, M.; Dressler, S.; Ouédraogo, A.; Hahn, K.; Zizka, G. Catalogue des plantes vasculaires du Burkina Faso. Boissiera 2012, 65, 1–391. [Google Scholar]
- Aynekulu, E.; Vagen, T.-G.; Shephard, K.D.; Winowiecki, L. A Protocol for Modeling, Measurement and Monitoring Soil Carbon Stocks in Agricultural Landscapes. Available online: http://www.worldagroforestry.org/publication/protocol-modeling-measurement-and-monitoring-soil-carbon-stocks-agricultural-landscapes (accessed on 21 October 2018).
- Chave, J.; Réjou-Méchain, M.; Búrquez, A.; Chidumayo, E.; Colgan, M.; Delitti, W.; Duque, A.; Eid, T.; Fearnside, P.; Goodman, R.; et al. Improved allometric models to estimate the aboveground biomass of tropical trees. Glob. Chang. Biol. 2014, 20, 3177–3190. [Google Scholar] [CrossRef] [PubMed]
- Zanne, A.E.; Lopez-Gonzalez, G.; Coomes, D.A.; Ilic, J.; Jansen, S.; Lewis, S.L.; Miller, R.B.; Swenson, N.G.; Wiemann, M.C.; Chave, J. Global Wood Density Database; University College of London: London, UK, 2009. [Google Scholar]
- Réjou-Méchain, M.; Tanguy, A.; Piponiot, C.; Chave, J.; Hérault, B. Biomass: An R Package for Estimating Above-Ground Biomass and Its Uncertainty in Tropical Forests. Methods Ecol. Evol. 2017, 8, 1163–1167. [Google Scholar] [CrossRef]
- Takimoto, A.; Nair, P.K.R.; Nair, V.D. Carbon stock and sequestration potential of traditional and improved agroforestry systems in the West African Sahel. Agric. Ecosyst. Environ. 2008, 125, 159–166. [Google Scholar] [CrossRef]
- IPCC. Guidelines for National Greenhouse Gas Inventories (Institute for Global Environmental Strategies, Japan, 2006); IPCC: Geneva, Switzerland, 2006. [Google Scholar]
- Giorgetta, M.A.; Jungclaus, J.; Reick, C.H.; Legutke, S.; Bader, J.; Böttinger, M.; Brovkin, V.; Crueger, T.; Esch, M.; Fieg, K. Climate and carbon cycle changes from 1850 to 2100 in MPI-ESM simulations for the Coupled Model Intercomparison Project phase 5. J. Adv. Model. Earth Syst. 2013, 5, 572–597. [Google Scholar] [CrossRef] [Green Version]
- Fandohan, A.B.; Oduor, A.M.O.; Sodé, A.I.; Wu, L.; Cuni-Sanchez, A.; Assédé, E.; Gouwakinnou, G.N. Modeling vulnerability of protected areas to invasion by Chromolaena odorata under current and future climates. Ecosyst. Health Sustain. 2015, 1, 1–2. [Google Scholar] [CrossRef]
- Forkuor, G.; Hounkpatin, O.K.L.; Welp, G.; Thiel, M. High Resolution Mapping of Soil Properties Using Remote Sensing Variables in South-Western Burkina Faso: A Comparison of Machine Learning and Multiple Linear Regression Models. PLoS ONE 2017, 12, e0170478. [Google Scholar] [CrossRef] [PubMed]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Freeman, E.A.; Moisen, G.G.; Coulston, J.W.; Wilson, B.T. Random forests and stochastic gradient boosting for predicting tree canopy cover: Comparing tuning processes and model performance. Can. J. For. Res. 2016, 46, 323–339. [Google Scholar] [CrossRef]
- Forkuor, G.; Dimobe, K.; Serme, I.; Tondoh, J.E. Landsat-8 vs. Sentinel-2: Examining the added value of sentinel-2’s red-edge bands to land-use and land-cover mapping in Burkina Faso. GISci. Remote Sens. 2018, 55, 331–354. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2015. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Fournier, A. Cycle saisonnier et production nette de la matière végétale herbacée en savanes soudaniennes pâturées: Les jachères de la région de Bondoukuy (Burkina Faso). Ecologie 1994, 25, 173. [Google Scholar]
- Sawadogo, L.; Tiveau, D.; Nygå ard, R. Influence of selective tree cutting, livestock and prescribed fire on herbaceous biomass in the savannah woodlands of Burkina Faso, West Africa. Agric. Ecosyst. Environ. 2005, 105, 335–345. [Google Scholar] [CrossRef]
- Knauer, K.; Gessner, U.; Fensholt, R.; Forkuor, G.; Kuenzer, C. Monitoring agricultural expansion in Burkina Faso over 14 years with 30 m resolution time series: The role of population growth and implications for the environment. Remote Sens. 2017, 9. [Google Scholar] [CrossRef]
- Zoungrana, B.J.B.; Conrad, C.; Amekudzi, L.K.; Thiel, M.; Da, E.D.; Forkuor, G.; Löw, F. Multi-Temporal Landsat Images and Ancillary Data for Land Use/Cover Change (LULCC) Detection in the Southwest of Burkina Faso, West Africa. Remote Sens. 2015, 7, 12076–12102. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Galiano, V.F.; Chica-Olmo, M.; Abarca-Hernandez, F.; Atkinson, P.M.; Jeganathan, C. Random Forest classification of Mediterranean land cover using multi-seasonal imagery and multi-seasonal texture. Remote Sens. Environ. 2014, 121, 93–107. [Google Scholar] [CrossRef]
- Inglada, J.; Arias, M.; Tardy, B.; Hagolle, O.; Valero, S.; Morin, D.; Dedieu, G.; Sepulcre, G.; Bontemps, S.; Defourny, P.; et al. Assessment of an Operational System for Crop Type Map Production Using High Temporal and Spatial Resolution Satellite Optical Imagery. Remote Sens. 2015, 7, 12356–12379. [Google Scholar] [CrossRef] [Green Version]
- Adam, E.; Mutanga, O.; Odindi, J.; Abdel-Rahman, E.M. Land-use/cover classification in a heterogeneous coastal landscape using RapidEye imagery: Evaluating the performance of random forest and support vector machines classifiers. Int. J. Remote Sens. 2014, 35, 3440–3458. [Google Scholar] [CrossRef]
- Blin, J.; Sidibe, S. Caractérisation et Amélioration d’un Foyer de Cuisson de” dolo” Équipé d’un Brûleur à Huile Végétale (Jatropha). 2012. Available online: http://agritrop.cirad.fr/577011/1/ID%20577011.pdf (accessed on 7 August 2018).
- Dimobe, K.; Wala, K.; Dourma, M.; Kiki, M.; Woegan, Y.; Folega, F.; Batawila, K.; Akpagana, K. Disturbance and Population Structure of Plant Communities in the Wildlife Reserve of Oti-Mandouri in Togo (West Africa). Annu. Res. Rev. Biol. 2014, 4, 2501–2516. [Google Scholar]
- Traore, L.; Ouedraogo, I.; Ouedraogo, A.; Thiombiano, A. Perceptions, usages et vulnérabilité des ressources végétales ligneuses dans le Sud-Ouest du Burkina Faso. Int. J. Biol. Chem. Sci. 2011, 5. [Google Scholar] [CrossRef]
- Goussanou, C.A.; Guendehou, S.; Assogbadjo, A.E.; Sinsin, B. Application of site-specific biomass models to quantify spatial distribution of stocks and historical emissions from deforestation in a tropical forest ecosystem. J. For. Res. 2018, 29, 205–213. [Google Scholar] [CrossRef]
- Madoffe, S.S.; Rija, A.A.; Midtgaard, F.; Katani, J.Z.; Mbeyale, G.; Zahabu, F.; Liwenga, F.; Christopher, B.C. Preliminary Assessment of Forest Structure, Management and Carbon Stocking in Tanzania Miombo Woodland. In Proceedings of the first Climate Change Impacts, Mitigation and Adaptation Programme Scientific Conference, Dar Es Salaam, Tanzania, 2–3 January 2012; pp. 106–117. [Google Scholar]
- Ribeiro, N.S.; Matos, C.N.; Moura, I.R.; Washington-Allen, R.A.; Ribeiro, A.I. Monitoring vegetation dynamics and carbon stock density in miombo woodlands. Carbon Balance Manag. 2013, 8, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimobe, K.; Ouédraogo, A.; Soma, S.; Goetze, D.; Porembski, S.; Thiombiano, A. Identification of driving factors of land degradation and deforestation in the Wildlife Reserve of Bontioli (Burkina Faso, West Africa). Glob. Ecol. Conserv. 2015, 4, 559–571. [Google Scholar] [CrossRef]
- Lindner, A.; Sattler, D. Biomass estimations in forests of different disturbance history in the Atlantic Forest of Rio de Janeiro, Brazil. New For. 2012, 43, 287–301. [Google Scholar] [CrossRef]
- Lin, D.; Lai, J.; Yang, B.; Song, P.; Li, N.; Ren, H.; Ma, K. Forest biomass recovery after different anthropogenic disturbances: Relative importance of changes in stand structure and wood density. Eur. J. For. Res. 2015, 134, 769–780. [Google Scholar] [CrossRef]
- Qasim, M.; Porembski, S.; Sattler, D.; Stein, K.; Thiombiano, A.; Lindner, A. Vegetation Structure and Carbon Stocks of Two Protected Areas Within the South-Sudanian Savannas of Burkina Faso, West Africa. Environment 2016, 3, 1–16. [Google Scholar] [CrossRef]
- Boakye-Danquah, J.; Antwi, E.K.; Saito, O.; Abekoe, M.K. Impact of farm management practices and agricultural land use on soil organic carbon storage potential in the savannah ecological zone of Northern Ghana. J. Disaster Res. 2014, 9, 484–500. [Google Scholar] [CrossRef]
- Hien, E.; Ganry, F.; Oliver, R. Carbon sequestration in a savannah soil in southwestern Burkina as affected by cropping and cultural practices. Arid Land Res. Manag. 2006, 20, 133–146. [Google Scholar] [CrossRef]
- Lal, R. Carbon sequestration in dryland ecosystems. Environ. Manag. 2004, 33, 528–544. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.M.; Nair, P.R. Carbon Sequestration Potential of Agroforestry Systems: Opportunities and Challenges; Springer Science & Business Media: Berlin, Germany, 2011. [Google Scholar]
- Dayamba, S.D.; Djoudi, H.; Zida, M.; Sawadogo, L.; Verchot, L. Biodiversity and carbon stocks in different land use types in the Sudanian Zone of Burkina Faso, West Africa. Agric. Ecosyst. Environ. 2015, 216, 61–72. [Google Scholar] [CrossRef]
- Thuiller, W. Patterns and uncertainties of species’ range shifts under climate change. Glob. Chang. Biol. 2004, 10, 2020–2027. [Google Scholar] [CrossRef]
- Pittock, B.A. Climate Change: The Science, Impacts and Solutions, 2nd ed.; CSIRO: London, UK, 2009. [Google Scholar]
- Harris, N.L.; Brown, S.; Hagen, S.C.; Saatchi, S.S.; Petrova, S.; Salas, W.; Hansen, M.C.; Potapov, P.V.; Lotsch, A. Baseline map of carbon emissions from deforestation in tropical regions. Science 2012, 336, 1573–1576. [Google Scholar] [CrossRef] [PubMed]
- Berenguer, E.; Ferreira, J.; Gardner, T.A.; Aragão, L.E.O.C.; De Camargo, P.B.; Cerri, C.E.; Durigan, M.; De Oliveira, R.C.; Vieira, I.C.G.; Barlow, J. A large-scale field assessment of carbon stocks in human-modified tropical forests. Glob. Chang. Biol. 2014, 20, 3713–3726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burivalova, Z.; Şekercioğlu, Ç.H.; Koh, L.P. Thresholds of logging intensity to maintain tropical forest biodiversity. Curr. Biol. 2014, 24, 1893–1898. [Google Scholar] [CrossRef] [PubMed]
- Metz, B.; Davidson, O.R.; Bosch, P.R.; Dave, R.; Meyer, L.A. Contribution of Working Group III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, 2007; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Dimobe, K.; Goetze, D.; Ouédraogo, A.; Forkuor, G.; Wala, K.; Porembski, S.; Thiombiano, A. Spatio-temporal dynamics in land use and habitat fragmentation within a protected area dedicated to tourism in a sudanian savanna of West Africa. J. Landsc. Ecol. 2017, 10, 75–95. [Google Scholar] [CrossRef]
- Hannah, L.; Midgley, G.F.; Lovejoy, T.; Bond, W.J.; Bush, M.; Lovett, J.C.; Scott, D.; Woodward, F.I. Conservation of biodiversity in a changing climate. Conserv. Biol. 2002, 16, 264–268. [Google Scholar] [CrossRef]
- Zonneveld, M.; van Jarvis, A.; Dvorak, W.; Lema, G.; Leibing, C. Climate change impact predictions on Pinus patula and Pinus tecunumanii populations in Mexico and Central America. For. Ecol. Manag. 2009, 257, 1566–1576. [Google Scholar] [CrossRef]




| Spectral Bands | 1 | 2 | 3 | 4 | |
|---|---|---|---|---|---|
| S-2 Band Name | Blue (B) | Green (G) | Red (R) | Near Infra Red (NIR) | |
| Spectral Indices | Name | Formula | References | ||
| Brightness Index (BI) | ((R2 + G2 + B2)/3)0.5 | Ray et al., 2004 | |||
| Saturation Index (SI) | (R-B)/(R + B) | Ray et al., 2004 | |||
| Hue Index (HI) | (2 ∗ R-G-B)/(G-B) | Ray et al., 2004 | |||
| Coloration Index (CI) | (R-G)/(R + G) | Ray et al., 2004 | |||
| Redness Index (RI) | R2/(B ∗ G)3 | Ray et al., 2004 | |||
| Normalized Difference Vegetation Index (NDVI) | Huete et al., 2002 | ||||
| Enhanced Vegetation Index 2 (EVI2) | Jiang et al., 2008 | ||||
| Enhanced Vegetation Index (EVI) | Huete et al., 1997 | ||||
| Soil Adjusted Vegetation Index (SAVI) | Huete, 1988 | ||||
| Variables | Unit |
|---|---|
| Aspect | degree |
| Catchment area | m2 |
| Flow direction | - |
| Flow line curvature | Degree·m−1 |
| Plan curvature | Degree·m−1 |
| Slope | Radians/% |
| Terrain ruggedness | - |
| Topographic wetness index | - |
| Topographic position index | - |
| Total curvature | Degree·m−1 |
| Classified | References | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gallery Forest | Woodland | Tree Savanna | Shrub Savanna | Grass Savanna | Cropland | Bare Soil | Water Body | Settlement | Total Raw | |
| 2017 | ||||||||||
| Gallery forest | 11 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 21 |
| Woodland | 6 | 58 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 70 |
| Tree savannas | 0 | 4 | 233 | 0 | 0 | 1 | 0 | 0 | 0 | 238 |
| Shrub savannas | 0 | 0 | 0 | 95 | 5 | 0 | 0 | 0 | 0 | 100 |
| Grass savannas | 0 | 0 | 0 | 11 | 18 | 0 | 0 | 0 | 0 | 29 |
| Cropland | 0 | 0 | 0 | 0 | 3 | 186 | 0 | 0 | 0 | 189 |
| Bare soil | 0 | 0 | 0 | 1 | 0 | 0 | 31 | 0 | 2 | 34 |
| Water body | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 357 | 0 | 357 |
| Settlement | 0 | 0 | 0 | 0 | 13 | 2 | 0 | 0 | 67 | 82 |
| Column total | 17 | 72 | 239 | 107 | 39 | 189 | 31 | 357 | 69 | |
| Overall accuracy (%) | 94.29 | |||||||||
| Kappa coefficient (%) | 92.95 | |||||||||
| Vegetation Type | No. of Plots | Species Richness (S, Species) | DBH (cm) | Height (m) | Tree Density (Tree·ha−1) | Basal Area (m2·ha−1) | C Stock (Mg·ha−1) |
|---|---|---|---|---|---|---|---|
| Cropland | 20 | 14 | 36.4 ± 24.2 | 10.3 ± 4.4 | 32.6 | 12.2 ± 4.7 | 4.6 ± 1.6 |
| Shrub savanna | 12 | 13 | 12.5 ± 14.0 | 4.8 ± 2.6 | 116 | 3.2 ± 3.5 | 0.9 ± 1.2 |
| Tree savanna | 15 | 30 | 11.6 ± 8.1 | 5.7 ± 2.5 | 332.2 | 5.2 ± 1.6 | 1.6 ± 0.6 |
| Woodland | 8 | 54 | 13.3 ± 10.0 | 8.9 ± 4.9 | 773.8 | 18.7 ± 6.8 | 10.2 ± 6.4 |
| Gallery forest | 6 | 50 | 17.5 ± 16.8 | 7.5 ± 4.5 | 920 | 21.3 ± 9.9 | 7.8 ± 4 |
| LULC | Depth (cm) | SOC (Mg/ha) | Mean Total Carbon Stock (Mg·ha−1) |
|---|---|---|---|
| Gallery forest | 0–20 | 16.7 ± 8.5 | 38 ± 17.8 |
| 20–50 | 13.5 ± 8.5 | ||
| Woodland | 0–20 | 10.4 ± 1.2 | 32.2 ± 7.7 |
| 20–50 | 11.6 ± 8.4 | ||
| Tree savanna | 0–20 | 9.9 ± 4.9 | 23.0 ± 7.3 |
| 20–50 | 11.5 ± 4.5 | ||
| Shrub savanna | 0–20 | 9.2 ± 4.9 | 22.9 ± 7.1 |
| 20–50 | 12.9 ± 4.7 | ||
| Cropland | 0–20 | 6.2 ± 2.2 | 19.5 ± 6.3 |
| 20–50 | 8.7 ± 4.3 |
| Evaluation Index | Current | HadGEM2-ES | MPI-ESM-MR |
|---|---|---|---|
| MAE | 20.31 | 21.12 | 21.22 |
| RMSE | 12.41 | 13.10 | 12.57 |
| R2 (%) | 98.92 | 98.67 | 98.40 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimobe, K.; Kouakou, J.L.N.; Tondoh, J.E.; Zoungrana, B.J.-B.; Forkuor, G.; Ouédraogo, K. Predicting the Potential Impact of Climate Change on Carbon Stock in Semi-Arid West African Savannas. Land 2018, 7, 124. https://doi.org/10.3390/land7040124
Dimobe K, Kouakou JLN, Tondoh JE, Zoungrana BJ-B, Forkuor G, Ouédraogo K. Predicting the Potential Impact of Climate Change on Carbon Stock in Semi-Arid West African Savannas. Land. 2018; 7(4):124. https://doi.org/10.3390/land7040124
Chicago/Turabian StyleDimobe, Kangbéni, Jean Léandre N’djoré Kouakou, Jérôme E. Tondoh, Benewinde J.-B. Zoungrana, Gerald Forkuor, and Korotimi Ouédraogo. 2018. "Predicting the Potential Impact of Climate Change on Carbon Stock in Semi-Arid West African Savannas" Land 7, no. 4: 124. https://doi.org/10.3390/land7040124





