Effects of Pig Manure and Its Organic Fertilizer Application on Archaea and Methane Emission in Paddy Fields
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Experimental Condition
2.2. Design of the Experiment
2.3. Methods for Collecting and Analyzing Soil Samples
2.3.1. Collection of Soil Sample
- (1)
- Sampling time: According to the growth cycle of rice, three times of sampling are set, which were in late August (heading stage—CK3, PM3, OF3), early September (flowering stage—CK4, PM4, OF4) and mid-October (mature stage—CK5, PM5, OF5).
- (2)
- Sampling method: A five-point sampling method was used to collect 500 g soil per plot at 0–20 cm deep, repeated three times.
- (3)
- Sample storage and transportation: After removing debris, earthworms and plant residues, the sample was divided into four parts. One part of the sample was placed in a sterile Eppendorf (EP) tube, the other part in a sterile brown bottle. The rest was placed in two self-sealed bags and transported to each laboratory in a refrigerated box for microbiological and physiochemical properties detection.
2.3.2. Analysis of Soil Physical and Chemical Properties
2.3.3. Analysis of Soil Microorganism
2.3.4. Determination of CH4 Mission Flux
2.4. Statistical Analysis
3. Results
3.1. Soil Physiochemical Properties in Paddy Field Applied with Manure
3.2. Bacterial Richness and Diversity of Soil Archaea in Paddy Field Applied with Manure
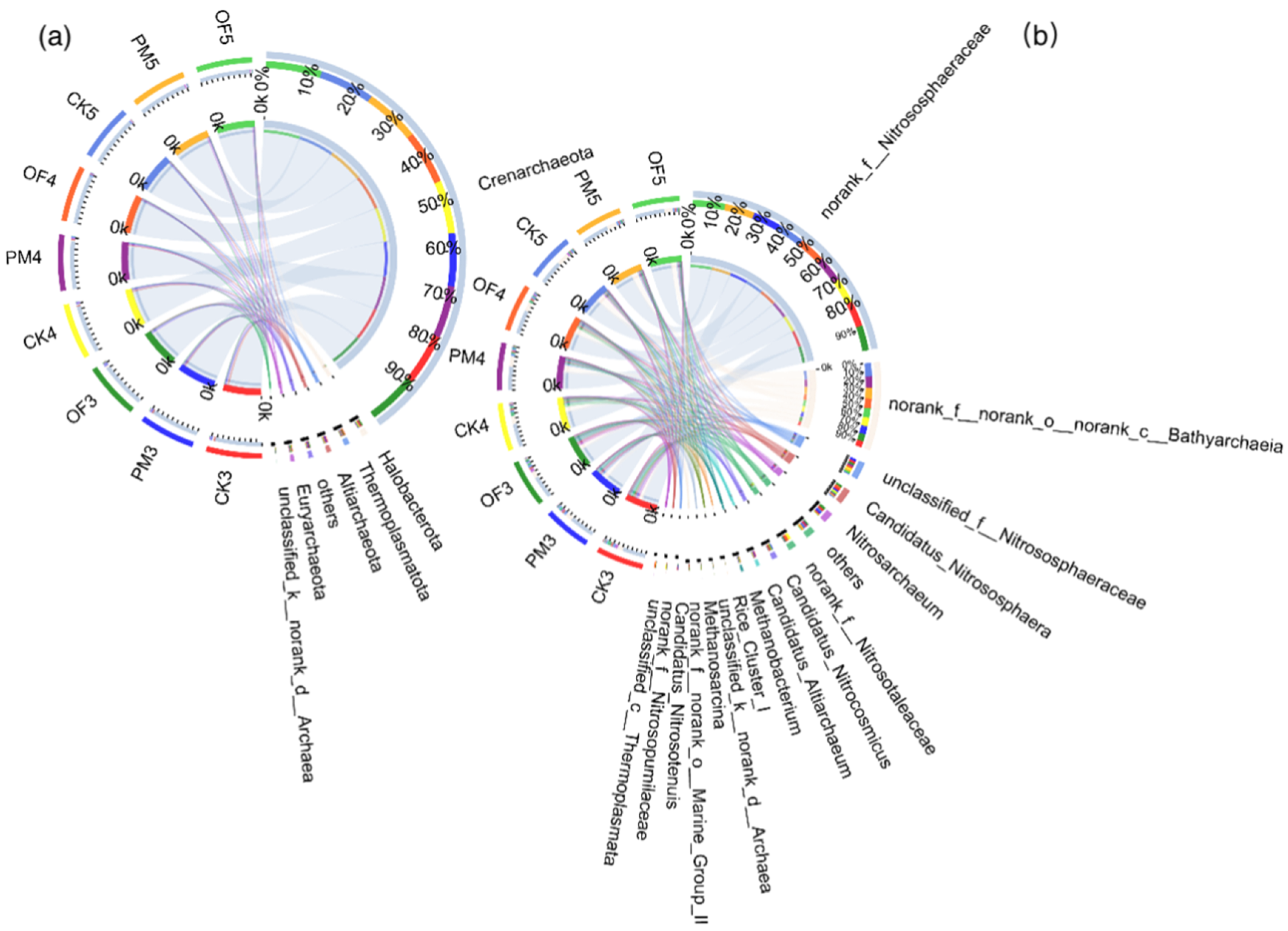
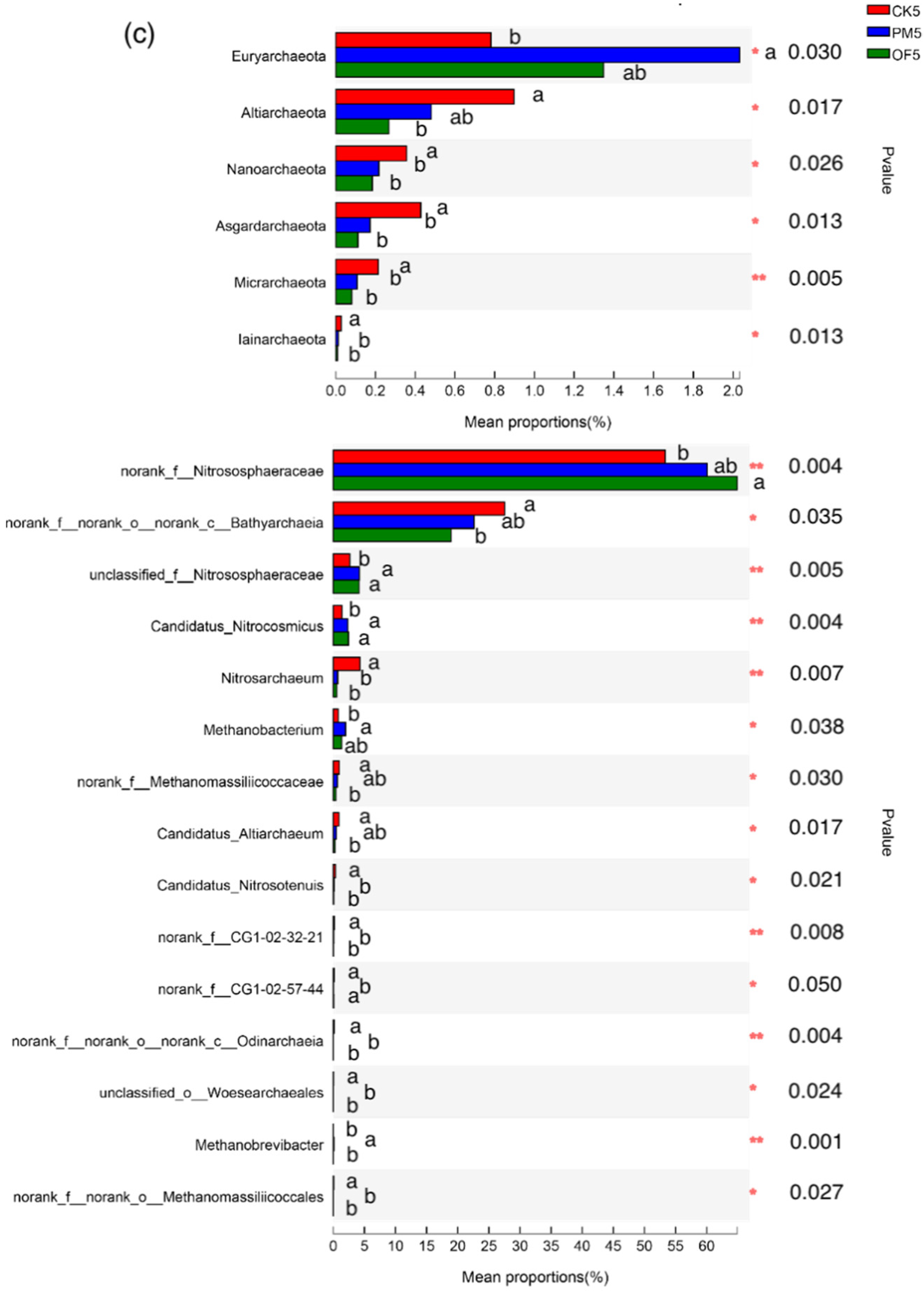
3.3. Taxonomic Composition of Soil Archaeal Microbial Communities in Paddy Field Applied with Manure
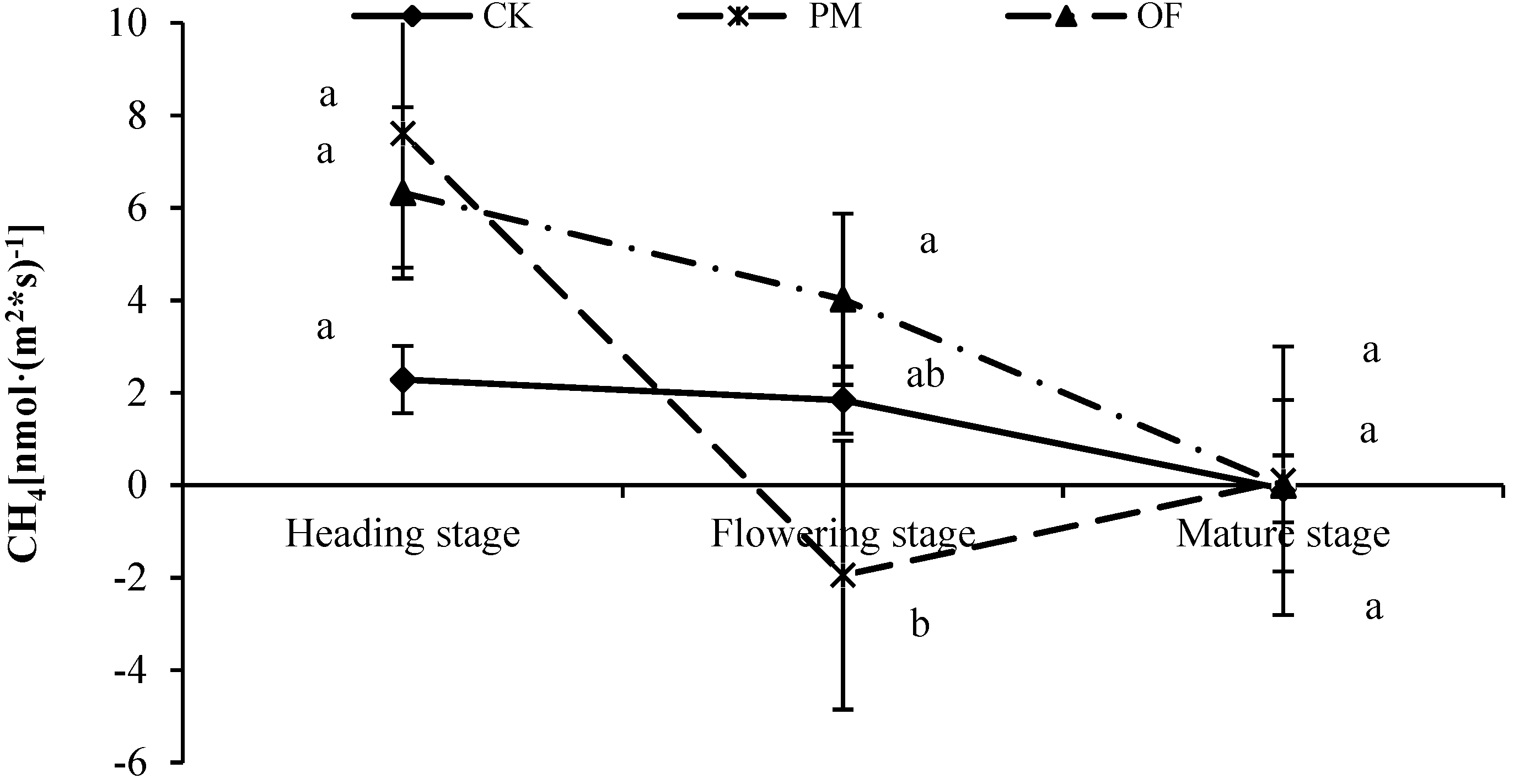
3.4. Effect of Manure Application on CH4 Emission from Paddy Soils
4. Discussion
4.1. Effect of Manure Application on Soil Archaeal Microorganisms in Paddy Fields
4.2. Effects of Changes in Environmental Factors on Soil Archaeal Microorganisms in Paddy Fields
4.3. Effects of Changes in Methanogens on CH4 Emission in Rice Fields after Manure Application
5. Conclusions
- (1)
- Compared with the control group, manure application increased the contents of Zn, Cr, and Ni in the paddy soil, but none of them were significantly different, and their contents gradually decreased with the growth of rice. Manure application increased the content of soil organic matter, TP and TK, which provided sufficient nutrients for rice growth.
- (2)
- Compared with the control group, manure application reduced the diversity and abundance of soil Archaea in paddy fields. TN, NN, TK and OM were the main environmental factors affecting the composition of soil methanogenic communities in the CK group; that in the PM group were EC, Cr, pH, Ni, TK and TN; and that in the OF group were TN, TK, Cr, Hg, Ni and As.
- (3)
- Methanobacterium, Methanobrevibacter, Methanosphaera, Methanosarcina and Rice_Cluster_I were the main methanogens in paddy soils after manure application, and the increase in the relative abundance of methanogens was probably the main reason for the increase in CH4 emission flux. Meanwhile, these Archaea are also involved in the carbon cycle of the paddy soil ecosystem.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nan, Q.; Xin, L.Q.; Qin, Y.; Waqas, M.; Wu, W.X. Exploring long-term effects of biochar on mitigating methane emissions from paddy soil: A review. Biochar 2021, 3, 125–134. [Google Scholar] [CrossRef]
- Nguyen, D.T.N.; Lee, O.K.; Nguyen, T.T.; Lee, E.Y. Type II methanotrophs: A promising microbial cell-factory platform for bioconversion of methane to chemicals. Biotechnol. Adv. 2021, 47, 107700. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.M.; Xiao, X.P.; Tang, W.G.; Sun, J.M.; Liu, J.W.; Ke, L.C.; Cheng, K.K.; Li, W.Y.; Sun, G. Changes of Multiple Cropping in Double Cropping Rice Area of Southern China and Its Policy Implications. J. Nat. Resour. 2017, 37, 7668–7678. [Google Scholar]
- Parry, M.L.; Canziani, O.F. Climate Change 2007: Impacts, Adapt-Ation and Vulnerability: Contribution of Working Group II to the Fourth Assessment Report of the INTERGOVERNMENTAL Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Das, S.; Adhya, T.K. Effect of combine application of organic manure and inorganic fertilizer on methane and nitrous oxide emissions from a tropical flooded soil planted to rice. Geoderma 2014, 213, 185–192. [Google Scholar] [CrossRef]
- Ma, J.; Xu, H.; Yagi, K.; Cai, Z.C. Methane emission from paddy soils as affected by wheat straw returning mode. Plant Soil. 2008, 313, 167–174. [Google Scholar] [CrossRef]
- Wang, J.Y.; Chen, Z.Z.; Ma, Y.C.; Sun, L.Y.; Xiong, Z.Q.; Huang, Q.W.; Sheng, Q.R. Methane and nitrous oxide emissions as affected by organic-inorganic mixed fertilizer from a rice paddy in southeast China. J. Soil. Sediment. 2013, 13, 1408–1417. [Google Scholar] [CrossRef]
- Yan, S.S. Effect of Straw Returning on Methane Emission and Methanogens in Rice Field in Cold Region; Northeast Agricultural University: Harbin, China, 2016. [Google Scholar]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Stackebrandt, E.; Goebel, B.M. Taxonomic Note: A Place for DNA-DNA Reassociation and 16S rRNA Sequence Analysis in the Present Species Definition in Bacteriology. Int. J. Syst. Bacteriol. 1994, 44, 846–849. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Q.Q.; Chen, L.Y.; Shi, J. Research Progress of the Mechanism of Action of Heavy Metal in Soil Environment by Biochar. Environ. Sci. Surv. 2017, 36, 12–18. [Google Scholar]
- Cheng, Y.L.; Zhou, D.Y. Effects of Fertilizer-residue on Soil Characteristics and Crop Production. Chin. J. Soil Sci. 2007, 1, 64–67. [Google Scholar]
- Xia, W.J. The Effect of Long-Term Fertilization on some Selected Physical, Chemical and Biological Characteristics in the Calcareous Fluvo-Aquic Soil; Chinese Academy of Agricultural Sciences: Beijing, China, 2007. [Google Scholar]
- Li, L.J. Effects of Long-term Compost Application on Soil Health in QuZhou Station; China Agricultural University: Beijing, China, 2017. [Google Scholar]
- Wang, R.; Zhu, J.; Jin, T.; Liu, Z.Y. Characteristics of ammonia oxidation microbial abundance and community structure in paddy soils of rice-crayfish symbiosis farming system. J. Plant Nutr. Fertil. 2019, 25, 1887–1899. [Google Scholar]
- Chen, J.W.; Ge, J.W.; Feng, L.; Zhou, Y.; Gan, J.; Li, Y.F.; Zhang, Z.Q. Methane Flux Characteristics and Its Relationship with Soil Archaeal community Composition of Dajiuhu Peatland in Shennongjia. Earth Sci. 2020, 45, 1082–1092. [Google Scholar]
- Fu, L.L.; Lu, Y.; Tang, L.; Hu, Y.J.; Xie, Q.Q.; Zhong, L.R.; Fan, C.Z.; Liu, Q.; Zhang, S.J. Dynamics of methane emission and archaeal archaeal community in paddy soil amended with different types of biochar. Appl. Soil. Ecol. 2021, 162, 103892. [Google Scholar] [CrossRef]
- Yuan, J.; Yi, X.M.; Cao, L.K. Three-Source Partitioning of Methane Emissions from Paddy Soil: Linkage to Methanogenic Community Structure. Int. J. Mol. Sci. 2019, 20, 1586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brauer, S.L.; Basiliko, N.; Siljanen, H.M.P.; Zinder, S.H. Methanogenic archaea in peatlands. FEMS Microbiol. Lett. 2020, 367, fnaa172. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liang, Y.; Zhang, H.C.; Liu, Y.; Zhu, J.; Xu, J.; Zhou, Z.M.; Ma, J.M.; Liu, K.H.; Yu, F.M. Variation, distribution, and diversity of canonical ammonia-oxidizing microorganisms and complete-nitrifying bacteria in highly contaminated ecological restoration regions in the Siding mine area. Ecotox. Envion. Safe 2021, 217, 112274. [Google Scholar] [CrossRef] [PubMed]
- Bernardes, F.S.; Pereira, M.A.D.; Hassan, I.A.I.; de Castro, A.P.; Roche, K.F.; Paulo, P.L. Change in microbial profile and environmental conditions in a constructed wetland system treating greywater. Environ. Sci. Pollut. Res. 2021, 28, 34539–34552. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.; Tang, J.; Wang, Y.Q.; Liu, X.M.; Yu, Z.; Zhou, S.G. Hyperthermophilic composting significantly decreases methane emissions: Insights into the microbial mechanism. Sci. Total Environ. 2021, 784, 147179. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.Y.; Zuo, M.X.; Yuan, Y.L.; Sun, J.; Gu, W.J.; Lu, Y.S.; Xie, K.Z.; Xu, P.Z. Effects of nitrogen fertilizer dosage optimization on nitrogen uptake content and utilization efficiency and microbial function genes of nitrogen cycle in sweet corn. J. South Agric. 2020, 51, 2919–2926. [Google Scholar]
- Cai, C.Y.; He, Z.F.; Hu, B.L. Microbial mechanisms of methane production and oxidation in terrestrial ecosystems. Acta Ecol. Sin. 2016, 42, 273–281. [Google Scholar]
- Valenzuela, E.I.; Padilla-Loma, C.; Gomez-Hernandez, N.; Lopez-Lozano, N.E.; Casas-Flores, S.; Cervantes, F.J. Humic Substances Mediate Anaerobic Methane Oxidation Linked to Nitrous Oxide Reduction in Wetland Sediments. Front. Microbiol. 2021, 11, 587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.H.; Conrad, R. In situ stable isotope probing of methanogenic archaea in the rice rhizosphere. Science 2005, 309, 1088–1090. [Google Scholar] [CrossRef] [PubMed]
- Pampillon-Gonzalez, L.; Ortiz-Cornejo, N.L.; Luna-Guido, M.; Dendooven, L.; Navarro-Noya, Y.E. Archaeal and Bacterial Community Structure in an Anaerobic Digestion Reactor (Lagoon Type) Used for Biogas Production at a Pig Farm. J. Mol. Microb. Biotech. 2017, 27, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.N.; Zang, H.D.; Xu, H.S.; Zhang, K.; Jiang, Y.; Hu, Y.G.; Zeng, Z.H. Methane emission and soil microbial communities in early rice paddy as influenced by urea-N fertilization. Plant Soil 2019, 445, 85–100. [Google Scholar] [CrossRef]
- Conrad, R.; Klose, M.; Noll, M.; Kemnitz, D.; Bodelier, P.L.E. Soil type links microbial colonization of rice roots to methane emission. Glob. Chang. Biol. 2008, 14, 657–669. [Google Scholar] [CrossRef]
- Fey, A.; Conrad, R. Effect of temperature on carbon and electron flow and on the archaeal community in methanogenic rice field soil. Appl. Environ. Microbiol. 2000, 66, 4790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kudo, Y.; Nakajima, T.; Miyaki, T.; Oyaizu, H. Methanogen flora of paddy soils in Japan. FEMS Microbiol. Ecol. 1997, 22, 39–48. [Google Scholar] [CrossRef]
- Jang, H.M.; Brady, J.; Kan, E.S. Succession of archaeal community in anaerobic digestion of dairy manure induced by manure-derived biochar. Environ. Eng. Res. 2021, 26, 200001. [Google Scholar] [CrossRef]
- Wang, H.H.; Li, X.; Li, X.Y.; Li, F.L.; Su, Z.C.; Zhang, H.W. Community Composition and Co-Occurrence Patterns of Diazotrophs along a Soil Profile in Paddy Fields of Three Soil Types in China. Microb. Ecol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.W.; Li, Z.X.; Chen, X.P.; Long, X.E. Effects of nickel and cobalt on methane production and methanogen abundance and diversity in paddy soil. PeerJ 2019, 7, e6274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Jiao, S.; Lu, Y.H. Biogeographic distribution of bacterial, archaeal and methanogenic communities and their associations with methanogenic capacity in Chinese wetlands. Sci. Total Environ. 2018, 622, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Bao, Q.L.; Xiao, K.Q.; Chen, Z.; Yao, H.Y.; Zhu, Y.G. Methane production and methanogenic archaeal communities in two types of paddy soil amended with different amounts of rice straw. FEMS Microbiol. Ecol. 2014, 88, 372–385. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, S.Y.; Kim, P.J.; Madsen, E.L.; Jeon, C.O. Methane emission and dynamics of methanotrophic and methanogenic communities in a flooded rice field ecosystem. FEMS Microbiol. Ecol. 2014, 88, 195–212. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.K.; Singh, A.; Watanabe, T.; Asakawa, S.; Singla, A.; Arai, H.; Inubushi, K. Methane production potential and methanogenic archaeal community structure in tropical irrigated Indian paddy soils. Biol. Fert. Soils 2014, 50, 369–379. [Google Scholar] [CrossRef]
- Nguyen, S.G.; Guevarra, R.B.; Kim, J.; Ho, C.T.; Trinh, M.V.; Unno, T. Impacts of initial fertilizers and irrigation systems on paddy methanogens and methane emission. Water Air Soil Poll. 2015, 226, 309. [Google Scholar] [CrossRef]
- He, X.Q.; Yin, H.J.; Sun, X.X.; Han, L.J.; Huang, G.Q. Effect of Different Particle-size Biochar on Methane Emissions during Pig Manure/Wheat Straw Aerobic Composting: Insights into Pore Characterization and Microbial Mechanisms. Bioresour. Technol. 2018, 268, 633–637. [Google Scholar] [CrossRef] [PubMed]


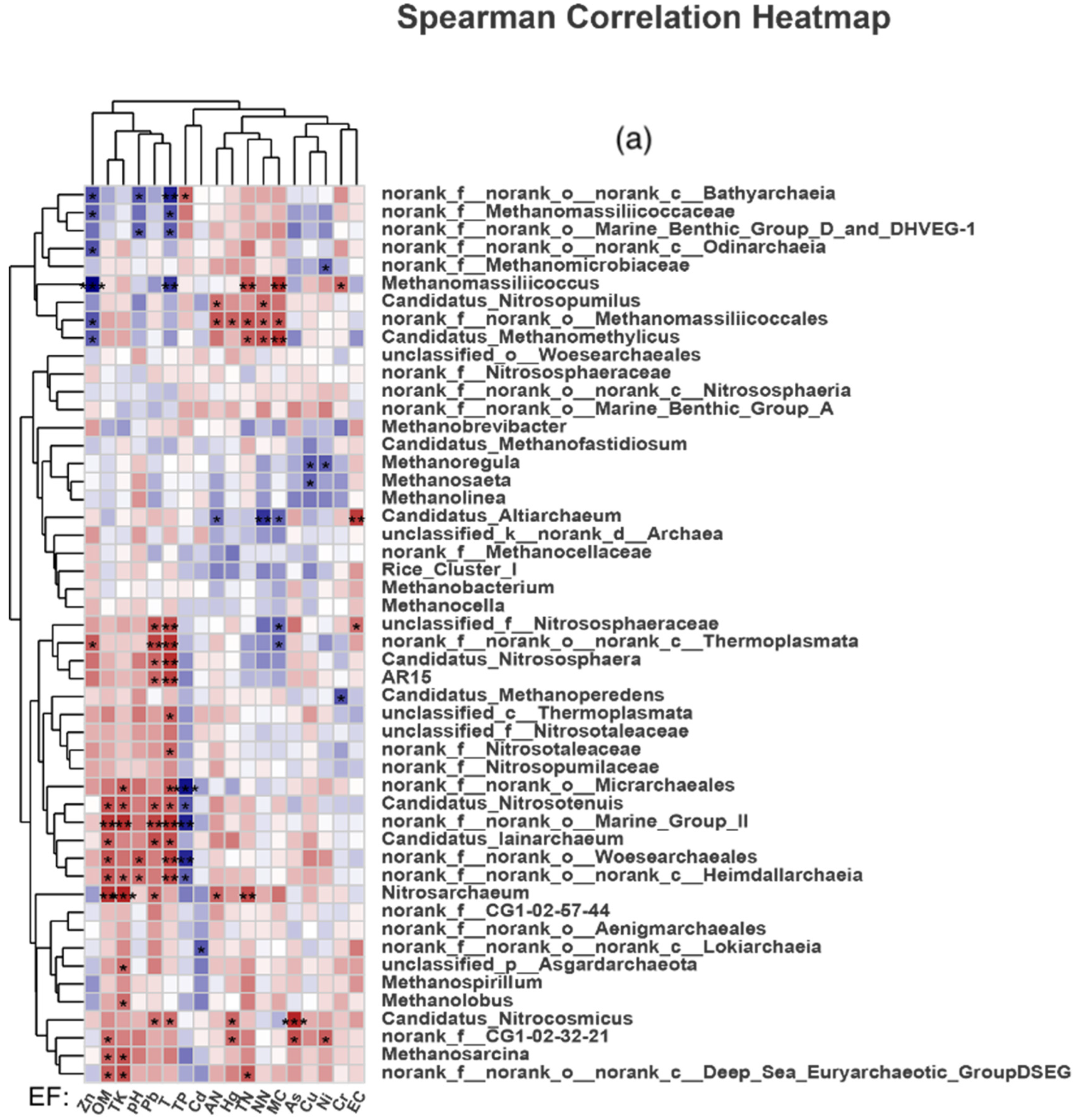
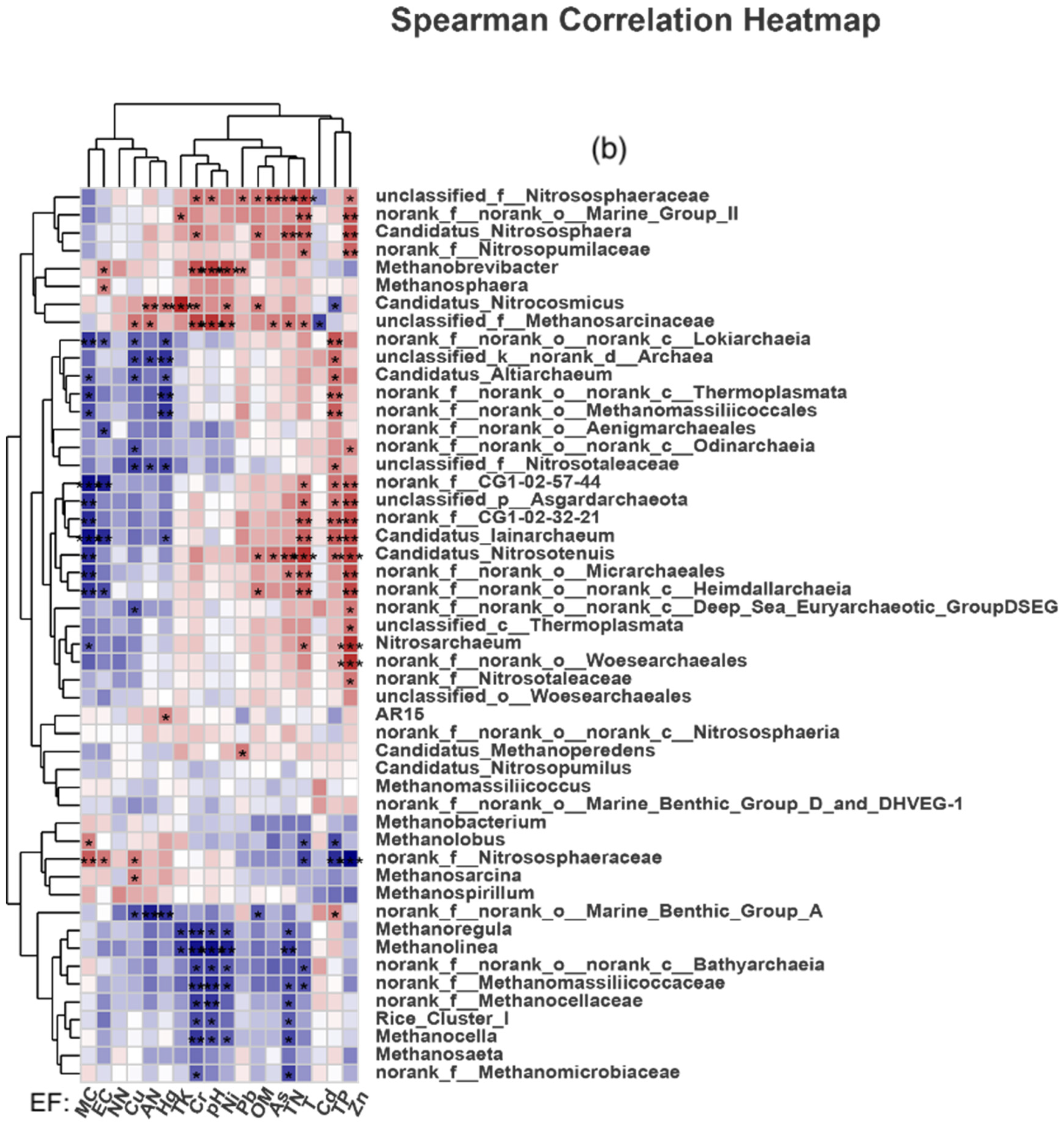
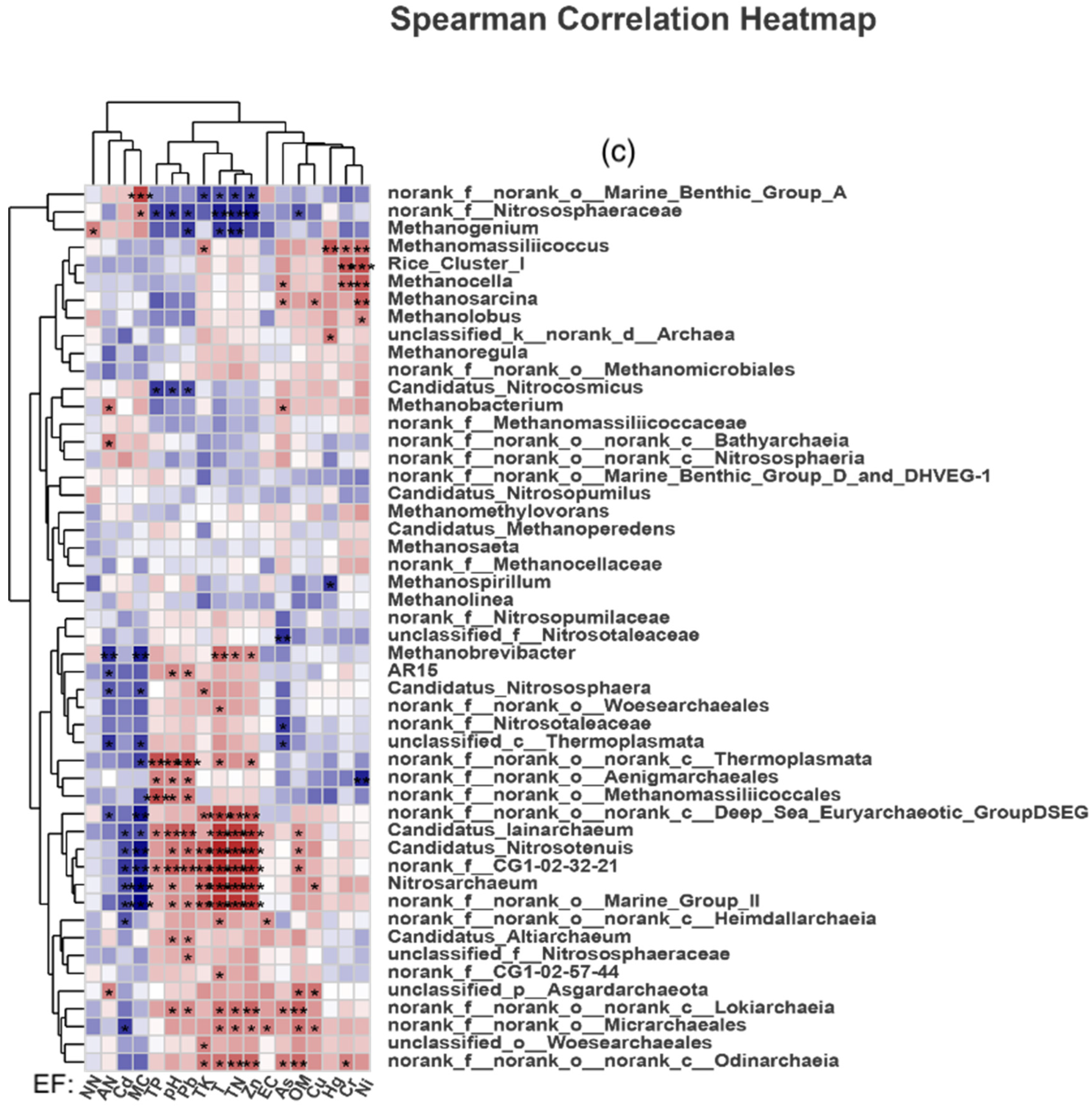
| Period | Simple | Cu | Pb | As | Cr | Hg | Zn | Cd | Ni |
|---|---|---|---|---|---|---|---|---|---|
| Heading stage | CK3 | 20.33 ± 0.58 a | 20.20 ± 1.97 a | 6.37 ± 1.40 a | 110.67 ± 59.28 a | 0.060 ± 0.010 a | 71.00 ± 2.65 b | 0.103 ± 0.006 a | 70.33 ± 18.58 a |
| PM3 | 20.00 ± 2.00 a | 20.03 ± 1.01 a | 5.83 ± 1.08 a | 188.00 ± 44.53 a | 0.054 ± 0.007 a | 74.00 ± 1.73 ab | 0.107 ± 0.012 a | 112.00 ± 27.62 a | |
| OF3 | 22.33 ± 2.89 a | 18.67 ± 0.65 a | 5.19 ± 0.78 a | 158.67 ± 73.28 a | 0.051 ± 0.007 a | 77.67 ± 3.79 a | 0.100 ± 0.000 a | 92.00 ± 31.24 a | |
| Flowering stage | CK4 | 18.00 ± 1.00 a | 15.07 ± 2.05 a | 5.36 ± 0.26 a | 65.67 ± 13.20 a | 0.050 ± 0.018 a | 72.67 ± 3.21 a | 0.110 ± 0.010 a | 51.00 ± 23.39 a |
| PM4 | 18.00 ± 1.00 a | 16.73 ± 3.42 a | 4.91 ± 0.06 a | 71.33 ± 17.39 a | 0.038 ± 0.061 a | 63.33 ± 21.22 a | 0.110 ± 0.000 a | 42.67 ± 9.07 a | |
| OF4 | 17.33 ± 1.15 a | 20.37 ± 3.92 a | 4.26 ± 1.12 a | 87.67 ± 8.33 a | 0.037 ± 0.002 a | 67.00 ± 2.65 a | 0.100 ± 0.060 a | 49.67 ± 4.93 a | |
| Mature stage | CK5 | 20.33 ± 2.52 a | 15.50 ± 1.77 a | 5.40 ± 0.55 a | 104.00 ± 21.93 a | 0.065 ± 0.020 a | 59.00 ± 6.56 a | 0.107 ± 0.012 a | 72.00 ± 25.53 a |
| PM5 | 19.67 ± 0.58 a | 14.57 ± 0.78 a | 4.44 ± 0.55 b | 73.67 ± 8.50 b | 0.069 ± 0.020 a | 61.67 ± 3.79 a | 0.110 ± 0.000 a | 50.67 ± 2.31 a | |
| OF5 | 18.33 ± 0.58 a | 14.03 ± 0.90 a | 4.61 ± 0.11 ab | 83.00 ± 7.55 ab | 0.047 ± 0.015 a | 58.67 ± 6.66 a | 0.107 ± 0.006 a | 54.67 ± 10.69 a |
| Period | Simple | pH (-) | Organic Matter (OM) (g·kg−1) | Total Nitrogen (TN) (g·kg−1) | Total Phosphorus (TP) (g·kg−1) | Total Potassium (TK) (g·kg−1) |
|---|---|---|---|---|---|---|
| Heading stage | CK3 | 8.4 ± 0.06 a | 30.33 ± 3.06 b | 1.35 ± 0.06 a | 1.00 ± 0.13 b | 25.90 ± 1.13 ab |
| PM3 | 8.32 ± 0.03 a | 35.33 ± 0.58 a | 1.46 ± 0.09 a | 1.18 ± 0.03 a | 27.80 ± 1.23 a | |
| OF3 | 8.23 ± 0.04 b | 30.33 ± 0.58 b | 1.46 ± 0.07 a | 1.18 ± 0.05 a | 24.70 ± 1.42 b | |
| Flowering stage | CK4 | 8.31 ± 0.28 a | 16.33 ± 0.58 a | 0.81 ± 0.24 a | 1.20 ± 0.14 b | 14.77 ± 3.74 b |
| PM4 | 8.17 ± 0.10 a | 19.33 ± 3.06 a | 0.97 ± 0.13 a | 1.31 ± 0.08 ab | 13.27 ± 2.11 b | |
| OF4 | 8.30 ± 0.18 a | 19.33 ± 2.52 a | 1.10 ± 0.13 a | 1.41 ± 0.08 a | 21.67 ± 3.76 a | |
| Mature stage | CK5 | 8.23 ± 0.02 a | 23.33 ± 2.08 a | 0.94 ± 0.06 b | 1.01 ± 0.07 b | 22.00 ± 3.03 a |
| PM5 | 8.22 ± 0.03 a | 20.33 ± 3.51 a | 0.86 ± 0.05 b | 1.11 ± 0.04 b | 22.27 ± 3.88 a | |
| OF5 | 8.13 ± 0.01 b | 20.33 ± 3.06 a | 1.35 ± 0.26 a | 1.23 ± 0.06 a | 23.23 ± 0.32 a |
| Period | Simple | Shannon | Ace | Coverage |
|---|---|---|---|---|
| Heading stage | CK3 | 3.5197 ± 0.1467 a | 428.4023 ± 75.3949 a | 0.9966 ± 0.0004 a |
| PM3 | 3.3375 ± 0.2366 a | 410.7037 ± 65.4228 a | 0.9968 ± 0.0005 a | |
| OF3 | 3.4470 ± 0.2200 a | 353.4041 ± 58.3528 a | 0.9972 ± 0.0005 a | |
| Flowering stage | CK4 | 3.3291 ± 0.2312 a | 443.5142 ± 89.6234 a | 0.9964 ± 0.0007 a |
| PM4 | 3.5109 ± 0.1334 a | 446.8897 ± 62.7242 a | 0.9964 ± 0.0004 a | |
| OF4 | 3.4087 ± 0.1180 a | 371.8767 ± 98.2980 a | 0.9968 ± 0.0009 a | |
| Mature stage | CK5 | 3.1943 ± 0.2263 a | 361.6134 ± 64.1589 a | 0.9971 ± 0.0005 a |
| PM5 | 3.2141 ± 0.0532 a | 358.8340 ± 43.6350 a | 0.9970 ± 0.0001 a | |
| OF5 | 3.1452 ± 0.1398 a | 353.8251 ± 104.0263 a | 0.9970 ± 0.0007 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Wang, M.; Li, P.; Shen, L.; Ma, M.; Xu, B.; Zhang, S.; Sha, C.; Ye, C.; Xiong, L.; et al. Effects of Pig Manure and Its Organic Fertilizer Application on Archaea and Methane Emission in Paddy Fields. Land 2022, 11, 499. https://doi.org/10.3390/land11040499
Wu J, Wang M, Li P, Shen L, Ma M, Xu B, Zhang S, Sha C, Ye C, Xiong L, et al. Effects of Pig Manure and Its Organic Fertilizer Application on Archaea and Methane Emission in Paddy Fields. Land. 2022; 11(4):499. https://doi.org/10.3390/land11040499
Chicago/Turabian StyleWu, Jianqiang, Min Wang, Peng Li, Leyang Shen, Mingyi Ma, Boyu Xu, Shuyuan Zhang, Chenyan Sha, Chunmei Ye, Lijun Xiong, and et al. 2022. "Effects of Pig Manure and Its Organic Fertilizer Application on Archaea and Methane Emission in Paddy Fields" Land 11, no. 4: 499. https://doi.org/10.3390/land11040499





