The Mineral Biochar Alters the Biochemical and Microbial Properties of the Soil and the Grain Yield of Hordeum vulgare L. under Drought Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description and Experimental Design
2.2. Soil Sampling and Measurements
Determination of Soil Biochemical and Microbial Properties
2.3. Statistical Analysis
3. Results
4. Discussion
4.1. Soil Organic Carbon Content
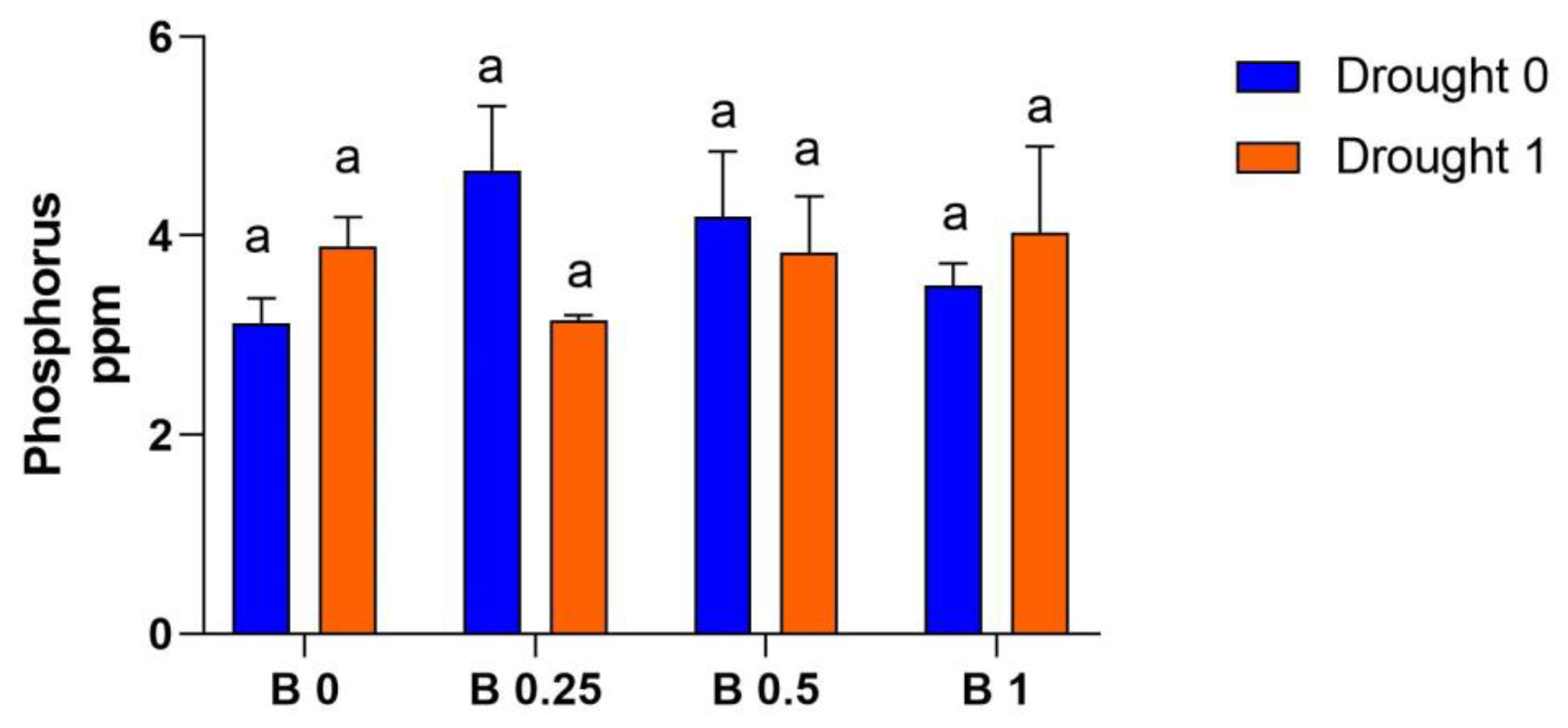
4.2. Soil Available Phosphorus
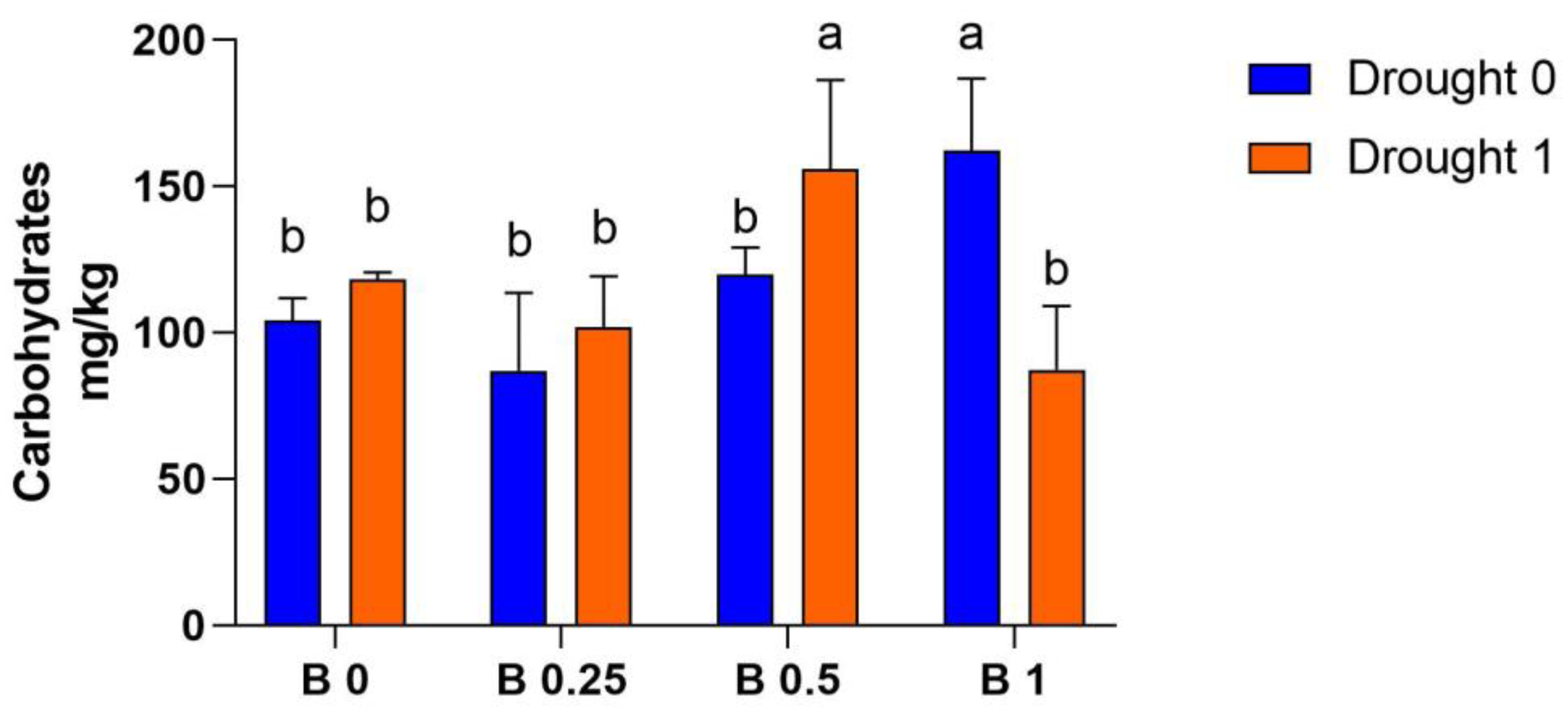
4.3. Soil Carbohydrate
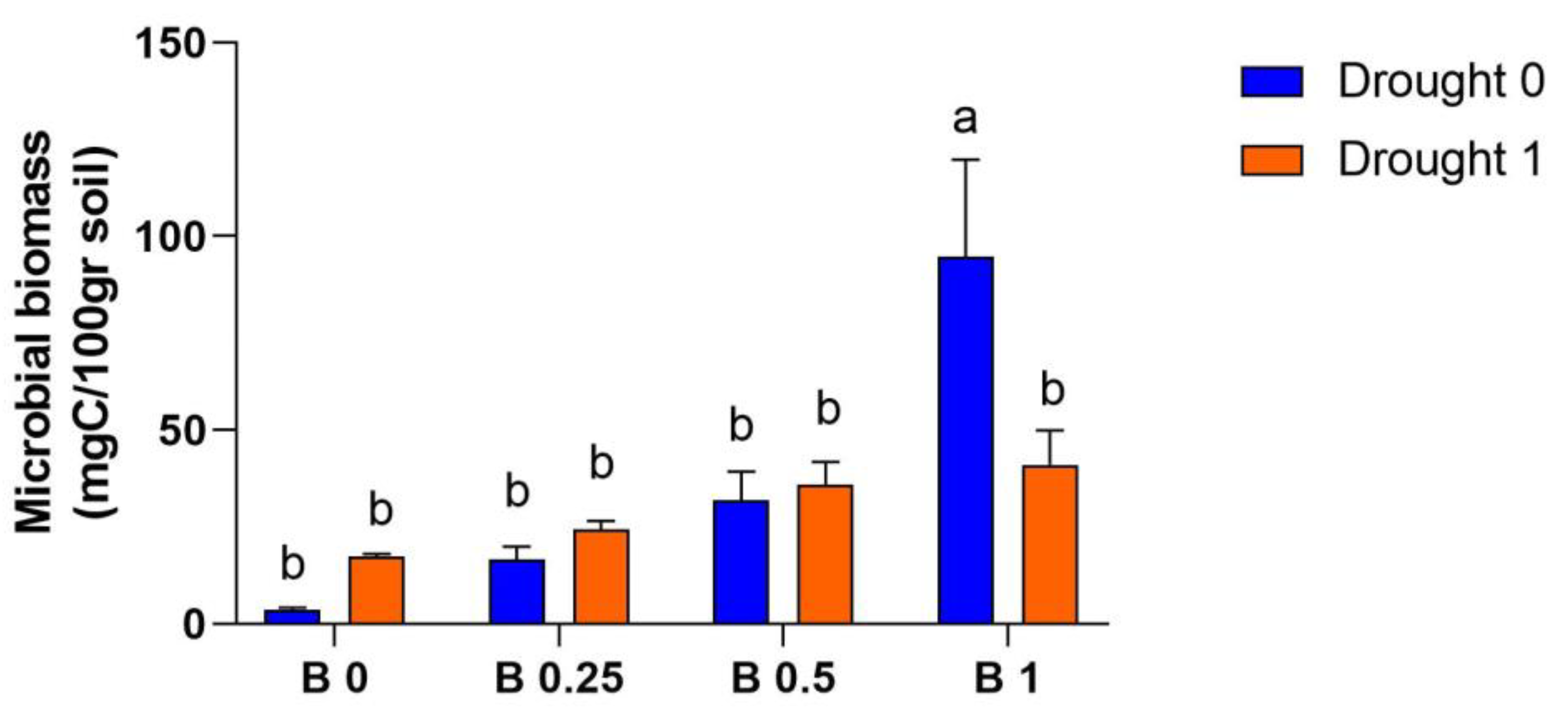
4.4. Soil Microbial Biomass
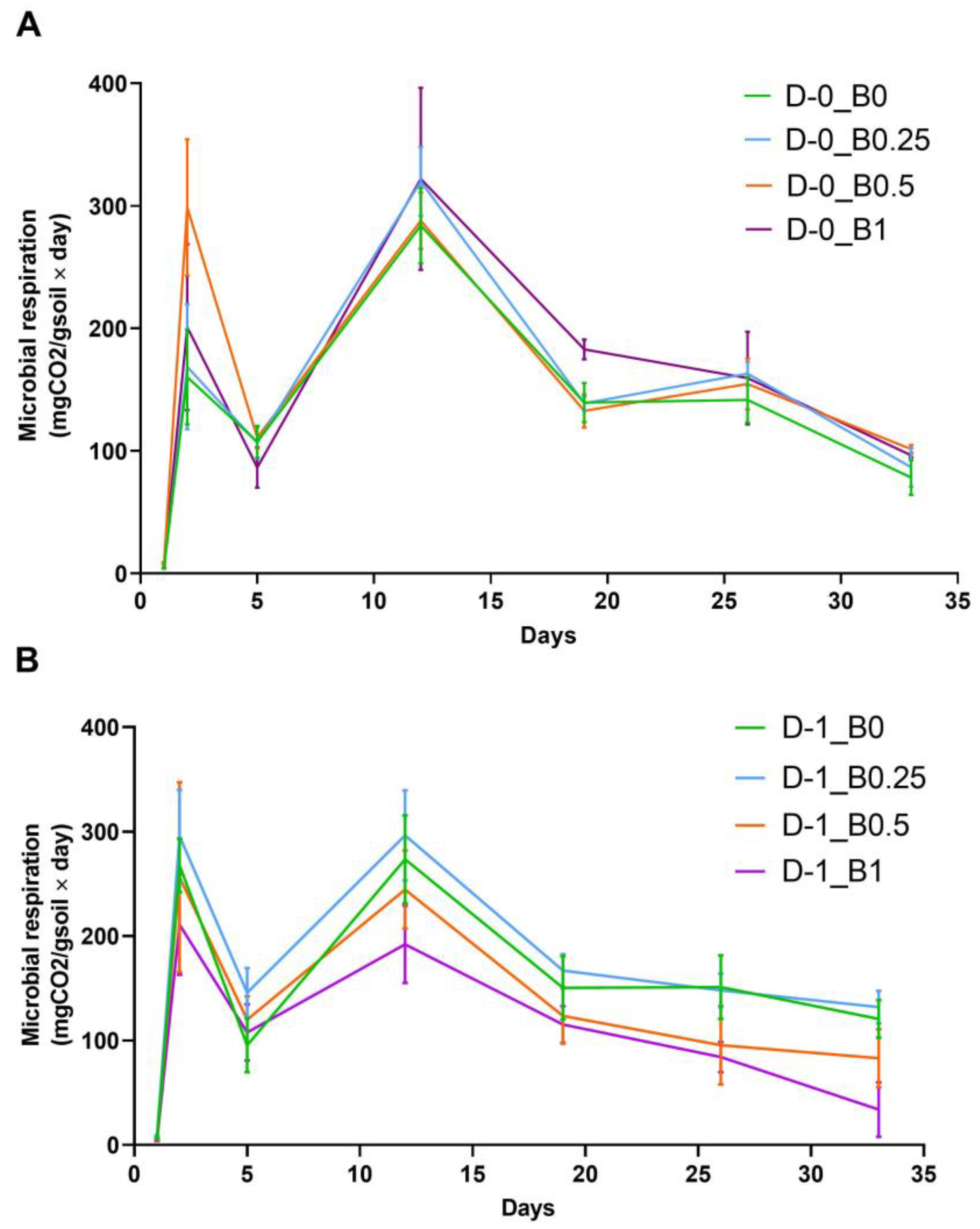
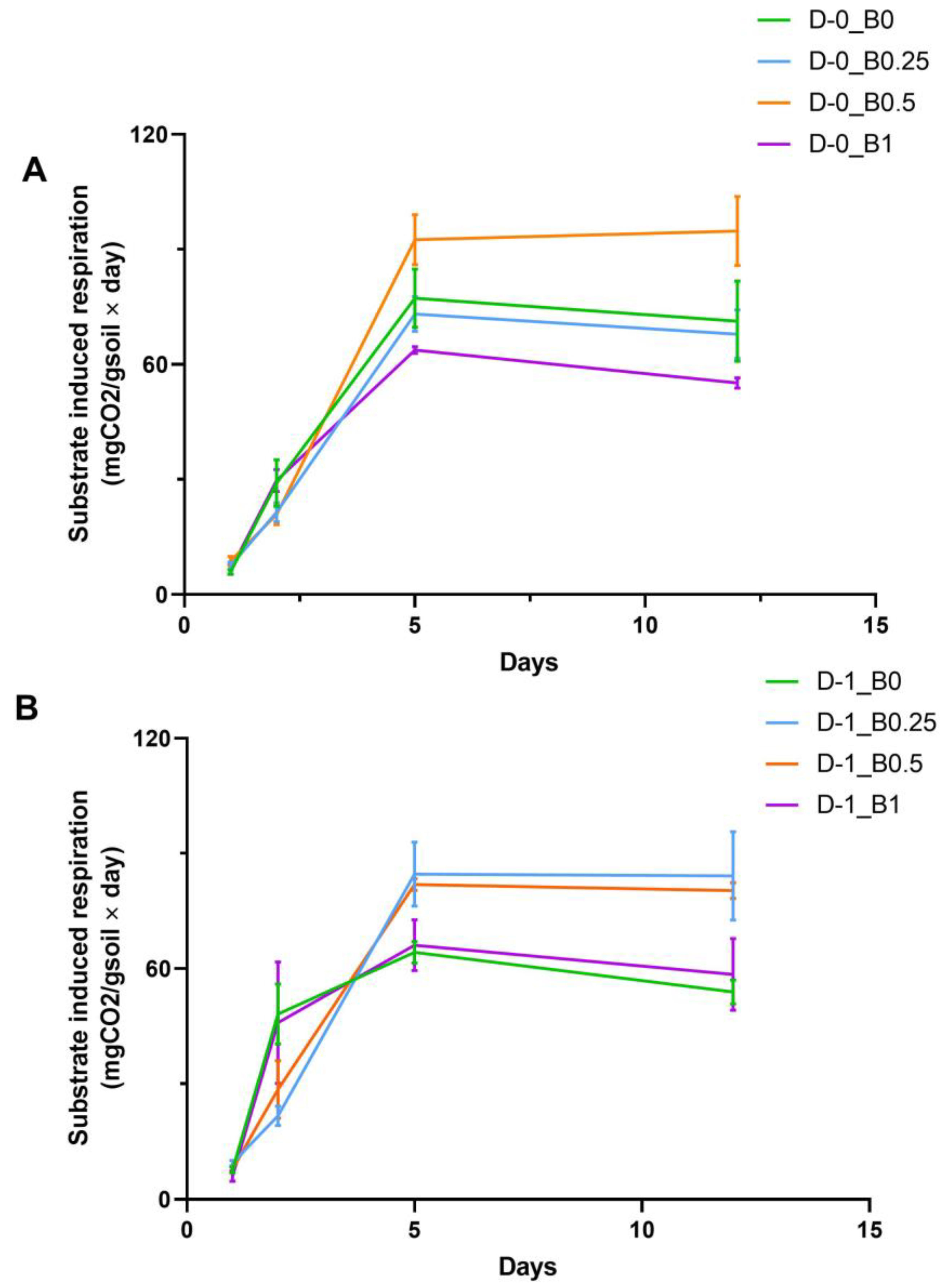
4.5. Microbial Respiration and Substrate Induced Respiration of the Soil
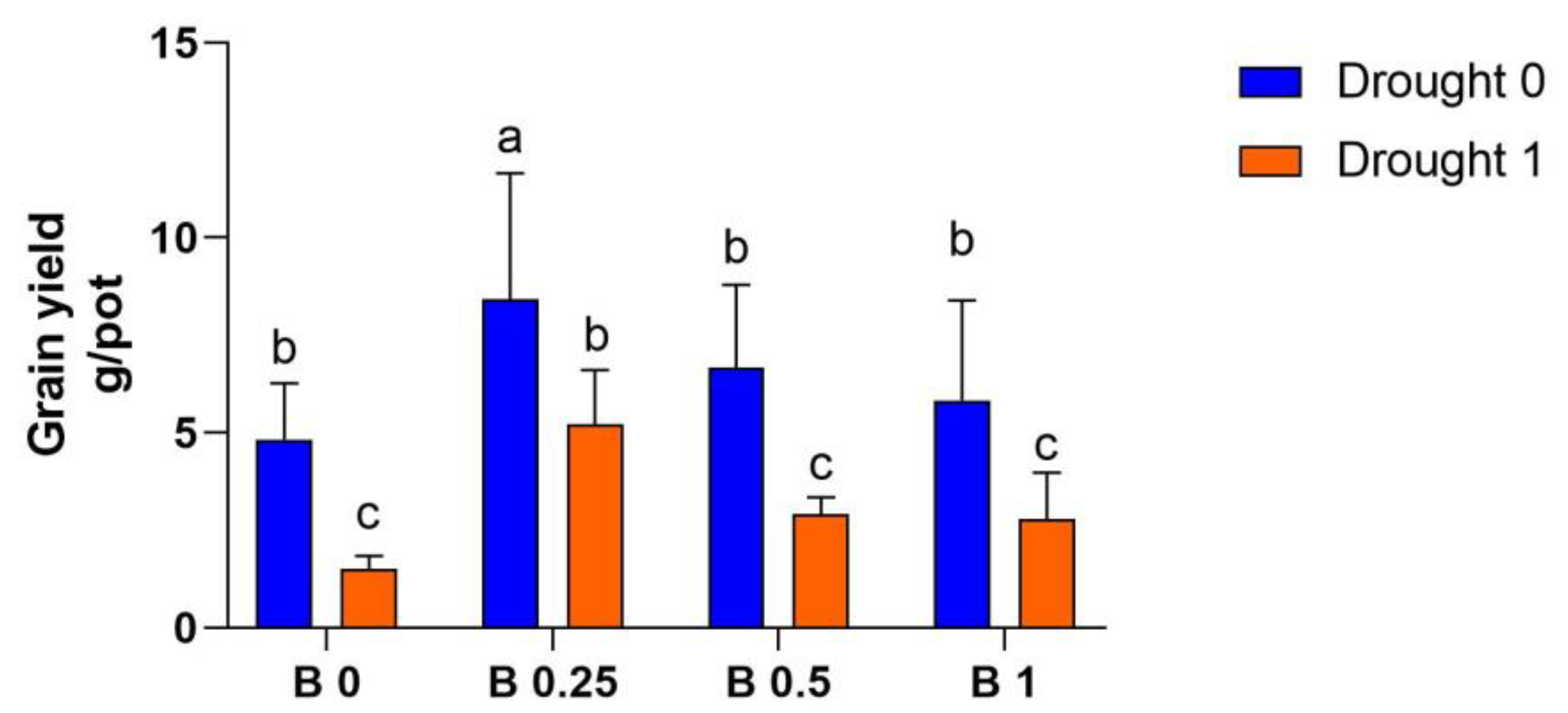
4.6. Grain Yield
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Meints, B.; Cuesta-Marcos, A.; Fisk, S.; Ross, A.; Hayes, P. Food Barley Quality Improvement and Germplasm Utilization. In Exploration, Identification and Utilization of Barley Germplasm; Elsevier: Amsterdam, The Netherlands, 2016; pp. 41–73. [Google Scholar] [CrossRef]
- Saddiq, M.S.; Wang, X.; Iqbal, S.; Hafeez, M.B.; Khan, S.; Raza, A.; Iqbal, J.; Maqbool, M.M.; Fiaz, S.; Qazi, M.A.; et al. Effect of Water Stress on Grain Yield and Physiological Characters of Quinoa Genotypes. Agronomy 2021, 11, 1934. [Google Scholar] [CrossRef]
- Ali, L.; Manzoor, N.; Li, X.Q.; Naveed, M.; Nadeem, S.M.; Waqas, M.R.; Khalid, M.; Abbas, A.; Ahmed, T.; Li, B.; et al. Impact of Corn Cob-Derived Biochar in Altering Soil Quality, Biochemical Status and Improving Maize Growth under Drought Stress. Agronomy 2021, 11, 2300. [Google Scholar] [CrossRef]
- Bayati, P.; Karimmojeni, H.; Razmjoo, J.; Pucci, M.; Abate, G.; Baldwin, T.C.; Mastinu, A. Physiological, Biochemical, and Agronomic Trait Responses of Nigella sativa Genotypes to Water Stress. Horticulturae 2022, 8, 193. [Google Scholar] [CrossRef]
- Biareh, V.; Shekari, F.; Sayfzadeh, S.; Zakerin, H.; Hadidi, E.; Beltrao, J.G.T.; Mastinu, A. Physiological and Qualitative Response of Cucurbita pepo L. to Salicylic Acid under Controlled Water Stress Conditions. Horticulturae 2022, 8, 79. [Google Scholar] [CrossRef]
- Naservafaei, S.; Sohrabi, Y.; Moradi, P.; Mac Sweeney, E.; Mastinu, A. Biological Response of Lallemantia iberica to Brassinolide Treatment under Different Watering Conditions. Plants 2021, 10, 496. [Google Scholar] [CrossRef]
- Bogati, K.; Walczak, M. The Impact of Drought Stress on Soil Microbial Community, Enzyme Activities and Plants. Agronomy 2022, 12, 189. [Google Scholar] [CrossRef]
- Aghajanlou, F.; Mirdavoudi, H.; Shojaee, M.; Mac Sweeney, E.; Mastinu, A.; Moradi, P. Rangeland Management and Ecological Adaptation Analysis Model for Astragalus curvirostris Boiss. Horticulturae 2021, 7, 67. [Google Scholar] [CrossRef]
- Mirdavoudi, H.; Ghorbanian, D.; Zarekia, S.; Soleiman, J.M.; Ghonchepur, M.; Sweeney, E.M.; Mastinu, A. Ecological Niche Modelling and Potential Distribution of Artemisia sieberi in the Iranian Steppe Vegetation. Land 2022, 11, 2315. [Google Scholar] [CrossRef]
- Moradi, P.; Aghajanloo, F.; Moosavi, A.; Monfared, H.H.; Khalafi, J.; Taghiloo, M.; Khoshzaman, T.; Shojaee, M.; Mastinu, A. Anthropic Effects on the Biodiversity of the Habitats of Ferula gummosa. Sustainability 2021, 13, 7874. [Google Scholar] [CrossRef]
- Danish, S.; Zafar-ul-Hye, M.; Fahad, S.; Saud, S.; Brtnicky, M.; Hammerschmiedt, T.; Datta, R. Drought Stress Alleviation by ACC Deaminase Producing Achromobacter xylosoxidans and Enterobacter cloacae, with and without Timber Waste Biochar in Maize. Sustainability 2020, 12, 6286. [Google Scholar] [CrossRef]
- Hanaka, A.; Ozimek, E.; Reszczynska, E.; Jaroszuk-Scisel, J.; Stolarz, M. Plant Tolerance to Drought Stress in the Presence of Supporting Bacteria and Fungi: An Efficient Strategy in Horticulture. Horticulturae 2021, 7, 390. [Google Scholar] [CrossRef]
- Danish, S.; Zafar-ul-Hye, M.; Hussain, M.; Shaaban, M.; Nunez-Delgado, A.; Hussain, S.; Qayyum, M.F. Rhizobacteria with ACC-Deaminase Activity Improve Nutrient Uptake, Chlorophyll Contents and Early Seedling Growth of Wheat under PEG-Induced Osmotic Stress. Int. J. Agric. Biol. 2019, 21, 1212–1220. [Google Scholar] [CrossRef]
- Anjum, S.A.; Wang, L.C.; Farooq, M.; Hussain, M.; Xue, L.L.; Zou, C.M. Brassinolide Application Improves the Drought Tolerance in Maize Through Modulation of Enzymatic Antioxidants and Leaf Gas Exchange. J. Agron. Crop Sci. 2011, 197, 177–185. [Google Scholar] [CrossRef]
- Taghvaei, M.; Nasrolahizadehi, A.; Mastinu, A. Effect of Light, Temperature, Salinity, and Halopriming on Seed Germination and Seedling Growth of Hibiscus sabdariffa under Salinity Stress. Agronomy 2022, 12, 2491. [Google Scholar] [CrossRef]
- Yousefi, A.R.; Rashidi, S.; Moradi, P.; Mastinu, A. Germination and Seedling Growth Responses of Zygophyllum fabago, Salsola kali L. and Atriplex canescens to PEG-Induced Drought Stress. Environments 2020, 7, 107. [Google Scholar] [CrossRef]
- Yousefvand, P.; Sohrabi, Y.; Heidari, G.; Weisany, W.; Mastinu, A. Salicylic Acid Stimulates Defense Systems in Allium hirtifolium Grown under Water Deficit Stress. Molecules 2022, 27, 3083. [Google Scholar] [CrossRef]
- Zafar-ul-Hye, M.; Danish, S.; Abbas, M.; Ahmad, M.; Munir, T.M. ACC Deaminase Producing PGPR Bacillus amyloliquefaciens and Agrobacterium fabrum along with Biochar Improve Wheat Productivity under Drought Stress. Agronomy 2019, 9, 343. [Google Scholar] [CrossRef] [Green Version]
- Zarei, A.R.; Mahmoudi, M.R. Evaluation of the Effect of Changes in Climatic Variables at Different Levels on the Rate of Potential Evapotranspiration in Arid and Semi-Arid Regions. Pure Appl. Geophys. 2022, 180, 421–437. [Google Scholar] [CrossRef]
- Yan, X.Y.; Zhang, Q.; Ren, X.Y.; Wang, X.Y.; Yan, X.M.; Li, X.Q.; Wang, L.; Bao, L.L. Climatic Change Characteristics towards the "Warming-Wetting" Trend in the Pan-Central-Asia Arid Region. Atmosphere 2022, 13, 467. [Google Scholar] [CrossRef]
- Khaleghnezhad, V.; Yousefi, A.R.; Tavakoli, A.; Farajmand, B.; Mastinu, A. Concentrations-dependent effect of exogenous abscisic acid on photosynthesis, growth and phenolic content of Dracocephalum moldavica L. under drought stress. Planta 2021, 253, 127. [Google Scholar] [CrossRef]
- Chaichi, M.; Nemati, A.; Dadrasi, A.; Heydari, M.; Hassanisaadi, M.; Yousefi, A.R.; Baldwin, T.C.; Mastinu, A. Germination of Triticum aestivum L.: Effects of Soil-Seed Interaction on the Growth of Seedlings. Soil Syst. 2022, 6, 37. [Google Scholar] [CrossRef]
- Zangani, E.; Afsahi, K.; Shekari, F.; Mac Sweeney, E.; Mastinu, A. Nitrogen and Phosphorus Addition to Soil Improves Seed Yield, Foliar Stomatal Conductance, and the Photosynthetic Response of Rapeseed (Brassica napus L.). Agriculture 2021, 11, 483. [Google Scholar] [CrossRef]
- Jam, B.J.; Shekari, F.; Andalibi, B.; Fotovat, R.; Jafarian, V.; Najafi, J.; Uberti, D.; Mastinu, A. Impact of Silicon Foliar Application on the Growth and Physiological Traits of Carthamus tinctorius L. Exposed to Salt Stress. Silicon-Neth 2022, 15, 1235–1245. [Google Scholar] [CrossRef]
- Fawad, M.; Khan, M.A.; Wahid, F.; Khan, H.; Gul, B.; Khattak, A.M.; Jamal, A.; Mastinu, A. Irrigation Scheduling and Weed Management: A Sustainable Approach for Managing Broomrape and Other Weeds in Tomato Crop. Horticulturae 2022, 8, 676. [Google Scholar] [CrossRef]
- Abdul Rahman, N.S.N.; Abdul Hamid, N.W.; Nadarajah, K. Effects of Abiotic Stress on Soil Microbiome. Int. J. Mol. Sci. 2021, 22, 9036. [Google Scholar] [CrossRef]
- Siebielec, S.; Siebielec, G.; Klimkowicz-Pawlas, A.; Galazka, A.; Grzadziel, J.; Stuczynski, T. Impact of Water Stress on Microbial Community and Activity in Sandy and Loamy Soils. Agronomy 2020, 10, 1429. [Google Scholar] [CrossRef]
- Motamedi, M.; Zahedi, M.; Karimmojeni, H.; Motamedi, H.; Mastinu, A. Effect of rhizosphere bacteria on antioxidant enzymes and some biochemical characteristics of Medicago sativa L. subjected to herbicide stress. Acta Physiol. Plant. 2022, 44, 84. [Google Scholar] [CrossRef]
- Nguyen, L.T.T.; Osanai, Y.; Anderson, I.C.; Bange, M.P.; Tissue, D.T.; Singh, B.K. Flooding and prolonged drought have differential legacy impacts on soil nitrogen cycling, microbial communities and plant productivity. Plant Soil 2018, 431, 371–387. [Google Scholar] [CrossRef]
- Zaheer, M.S.; Ali, H.H.; Soufan, W.; Iqbal, R.; Habib-ur-Rahman, M.; Iqbal, J.; Israr, M.; El Sabagh, A. Potential Effects of Biochar Application for Improving Wheat (Triticum aestivum L.) Growth and Soil Biochemical Properties under Drought Stress Conditions. Land 2021, 10, 1125. [Google Scholar] [CrossRef]
- Dai, P.G.; Cong, P.; Wang, P.; Dong, J.X.; Dong, Z.R.; Song, W.J. Alleviating Soil Acidification and Increasing the Organic Carbon Pool by Long-Term Organic Fertilizer on Tobacco Planting Soil. Agronomy 2021, 11, 2135. [Google Scholar] [CrossRef]
- Lu, J.J.; Li, S.P.; Liang, G.P.; Wu, X.P.; Zhang, Q.; Gao, C.H.; Li, J.H.; Jin, D.S.; Zheng, F.J.; Zhang, M.N.; et al. The Contribution of Microorganisms to Soil Organic Carbon Accumulation under Fertilization Varies among Aggregate Size Classes. Agronomy 2021, 11, 2126. [Google Scholar] [CrossRef]
- Foster, E.J.; Hansen, N.; Wallenstein, M.; Cotrufo, M.F. Biochar and manure amendments impact soil nutrients and microbial enzymatic activities in a semi-arid irrigated maize cropping system. Agric. Ecosyst. Environ. 2016, 233, 404–414. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Li, T.X.; Fu, Q.; Li, H.; Liu, D.; Ji, Y.; Li, Q.L.; Cai, Y.P. Biochar application for the improvement ofwater-soil environments and carbon emissions under freeze-thaw conditions: An in-situ field trial. Sci. Total Environ. 2020, 723, 138007. [Google Scholar] [CrossRef]
- Khademalrasoul, A.; Naveed, M.; Heckrath, G.; Kumari, K.G.I.D.; de Jonge, L.W.; Elsgaard, L.; Vogel, H.J.; Iversen, B.V. Biochar Effects on Soil Aggregate Properties Under No-Till Maize. Soil Sci. 2014, 179, 273–283. [Google Scholar] [CrossRef]
- Li, M.; Cai, L. Biochar and Arbuscular Mycorrhizal Fungi Play Different Roles in Enabling Maize to Uptake Phosphorus. Sustainability 2021, 13, 3244. [Google Scholar] [CrossRef]
- Saffari, N.; Hajabbasi, M.A.; Shirani, H.; Mosaddeghi, M.R.; Mamedov, A.I. Biochar type and pyrolysis temperature effects on soil quality indicators and structural stability. J. Environ. Manag. 2020, 261, 110190. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Onweremadu, E.U.; Onyia, V.N.; Anikwe, M.A.N. Carbon and nitrogen distribution in water-stable aggregates under two tillage techniques in Fluvisols of Owerri area, southeastern Nigeria. Soil Tillage Res. 2007, 97, 195–206. [Google Scholar] [CrossRef]
- Masto, R.E.; Kumar, S.; Rout, T.K.; Sarkar, P.; George, J.; Ram, L.C. Biochar from water hyacinth (Eichornia crassipes) and its impact on soil biological activity. Catena 2013, 111, 64–71. [Google Scholar] [CrossRef]
- Zhang, R.; Qu, Z.; Liu, L.; Yang, W.; Wang, L.; Li, J.; Zhang, D. Soil Respiration and Organic Carbon Response to Biochar and Their Influencing Factors. Atmosphere 2022, 13, 2038. [Google Scholar] [CrossRef]
- Bayu, D.; Tadesse, M.; Amsalu, N. Effect of biochar on soil properties and lead (Pb) availability in a military camp in South West Ethiopia. Afr. J. Environ. Sci. Technol. 2016, 10, 77–85. [Google Scholar] [CrossRef] [Green Version]
- Chun, J.-H.; Kang, Y.-G.; Lee, J.-H.; Yun, Y.-U.; Oh, T.-K.; Yoon, M.-H. The combined effect of nitrogen and biochar amendments on the yield and glucosinolate contents of the Chinese cabbage. J. King Saud Univ. Sci. 2022, 34, 101799. [Google Scholar] [CrossRef]
- Jabborova, D.; Annapurna, K.; Al-Sadi, A.M.; Alharbi, S.A.; Datta, R.; Zuan, A.T.K. Biochar and Arbuscular mycorrhizal fungi mediated enhanced drought tolerance in Okra (Abelmoschus esculentus) plant growth, root morphological traits and physiological properties. Saudi J. Biol. Sci. 2021, 28, 5490–5499. [Google Scholar] [CrossRef] [PubMed]
- Laird, D.A.; Fleming, P.; Davis, D.D.; Horton, R.; Wang, B.; Karlen, D.L. Impact of biochar amendments on the quality of a typical Midwestern agricultural soil. Geoderma 2010, 158, 443–449. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Zhao, C.; Zhao, X.; Wang, Y.; Lv, X.; Zhu, X.; Song, X. Beneficial Effects of Biochar Application with Nitrogen Fertilizer on Soil Nitrogen Retention, Absorption and Utilization in Maize Production. Agronomy 2022, 13, 113. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.J.; Novak, A.; Yang, Y.C.; Wang, J.W. Role of Biochar in Improving Sandy Soil Water Retention and Resilience to Drought. Water 2021, 13, 407. [Google Scholar] [CrossRef]
- Yang, S.; Chen, X.; Jiang, Z.; Ding, J.; Sun, X.; Xu, J. Effects of Biochar Application on Soil Organic Carbon Composition and Enzyme Activity in Paddy Soil under Water-Saving Irrigation. Int. J. Environ. Res. Public Health 2020, 17, 333. [Google Scholar] [CrossRef] [Green Version]
- Zhou, G.; Zhou, X.; Zhang, T.; Du, Z.; He, Y.; Wang, X.; Shao, J.; Cao, Y.; Xue, S.; Wang, H.; et al. Biochar increased soil respiration in temperate forests but had no effects in subtropical forests. For. Ecol. Manag. 2017, 405, 339–349. [Google Scholar] [CrossRef]
- Drover, D.P.; Manner, H. Comparison between Walkley-Black and a Dry Combustion Method for Determining Organic Carbon in a Humic Brown Soil, Papua-New-Guinea. Commun. Soil Sci. Plant Anal. 1975, 6, 495–500. [Google Scholar] [CrossRef]
- Adesodun, J.K.; Mbagwu, J.S.C.; Oti, N. Structural stability and carbohydrate contents of an ultisol under different management systems. Soil Tillage Res. 2001, 60, 135–142. [Google Scholar] [CrossRef]
- Nielsen, S.S. Phenol-Sulfuric Acid Method for Total Carbohydrates. In Food Analysis Laboratory Manual, 1st ed.; Nielsen, S.S., Ed.; Springer: Boston, MA, USA, 2010; pp. 47–53. [Google Scholar] [CrossRef]
- Whalley, W.R.; Clark, L.J.; Gowing, D.J.G.; Cope, R.E.; Lodge, R.J.; Leeds-Harrison, P.B. Does Soil Strength Play a Role in Wheat Yield Losses Caused by Soil Drying? Plant Soil 2006, 280, 279–290. [Google Scholar] [CrossRef]
- Nguyen, B.T.; Lehmann, J.; Kinyangi, J.; Smernik, R.; Riha, S.J.; Engelhard, M.H. Long-term black carbon dynamics in cultivated soil. Biogeochemistry 2008, 89, 295–308. [Google Scholar] [CrossRef]
- Liang, B.; Lehmann, J.; Solomon, D.; Sohi, S.; Thies, J.E.; Skjemstad, J.O.; Luizão, F.J.; Engelhard, M.H.; Neves, E.G.; Wirick, S. Stability of biomass-derived black carbon in soils. Geochim. Cosmochim. Acta 2008, 72, 6069–6078. [Google Scholar] [CrossRef]
- Deng, L.; Peng, C.; Zhu, G.; Chen, L.; Liu, Y.; Shangguan, Z. Positive responses of belowground C dynamics to nitrogen enrichment in China. Sci. Total Environ. 2018, 616–617, 1035–1044. [Google Scholar] [CrossRef]
- Deng, L.; Peng, C.H.; Kim, D.G.; Li, J.W.; Liu, Y.L.; Hai, X.Y.; Liu, Q.Y.; Huang, C.B.; Shangguan, Z.P.; Kuzyakov, Y. Drought effects on soil carbon and nitrogen dynamics in global natural ecosystems. Earth-Sci. Rev. 2021, 214, 103501. [Google Scholar] [CrossRef]
- Ren, C.; Zhao, F.; Shi, Z.; Chen, J.; Han, X.; Yang, G.; Feng, Y.; Ren, G. Differential responses of soil microbial biomass and carbon-degrading enzyme activities to altered precipitation. Soil Biol. Biochem. 2017, 115, 1–10. [Google Scholar] [CrossRef]
- Hoover, D.L.; Rogers, B.M. Not all droughts are created equal: The impacts of interannual drought pattern and magnitude on grassland carbon cycling. Glob. Chang. Biol. 2016, 22, 1809–1820. [Google Scholar] [CrossRef]
- Xiao, L.; Min, X.X.; Liu, G.B.; Li, P.; Xue, S. Effect of plant-plant interactions and drought stress on the response of soil nutrient contents, enzyme activities and microbial metabolic limitations. Appl. Soil Ecol. 2023, 181, 104666. [Google Scholar] [CrossRef]
- Hashem, A.; Kumar, A.; Al-Dbass, A.M.; Alqarawi, A.A.; Al-Arjani, A.F.; Singh, G.; Farooq, M.; Abd Allah, E.F. Arbuscular mycorrhizal fungi and biochar improves drought tolerance in chickpea. Saudi J. Biol. Sci. 2019, 26, 614–624. [Google Scholar] [CrossRef]
- Geng, N.; Kang, X.; Yan, X.; Yin, N.; Wang, H.; Pan, H.; Yang, Q.; Lou, Y.; Zhuge, Y. Biochar mitigation of soil acidification and carbon sequestration is influenced by materials and temperature. Ecotoxicol. Environ. Saf. 2022, 232, 113241. [Google Scholar] [CrossRef]
- Zhang, M.; Riaz, M.; Zhang, L.; El-desouki, Z.; Jiang, C. Biochar Induces Changes to Basic Soil Properties and Bacterial Communities of Different Soils to Varying Degrees at 25 mm Rainfall: More Effective on Acidic Soils. Front. Microbiol. 2019, 10, 1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spaccini, R.; Mbagwu, J.S.C.; Igwe, C.A.; Conte, P.; Piccolo, A. Carbohydrates and aggregation in lowland soils of Nigeria as influenced by organic inputs. Soil Tillage Res. 2004, 75, 161–172. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, L.; Wang, F.; Tang, J.; Yu, L.; Zhang, R. Effects of biochar amendment on soil aggregates and hydraulic properties. J. Soil Sci. Plant Nutr. 2013, 13, 991–1002. [Google Scholar] [CrossRef] [Green Version]
- Soinne, H.; Hovi, J.; Tammeorg, P.; Turtola, E. Effect of biochar on phosphorus sorption and clay soil aggregate stability. Geoderma 2014, 219, 162–167. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Stromberger, M.E.; Lentz, R.D.; Dungan, R.S. Hardwood Biochar Influences Calcareous Soil Physicochemical and Microbiological Status. J. Environ. Qual. 2014, 43, 681–689. [Google Scholar] [CrossRef] [Green Version]
- Khadem, A.; Raiesi, F. Responses of microbial performance and community to corn biochar in calcareous sandy and clayey soils. Appl. Soil Ecol. 2017, 114, 16–27. [Google Scholar] [CrossRef]
- Jabborova, D.; Wirth, S.; Kannepalli, A.; Narimanov, A.; Desouky, S.; Davranov, K.; Sayyed, R.Z.; El Enshasy, H.; Malek, R.A.; Syed, A.; et al. Co-Inoculation of Rhizobacteria and Biochar Application Improves Growth and Nutrientsin Soybean and Enriches Soil Nutrients and Enzymes. Agronomy 2020, 10, 1142. [Google Scholar] [CrossRef]
- Qin, M.S.; Zhang, Q.; Pan, J.B.; Jiang, S.J.; Liu, Y.J.; Bahadur, A.; Peng, Z.L.; Yang, Y.; Feng, H.Y. Effect of arbuscular mycorrhizal fungi on soil enzyme activity is coupled with increased plant biomass. Eur. J. Soil Sci. 2020, 71, 84–92. [Google Scholar] [CrossRef]
- Liu, L.; Estiarte, M.; Bengtson, P.; Li, J.; Asensio, D.; Wallander, H.; Penuelas, J. Drought legacies on soil respiration and microbial community in a Mediterranean forest soil under different soil moisture and carbon inputs. Geoderma 2022, 405, 115425. [Google Scholar] [CrossRef]
- Ölinger, R.; Beck, T.; Heilmann, B.; Beese, F. Soil Respiration. In Methods in Soil Biology; Springer: Berlin/Heidelberg, Germany, 1996; pp. 93–110. [Google Scholar] [CrossRef]
- Sackett, T.E.; Basiliko, N.; Noyce, G.L.; Winsborough, C.; Schurman, J.; Ikeda, C.; Thomas, S.C. Soil and greenhouse gas responses to biochar additions in a temperate hardwood forest. GCB Bioenergy 2015, 7, 1062–1074. [Google Scholar] [CrossRef]
- Schindlbacher, A.; Wunderlich, S.; Borken, W.; Kitzler, B.; Zechmeister-Boltenstern, S.; Jandl, R. Soil respiration under climate change: Prolonged summer drought offsets soil warming effects. Glob. Chang. Biol. 2012, 18, 2270–2279. [Google Scholar] [CrossRef] [Green Version]
- Schimel, J.P. Life in Dry Soils: Effects of Drought on Soil Microbial Communities and Processes. Annu. Rev. Ecol. Evol. Syst. 2018, 49, 409–432. [Google Scholar] [CrossRef]
- Meisner, A.; Jacquiod, S.; Snoek, B.L.; ten Hooven, F.C.; van der Putten, W.H. Drought Legacy Effects on the Composition of Soil Fungal and Prokaryote Communities. Front. Microbiol. 2018, 9, 294. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Lei, H.; Chang, S.X. Drought differentially affects autotrophic and heterotrophic soil respiration rates and their temperature sensitivity. Biol. Fertil. Soils 2019, 55, 275–283. [Google Scholar] [CrossRef]
- Hinojosa, M.B.; Laudicina, V.A.; Parra, A.; Albert-Belda, E.; Moreno, J.M. Drought and its legacy modulate the post-fire recovery of soil functionality and microbial community structure in a Mediterranean shrubland. Glob. Chang. Biol. 2019, 25, 1409–1427. [Google Scholar] [CrossRef]
- Ficken, C.D.; Warren, J.M. The carbon economy of drought: Comparing respiration responses of roots, mycorrhizal fungi, and free-living microbes to an extreme dry-rewet cycle. Plant Soil 2019, 435, 407–422. [Google Scholar] [CrossRef]
- Liu, S.W.; Zhang, Y.J.; Zong, Y.J.; Hu, Z.Q.; Wu, S.; Zhou, J.; Jin, Y.G.; Zou, J.W. Response of soil carbon dioxide fluxes, soil organic carbon and microbial biomass carbon to biochar amendment: A meta-analysis. GCB Bioenergy 2016, 8, 392–406. [Google Scholar] [CrossRef]
- Hussain, T.; Hussain, N.; Tahir, M.; Raina, A.; Ikram, S.; Maqbool, S.; Fraz Ali, M.; Duangpan, S. Impacts of Drought Stress on Water Use Efficiency and Grain Productivity of Rice and Utilization of Genotypic Variability to Combat Climate Change. Agronomy 2022, 12, 2518. [Google Scholar] [CrossRef]
- Wu, B.J.; Lin, X.; Ali, M.F.; Wang, D. Development of an irrigation regime for winter wheat to save water resources by avoiding irrigation at anthesis stage. J. Agron. Crop Sci. 2023, 209, 188–203. [Google Scholar] [CrossRef]
- Guo, J.; Qu, L.; Wang, L.; Lu, W.; Lu, D. Effects of post-silking drought stress degree on grain yield and quality of waxy maize. J. Sci. Food Agric. 2022, 103, 1530–1540. [Google Scholar] [CrossRef]
- Iseghohi, I.; Abe, A.; Meseka, S.; Mengesha, W.; Gedil, M.; Menkir, A. Effects of drought stress on grain yield, agronomic performance, and heterosis of marker-based improved provitamin-A maize synthetics and their hybrids. J. Crop Improv. 2021, 36, 239–259. [Google Scholar] [CrossRef]
- Saikumar, S.; Varma, C.M.K.; Saiharini, A.; Kalmeshwer, G.P.; Nagendra, K.; Lavanya, K.; Ayyappa, D. Grain yield responses to varied level of moisture stress at reproductive stage in an interspecific population derived from Swarna/O. glaberrima introgression line. NJAS-Wagening. J. Life Sci. 2016, 78, 111–122. [Google Scholar] [CrossRef]
- Duvnjak, J.; Lončarić, A.; Brkljačić, L.; Šamec, D.; Šarčević, H.; Salopek-Sondi, B.; Španić, V. Morpho-Physiological and Hormonal Response of Winter Wheat Varieties to Drought Stress at Stem Elongation and Anthesis Stages. Plants 2023, 12, 418. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.J.; Li, H.B.; Xiao, J.N.; Sun, J.L.; Liu, R.L.; Zhang, A.P. Soil multifunctionality of paddy field is explained by soil pH rather than microbial diversity after 8-years of repeated applications of biochar and nitrogen fertilizer. Sci. Total Environ. 2022, 853, 158620. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.C.; Gale, N. Biochar and forest restoration: A review and meta-analysis of tree growth responses. New For. 2015, 46, 931–946. [Google Scholar] [CrossRef]
- Phares, C.A.; Atiah, K.; Frimpong, K.A.; Danquah, A.; Asare, A.T.; Aggor-Woananu, S. Application of biochar and inorganic phosphorus fertilizer influenced rhizosphere soil characteristics, nodule formation and phytoconstituents of cowpea grown on tropical soil. Heliyon 2020, 6, e05255. [Google Scholar] [CrossRef]
- Liu, Y.X.; Lu, H.H.; Yang, S.M.; Wang, Y.F. Impacts of biochar addition on rice yield and soil properties in a cold waterlogged paddy for two crop seasons. Field Crop. Res. 2016, 191, 161–167. [Google Scholar] [CrossRef]
- Simiele, M.; Argentino, O.; Baronti, S.; Scippa, G.S.; Chiatante, D.; Terzaghi, M.; Montagnoli, A. Biochar Enhances Plant Growth, Fruit Yield, and Antioxidant Content of Cherry Tomato (Solanum lycopersicum L.) in a Soilless Substrate. Agriculture 2022, 12, 1135. [Google Scholar] [CrossRef]
- Iacomino, G.; Sarker, T.C.; Ippolito, F.; Bonanomi, G.; Vinale, F.; Staropoli, A.; Idbella, M. Biochar and Compost Application either Alone or in Combination Affects Vegetable Yield in a Volcanic Mediterranean Soil. Agronomy 2022, 12, 1996. [Google Scholar] [CrossRef]
- Achakzai, A.G.; Gul, S.; Buriro, A.H.; Khan, H.; Mushtaq, A.; Bano, A.; Agha, S.; Kamran, K.; Ponya, Z.; Ismail, T. Biochar-fertilizer mixture: Does plant life history trait determine fertilizer application rate? Environ. Pollut. Bioavailab. 2023, 35, 2170282. [Google Scholar] [CrossRef]
- Honvault, N.; Houben, D.; Lebrun, M.; Vedere, C.; Nobile, C.; Guidet, J.; Kervroedan, L.; Aubertin, M.L.; Rumpel, C.; Faucon, M.P.; et al. Positive or neutral effects of biochar-compost mixtures on earthworm communities in a temperate cropping system. Appl. Soil Ecol. 2023, 182, 104684. [Google Scholar] [CrossRef]
- Vedere, C.; Lebrun, M.; Biron, P.; Planchais, S.; Bordenave-Jacquemin, M.; Honvault, N.; Firmin, S.; Savoure, A.; Houben, D.; Rumpel, C. The older, the better: Ageing improves the efficiency of biochar-compost mixture to alleviate drought stress in plant and soil. Sci. Total Environ. 2023, 856, 158920. [Google Scholar] [CrossRef]
- Jia, H.J.; Chu, D.P.; You, X.W.; Li, Y.Q.; Huang, C.J.; Zhang, J.L.; Zeng, X.N.; Yao, H.; Zhou, Z.F. Biochar improved the composting quality of seaweeds and cow manure mixture and altered the microbial community. Front. Microbiol. 2022, 13, 4622. [Google Scholar] [CrossRef]

| Unit | Fe mg/kg | Mn mg/kg | Zn mg/kg | Cu mg/kg | EC × 103 mS/cm | pH | O.C % | K mg/kg | S.P mg/kg | N % | Sand % | Silt % | Clay % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Quantity | 2.79 | 4.20 | 0.38 | 0.28 | 1.20 | 8.17 | 0.12 | 181 | 5.6 | 0.012 | 72 | 16 | 12 |
| Unit | Pb mg/kg | N % | SP % | K % | O.C % | pH | EC × 103 mS/cm | Cd mg/kg | Cu mg/kg | Zn mg/kg | Mn mg/kg | Fe mg/kg |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Quantity | 0.58 | 1.38 | 0.15 | 0.13 | 13.85 | 6.4 | 1.23 | 0.02 | 0.56 | 1.74 | 3.42 | 4.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasiri, S.; Andalibi, B.; Tavakoli, A.; Delavar, M.A.; El-Keblawy, A.; Zwieten, L.V.; Mastinu, A. The Mineral Biochar Alters the Biochemical and Microbial Properties of the Soil and the Grain Yield of Hordeum vulgare L. under Drought Stress. Land 2023, 12, 559. https://doi.org/10.3390/land12030559
Nasiri S, Andalibi B, Tavakoli A, Delavar MA, El-Keblawy A, Zwieten LV, Mastinu A. The Mineral Biochar Alters the Biochemical and Microbial Properties of the Soil and the Grain Yield of Hordeum vulgare L. under Drought Stress. Land. 2023; 12(3):559. https://doi.org/10.3390/land12030559
Chicago/Turabian StyleNasiri, Sajjad, Babak Andalibi, Afshin Tavakoli, Mohammad Amir Delavar, Ali El-Keblawy, Lukas Van Zwieten, and Andrea Mastinu. 2023. "The Mineral Biochar Alters the Biochemical and Microbial Properties of the Soil and the Grain Yield of Hordeum vulgare L. under Drought Stress" Land 12, no. 3: 559. https://doi.org/10.3390/land12030559







