Generalized Linear Models to Identify Key Hydromorphological and Chemical Variables Determining the Occurrence of Macroinvertebrates in the Guayas River Basin (Ecuador)
Abstract
:1. Introduction
2. Materials and Methods
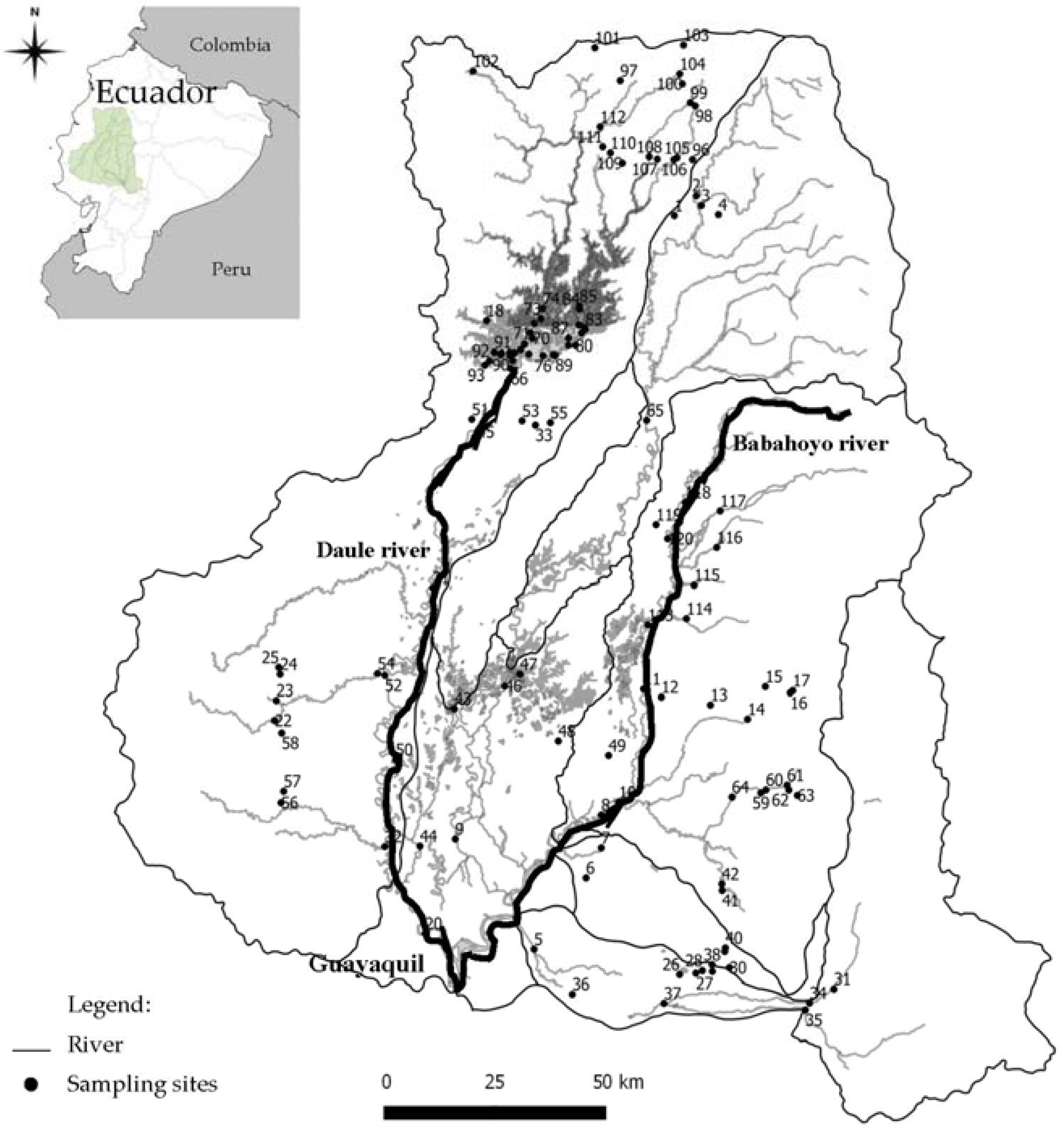
2.1. Study Area
2.2. Data Collection
2.3. Biotic Integrity
2.4. Statistical Model
2.5. Sensitivity Analysis: Identifying Potential Restoration Actions
3. Results
3.1. Bioassessment Indices and Biotic Integrity
3.2. Statistical Model
3.3. Sensitivity Analysis
4. Discussion
4.1. Biotic Integrity and Potential Restoration Actions
4.2. Model Development and Validation
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BMWP | Biological Monitoring Working Party |
| BMWP-Col | Biological Monitoring Working Party adapted for Colombia |
| IMEERA | Índice Multimétrico del Estado Ecológico para Ríos Altoandinos |
| ABI | Andean Biotic Index |
| NLSMI | Neotropical Low-land Stream Multimetric Index |
| ASPT | average score per taxon |
| GLM | generalized linear model |
| ANN | artificial neural networks |
| BBN | Bayesian belief networks |
| TDS | total dissolved solids |
| DO | dissolved oxygen |
| COD | chemical oxygen demand |
| Total N | total nitrogen |
| Total P | total phosphorus |
| Std dev | standard deviation |
| AUSRIVAS | Australian River Assessment System |
| RHS | the United Kingdom and the Isle of Man River Habitat Survey |
| FFG | functional feeding group |
| VIF | variance inflation factor |
| EQR | ecological quality ratio |
| AIC | Akaike information criterion |
| RCC | river continuum concept |
| CPOM | coarse particulate organic matter |
| FPOM | fine particulate organic matter |
| MAE | Ministerio del Ambiente del Ecuador |
References
- Goethals, P.L.M. Sustainability of water quality and ecology: Easier said than defined and implemented. Sustain. Water Qual. Ecol. 2013, 1, 1–2. [Google Scholar] [CrossRef]
- Helson, J.E.; Williams, D.D. Development of a macroinvertebrate multimetric index for the assessment of low-land streams in the neotropics. Ecol. Indic. 2013, 29, 167–178. [Google Scholar] [CrossRef]
- Arimoro, F.O.; Odume, O.N.; Uhunoma, S.I.; Edegbene, A.O. Anthropogenic impact on water chemistry and benthic macroinvertebrate associated changes in a southern Nigeria stream. Environ. Monit. Assess. 2015, 187, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bruno, D.; Belmar, O.; Sanchez-Fernandez, D.; Guareschi, S.; Millan, A.; Velasco, J. Responses of Mediterranean aquatic and riparian communities to human pressures at different spatial scales. Ecol. Indic. 2014, 45, 456–464. [Google Scholar] [CrossRef]
- Frankforter, J.D.; Weyers, H.S.; Bales, J.D.; Moran, P.W.; Calhoun, D.L. The relative influence of nutrients and habitat on stream metabolism in agricultural streams. Environ. Monit. Assess. 2010, 168, 461–479. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.M.L.; Camargo, P.B.; Mcdowell, W.H.; Vieira, I.; Salomao, M.S.M.B.; Martinelli, L.A. Influence of land use changes on water chemistry in streams in the State of Sao Paulo, southeast Brazil. An. Acad. Bras. Cienc. 2012, 84, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Karr, J.R. Biological Integrity—A Long-Neglected Aspect of Water-Resource Management. Ecol. Appl. 1991, 1, 66–84. [Google Scholar] [CrossRef]
- Blanchette, M.L.; Pearson, R.G. Dynamics of habitats and macroinvertebrate assemblages in rivers of the Australian dry tropics. Freshw. Biol. 2013, 58, 742–757. [Google Scholar] [CrossRef]
- Hughes, S.J.; Santos, J.M.; Ferreira, M.T.; Caraca, R.; Mendes, A.M. Ecological assessment of an intermittent Mediterranean river using community structure and function: Evaluating the role of different organism groups. Freshw. Biol. 2009, 54, 2383–2400. [Google Scholar] [CrossRef]
- Everaert, G.; De Neve, J.; Boets, P.; Dominguez-Granda, L.; Mereta, S.T.; Ambelu, A.; Hoang, T.H.; Goethals, P.L.M.; Thas, O. Comparison of the Abiotic Preferences of Macroinvertebrates in Tropical River Basins. PLoS ONE 2014, 9, e108898. [Google Scholar] [CrossRef] [PubMed]
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.I.; Knowler, D.J.; Leveque, C.; Naiman, R.J.; Prieur-Richard, A.H.; Soto, D.; Stiassny, M.L.J.; et al. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. 2006, 81, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Mieles, G.; Irvine, K.; Griensven, A.V.; Arias-Hidalgo, M.; Torres, A.; Mynett, A.E. Relationships between aquatic biotic communities and water quality in a tropical river-wetland system (Ecuador). Environ. Sci. Policy 2013, 34, 115–127. [Google Scholar] [CrossRef]
- Knee, K.L.; Encalada, A.C. Land Use and Water Quality in a Rural Cloud Forest Region (Intag, Ecuador). River Res. Appl. 2014, 30, 385–401. [Google Scholar] [CrossRef]
- Rezende, R.S.; Santos, A.M.; Henke-Oliveira, C.; Goncalves, J.F. Effects of spatial and environmental factors on benthic a macroinvertebrate community. Zoologia 2014, 31, 426–434. [Google Scholar] [CrossRef]
- Villamarin, C.; Rieradevall, M.; Paul, M.J.; Barbour, M.T.; Prat, N. A tool to assess the ecological condition of tropical high Andean streams in Ecuador and Peru: The IMEERA index. Ecol. Indic. 2013, 29, 79–92. [Google Scholar] [CrossRef]
- Rios-Touma, B.; Encalada, A.C.; Fornells, N.P. Macroinvertebrate Assemblages of an Andean High-Altitude Tropical Stream: The Importance of Season and Flow. Int. Rev. Hydrobiol. 2011, 96, 667–685. [Google Scholar] [CrossRef]
- Arias-Hidalgo, M.; Villa-Cox, G.; Griensven, A.V.; Solorzano, G.; Villa-Cox, R.; Mynett, A.E.; Debels, P. A decision framework for wetland management in a river basin context: The “Abras de Mantequilla” case study in the Guayas River Basin, Ecuador. Environ. Sci. Policy 2013, 34, 103–114. [Google Scholar] [CrossRef]
- Everaert, G.; Pauwels, I.S.; Boets, P.; Verduin, E.; de la Haye, M.A.A.; Blom, C.; Goethals, P.L.M. Model-based evaluation of ecological bank design and management in the scope of the European Water Framework Directive. Ecol. Eng. 2013, 53, 144–152. [Google Scholar] [CrossRef]
- Hoang, T.H.; Lock, K.; Mouton, A.; Goethals, P.L.M. Application of classification trees and support vector machines to model the presence of macroinvertebrates in rivers in Vietnam. Ecol. Inform. 2010, 5, 140–146. [Google Scholar] [CrossRef]
- Forio, M.A.E.; Landuyt, D.; Bennetsen, E.; Lock, K.; Nguyen, T.H.T.; Damanik-Ambarita, M.N.; Musonge, P.L.S.; Boets, P.; Everaert, G.; Dominguez-Granda, L.; et al. Bayesian belief network models to analyse and predict ecological water quality in rivers. Ecol. Model. 2015, 312, 222–238. [Google Scholar] [CrossRef]
- Arriaga, L. The Daule-Peripe dam project, urban development of Guayaquil and their impact on shrimp mariculture. In A Sustainable Shrimp Mariculture Industry for Ecuador; Olsen, S., Arriaga, L., Eds.; Coastal Resources Center, University of Rhode Island: Narragansett, RI, USA, 1989. [Google Scholar]
- Nguyen, T.H.T.; Boets, P.; Lock, K.; Damanik-Ambarita, M.N.; Forio, M.A.E.; Sasha, P.; Dominguez-Granda, L.E.; Hoang, T.H.T.; Everaert, G.; Goethals, P.L.M. Habitat suitability of the invasive water hyacinth and its relation to water quality and macroinvertebrate diversity in a tropical reservoir. Limnologica 2015, 52, 67–74. [Google Scholar] [CrossRef]
- United States Army Corps of Engineers (USACE). Water Resources Assessment of Ecuador; USACE: Mobile, AL, USA, 1998. [Google Scholar]
- Corporación Eléctrica del Ecuador (CELEC). Revista 25 Años de la Presa Daule-Peripa; CELEC: Cuenca, Ecuador, 2013. [Google Scholar]
- Kang, S.M.; Seager, R.; Frierson, D.M.W.; Liu, X.J. Croll revisited: Why is the northern hemisphere warmer than the southern hemisphere? Clim. Dyn. 2015, 44, 1457–1472. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (USEPA). Stream flow. In Water: Monitoring & Assessment; USEPA: Washington, DC, USA, 2012. [Google Scholar]
- Parsons, M.; Thoms, M.; Norris, R. Australian River Assessment System: AusRivAS Physical Assessment Protocol; Monitoring River Health Initiative Technical Report, Report No. 22; Environment Australia, Commonwealth of Australia and University of Canberra: Canberra, Australia, 2002. [Google Scholar]
- Raven, P.J.; Holmes, N.T.H.; Dawson, F.H.; Fox, P.J.A.; Everard, M.; Fozzard, I.R.; Rouen, K.J. River Habitat Quality: The Physical Character of Rivers and Streams in the UK and Isle of Man; River Habitat Survey, Report No. 2; Environment Agency: Bristol, UK, 1998. [Google Scholar]
- Gabriels, W.; Lock, K.; De Pauw, N.; Goethals, P.L.M. Multimetric Macroinvertebrate Index Flanders (MMIF) for biological assessment of rivers and lakes in Flanders (Belgium). Limnologica 2010, 40, 199–207. [Google Scholar] [CrossRef]
- De Pauw, N.; Van Damme, D.; Bij De Vaate, A. Integrated programme for implementation of the recommended transnational monitoring strategy for the Danube river basin, CEC PHARE/TACIS project. In Manual for Macroinvertebrate Identification and Water Quality Assesment; University of Ghent: Ghent, Belgium, 1996. [Google Scholar]
- Domínguez, E.; Fernández, H.R. Macroinvertebrados Bentónicos Sudamericanos: Sistemática y Biología; Fundación Miguel Lillo: San Miguel de Tucumán, Argentina, 2009; Volume XV, p. 654. [Google Scholar]
- Mereta, S.T.; Boets, P.; De Meester, L.; Goethals, P.L.M. Development of a multimetric index based on benthic macroinvertebrates for the assessment of natural wetlands in Southwest Ethiopia. Ecol. Indic. 2013, 29, 510–521. [Google Scholar] [CrossRef]
- Barbour, M.T.; Gerritsen, J.; Snyder, B.D.; Stribling, J.B. Rapid Bioassessment Protocols for Use in Streams and Wadeable Rivers: Periphyton, Benthic Macroinvertebrates, and Fish, 2nd ed.; U.S. Environmental Protection Agency, Office of Water: Washington, DC, USA, 1999. [Google Scholar]
- Vannote, R.L.; Minshall, G.W.; Cummins, K.W.; Sedell, J.R.; Cushing, C.E. River Continuum Concept. Can. J. Fish. Aquat. Sci. 1980, 37, 130–137. [Google Scholar] [CrossRef]
- Roldán Pérez, G. Bioindicación de la Calidad del agua en Colombia: Propuesta para el Uso del Método BMWP/Col, 1st ed.; Ciencia y tecnología; Editorial Universidad de Antioquia: Medellín, Colombia, 2003; Volume XVIII, p. 170. [Google Scholar]
- Alvarez, A.L.F. Metodologia para la Evaluacion de los Macroinvertebrados Acuaticos Como Indicadores de los Recursos Hidrobiologicos; Instituto Alexander von Humboldt: Medellín, Colombia, 2005. [Google Scholar]
- Dominguez-Granda, L.; Lock, K.; Goethals, P.L.M. Using multi-target clustering trees as a tool to predict biological water quality indices based on benthic macroinvertebrates and environmental parameters in the Chaguana watershed (Ecuador). Ecol. Inform. 2011, 6, 303–308. [Google Scholar] [CrossRef]
- Damanik-Ambarita, M.N.; Lock, K.; Boets, P.; Everaert, G.; Nguyen, T.H.T.; Forio, M.A.E.; Musonge, P.L.S.; Suhareva, N.; Bennetsen, E.; Landuyt, D.; et al. Ecological water quality analysis of the Guayas river basin (Ecuador) based on macroinvertebrates indices. Limnologica 2016, 57, 27–59. [Google Scholar] [CrossRef]
- Armitage, P.D.; Moss, D.; Wright, J.F.; Furse, M.T. The Performance of a New Biological Water-Quality Score System Based on Macroinvertebrates over a Wide-Range of Unpolluted Running-Water Sites. Water Res. 1983, 17, 333–347. [Google Scholar] [CrossRef]
- Mandaville, S.M. Benthic Macroinvertebrates in Freshwaters-Taxa Tolerance Values, Metrics, and Protocols. In Soil & Water Conservation Society of Metro Halifax (Project H-1); Chebucto Community Net (CCN): Halifax, NS, Canada, 2002; pp. 1–128. [Google Scholar]
- Hruby, T. Washington State wetland rating system for eastern Washington—Revised; Ecology Publication # 04-06-15; Washington State Department of Ecology: Olympia, WA, USA, 2004. [Google Scholar]
- United States Environmental Protection Agency (USEPA). Methods for Evaluating Wetland Condition: Developing Metrics and Indexes of Biological Integrity; EPA-822-R-02-016; Office of Water, U.S. Environmental Protection Agency: Washington, DC, USA, 2002. [Google Scholar]
- Zuur, A.F. Mixed Effects Models and Extensions in Ecology with R; Statistics for Biology and Health; Springer: New York, NY, USA, 2009; Volume XXII, pp. 1–574. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Smith, G.M. Analysing Ecological Data; Statistics for Biology and Health; Springer: New York, NY, USA; London, UK, 2007; Volume XXVI, pp. 1–672. [Google Scholar]
- Weirich, S.R.; Silverstein, J.; Rajagopalan, B. Effect of average flow and capacity utilization on effluent water quality from US municipal wastewater treatment facilities. Water Res. 2011, 45, 4279–4286. [Google Scholar] [CrossRef] [PubMed]
- Guisan, A.; Lehmann, A.; Ferrier, S.; Austin, M.; Overton, J.M.C.; Aspinall, R.; Hastie, T. Making better biogeographical predictions of species’ distributions. J. Appl. Ecol. 2006, 43, 386–392. [Google Scholar] [CrossRef]
- Thuiller, W. BIOMOD—Optimizing predictions of species distributions and projecting potential future shifts under global change. Glob. Chang. Biol. 2003, 9, 1353–1362. [Google Scholar] [CrossRef]
- Dedecker, A.P.; Goethals, P.L.M.; D’Heygere, T.; Gevrey, M.; Lek, S.; De Pauw, N. Application of artificial neural network models to analyse the relationships between Gammarus pulex L. (Crustacea, Amphipoda) and river characteristics. Environ. Monit. Assess. 2005, 111, 223–241. [Google Scholar] [CrossRef] [PubMed]
- R-Core-Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Everaert, G.; Pauwels, I.S.; Goethals, P.L.M. Development of data-driven models for the assessment of macroinvertebrates in rivers in Flanders. Int. Congr. Environ. Model. Softw. Soc. (iEMSs) 2010, 3, 1948–1956. [Google Scholar]
- Goethals, P.L.M.; Dedecker, A.P.; Gabriels, W.; Lek, S.; De Pauw, N. Applications of artificial neural networks predicting macroinvertebrates in freshwaters. Aquat. Ecol. 2007, 41, 491–508. [Google Scholar] [CrossRef]
- Mouton, A.M.; Dedecker, A.P.; Lek, S.; Goethals, P.L.M. Selecting Variables for Habitat Suitability of Asellus (Crustacea, Isopoda) by Applying Input Variable Contribution Methods to Artificial Neural Network Models. Environ. Model. Assess. 2010, 15, 65–79. [Google Scholar] [CrossRef]
- Malmqvist, B.; Maki, M. Benthic Macroinvertebrate Assemblages in North Swedish Streams—Environmental Relationships. Ecography 1994, 17, 9–16. [Google Scholar] [CrossRef]
- Younes-Baraille, Y.; Garcia, X.F.; Gagneur, J. Impact of the longitudinal and seasonal changes of the water quality on the benthic macroinvertebrate assemblages of the Andorran streams. C. R. Biol. 2005, 328, 963–976. [Google Scholar] [CrossRef] [PubMed]
- Compin, A.; Cereghino, R. Spatial patterns of macroinvertebrate functional feeding groups in streams in relation to physical variables and land-cover in Southwestern France. Landsc. Ecol. 2007, 22, 1215–1225. [Google Scholar] [CrossRef]
- Grubaugh, J.W.; Wallace, J.B.; Houston, E.S. Longitudinal changes of macroinvertebrate communities along an Appalachian stream continuum. Can. J. Fish. Aquat. Sci. 1996, 53, 896–909. [Google Scholar] [CrossRef]
- Rios, S.L.; Bailey, R.C. Relationship between riparian vegetation and stream benthic communities at three spatial scales. Hydrobiologia 2006, 553, 153–160. [Google Scholar] [CrossRef]
- Strayer, D.L.; Beighley, R.E.; Thompson, L.C.; Brooks, S.; Nilsson, C.; Pinay, G.; Naiman, R.J. Effects of land cover on stream ecosystems: Roles of empirical models and scaling issues. Ecosystems 2003, 6, 407–423. [Google Scholar] [CrossRef]
- Kasangaki, A.; Chapman, L.J.; Balirwa, J. Land use and the ecology of benthic macroinvertebrate assemblages of high-altitude rainforest streams in Uganda. Freshw. Biol. 2008, 53, 681–697. [Google Scholar] [CrossRef]
- Townsend, C.R.; Arbuckle, C.J.; Crowl, T.A.; Scarsbrook, M.R. The relationship between land use and physicochemistry, food resources and macroinvertebrate communities in tributaries of the Taieri River, New Zealand: A hierarchically scaled approach. Freshw. Biol. 1997, 37, 177–191. [Google Scholar] [CrossRef]
- Sweeney, B.W.; Bott, T.L.; Jackson, J.K.; Kaplan, L.A.; Newbold, J.D.; Standley, L.J.; Hession, W.C.; Horwitz, R.J. Riparian deforestation, stream narrowing, and loss of stream ecosystem services. Proc. Natl. Acad. Sci. USA 2004, 101, 14132–14137. [Google Scholar] [CrossRef] [PubMed]
- Ellison, C.A.; Skinner, Q.D.; Hicks, L.S. Assessment of Best-Management Practice Effects on Rangeland Stream Water Quality Using Multivariate Statistical Techniques. Rangel. Ecol. Manag. 2009, 62, 371–386. [Google Scholar] [CrossRef]
- Hutchens, J.J.; Schuldt, J.A.; Richards, C.; Johnson, L.B.; Host, G.E.; Breneman, D.H. Multi-scale mechanistic indicators of Midwestern USA stream macroinvertebrates. Ecol. Indic. 2009, 9, 1138–1150. [Google Scholar] [CrossRef]
- Von Sperling, M.; Chernicharo, C.A.L. Urban wastewater treatment technologies and the implementation of discharge standards in developing countries. Urban Water 2002, 4, 105–114. [Google Scholar] [CrossRef]
- Chapman, D. Water Quality Assessments—A Guide to Use of Biota, Sediments and Water in Environmental Monitoring, 2nd ed.; E&FN Spon on Behalved of UNESCO/WHO/UNEP: London, UK, 1996; pp. 1–651. [Google Scholar]
- Ballance, R. Physical and chemical analyses. In Water Quality Monitoring—A Practical Guide to the Design and Implementation of Freshwater Quality Studies and Monitoring Programmes; Bartram, J., Ballance, R., Eds.; E&FN Spon on Behalved of UNESCO/WHO/UNEP: London, UK, 1996; Chapter 7. [Google Scholar]
- Garcia, E.A.; Pettit, N.E.; Warfe, D.M.; Davies, P.M.; Kyne, P.M.; Novak, P.; Douglas, M.M. Temporal variation in benthic primary production in streams of the Australian wet-dry tropics. Hydrobiologia 2015, 760, 43–55. [Google Scholar] [CrossRef]
- O’Toole, C.; Donohue, I.; Moe, S.J.; Irvine, K. Nutrient optima and tolerances of benthic invertebrates, the effects of taxonomic resolution and testing of selected metrics in lakes using an extensive European data base. Aquat. Ecol. 2008, 42, 277–291. [Google Scholar] [CrossRef]
- European Union (EU). EC—Drinking Water Directive—DWD—98/83/EC; European Union: Brussels, Belgium, 1998. [Google Scholar]
- Ministerio del Ambiente del Ecuador (MAE). Reforma del Libro VI del Texto Unificado de Legislación Secundaria; Ministerio del Ambiente del Ecuador (MAE): Quito, Ecuador, 2015. [Google Scholar]
- Kincheloe, J.W.; Wedemeyer, G.A.; Koch, D.L. Tolerance of Developing Salmonid Eggs and Fry to Nitrate Exposure. Bull. Environ. Contam. Toxicol. 1979, 23, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Camargo, J.A.; Alonso, A.; Salamanca, A. Nitrate toxicity to aquatic animals: A review with new data for freshwater invertebrates. Chemosphere 2005, 58, 1255–1267. [Google Scholar] [CrossRef] [PubMed]
- Borbor-Cordova, M.J.; Boyer, E.W.; Mcdowell, W.H.; Hall, C.A. Nitrogen and phosphorus budgets for a tropical watershed impacted by agricultural land use: Guayas, Ecuador. Biogeochemistry 2006, 79, 135–161. [Google Scholar] [CrossRef]
- Holomuzki, J.R.; Biggs, B.J.F. Sediment texture mediates high-flow effects on lotic macroinvertebrates. J. N. Am. Benthol. Soc. 2003, 22, 542–553. [Google Scholar] [CrossRef]
- Flügel, E. Microfacies of Carbonate Rocks: Analysis, Interpretation and Application; Springer: Berlin, Germany; New York, NY, USA, 2004; Volume XIX, pp. 1–976. [Google Scholar]
- Raymond, K.L.; Vondracek, B. Relationships among rotational and conventional grazing systems, stream channels, and macroinvertebrates. Hydrobiologia 2011, 669, 105–117. [Google Scholar] [CrossRef]
- Lester, R.E.; Boulton, A.J. Rehabilitating agricultural streams in Australia with wood: A review. Environ. Manag. 2008, 42, 310–326. [Google Scholar] [CrossRef] [PubMed]
- Wyzga, B.; Amirowicz, A.; Oglecki, P.; Hajdukiewicz, H.; Radecki-Pawlik, A.; Zawiejska, J.; Mikus, P. Response of fish and benthic invertebrate communities to constrained channel conditions in a mountain river: Case study of the Biala, Polish Carpathians. Limnologica 2014, 46, 58–69. [Google Scholar] [CrossRef]
- Wyzga, B.; Amirowicz, A.; Radecki-Pawlik, A.; Zawiejska, J. Hydromorphological Conditions, Potential Fish Habitats and the Fish Community in a Mountain River Subjected to Variable Human Impacts, the Czarny Dunajec, Polish Carpathians. River Res. Appl. 2009, 25, 517–536. [Google Scholar] [CrossRef]
- Boulton, A.J.; Scarsbrook, M.R.; Quinn, J.M.; Burrell, G.P. Land-use effects on the hyporheic ecology of five small streams near Hamilton, New Zealand. N. Z. J. Mar. Freshw. Res. 1997, 31, 609–622. [Google Scholar] [CrossRef]
- Collins, A.L.; Walling, D.E. Fine-grained bed sediment storage within the main channel systems of the Frome and Piddle catchments, Dorset, UK. Hydrol. Process. 2007, 21, 1448–1459. [Google Scholar] [CrossRef]
- Fornaroli, R.; Cabrini, R.; Sartori, L.; Marazzi, F.; Vracevic, D.; Mezzanotte, V.; Annala, M.; Canobbio, S. Predicting the constraint effect of environmental characteristics on macroinvertebrate density and diversity using quantile regression mixed model. Hydrobiologia 2015, 742, 153–167. [Google Scholar] [CrossRef]
- Kairo, K.; Timm, H.; Haldna, M.; Virro, T. Biological Quality on the Basis of Macroinvertebrates in Dammed Habitats of Some Estonian Streams, Central—Baltic Europe. Int. Rev. Hydrobiol. 2012, 97, 497–508. [Google Scholar] [CrossRef]
- Greenwood, M.J.; Booker, D.J. The influence of antecedent floods on aquatic invertebrate diversity, abundance and community composition. Ecohydrology 2015, 8, 188–203. [Google Scholar] [CrossRef]
- Solomatine, D.P.; Ostfeld, A. Data-driven modelling: Some past experiences and new approaches. J. Hydroinform. 2008, 10, 3–22. [Google Scholar] [CrossRef]
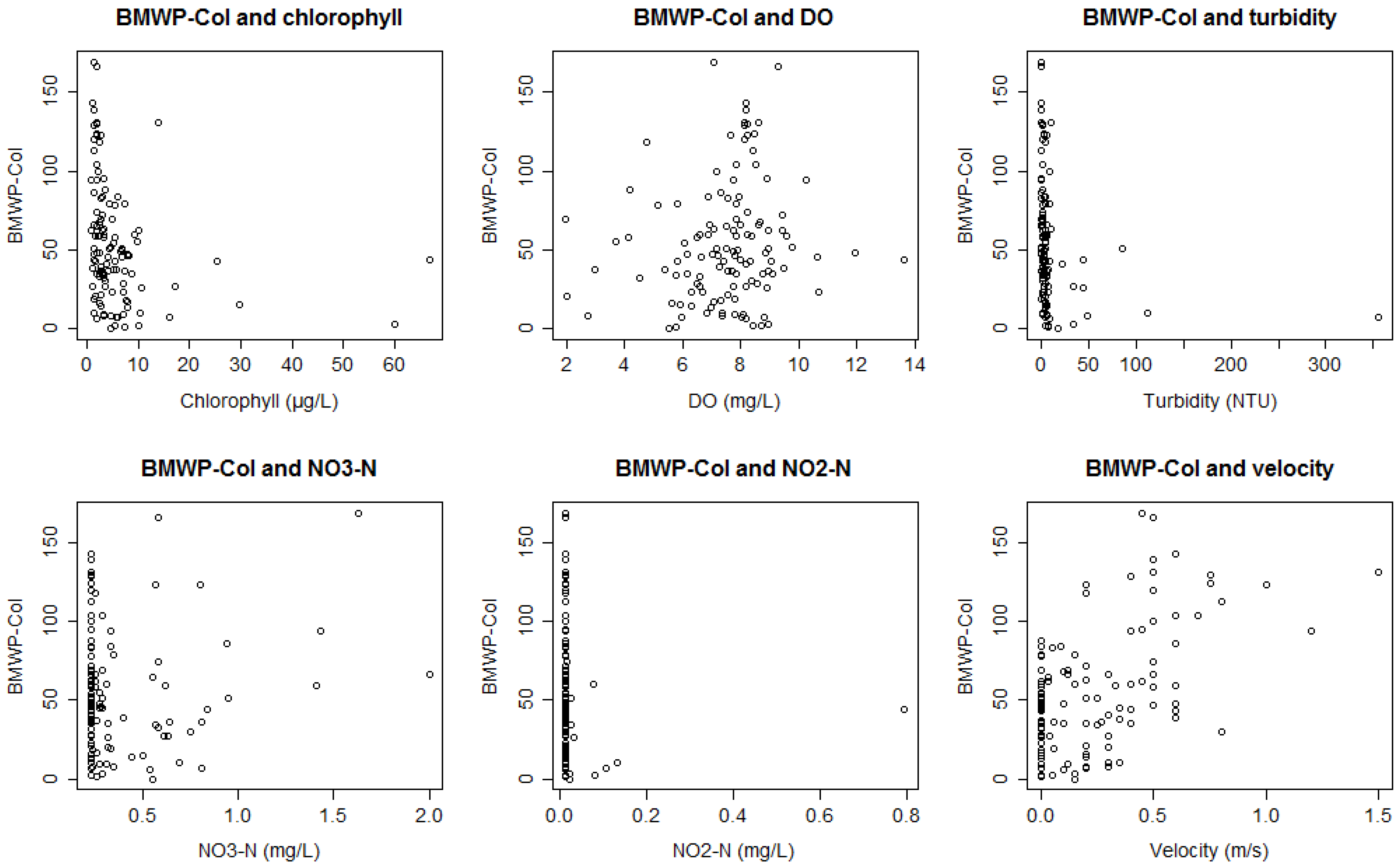
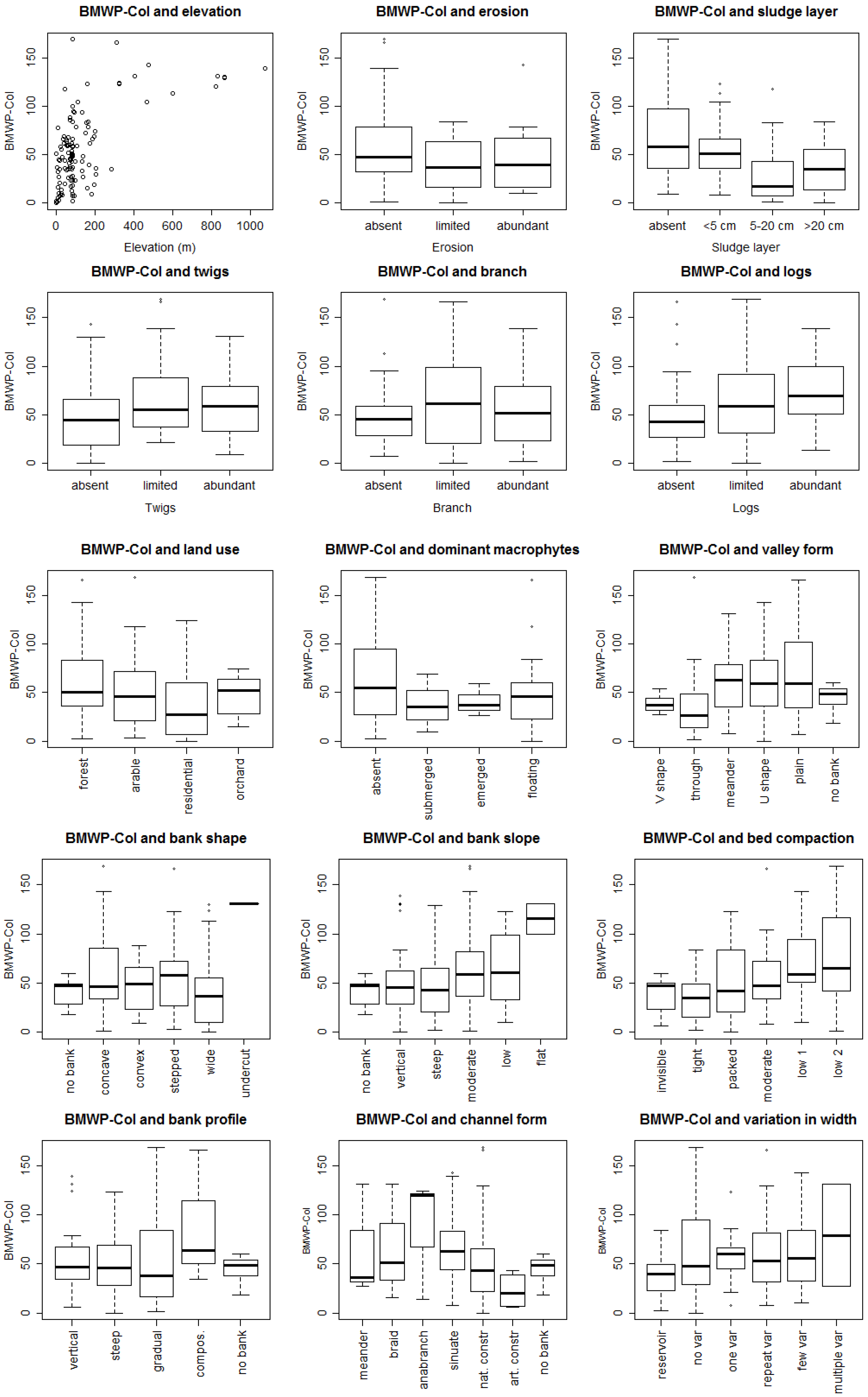

| Variables | Unit | Mean | Median | Min | Max | Std Dev | # of Missing Values |
|---|---|---|---|---|---|---|---|
| Total N a | mg/L | 1.1 | 1.0 | 1.0 * | 7.7 | 0.6 | - |
| Total P a | mg/L | 0.5 | 0.5 | 0.5 * | 4.5 | 0.4 | - |
| Nitrate-N | mg/L | 0.4 | 0.2 | 0.23 * | 2.0 | 0.3 | - |
| Nitrite-N | mg/L | 0.0 | 0.0 | 0.02 * | 0.8 | 0.1 | - |
| Ammonium-N a | mg/L | 0.2 | 0.1 | 0.02 * | 8.8 | 0.8 | - |
| DO | mg/L | 7.5 | 7.8 | 2.0 | 13.6 | 1.7 | - |
| COD b | mg/L | 17.0 | 13.3 | 5.0 * | 117.6 | 14.9 | 30 |
| Chlorophyll | µg/L | 5.6 | 3.1 | 0.7 | 66.8 | 8.7 | - |
| pH a | 7.7 | 7.6 | 6.6 | 8.9 | 0.5 | - | |
| Chloride a | mg/L | 7.3 | 2.5 | 0.5 | 181.7 | 22.8 | - |
| Conductivity a | µS/cm | 200 | 123 | 37 | 1981 | 238 | - |
| Temperature a | °C | 26.0 | 26.0 | 19.0 | 34.0 | 2.5 | - |
| TDS a | g/L | 0.13 | 0.08 | 0.05 | 1.27 | 0.15 | - |
| Turbidity | Nephelometric Turbidity Units | 9.8 | 3.4 | 0.0 | 355.6 | 35.1 | - |
| Velocity | m/s | 0.2 | 0.2 | 0.0 | 1.5 | 0.3 | - |
| Elevation | m | 135 | 82 | 2 | 1075 | 187 | - |
| Average stream width b | m | 22.5 | 12.0 | 1.5 | 230.0 | 32.1 | 32 |
| Average water depth b | m | 0.40 | 0.36 | 0.03 | 1.00 | 0.22 | 40 |
| No. | Variables | Categories | Definition |
|---|---|---|---|
| 1 | Main land use | 1. forest | land covered by high density of trees, includes primary, secondary and tertiary forests |
| 2. arable | land used for agriculture or farm (e.g., maize) | ||
| 3. residential | land used for residential houses | ||
| 4. orchard | land used for fruits production (e.g., cacao, banana, mango) | ||
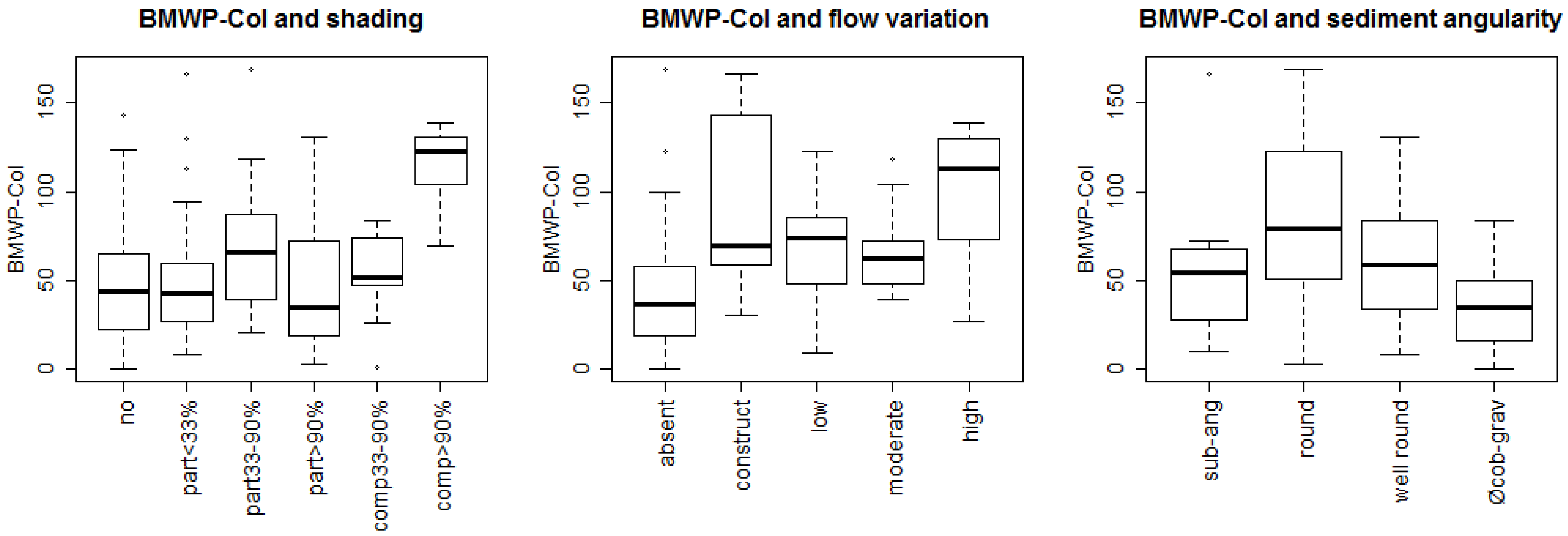
| 2 | Shading | 0. no shading | no shading at the sampling sites |
| 1. partly shaded, limited stretch <33% | less than 33% of the sampling site is partly shaded | ||
| 2. partly shaded, longer stretch 33%–90% | about 33%–90% of the sampling site is partly shaded | ||
| 3. partly shaded, whole stretch >90% | more than 90% of the sampling site is partly shaded | ||
| 4. completely shaded, limited stretch <33% | less than 33% of the sampling site is completely shaded | ||
| 5. completely shaded, longer stretch 33%–90% | about 33%–90% of the sampling site is completely shaded | ||
| 6. completely shaded, whole stretch >90% | more than 90% of the sampling site is completely shaded | ||
| 3 | Type of macrophyte cover a | 0. no macrophyte | macrophytes are absent |
| 1. interrupted | macrophytes are not sharing a common border at more than one intersection | ||
| 2. contiguous | macrophytes are sharing a common border at more than one intersection | ||
| 4 | Main macrophytes | 0. absent | macrophytes are not present |
| 1. submerged macrophytes | macrophytes rooted in the bottom substrate with vegetative parts predominantly immerse | ||
| 2. emerged macrophytes | macrophytes rooted in the bottom substrate with vegetative parts emerging above the water surface | ||
| 3. floating macrophytes | macrophytes with roots, if present, hang on water surface | ||
| 5 | Valley form | 1. canyon |  |
| 2. V-shaped valley |  | ||
| 3. trough |  | ||
| 4. meander valley |  | ||
| 5. U-shaped valley |  | ||
| 6. plain floodplain |  | ||
| 7. no bank | macroinvertebrates were collected from macrophytes, away from the bank | ||
| 6 | Channel form | 1. meandering |  |
| 2. braided |  | ||
| 3. anabranching |  | ||
| 4. sinuate |  | ||
| 5. constrained (natural) |  | ||
| 6. constrained (artificial) |  | ||
| 7. no bank | macroinvertebrates were collected from macrophytes, away from the bank | ||
| 7 | Variation in width | 0 | data collected at the reservoir |
| 1 |  | ||
| 2 |  | ||
| 3 |  | ||
| 4 |  | ||
| 5 |  | ||
| 8 | Extent of erosion | 0. absent | erosion is not present |
| 1. limited | less than 30% is eroded | ||
| 2. abundant | more than 30% is eroded | ||
| 9 | Bank profile | 1. vertical |  |
| 2. steep (>45°) |  | ||
| 3. gradually not trampled |  | ||
| 4. composite not trampled |  | ||
| 5. no bank | macroinvertebrates were collected from macrophytes, away from the bank | ||
| 10 | Variation in flow | 0. absent | no variation in flow |
| 1. at human constructions | flow is varied at human constructions | ||
| 2. low | variation in flow is less than 20% | ||
| 3. moderate | variation in flow is about 20%–50% | ||
| 4. high | variation in flow is more than 50% | ||
| 11 | Sludge layer | 0. absent | sludge layer is absent |
| 1. <5 cm | sludge is accumulated for less than 5 cm | ||
| 2. 5–20 cm | sludge is accumulated about 5–20 cm | ||
| 3. >20 cm | sludge is accumulated for more than 5 cm | ||
| Dead wood | similar categories and definition for twigs, branch, logs | ||
| 12 | - twigs d < 3 cm | 0. absent | dead wood is not present |
| 13 | - branch 3–30 cm | 1. limited | presence of dead wood is less than 5% |
| 14 | - logs d > 30 cm | 2. abundant | presence of dead wood is more than 5% |
| 15 | Pool/Riffle class a | 1. Class 1 | pool-riffle pattern is (nearly) pristine: extensive sequences of pools and riffles |
| 2. Class 2 | pool-riffle pattern is well developed: high variety in pools and riffles | ||
| 3. Class 3 | pool-riffle pattern is moderately developed: variety in pools and riffles but locally | ||
| 4. Class 4 | pool-riffle pattern is poorly developed: low variety in pools and riffles | ||
| 5. Class 5 | pool-riffle pattern is absent: uniform pool-riffle pattern | ||
| 6. Class 6 | pool-riffle pattern is absent due to structural changes: uniform pool-riffle pattern due to reinforced bank and bed structures | ||
| 16 | Bank shape | 0. no bank | macroinvertebrates were collected from macrophytes, away from the bank |
| 1. concave |  | ||
| 2. convex |  | ||
| 3. stepped |  | ||
| 4. wide lower bench |  | ||
| 5. undercut |  | ||
| 17 | Bank slope | 0. no bank | macroinvertebrates were collected from macrophytes, away from the bank |
| 1. vertical | 80°–90° bank sloping | ||
| 2. steep | 60°–80° bank sloping | ||
| 3. moderate | 30°–60° bank sloping | ||
| 4. low | 10°–30° bank sloping | ||
| 5. flat | less than 10° bank sloping | ||
| 18 | Bed compaction | 0. invisible | bed is not visible |
| 1. tightly packed | array of sediment sizes overlapping, tightly packed and very hard to dislodge | ||
| 2. packed | array of sediment sizes overlapping, tightly packed but can be dislodged moderately | ||
| 3. moderate compaction | array of sediment sizes little overlapping, some packing but can be dislodged moderately | ||
| 4. low compaction (1) | limited range of sediment sizes, little overlapping, some packing and structure but can be dislodged very easily | ||
| 5. low compaction (2) | loose array of fine sediments, no overlapping, no packing and structure, and can be dislodged very easily | ||
| 19 | Sediment matrix a | 1. bedrock | formation of bedrock |
| 2. open framework | 0%–5% fine sediment, high availability of interstitial spaces | ||
| 3. matrix-filled contact | 5%–32% fine sediment, moderate availability of interstitial spaces | ||
| 4. framework dilated | 32%–60% fine sediment, low availability of interstitial spaces | ||
| 5. matrix dominated | more than 60% fine sediment, interstitial spaces virtually absent | ||
| 20 | Sediment angularity | 1. very angular |  |
| 2. angular |  | ||
| 3. sub-angular |  | ||
| 4. rounded |  | ||
| 5. well-rounded |  | ||
| 6. cobble, pebble and gravel fractions not present |  | ||
| 21 | Main sediment type a | 1. boulder | sediment composed of substrates with diameter larger than 256 mm |
| 2. cobble | sediment composed of substrates with diameter about 64–256 mm | ||
| 3. gravel | sediment composed of substrates with diameter about 2–64 mm | ||
| 4. sand | sediment composed of substrates with diameter about 0.062–2 mm | ||
| 5. silt and clay | sediment composed of substrates with diameter about 0.24–62 µm |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damanik-Ambarita, M.N.; Everaert, G.; Forio, M.A.E.; Nguyen, T.H.T.; Lock, K.; Musonge, P.L.S.; Suhareva, N.; Dominguez-Granda, L.; Bennetsen, E.; Boets, P.; et al. Generalized Linear Models to Identify Key Hydromorphological and Chemical Variables Determining the Occurrence of Macroinvertebrates in the Guayas River Basin (Ecuador). Water 2016, 8, 297. https://doi.org/10.3390/w8070297
Damanik-Ambarita MN, Everaert G, Forio MAE, Nguyen THT, Lock K, Musonge PLS, Suhareva N, Dominguez-Granda L, Bennetsen E, Boets P, et al. Generalized Linear Models to Identify Key Hydromorphological and Chemical Variables Determining the Occurrence of Macroinvertebrates in the Guayas River Basin (Ecuador). Water. 2016; 8(7):297. https://doi.org/10.3390/w8070297
Chicago/Turabian StyleDamanik-Ambarita, Minar Naomi, Gert Everaert, Marie Anne Eurie Forio, Thi Hanh Tien Nguyen, Koen Lock, Peace Liz Sasha Musonge, Natalija Suhareva, Luis Dominguez-Granda, Elina Bennetsen, Pieter Boets, and et al. 2016. "Generalized Linear Models to Identify Key Hydromorphological and Chemical Variables Determining the Occurrence of Macroinvertebrates in the Guayas River Basin (Ecuador)" Water 8, no. 7: 297. https://doi.org/10.3390/w8070297