Development, Characterization, and Validation of a Cold Stage-Based Ice Nucleation Array (PKU-INA)
Abstract
:1. Introduction
2. Experiments
2.1. Description of the Instrument
2.2. Temperature Calibration
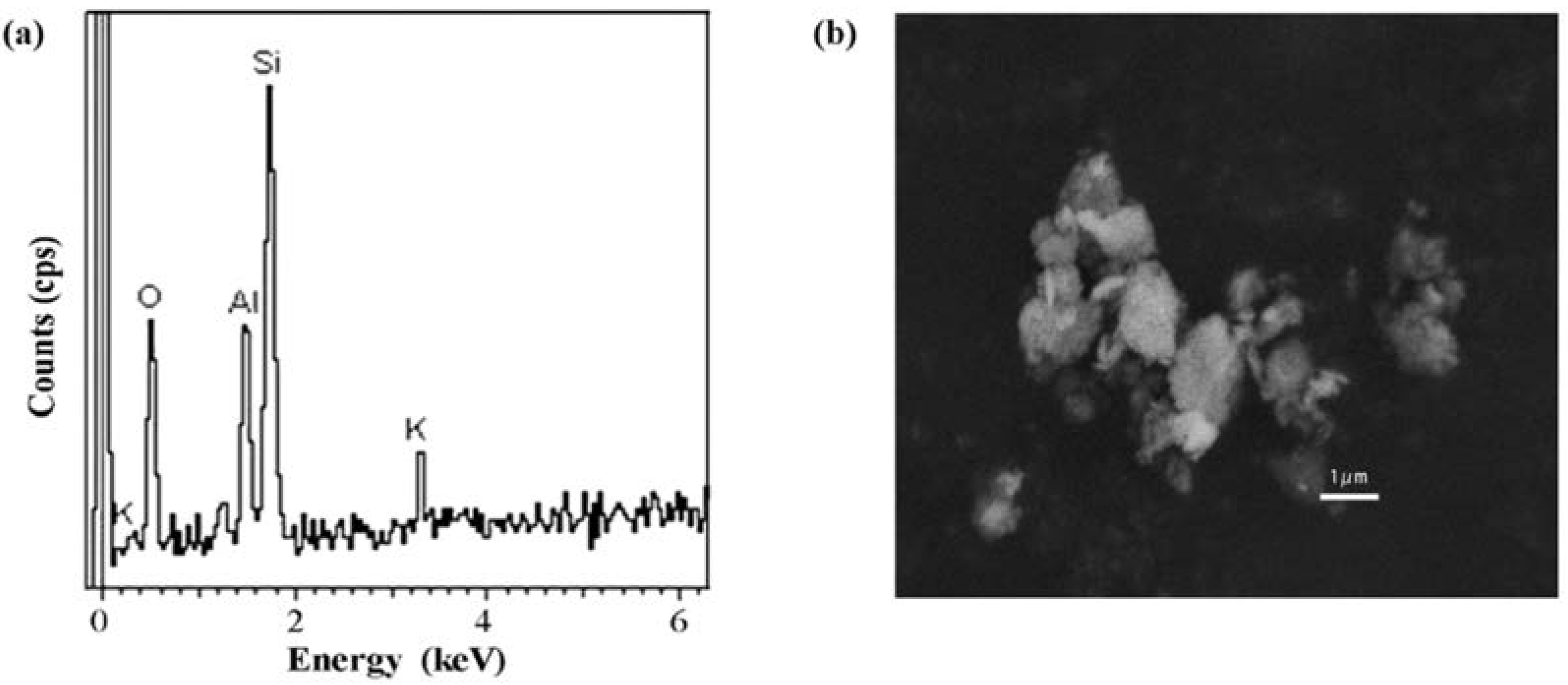
2.3. Illite NX Characterization
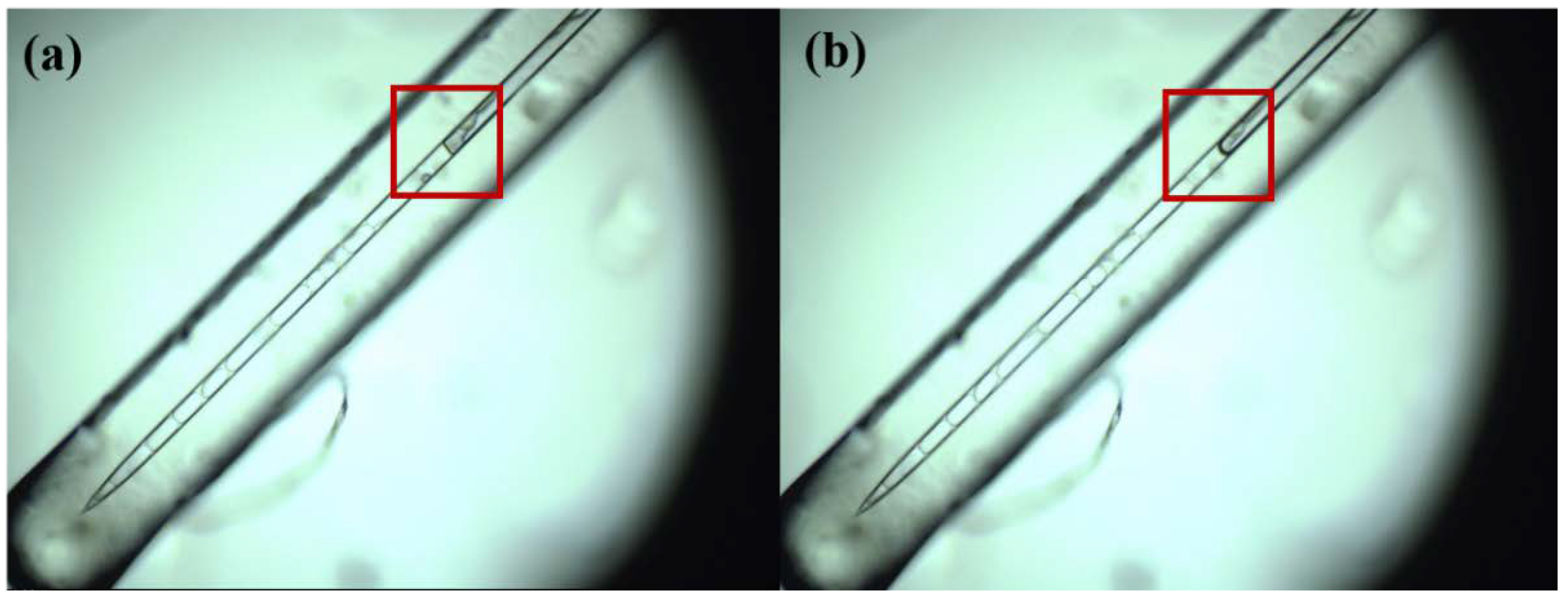
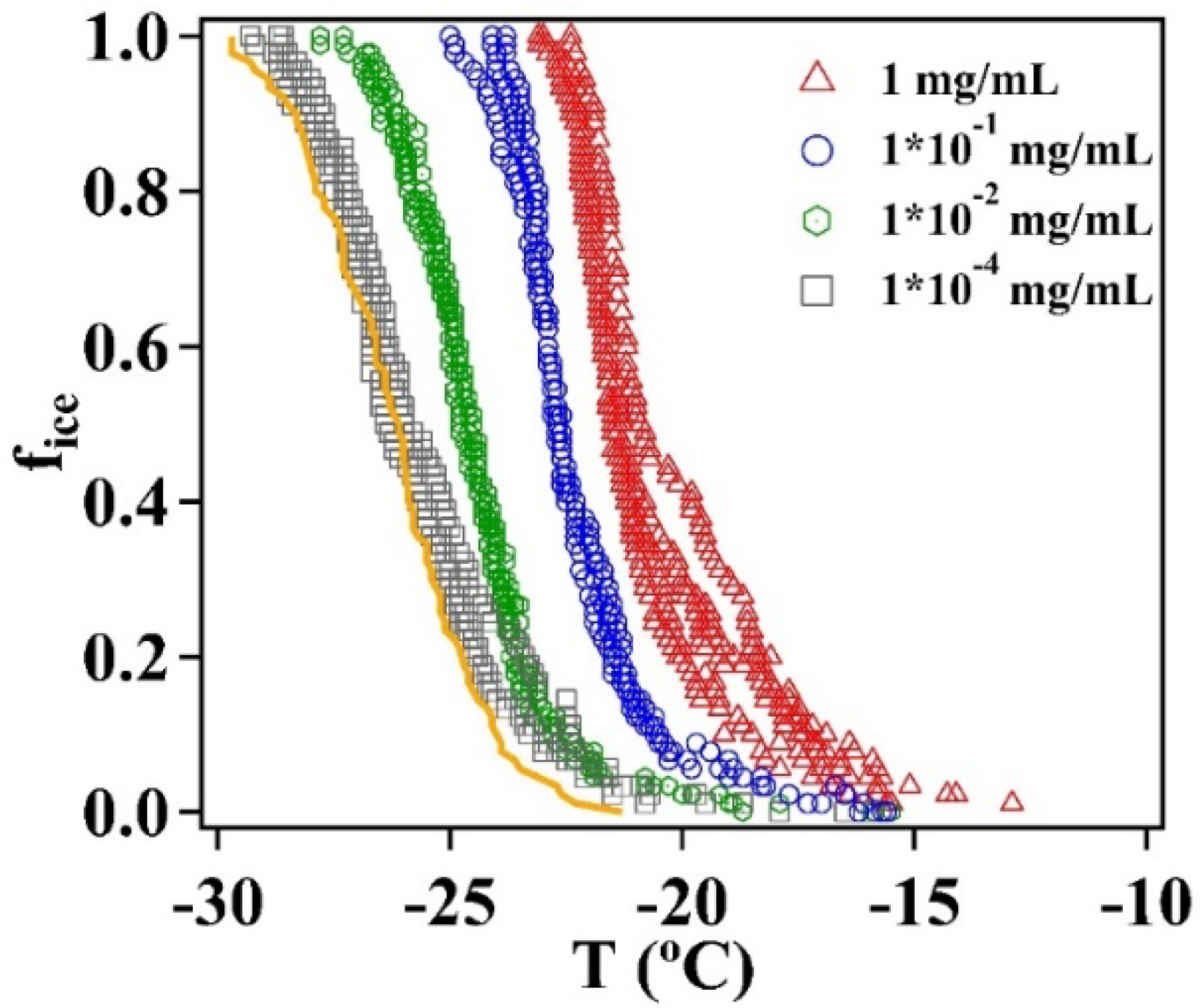
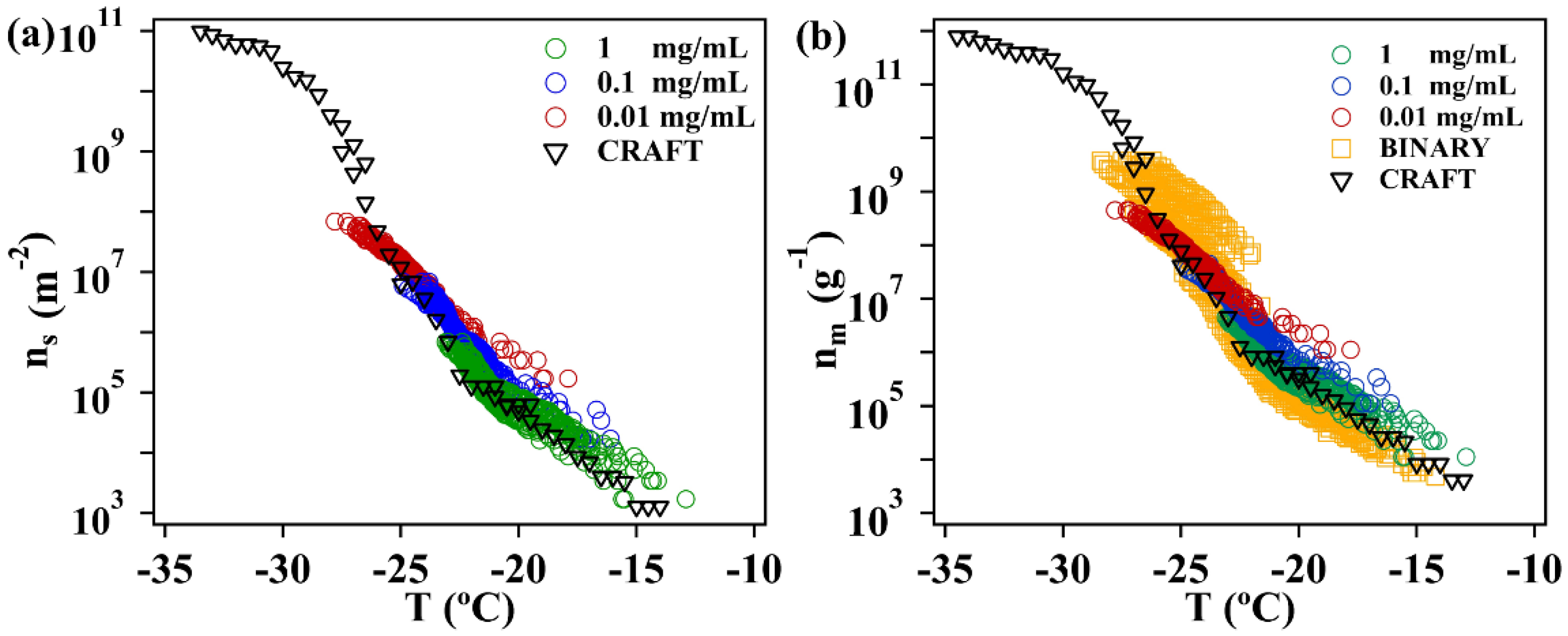
2.4. Freezing Experiments of Illite NX
2.5. Distilled Water Experiments
3. Results and Discussions
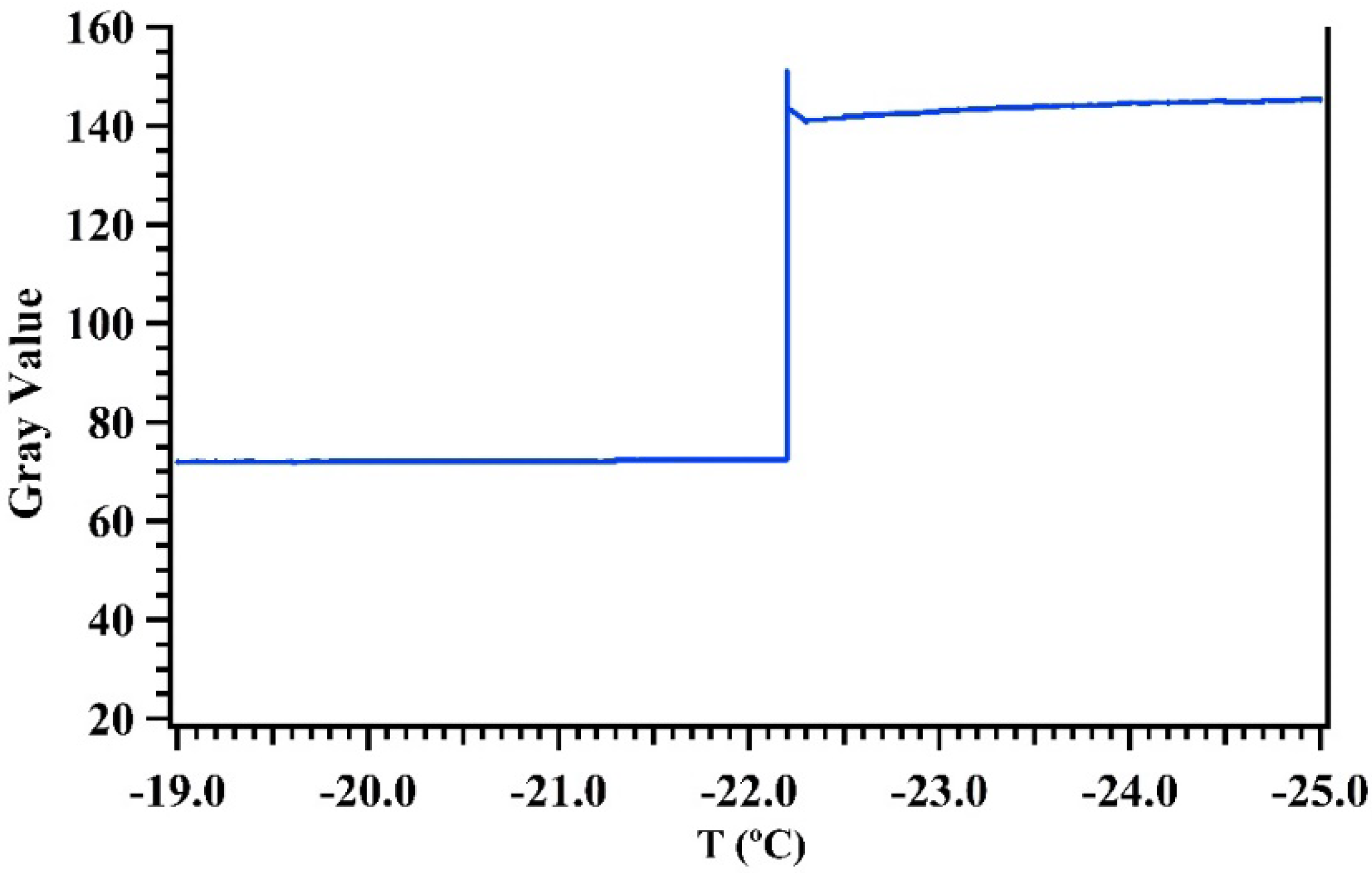
3.1. Temperature Calibration
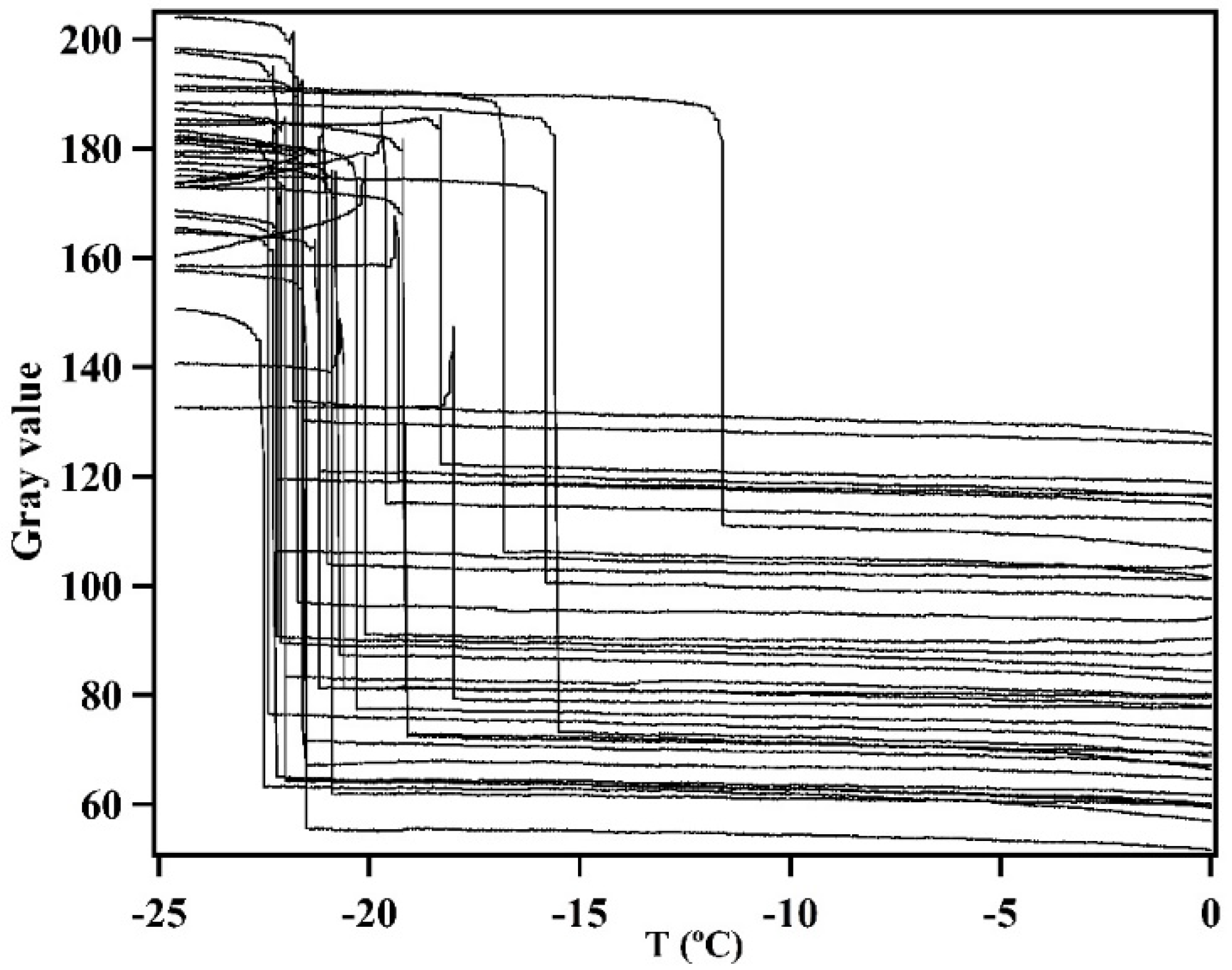
3.2. Immersion Freezing of Illite NX
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

References
- DeMott, P.J.; Prenni, A.J.; Liu, X.; Kreidenweis, S.M.; Petters, M.D.; Twohy, C.H.; Richardson, M.S.; Eidhammer, T.; Rogers, D.C. Predicting global atmospheric ice nuclei distributions and their impacts on climate. Proc. Natl. Acad. Sci. USA 2010, 107, 11217–11222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, T.W.; Ladino, L.A.; Alpert, P.A.; Breckels, M.N.; Brooks, I.M.; Browse, J.; Burrows, S.M.; Carslaw, K.S.; Huffman, J.A.; Judd, C.; et al. A marine biogenic source of atmospheric ice-nucleating particles. Nature 2015, 525, 234–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanji, Z.A.; Ladino, L.A.; Wex, H.; Boose, Y.; Burkert-Kohn, M.; Cziczo, D.J.; Krämer, M. Chapter 1: Overview of ice nucleating particles. Am. Meteorol. Soc. 2017, 58, 1–33. [Google Scholar]
- Pruppacher, H.R.; Klett, J.D.; Wang, P.K. Microphysics of clouds and precipitation. Aerosol Sci. Technol. 1998, 28, 381–382. [Google Scholar] [CrossRef]
- Cantrell, W.; Heymsfield, A. Production of ice in tropospheric clouds: A review. Bull. Am. Meteorol. Soc. 2005, 86, 795–808. [Google Scholar] [CrossRef]
- Vali, G.; DeMott, P.J.; Möhler, O.; Whale, T.F. Technical note: A proposal for ice nucleation terminology. Atmos. Chem. Phys. 2015, 15, 10263–10270. [Google Scholar] [CrossRef]
- Hoose, C.; Möhler, O. Heterogeneous ice nucleation on atmospheric aerosols: A review of results from laboratory experiments. Atmos. Chem. Phys. 2012, 12, 9817–9854. [Google Scholar] [CrossRef]
- Tang, M.; Cziczo, D.J.; Grassian, V.H. Interactions of water with mineral dust aerosol: Water adsorption, hygroscopicity, cloud condensation, and ice nucleation. Chem. Rev. 2016, 116, 4205–4259. [Google Scholar] [CrossRef] [PubMed]
- Murray, B.J.; O’Sullivan, D.; Atkinson, J.D.; Webb, M.E. Ice nucleation by particles immersed in supercooled cloud droplets. Chem. Soc. Rev. 2012, 41, 6519–6554. [Google Scholar] [CrossRef] [PubMed]
- Westbrook, C.D.; Illingworth, A.J. The formation of ice in a long-lived supercooled layer cloud. The formation of ice in a long-lived supercooled layer cloud. Q. J. R. Meterol. Soc. 2013, 139, 2209–2221. [Google Scholar] [CrossRef]
- Hiranuma, N.; Hoffmann, N.; Steinke, I.; Kiselev, A.; Dreyer, A.; Zhang, K.; Kulkarni, G.; Koop, T.; Möhler, O. Influence of surface morphology on the heterogeneous ice nucleation efficiency of hematite articles. Atmos. Chem. Phys. 2014, 14, 2315–2324. [Google Scholar] [CrossRef]
- Knopf, D.A.; Alpert, P.A.; Wang, B.; Aller, J.Y. Stimulation of ice nucleation by marine diatoms. Nat. Geosci. 2010, 4, 88. [Google Scholar] [CrossRef]
- Wang, B.; Knopf, D.A. Heterogeneous ice nucleation on particles composed of humic-like substances impacted by O3. J. Geophysi. Res. 2011, 116. [Google Scholar] [CrossRef]
- Hiranuma, N.; Brooks, S.D.; Moffet, R.C.; Glen, A.; Laskin, A.; Gilles, M.K.; Liu, P.; Macdonald, A.M.; Strapp, J.W.; McFarquhar, G.M. Chemical characterization of individual particles and residuals of cloud droplets and ice crystals collected on board research aircraft in the isdac 2008 study. J. Geophysi. Res. 2013, 118, 6564–6579. [Google Scholar] [CrossRef]
- Hiranuma, N.; Augustin-Bauditz, S.; Bingemer, H.; Budke, C.; Curtius, J.; Danielczok, A.; Diehl, K.; Dreischmeier, K.; Ebert, M.; Frank, F.; et al. A comprehensive laboratory study on the immersion freezing behavior of illite nx particles: A comparison of 17 ice nucleation measurement techniques. Atmos. Chem. Phys. 2015, 15, 2489–2518. [Google Scholar] [CrossRef]
- Wex, H.; Augustin-Bauditz, S.; Boose, Y.; Budke, C.; Curtius, J.; Diehl, K.; Dreyer, A.; Frank, F.; Hartmann, S.; Hiranuma, N.; et al. Intercomparing different devices for the investigation of ice nucleating particles using snomax® as test substance. Atmos. Chem. Phys. 2015, 15, 1463–1485. [Google Scholar] [CrossRef]
- Stopelli, E.; Conen, F.; Zimmermann, L.; Alewell, C.; Morris, C.E. Freezing nucleation apparatus puts new slant on study of biological ice nucleators in precipitation. Atmos. Meas. Tech. 2014, 7, 129–134. [Google Scholar] [CrossRef] [Green Version]
- Rogers, D.C. Development of a continuous flow thermal gradient diffusion chamber for ice nucleation studies. Atmos. Res. 1988, 22, 149–181. [Google Scholar] [CrossRef]
- Stetzer, O.; Baschek, B.; Lüönd, F.; Lohmann, U. The zurich ice nucleation chamber (zinc)-a new instrument to investigate atmospheric ice formation. Aerosol Sci. Technol. 2008, 42, 64–74. [Google Scholar] [CrossRef]
- Chou, C.; Stetzer, O.; Weingartner, E.; Jurányi, Z.; Kanji, Z.A.; Lohmann, U. Ice nuclei properties within a saharan dust event at the jungfraujoch in the swiss alps. Atmos. Chem. Phys. 2011, 11, 4725–4738. [Google Scholar] [CrossRef]
- Garimella, S.; Kristensen, T.B.; Ignatius, K.; Welti, A.; Voigtlander, J.; Kulkarni, G.R.; Sagan, F.; Kok, G.L.; Dorsey, J.; Nichman, L. The spectrometer for ice nuclei (spin): An instrument to investigate ice nucleation. Atmos. Meas. Tech. 2016, 9, 2781–2795. [Google Scholar] [CrossRef]
- Bigg, E.K. The supercooling of water. Proc. Phys. Soc. 1953, 66, 688. [Google Scholar] [CrossRef]
- Conen, F.; Henne, S.; Morris, C.E.; Alewell, C. Atmospheric ice nucleators active ≥12 °C can be quantified on pm10 filters. Atmos. Meas. Tech. 2012, 5, 321–327. [Google Scholar] [CrossRef] [Green Version]
- Budke, C.; Koop, T. Binary: An optical freezing array for assessing temperature and time dependence of heterogeneous ice nucleation. Atmos. Meas. Tech. 2015, 8, 689–703. [Google Scholar] [CrossRef]
- Tobo, Y. An improved approach for measuring immersion freezing in large droplets over a wide temperature range. Sci. Rep. 2016, 6, 32930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, B.J.; Broadley, S.L.; Wilson, T.W.; Bull, S.J.; Wills, R.H.; Christenson, H.K.; Murray, E.J. Kinetics of the homogeneous freezing of water. Phys. Chem. Chem. Phys. 2010, 12, 10380–10387. [Google Scholar] [CrossRef] [PubMed]
- Reicher, N.; Segev, L.; Rudich, Y. The weizmann supercooled droplets observation on a microarray (wisdom) and application for ambient dust. Atmos. Meas. Tech. 2018, 11, 233–248. [Google Scholar] [CrossRef]
- DeMott, P.J.; Möhler, O.; Stetzer, O.; Vali, G.; Levin, Z.; Petters, M.D.; Murakami, M.; Leisner, T.; Bundke, U.; Klein, H.; et al. Resurgence in ice nuclei measurement research. Bull. Am. Meteorol. Soc. 2011, 92, 1623–1635. [Google Scholar] [CrossRef]
- Polen, M.; Lawlis, E.; Sullivan, R.C. The unstable ice nucleation properties of snomax® bacterial particles. J. Geophysi. Res. 2016, 121. [Google Scholar] [CrossRef]
- Jung, S.; Tiwari, M.K.; Poulikakos, D. Frost halos from supercooled water droplets. Proc. Natl. Acad. Sci. USA 2012, 109, 16073–16078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarge, S.M.; Höhne, G.W.H.; Cammenga, H.K.; Eysel, W.; Gmelin, E. Temperature, heat and heat flow rate calibration of scanning calorimeters in the cooling mode. Thermochim. Acta. 2000, 361, 1–20. [Google Scholar] [CrossRef]
- Gatta, G.D.; Richardson, M.J.; Sarge, S.M.; Stolen, S. Standards, calibration, and guidelines in microcalorimetry. Part 2. Calibration standards for differential calorimetry. Pure Appl. Chem. 2006, 78, 1455–1476. [Google Scholar]
- Mondieig, D.; Rajabalee, F.; Metivaud, V.; Oonk, H.A.J.; Cuevas-Diarte, M. N-alkane binary molecular alloys. Chem. Mater. 2004, 16, 786–798. [Google Scholar] [CrossRef]
- Messerly, J.F.; Guthrie, G.B.; Todd, S.S.; Finke, H.L. Low-temperature thermal data for pentane, n-heptadecane, and n-octadecane. Revised thermodynamic functions for the n-alkanes, c5-c18. J. Chem. Eng. Data 1967, 12, 338–346. [Google Scholar] [CrossRef]
- Wright, T.P.; Petters, M.D. The role of time in heterogeneous freezing nucleation. J. Geophys. Res. 2013, 118, 3731–3743. [Google Scholar] [CrossRef] [Green Version]
- Polen, M.; Brubaker, T.; Somers, J.; Sullivan, R.C. Cleaning up our water: Reducing interferences from non-homogeneous freezing of pure water in droplet freezing assays of ice nucleating particles. Atmos. Meas. Tech. Discuss. 2018. in review. [Google Scholar] [CrossRef]
- Broadley, S.L.; Murray, B.J.; Herbert, R.J.; Atkinson, J.D.; Dobbie, S.; Malkin, T.L.; Condliffe, E.; Neve, L. Immersion mode heterogeneous ice nucleation by an illite rich powder representative of atmospheric mineral dust. Atmos. Chem. Phys. 2012, 12, 287–307. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Wu, Z.; Augustin-Bauditz, S.; Grawe, S.; Hartmann, M.; Pei, X.; Liu, Z.; Ji, D.; Wex, H. Ice-nucleating particle concentrations unaffected by urban air pollution in beijing, china. Atmos. Chem. Phys. 2018, 18, 3523–3539. [Google Scholar] [CrossRef]
- Whale, T.F.; Murray, B.J.; O’Sullivan, D.; Wilson, T.W.; Umo, N.; Baustian, K.J.; Atkinson, J.D.; Workneh, D.; Morris, G. A technique for quantifying heterogeneous ice nucleation in microlitre supercooled water droplets. Atmos. Meas. Tech. 2015, 8, 2437–2447. [Google Scholar] [CrossRef] [Green Version]

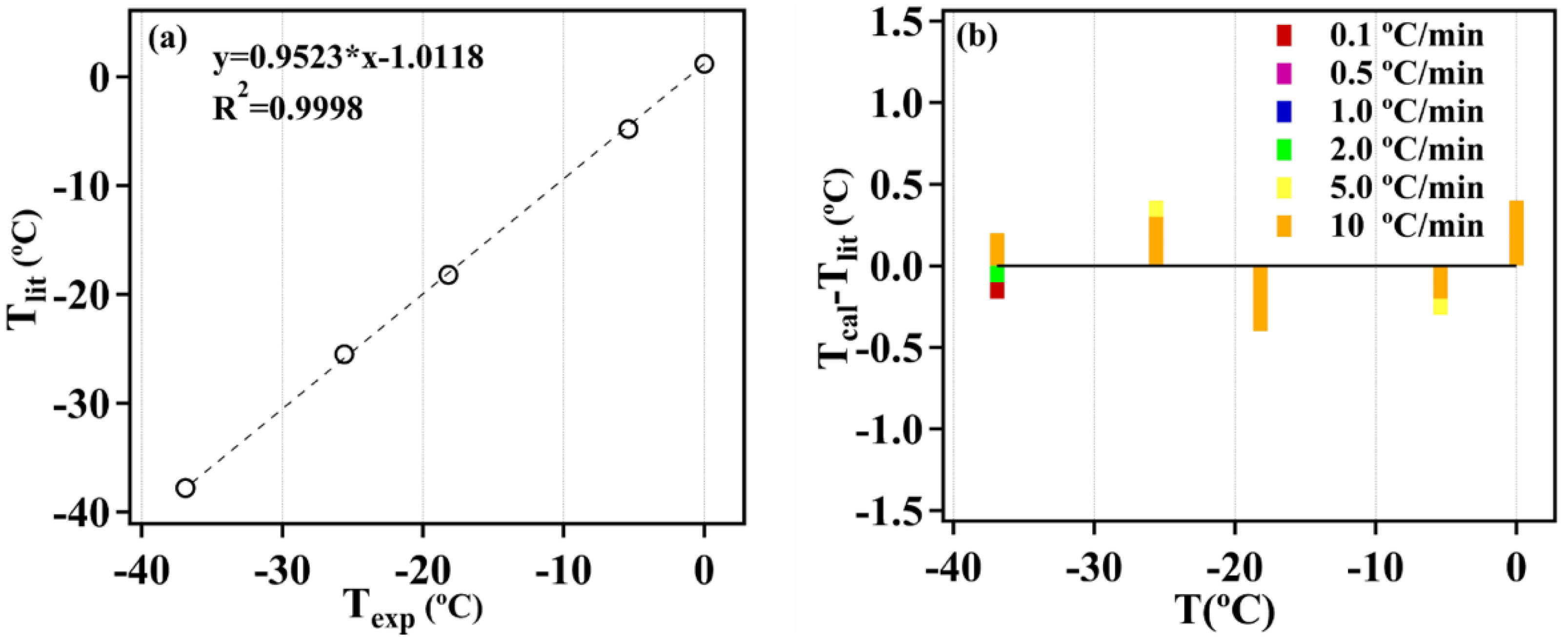
| Heating Rate (°C/min) | 0.1 | 0.5 | 1 | 2 | 5 | 10 | |
|---|---|---|---|---|---|---|---|
| T (°C) | Tlit | Texp | Texp | Texp | Texp | Texp | Texp |
| Water | 0 | 0.9 | 1.2 | 1.5 | 1.7 | 2.3 | 2.7 |
| Tridecane (sl) | −5.4 | −5 | −4.8 | −4.7 | −4.5 | −4.2 | −3.7 |
| Tridecane (ss) | −18.2 | −18.4 | −18.3 | −18.2 | −18.1 | −17.9 | −17.5 |
| Undecane (sl) | −25.6 | −25.6 | −25.5 | −25.4 | −25.1 | −24.9 | −24.6 |
| Undecane (ss) | −36.9 | −38 | −37.8 | −37.7 | −37.6 | −37.3 | −36.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Pei, X.; Wang, H.; Chen, J.; Zhu, Y.; Tang, M.; Wu, Z. Development, Characterization, and Validation of a Cold Stage-Based Ice Nucleation Array (PKU-INA). Atmosphere 2018, 9, 357. https://doi.org/10.3390/atmos9090357
Chen J, Pei X, Wang H, Chen J, Zhu Y, Tang M, Wu Z. Development, Characterization, and Validation of a Cold Stage-Based Ice Nucleation Array (PKU-INA). Atmosphere. 2018; 9(9):357. https://doi.org/10.3390/atmos9090357
Chicago/Turabian StyleChen, Jie, Xiangyu Pei, Hong Wang, Jingchuan Chen, Yishu Zhu, Mingjin Tang, and Zhijun Wu. 2018. "Development, Characterization, and Validation of a Cold Stage-Based Ice Nucleation Array (PKU-INA)" Atmosphere 9, no. 9: 357. https://doi.org/10.3390/atmos9090357





