Nitrogen Pollution and the Meltdown of Urban Ecosystems
Abstract
:1. Urbanisation
2. Urban Ecosystems Are Nitrogen Enriched
3. Potential Effects of Nitrogen Enrichment on Ecological Processes
4. Predictions
- (1)
- Nitrogen (N) fertilisation will cause shifts in patterns of biomass allocation in native perennials. This will increase the vulnerability of perennial native flora to environmental variability (i.e., drought). N fertilisation will also affect biomass allocation in exotic and/or ephemeral species, but the fitness consequences will be less extreme. With regard to the ephemeral species, it should not matter if the ephemerals are native or exotic [61]. The life history schedule should be all that matters.
- (2)
- N fertilisation will increase exotic species growth and exotic species will have direct negative impacts on the native flora. This competitive effect should be most important in preventing seedling establishment.
- (3)
- N fertilisation will increase the water use efficiency of vegetation, and thereby increase soil water availability. This in turn will promote growth of soil pathogens that will exert a more negative effect on native flora in relative terms.
- (4)
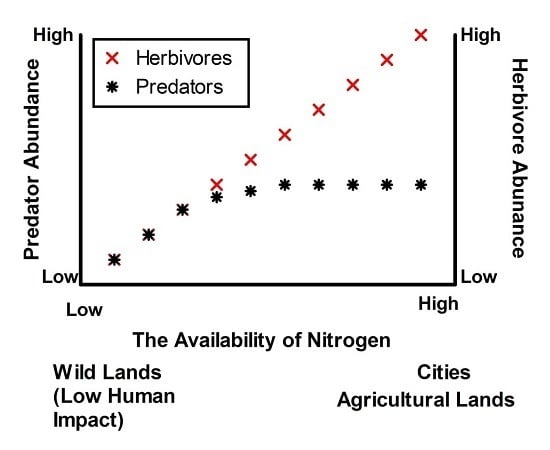
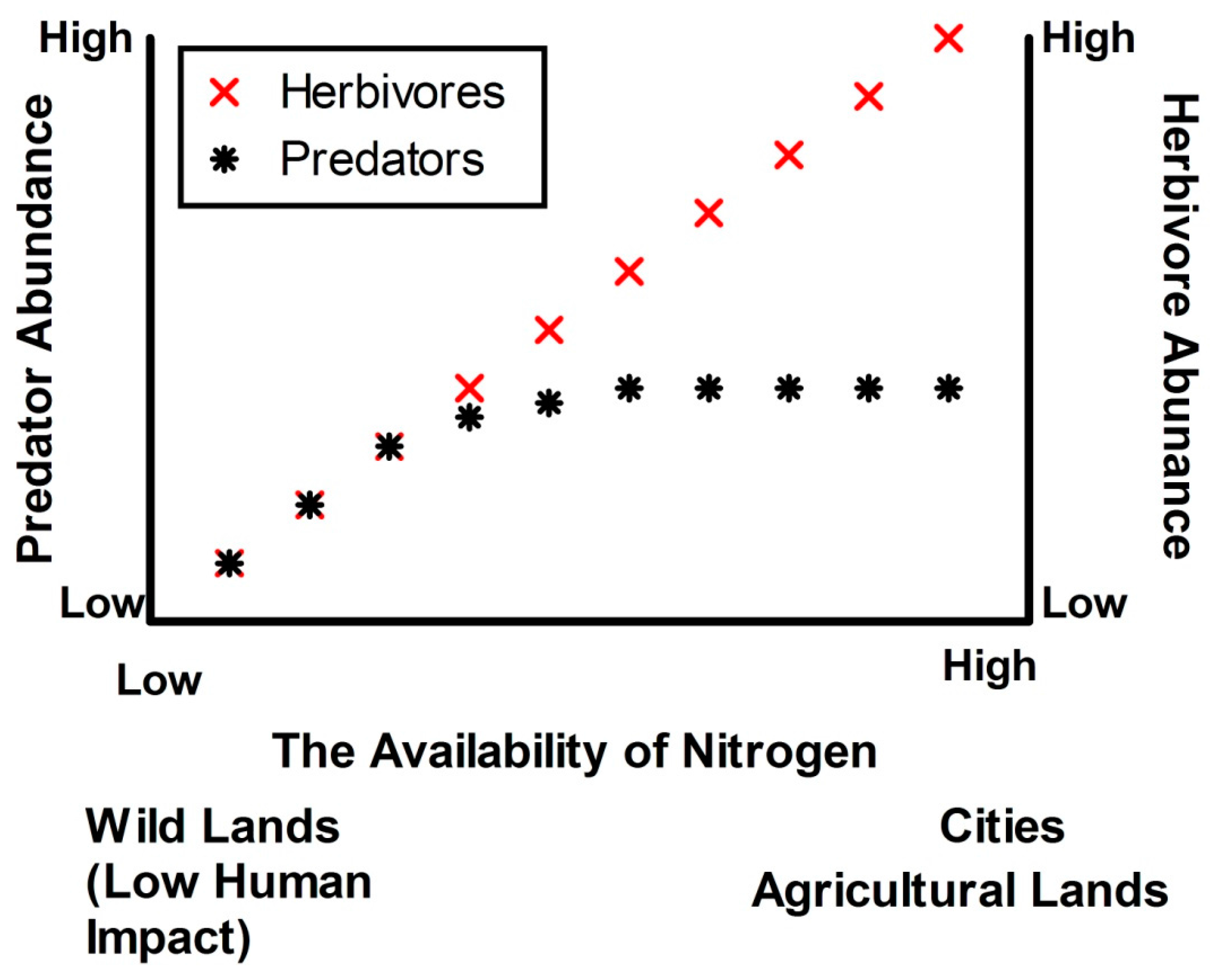
- N fertilisation in urban ecosystems will decouple herbivore and predator production (see Figure 1) and this will limit recruitment of native species. These changes will be due to: (a) relatively dense swards of grasses affording herbivorous invertebrates protection from predators; and (b) changes in herbivore body size following fertilisation, placing herbivores beyond the size handling class of many predators. High herbivore loads will in turn limit recruitment of the indigenous flora more than the exotic flora.
5. Solutions
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Luck, G.W. A review of the relationships between human population density and biodiversity. Biol. Rev. 2007, 82, 607–645. [Google Scholar] [CrossRef] [PubMed]
- Williams, N.S.G.; Schwartz, M.W.; Vesk, P.A.; McCarthy, M.A.; Hahs, A.K.; Clemants, S.E.; Corlett, R.T.; Duncan, R.P.; Norton, B.A.; Thompson, K.; et al. A conceptual framework for predicting the effects of urban environments on floras. J. Ecol. 2009, 97, 4–9. [Google Scholar] [CrossRef]
- Hahs, A.K.; McDonnell, M.J.; McCarthy, M.A.; Vesk, P.A.; Corlett, R.T.; Norton, B.A.; Clemants, S.E.; Duncan, R.P.; Thompson, K.; Schwartz, M.W.; et al. A global synthesis of plant extinction rates in urban areas. Ecol. Lett. 2009, 12, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- McKinney, M.L. Urbanization as a major cause of biotic homogenization. Biol. Conserv. 2005, 127, 247–260. [Google Scholar] [CrossRef]
- Miller, J.R. Restoration, reconciliation, and reconnecting with nature nearby. Biol. Conserv. 2006, 127, 356–361. [Google Scholar] [CrossRef]
- McCarthy, H.; Pataki, D. Drivers of variability in water use of native and non-native urban trees in the greater los angeles area. Urban Ecosyst. 2010, 13, 393–414. [Google Scholar] [CrossRef]
- Kaye, J.P.; Groffman, P.M.; Grimm, N.B.; Baker, L.A.; Pouyat, R.V. A distinct urban biogeochemistry? Trends Ecol. Evol. 2006, 21, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Alberti, M. Maintaining ecological integrity and sustaining ecosystem function in urban areas. Curr. Opin. Environ. Sustain. 2010, 2, 178–184. [Google Scholar] [CrossRef]
- Grimm, N.B.; Foster, D.; Groffman, P.; Grove, J.M.; Hopkinson, C.S.; Nadelhoffer, K.J.; Pataki, D.E.; Peters, D.P. The changing landscape: Ecosystem responses to urbanization and pollution across climatic and societal gradients. Front. Ecol. Environ. 2008, 6, 264–272. [Google Scholar] [CrossRef]
- Pickett, S.T.; Cadenasso, M.L.; Grove, J.M.; Groffman, P.M.; Band, L.E.; Boone, C.G.; Burch, W.R.; Grimmond, C.S.B.; Hom, J.; Jenkins, J.C. Beyond urban legends: An emerging framework of urban ecology, as illustrated by the Baltimore Ecosystem Study. BioScience 2008, 58, 139–150. [Google Scholar] [CrossRef]
- White, T.C.R. The Inadequate Environment: Nitrogen and the Abundance of Animals; Springer-Verlag: Berlin, Germany, 1993. [Google Scholar]
- McKane, R.B.; Johnson, L.C.; Shaver, G.R.; Nadelhoffer, K.J.; Rastetter, E.B.; Fry, B.; Giblin, A.E.; Kielland, K.; Kwiatkowski, B.L.; Laundre, J.A.; et al. Resource-based niches provide a basis for plant species diversity and dominance in arctic tundra. Nature 2002, 415, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Näsholm, T.; Kielland, K.; Ganeteg, U. Uptake of organic nitrogen by plants. New Phytol. 2009, 182, 31–48. [Google Scholar] [CrossRef] [PubMed]
- Galloway, J.N.; Aber, J.D.; Erisman, J.W.; Seitzinger, S.P.; Howarth, R.W.; Cowling, E.B.; Cosby, B.J. The nitrogen cascade. BioScience 2003, 53, 341–356. [Google Scholar] [CrossRef]
- Houlton, B.Z.; Sigman, D.M.; Schuur, E.A.G.; Hedin, L.O. A climate-driven switch in plant nitrogen acquisition within tropical forest communities. Proc. Natl. Acad. Sci. USA 2007, 104, 8902–8906. [Google Scholar] [CrossRef] [PubMed]
- Pouyat, R.V.; Carreiro, M.M.; McDonnell, M.J.; Pickett, S.T.A.; Groffman, P.M.; Parmelee, R.W.; Medley, K.E.; Zipperer, W.C. Carbon and nitrogen dynamics in oak stands along an urban-rural gradient. In Carbon Forms and Functions in Forest Soils; Kelly, J.M., Mcfee, W.W., Eds.; Soil Science Soc. Am.: Madison, WI, USA, 1995; pp. 569–587. [Google Scholar]
- Zhu, W.-X.; Carreiro, M.M. Temporal and spatial variations in nitrogen transformations in deciduous forest ecosystems along an urban-rural gradient. Soil Biol. Biochem. 2004, 36, 267–278. [Google Scholar] [CrossRef]
- Baxter, J.W.; Pickett, S.T.A.; Dighton, J.; Carreiro, M.M. Nitrogen and phosphorus availability in oak forest stands exposed to contrasting anthropogenic impacts. Soil Biol. Biochem. 2002, 34, 623–633. [Google Scholar] [CrossRef]
- Bidwell, S.; Attiwill, P.M.; Adams, M.A. Nitrogen availability and weed invasion in a remnant native woodland in urban melbourne. Austral. Ecol. 2006, 31, 262–270. [Google Scholar] [CrossRef]
- Granger, L.; Kasel, S.; Adams, M.A. Tree decline in southeastern australia-nitrate reductase-activity and indications of unbalanced nutrition in eucalyptus-ovata (labill.) and e. Camphora (r.T. Baker) communities at yellingbo, victoria. Oecologia 1994, 98, 221–228. [Google Scholar] [CrossRef]
- Fenn, M.E.; Baron, J.S.; Allen, E.B.; Rueth, H.M.; Nydick, K.R.; Geiser, L.; Bowman, W.D.; Sickman, J.O.; Meixner, T.; Johnson, D.W.; et al. Ecological effects of nitrogen deposition in the western united states. BioScience 2003, 53, 404–420. [Google Scholar] [CrossRef]
- Bobbink, R.; Hicks, K.; Galloway, J.; Spranger, T.; Alkemade, R.; Ashmore, M.; Bustamante, M.; Cinderby, S.; Davidson, E.; Dentener, F.; et al. Global assessment of nitrogen deposition effects on terrestrial plant diversity: A synthesis. Ecol. Appl. 2010, 20, 30–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Köchy, M.; Wilson, S.D. Nitrogen deposition and forest expansion in the northern great plains. J. Ecol. 2001, 89, 807–817. [Google Scholar] [CrossRef]
- Bertness, M.D.; Ewanchuk, P.J.; Silliman, B.R. Anthropogenic modification of new england salt marsh landscapes. Proc. Natl. Acad. Sci. USA 2002, 99, 1395–1398. [Google Scholar] [CrossRef] [PubMed]
- Jenerette, G.D.; Wu, J.; Grimm, N.B.; Hope, D. Points, patches, and regions: Scaling soil biogeochemical patterns in an urbanized arid ecosystem. Glob. Chang. Biol. 2006, 12, 1532–1544. [Google Scholar] [CrossRef]
- Newbound, M.; McCarthy, M.A.; Lebel, T. Fungi and the urban environment: A review. Landsc. Urban Plan. 2010, 96, 138–145. [Google Scholar] [CrossRef]
- Groffman, P.M.; Law, N.L.; Belt, K.T.; Band, L.E.; Fisher, G.T. Nitrogen fluxes and retention in urban watershed ecosystems. Ecosystems 2004, 7, 393–403. [Google Scholar] [CrossRef]
- Bernhardt, E.S.; Band, L.E.; Walsh, C.J.; Berke, P.E. Understanding, managing, and minimizing urban impacts on surface water nitrogen loading. Ann. N. Y. Acad. Sci. 2008, 1134, 61–96. [Google Scholar] [CrossRef] [PubMed]
- Lovett, G.M.; Traynor, M.M.; Pouyat, R.V.; Carreiro, M.M.; Zhu, W.X.; Baxter, J.W. Atmospheric deposition to oak forests along an urban-rural gradient. Environ. Sci. Technol. 2000, 34, 4294–4300. [Google Scholar] [CrossRef]
- Carreiro, M.M.; Tripler, C.E. Forest remnants along urban-rural gradients: Examining their potential for global change research. Ecosystems 2005, 8, 568–582. [Google Scholar] [CrossRef]
- Weiss, S.B. Cars, cows, and checkerspot butterflies: Nitrogen deposition and management of nutrient-poor grasslands for a threatened species. Conserv. Biol. 1999, 13, 1476–1486. [Google Scholar] [CrossRef]
- Lewis, D.B.; Kaye, J.P.; Gries, C.; Kinzig, A.P.; Redman, C.L. Agrarian legacy in soil nutrient pools of urbanizing arid lands. Glob. Chang. Biol. 2006, 12, 703–709. [Google Scholar] [CrossRef]
- Stallard, R.F. Terrestrial sedimentation and the carbon cycle: Coupling weathering and erosion to carbon burial. Glob. Biogeochem. Cycles 1998, 12, 231–257. [Google Scholar] [CrossRef]
- Groffman, P.M.; Bain, D.J.; Band, L.E.; Belt, K.T.; Brush, G.S.; Grove, J.M.; Pouyat, R.V.; Yesilonis, I.C.; Zipperer, W.C. Down by the riverside: Urban riparian ecology. Front. Ecol. Environ. 2003, 1, 315–321. [Google Scholar] [CrossRef]
- Vanhala, P.; Karhu, K.; Tuomi, M.; Sonninen, E.; Jungner, H.; Fritze, H.; Liski, J. Old soil carbon is more temperature sensitive than the young in an agricultural field. Soil Biol. Biochem. 2007, 39, 2967–2970. [Google Scholar] [CrossRef]
- Hall, S.J.; Sponseller, R.A.; Grimm, N.B.; Huber, D.; Kaye, J.P.; Clark, C.; Collins, S.L. Ecosystem response to nutrient enrichment across an urban airshed in the sonoran desert. Ecol. Appl. 2011, 21, 640–660. [Google Scholar] [CrossRef] [PubMed]
- Poorter, H.; Nagel, O. The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: A quantitative review. Aust. J. Plant. Physiol. 2000, 27, 595–607. [Google Scholar] [CrossRef]
- Nilsen, P. Effect of nitrogen on drought strain and nutrient-uptake in norway spruce picea abies (l.) karst) trees. Plant. Soil 1995, 172, 73–85. [Google Scholar] [CrossRef]
- Sutherland, S. What makes a weed a weed: Life history traits of native and exotic plants in the USA. Oecologia 2004, 141, 24–39. [Google Scholar] [CrossRef] [PubMed]
- Grotkopp, E.; Rejmanek, M.; Rost, T.L. Toward a causal explanation of plant invasiveness: Seedling growth and life-history strategies of 29 pine (pinus) species. Am. Nat. 2002, 159, 396–419. [Google Scholar] [CrossRef] [PubMed]
- Craine, J.M. Resource Stategies of Wild Plants, 1st, ed.; Princeton University Press: Princeton, NJ, USA, 2009. [Google Scholar]
- Davis, M.A.; Grime, J.P.; Thompson, K. Fluctuating resources in plant communities: A general theory of invasibility. J. Ecol. 2000, 88, 528–534. [Google Scholar] [CrossRef]
- Levine, J.M.; Vila, M.; D’Antonio, C.M.; Dukes, J.S.; Grigulis, K.; Lavorel, S. Mechanisms underlying the impacts of exotic plant invasions. Proc. R. Soc. Lond. Ser. B-Biol. Sci. 2003, 270, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Huenneke, L.F.; Hamburg, S.P.; Koide, R.; Mooney, H.A.; Vitousek, P.M. Effects of soil resources on plant invasion and community structure in californian serpentine grassland. Ecology 1990, 71, 478–491. [Google Scholar] [CrossRef]
- McDonald, E.P.; Erickson, J.E.; Kruger, E.L. Can decreased transpiration limit plant nitrogen acquisition in elevated CO2? Funct. Plant Biol. 2002, 29, 1115–1120. [Google Scholar] [CrossRef]
- Gilliam, F.S. Response of the herbaceous layer of forest ecosystems to excess nitrogen deposition. J. Ecol. 2006, 94, 1176–1191. [Google Scholar] [CrossRef]
- Chun, Y.J.; van Kleunen, M.; Dawson, W. The role of enemy release, tolerance and resistance in plant invasions: Linking damage to performance. Ecol. Lett. 2010, 13, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Engelkes, T.; Morrien, E.; Verhoeven, K.J.F.; Bezemer, T.M.; Biere, A.; Harvey, J.A.; McIntyre, L.M.; Tamis, W.L.M.; van der Putten, W.H. Successful range-expanding plants experience less above-ground and below-ground enemy impact. Nature 2008, 456, 946–948. [Google Scholar] [CrossRef] [PubMed]
- Cuesta, D.; Taboada, A.; Calvo, L.; Salgado, J.M. Short- and medium-term effects of experimental nitrogen fertilization on arthropods associated with calluna vulgaris heathlands in north-west spain. Environ. Pollut. 2008, 152, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.M.; Rosemond, A.D.; Eggert, S.L.; Cross, W.F.; Wallace, J.B. Long-term nutrient enrichment decouples predator and prey production. Proc. Natl. Acad. Sci. USA 2010, 107, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Aber, J.D.; Nadelhoffer, K.J.; Steudler, P.; Melillo, J.M. Nitrogen saturation in northern forest ecosystems: Excess nitrogen from fossil fuel combustion may stress the biosphere. BioScience 1989, 39, 378–379. [Google Scholar] [CrossRef]
- Raupp, M.J.; Shrewsbury, P.M.; Herms, D.A. Ecology of herbivorous arthropods in urban landscapes. Annu. Rev. Entomol. 2010, 55, 19–38. [Google Scholar] [CrossRef] [PubMed]
- Morin, P.J.; Lawler, S.P.; Johnson, E.A. Competition between aquatic insects and vertebrates: Interaction strength and higher order interactions. Ecology 1988, 69, 1401–1409. [Google Scholar] [CrossRef]
- Shrewsbury, P.M.; Raupp, M.J. Do top-down or bottom-up forces determine stephanitis pyrioides abundance in urban landscapes? Ecol. Appl. 2006, 16, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Ladd, B.M.; Facelli, J.M. Effects of competition, resource availability and invertebrates on tree seedling establishment. J. Ecol. 2005, 93, 968–977. [Google Scholar] [CrossRef]
- Faeth, S.H.; Warren, P.S.; Shochat, E.; Marussich, W.A. Trophic dynamics in urban communities. BioScience 2005, 55, 399–407. [Google Scholar] [CrossRef]
- Christie, F.J.; Hochuli, D.F. Elevated levels of herbivory in urban landscapes: Are declines in tree health more than an edge effect? Ecol. Soc. 2005, 10, 10. [Google Scholar]
- Holland, K.D.; McDonnell, M.J.; Williams, N.S.G. Abundance, species richness and feeding preferences of introduced molluscs in native grasslands of victoria, australia. Austral Ecol. 2007, 32, 626–634. [Google Scholar] [CrossRef]
- Marussich, W.; Faeth, S. Effects of urbanization on trophic dynamics of arthropod communities on a common desert host plant. Urban Ecosyst. 2009, 12, 265–286. [Google Scholar] [CrossRef]
- Shochat, E.; Lerman, S.B.; Anderies, J.M.; Warren, P.S.; Faeth, S.H.; Nilon, C.H. Invasion, competition, and biodiversity loss in urban ecosystems. BioScience 2010, 60, 199–208. [Google Scholar] [CrossRef]
- Davis, M.A.; Pelsor, M. Experimental support for a resource-based mechanistic model of invasibility. Ecol. Lett. 2001, 4, 421–428. [Google Scholar] [CrossRef]
- Bond, W.J. Large parts of the world are brown or black: A different view on the ‘green world’ hypothesis. J. Veg. Sci. 2005, 16, 261–266. [Google Scholar] [CrossRef]
- Williams, N.S.G.; Morgan, J.W.; McCarthy, M.A.; McDonnell, M.J. Local extinction of grassland plants: The landscape matrix is more important than patch attributes. Ecology 2006, 87, 3000–3006. [Google Scholar] [CrossRef]
- Geurts, J.J.; van de Wouw, P.A.; Smolders, A.J.; Roelofs, J.G.; Lamers, L.P. Ecological restoration on former agricultural soils: Feasibility of in situ phosphate fixation as an alternative to top soil removal. Ecol. Eng. 2011, 37, 1620–1629. [Google Scholar]
- Moles, A.T.; Gruber, M.A.M.; Bonser, S.P. A new framework for predicting invasive plant species. J. Ecol. 2008, 96, 13–17. [Google Scholar] [CrossRef]
- Sohi, S.P.; Krull, E.; Lopez-Capel, E.; Bol, R. A review of biochar and its use and function in soil. Adv. Agron. 2010, 105, 47–82. [Google Scholar] [CrossRef]
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.C.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Hochuli, D.F.; Gibb, H.; Burrows, S.E.; Christie, F.J. Ecology of sydney’s urban fragments: Has fragmentation taken the sting out of insect herbivory? In Urban Wildlife: More Than Meets the Eye; Lunney, D., Burgin, S., Eds.; Royal Zoological Society of NSW: Sydney, Australia, 2004; pp. 63–69. [Google Scholar]
- Ramalho, C.E.; Hobbs, R.J. Time for a change: Dynamic urban ecology. Trends Ecol. Evol. 2012, 27, 179–188. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ladd, B. Nitrogen Pollution and the Meltdown of Urban Ecosystems. Land 2016, 5, 23. https://doi.org/10.3390/land5030023
Ladd B. Nitrogen Pollution and the Meltdown of Urban Ecosystems. Land. 2016; 5(3):23. https://doi.org/10.3390/land5030023
Chicago/Turabian StyleLadd, Brenton. 2016. "Nitrogen Pollution and the Meltdown of Urban Ecosystems" Land 5, no. 3: 23. https://doi.org/10.3390/land5030023
APA StyleLadd, B. (2016). Nitrogen Pollution and the Meltdown of Urban Ecosystems. Land, 5(3), 23. https://doi.org/10.3390/land5030023