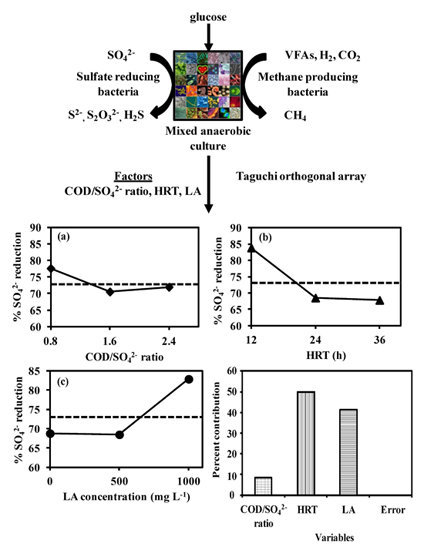
Using a Statistical Model to Examine the Effect of COD: SO42− Ratio, HRT and LA Concentration on Sulfate Reduction in an Anaerobic Sequencing Batch Reactor
Abstract
:1. Introduction
| Reaction No. | Stoichiometric Reaction | ΔG°' (kJ mol−1) |
|---|---|---|
| (1) | 2FeS2 + 7O2 + 2H2O→2Fe2+ + 4SO42− + 4H+ | −2168.0 |
| (2) | ZnS + 2O2→Zn2+ + SO42− | −690.0 |
| (3) | 4H2 + HCO3− + H+→CH4 + 3H2O | −135.6 |
| (4) | 4H2 + H+ + SO42−→4H2O + HS− | −152.2 |
| (5) | 8H2 + 2SO42−→H2S + HS− + 5H2O + 3OH− | −146.9 |
2. Materials and Methods
2.1. Inoculum Source
2.2. Sulfate Reduction Studies
2.3. Analytical Methods
| Exp. No. | COD/SO42− Ratio 1 | HRT (h) | LA conc. (mg L−1) | Experimental SO42− Reduction (%) 2 | Predicted SO42− Reduction (%) | |||
|---|---|---|---|---|---|---|---|---|
| X1 | Level | X2 | Level | X3 | Level | |||
| 1 | 0.8 | 1 | 12 | 1 | 0 | 1 | 86.5 ± 2.6 | 83.5 |
| 2 | 0.8 | 1 | 24 | 2 | 500 | 2 | 65.8 ± 1.9 | 67.8 |
| 3 | 0.8 | 1 | 36 | 3 | 1000 | 3 | 80.6 ± 0.7 | 81.6 |
| 4 | 1.6 | 2 | 12 | 1 | 500 | 2 | 75.1 ± 1.9 | 76.1 |
| 5 | 1.6 | 2 | 24 | 2 | 1000 | 3 | 78.2 ± 3.7 | 75.2 |
| 6 | 1.6 | 2 | 36 | 3 | 0 | 1 | 58.3 ± 2.7 | 60.3 |
| 7 | 2.4 | 3 | 12 | 1 | 1000 | 3 | 89.9 ± 6.0 | 91.9 |
| 8 | 2.4 | 3 | 24 | 2 | 0 | 1 | 61.5 ± 8.6 | 62.5 |
| 9 | 2.4 | 3 | 36 | 3 | 500 | 2 | 64.5 ± 2.9 | 61.5 |
2.4. Taguchi Design
2.4.1. Fractional Factorial Design of Experiments (FFDOE) (Phase 1)
2.4.2. Sulfate Removal ASBR Experiments with Selected Factors and Levels (Phase 2)
2.4.3. Analysis of Experimental Data (AED) and Prediction of Performance (POP) (Phase 3)
3. Results and Discussion
3.1. Experimental Design Analysis
3.2. Analysis of Variance
| Factor | DOF (f) | Sum of Squares (s) | Mean Squares | Variance (v) | F ratio (F) 1 | Pure Sum (S’) | Percent p (%) 2 |
|---|---|---|---|---|---|---|---|
| COD/SO42− | 2 | 84.57 | 42.29 | 42.29 | 845,749.5 3 | 84.57 | 8.58 |
| HRT | 2 | 492.67 | 246.33 | 246.33 | 4,926,686.4 3 | 492.67 | 50.00 |
| LA | 2 | 408.16 | 204.08 | 204.08 | 4,081,612.0 3 | 408.16 | 41.42 |
| Error | 2 | 0.001 | 0.0005 | 0.001 | 0.002 | ||
| Total | 8 | 985.40 | 492.70 | 100.00 |
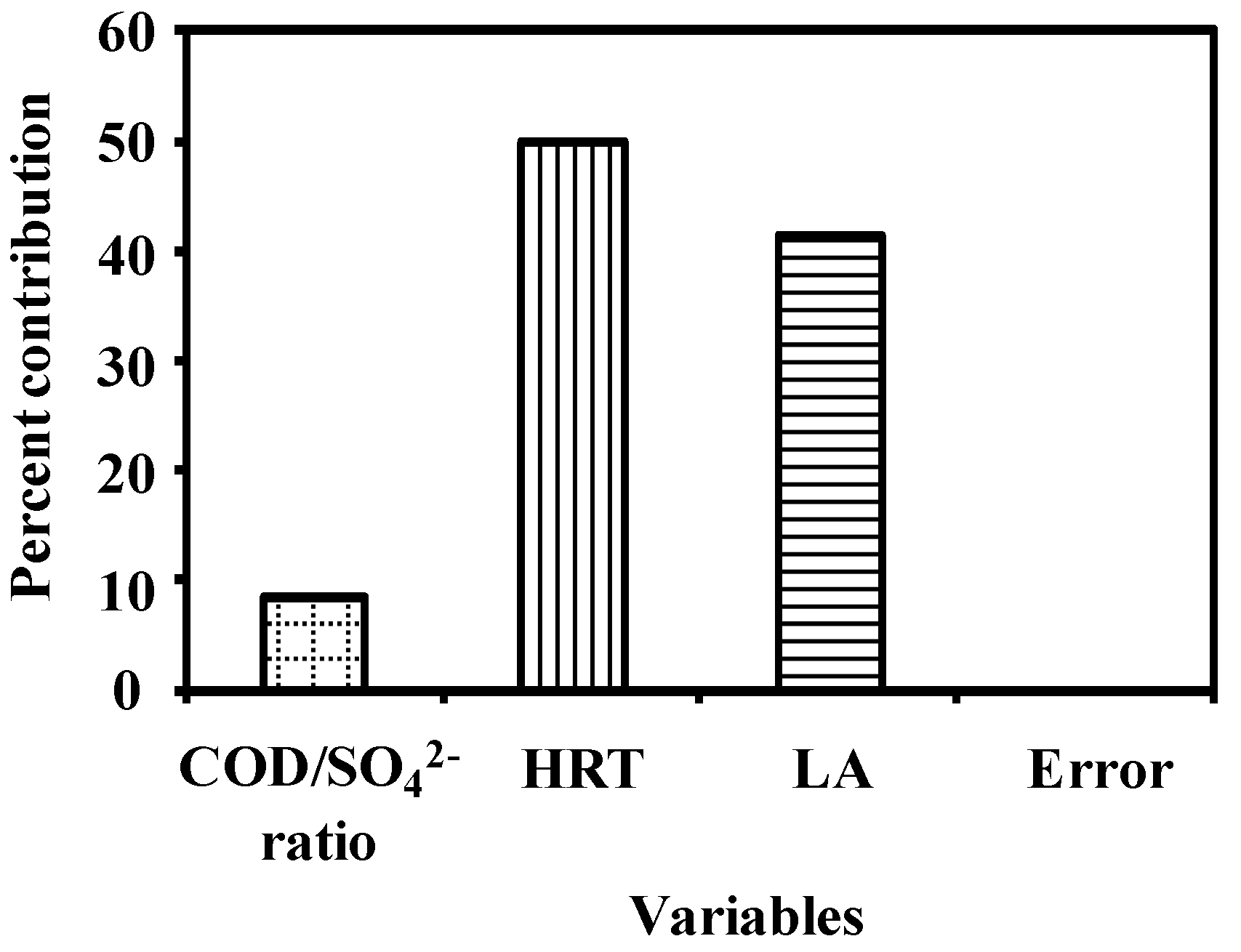
3.3. Effect of Factors on the Response Variables
3.3.1. Main Effects Plot
Effect of COD/SO42− Ratio
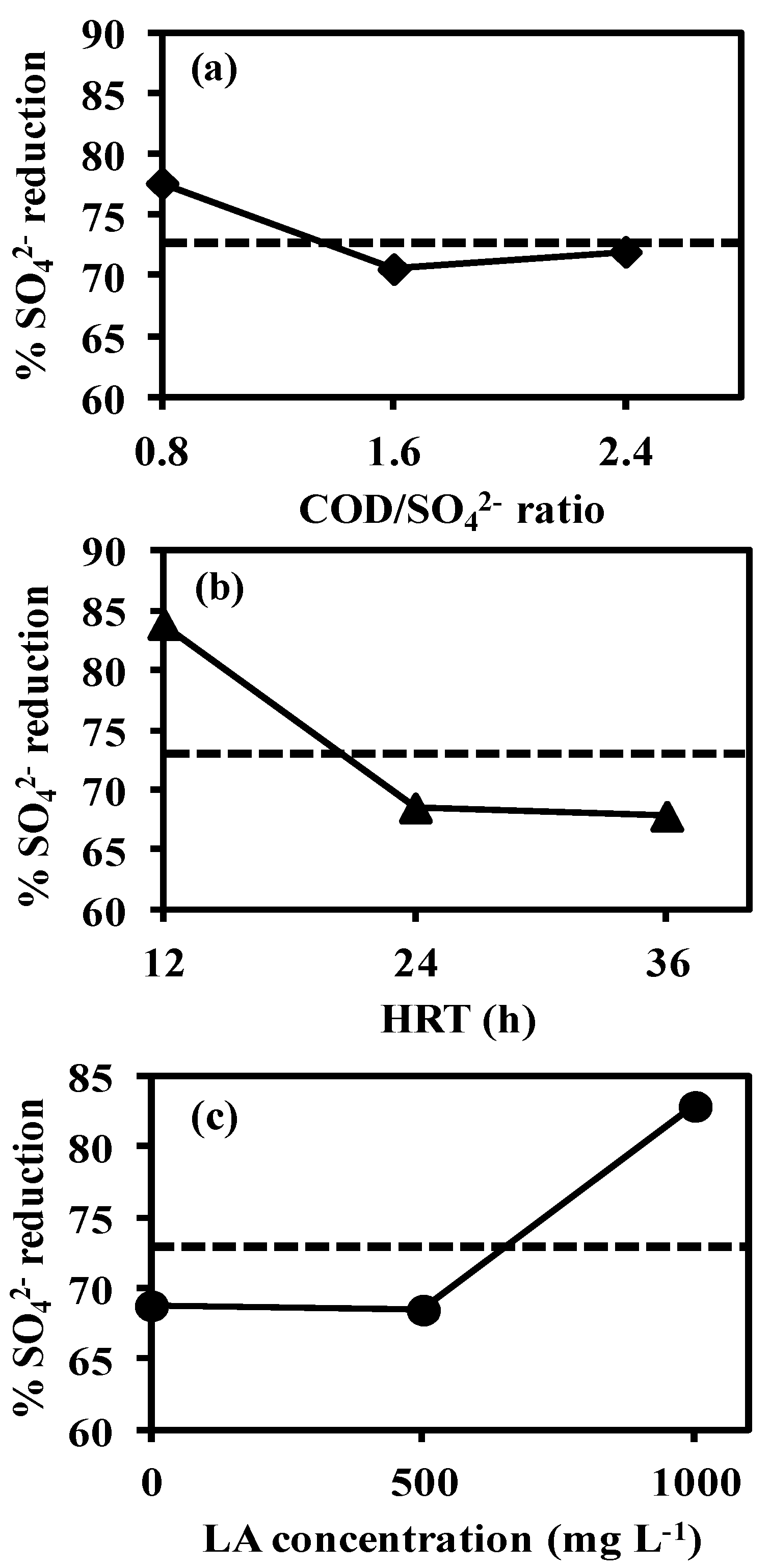
Effect of Hydraulic Retention Time
Effect of Linoleic Acid Concentration
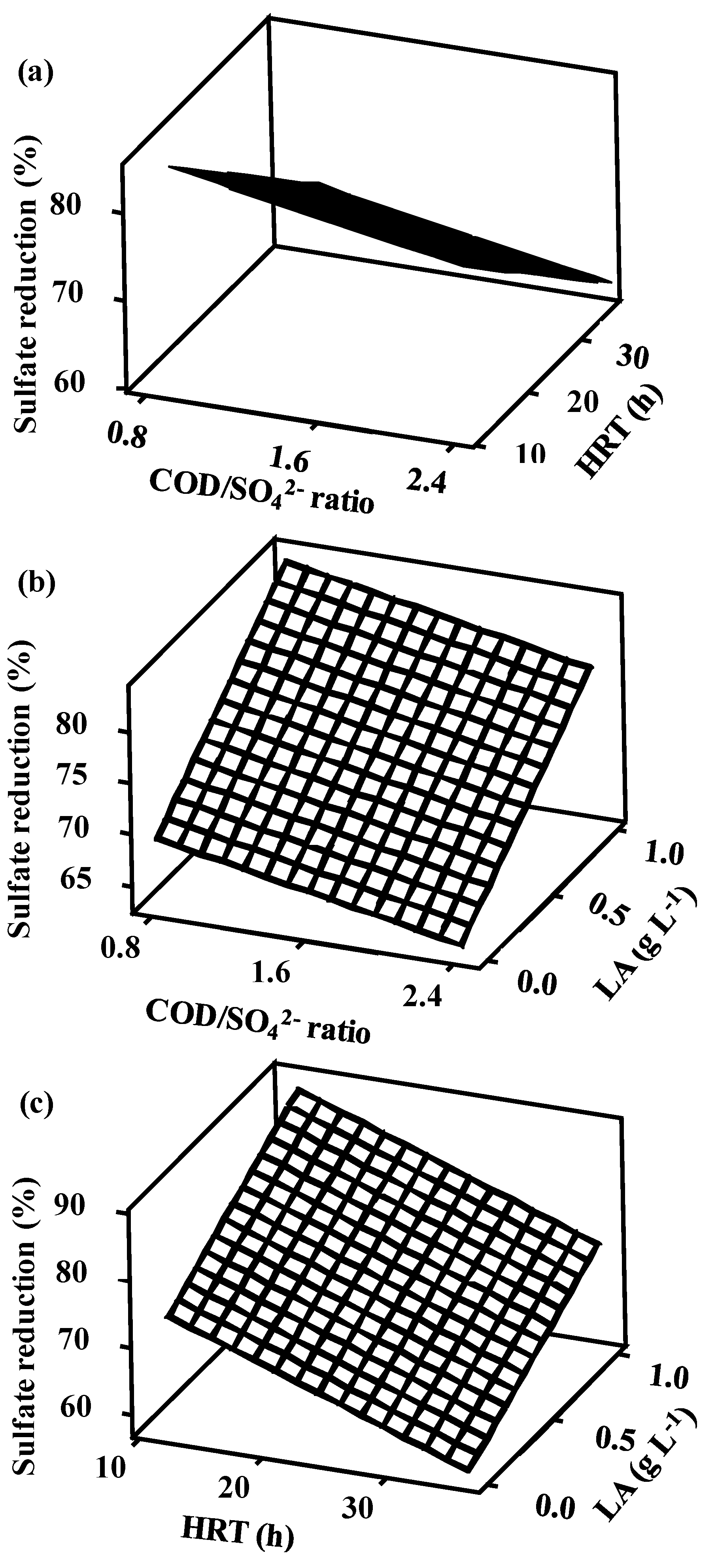
3.3.2. Surface Plots
3.4. Model Verification
| COD/SO42− Ratio | Reactor Type; Mode of Operation | Temp. (°C) | pH | SO42− Reduction (%) | Substrate | HRT | Ref. |
|---|---|---|---|---|---|---|---|
| 2.5 | UASBR; Continuous | 30 | 7.0 ± 0.5 | 94 ± 1 | Ethanol | 4 d | [36] |
| 4 | FBR; Continuous | 35 | 7.4 ± 0.2 | 90 | Ethanol | 6.5 h | [32] |
| 3.2, 4, 5 | UASBR; Continuous | 30–33 | 7.3 ± 0.7 | 70, 81, 74 | Glucose | 24 h | [10] |
| 3.15, 2.7 | CSTR; Continuous | 30 | NR | 29, 28 | Glucose | NR | [10] |
| 2.7, 1.23, 0.6 | Serum bottle; Batch | 30 | NR | 9, 4, 4.5 | Acetate | NA | [10] |
| 0.41, 1.03, 2.07 | Serum bottle; Batch | 35 ± 1 | 7.3 ± 0.1 | 26, 60, 93 | Propionate | NA | [11] |
| 1, 4 | UASBR; Continuous | 55 | 6.0 | 25–35, 65 | Sucrose | 10 h | [42] |
| 6.67 | UASBR; Continuous | 35 ± 1 | 7.0–7.5 | 80–86 | Sulfate rich vinasse | 4.86 days | [43] |
| 4 | AFR; Continuous | 37 ± 0.5 | 9.5 | 97.8 ± 1.1 | Ethanol | 18 h | [39] |
| 0.8, 1.6, 2.4 | ASBR; Sequencing batch | 37 ± 0.1 | 6.5 ± 0.1 | 87 ± 3, 58 ± 3, 62 ± 9 | Glucose | 12, 36, 24 h | This study * |
3.5. Factor Interactions and Their Influence on Sulfate Reduction
3.6. Optimum Conditions for Sulfate Reduction
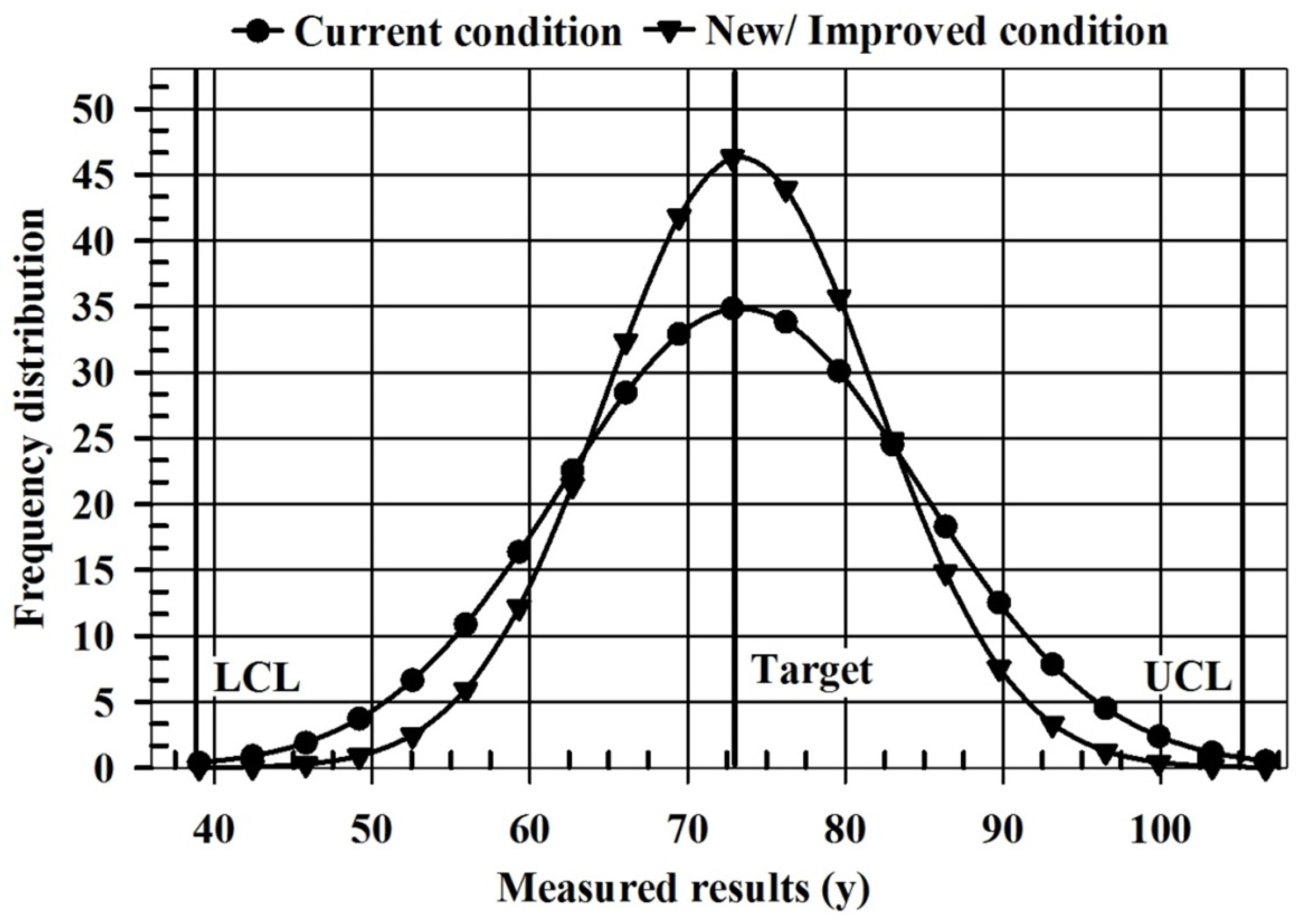
| Parameters | Current Condition | New/Improved Condition |
|---|---|---|
| Mean | 72.49 | 72.49 |
| Standard deviation | 11.50 | 7.93 |
| Cp | 1.00 | 1.45 |
| Cpk | 1.00 | 1.45 |
| Quality characteristic (QC) | Bigger is better | Bigger is better |
| Lower control limit (LCL) | 37.99 | 37.99 |
| Upper control limit (UCL) | 106.99 | 106.99 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Austin, G.T. Shreve’s Chemical Process Industries, 1st ed.; McGraw Hill: New York, NY, USA, 1984. [Google Scholar]
- Shin, H.S.; Sae-Eun, O.; Chae-Young, L. Influence of sulphur compounds and heavy metals on the methanisation of tannery wastewater. Water Sci. Technol. 1997, 35, 239–245. [Google Scholar] [CrossRef]
- Pol, L.W.H.; Lens, P.N.L.; Stams, A.J.M.; Lettinga, G. Anaerobic treatment of sulphate-rich wastewaters. Biodegradation 1998, 9, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Dugan, P.R. Bacterial ecology of strip mine areas and its relationship to the production of acidic mine drainage. Ohio J. Sci. 1975, 75, 266–279. [Google Scholar]
- Foucher, S.; Battaglia-Brunet, F.; Ignatiadis, I.; Morin, D. Treatment by sulfate-reducing bacteria of Chessy acid-mine drainage and metals recovery. Chem. Eng. Sci. 2001, 56, 1639–1645. [Google Scholar] [CrossRef]
- Johnson, D.B.; Hallberg, K.B. Pitfalls of passive mine water treatment. Rev. Environ. Sci. Biotechnol. 2002, 1, 335–343. [Google Scholar] [CrossRef]
- Moon, C.; Singh, R.; Chaganti, S.R.; Lalman, J.A. Modeling sulfate removal by inhibited mesophilic mixed anaerobic communities using a statistical approach. Water Res. 2013, 47, 2341–2351. [Google Scholar] [CrossRef] [PubMed]
- Thauer, R.K.; Jungermann, K.; Decker, K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol. Rev. 1977, 41, 100–180. [Google Scholar] [PubMed]
- Choi, E.; Rim, J.M. Competition and inhibition of sulfate reducers and methane producers in anaerobic treatment. Water Sci. Technol. 1991, 23, 1259–1264. [Google Scholar]
- Erdirencelebi, D.; Ozturk, I.; Cokgor, E.U.; Tonuk, G.U. Degree of sulfate-reducing activities on COD removal in various reactor configurations in anaerobic glucose and acetate-fed reactors. Clean Soil Air Water 2007, 35, 178–182. [Google Scholar] [CrossRef]
- Uberoi, V.; Bhattacharya, S.K. Interactions among sulfate reducers, acetogens, and methanogens in anaerobic propionate systems. Water Environ. Res. 1995, 67, 330–339. [Google Scholar] [CrossRef]
- Omil, F.; Bakker, C.D.; Pol, L.W.H.; Lettinga, G. Effect of pH and low temperature shocks on the competition between sulphate reducing bacteria and methane producing bacteria in UASB reactors. Environ. Technol. 1997, 18, 255–264. [Google Scholar] [CrossRef]
- Hao, O.J.; Chen, J.M.; Huang, L.; Buglass, R.L. Sulfate-reducing bacteria. Crit. Rev. Environ. Sci. Technol. 1996, 26, 155–187. [Google Scholar] [CrossRef]
- Okabe, S.; Nielsen, P.H.; Jones, W.L.; Characklis, W.G. Sulfide product inhibition of Desulfovibrio desulfuricans in batch and continuous cultures. Water Res. 1995, 29, 571–578. [Google Scholar] [CrossRef]
- Widdel, F.; Pfennig, N. Sporulation and further nutritional characteristics of Desulfotomaculum acetoxidans. Arch. Microbiol. 1981, 129, 401–402. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R.; Mah, R.A. Growth and methanogenesis by Methanosarcina Strain 227 on acetate and methanol. Appl. Environ. Microbiol. 1978, 36, 870–879. [Google Scholar] [PubMed]
- Oh, S.E.; van Ginkel, S.; Logan, B.E. The relative effectiveness of pH control and heat treatment for enhancing biohydrogen gas production. Environ. Sci. Technol. 2003, 37, 5186–5190. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, S.R.; Chaganti, S.R.; Lalman, J.A.; Heath, D.D. Effect of inhibitors on hydrogen consumption and microbial population dynamics in mixed anaerobic cultures. Int. J. Hydrogen Energy 2014, 39, 249–257. [Google Scholar] [CrossRef]
- Galbraith, H.; Miller, T.B. Physicochemical effects of long-chain fatty acids on bacterial cells and their protoplasts. J. Appl. Bacteriol. 1973, 36, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Lalman, J.; Bagley, D.M. Effects of C18 long chain fatty acids on glucose, butyrate and hydrogen degradation. Water Res. 2002, 36, 3307–3313. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.S.; Kumar, C.G.; Prakasham, R.S.; Hobbs, P.J. The Taguchi methodology as a statistical tool for biotechnological applications: A critical appraisal. Biotechnol. J. 2008, 3, 510–523. [Google Scholar] [CrossRef] [PubMed]
- Myer, R.H.; Montogomery, D.C. Response Surface Methodology: Process and Product Optimization Using Designed Experiment, 2nd ed.; John Wiley and Sons: New York, NY, USA, 2002. [Google Scholar]
- Prakasham, R.S.; Rao, C.S.; Rao, R.S.; Rajesham, S.; Sarma, P.N. Optimization of alkaline protease production by Bacillus sp. using Taguchi methodology. Appl. Biochem. Biotechnol. 2005, 120, 133–144. [Google Scholar] [CrossRef]
- Prakasham, R.S.; Rao, C.S.; Rao, R.S.; Sarma, P.N. Enhancement of acid amylase production by an isolated Aspergillus awamori. J. Appl. Microbiol. 2007, 102, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Antony, F.; Warwood, S.; Fernandes, K.; Rowlands, H. Process optimization using Taguchi methods of experimental design. Work Study 2001, 50, 51–57. [Google Scholar] [CrossRef]
- Roy, R.K. Design of Experiments Using Taguchi Approach:16 Steps to Product and Process Improvement; John Wiley and Sons: New York, NY, USA, 2001. [Google Scholar]
- Ray, S.; Chowdhury, N.; Lalman, J.A.; Seth, R.; Biswas, N. Impact of initial pH and linoleic acid (C18:2) on hydrogen production by a mesophilic anaerobic mixed culture. J. Environ. Eng. ASCE 2008, 134, 110–117. [Google Scholar] [CrossRef]
- Wiegant, W.M.; Lettinga, G. Thermophilic anaerobic digestion of sugars in upflow anaerobic sludge blanket reactors. Biotechnol. Bioeng. 1985, 27, 1603–1607. [Google Scholar] [CrossRef] [PubMed]
- Shizas, L.; Bagley, D.M. Improving anaerobic sequencing batch reactor performance by modifying operational parameters. Water Res. 2002, 36, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Speece, R.E. Gas Flow Totalizer. U.S. Patent 4064750 A, 27 December 1977. [Google Scholar]
- American Publishers Health Association (APHA). Standard Methods for the Examination of Water and Wastewater, 20th ed.; APHA: Washington, DC, USA, 1999. [Google Scholar]
- Kaksonen, A.H.; Franzmann, P.D.; Puhakka, J.A. Effects of hydraulic retention time and sulfide toxicity on ethanol and acetate oxidation in sulfate-reducing metal-precipitating fluidized-bed reactor. Biotechnol. Bioeng. 2004, 86, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.X.; Roush, M.L. What Every Engineer Should Know about Risk Engineering and Management; Marcel Dekker, Inc.: New York, NY, USA, 2000. [Google Scholar]
- Evangelaras, H.; Koukouvinos, C.; Lappas, E. An efficient algorithm for the identification of isomorphic orthogonal arrays. J. Discrete Math. Sci. Cryptogr. 2006, 9, 125–132. [Google Scholar] [CrossRef]
- Phadke, M.S.; Dehnad, K. Optimization of product and process design for quality and cost. Qual. Reliab. Eng. Int. 1988, 4, 105–112. [Google Scholar] [CrossRef]
- Velasco, A.; Ramirez, M.; Volke-Sepulveda, T.; Gonzalez-Sanchez, A.; Revah, S. Evaluation of feed COD/sulfate ratio as a control criterion for the biological hydrogen sulfide production and lead precipitation. J. Hazard. Mater. 2008, 151, 407–413. [Google Scholar] [CrossRef] [PubMed]
- El Bayoumy, M.A.; Bewtra, J.K.; Ali, H.I.; Biswas, N. Sulfide production by sulfate reducing bacteria with lactate as feed in an upflow anaerobic fixed film reactor. Water Air Soil Poll. 1999, 112, 67–84. [Google Scholar]
- Neculita, C.M.; Zagury, G.J.; Bussiere, B. Effectiveness of sulfate-reducing passive bioreactors for treating highly contaminated acid mine drainage: I. Effect of hydraulic retention time. Appl. Geochem. 2008, 23, 3442–3451. [Google Scholar]
- Ray, S.; Saady, N.M.C.; Lalman, J.A. Diverting electron fluxes to hydrogen in mixed anaerobic communities fed with glucose and unsaturated C18 long chain fatty acids. J. Environ. Eng. ASCE 2010, 136, 568–575. [Google Scholar] [CrossRef]
- Chowdhury, N.; Lalman, J.A.; Seth, R.; Ndegwa, P. Biohydrogen production by mesophilic anaerobic fermentation of glucose in the presence of linoleic acid. J. Environ. Eng. 2007, 133, 1145–1152. [Google Scholar] [CrossRef]
- Lopes, S.I.C.; Wang, X.; Capela, M.I.; Lens, P.N.L. Effect of COD/SO42− ratio and sulfide on thermophilic (55 °C) sulfate reduction during the acidification of sucrose at pH 6. Water Res. 2007, 41, 2379–2392. [Google Scholar] [CrossRef] [PubMed]
- Barrera, E.L.; Spanjers, H.; Romero, O.; Rosa, E.; Dewulf, J. Characterization of the sulfate reduction process in the anaerobic digestion of a very high strength and sulfate rich vinasse. Chem. Eng. J. 2014, 248, 383–393. [Google Scholar] [CrossRef]
- Zhou, J.M.; Song, Z.Y.; Yan, D.J.; Liu, Y.L.; Yang, M.H.; Cao, H.B.; Xing, J.M. Performance of a haloalkaliphilic bioreactor and bacterial community shifts under different COD/SO42− ratios and hydraulic retention times. J. Hazard. Mater. 2014, 274, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Van Aartsengel, A.; Kurtoglu, S. Handbook on Continuous Improvement Transformation: The Lean Six Sigma Framework and Systematic Methodology for Implementation; Springer: New York, NY, USA, 2013. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, R.; Moon, C.; Veeravalli, S.S.; Shanmugam, S.R.; Chaganti, S.R.; Lalman, J.A. Using a Statistical Model to Examine the Effect of COD: SO42− Ratio, HRT and LA Concentration on Sulfate Reduction in an Anaerobic Sequencing Batch Reactor. Water 2014, 6, 3478-3494. https://doi.org/10.3390/w6113478
Singh R, Moon C, Veeravalli SS, Shanmugam SR, Chaganti SR, Lalman JA. Using a Statistical Model to Examine the Effect of COD: SO42− Ratio, HRT and LA Concentration on Sulfate Reduction in an Anaerobic Sequencing Batch Reactor. Water. 2014; 6(11):3478-3494. https://doi.org/10.3390/w6113478
Chicago/Turabian StyleSingh, Rajesh, Chungman Moon, Sathyanarayan S. Veeravalli, Saravanan R. Shanmugam, Subba Rao Chaganti, and Jerald A. Lalman. 2014. "Using a Statistical Model to Examine the Effect of COD: SO42− Ratio, HRT and LA Concentration on Sulfate Reduction in an Anaerobic Sequencing Batch Reactor" Water 6, no. 11: 3478-3494. https://doi.org/10.3390/w6113478