Model Performance Differences in Sulfate Aerosol in Winter over Japan Based on Regional Chemical Transport Models of CMAQ and CAMx
Abstract
:1. Introduction
2. Modeling Design
3. Results and Discussion
3.1. Model Performance during Winter Haze
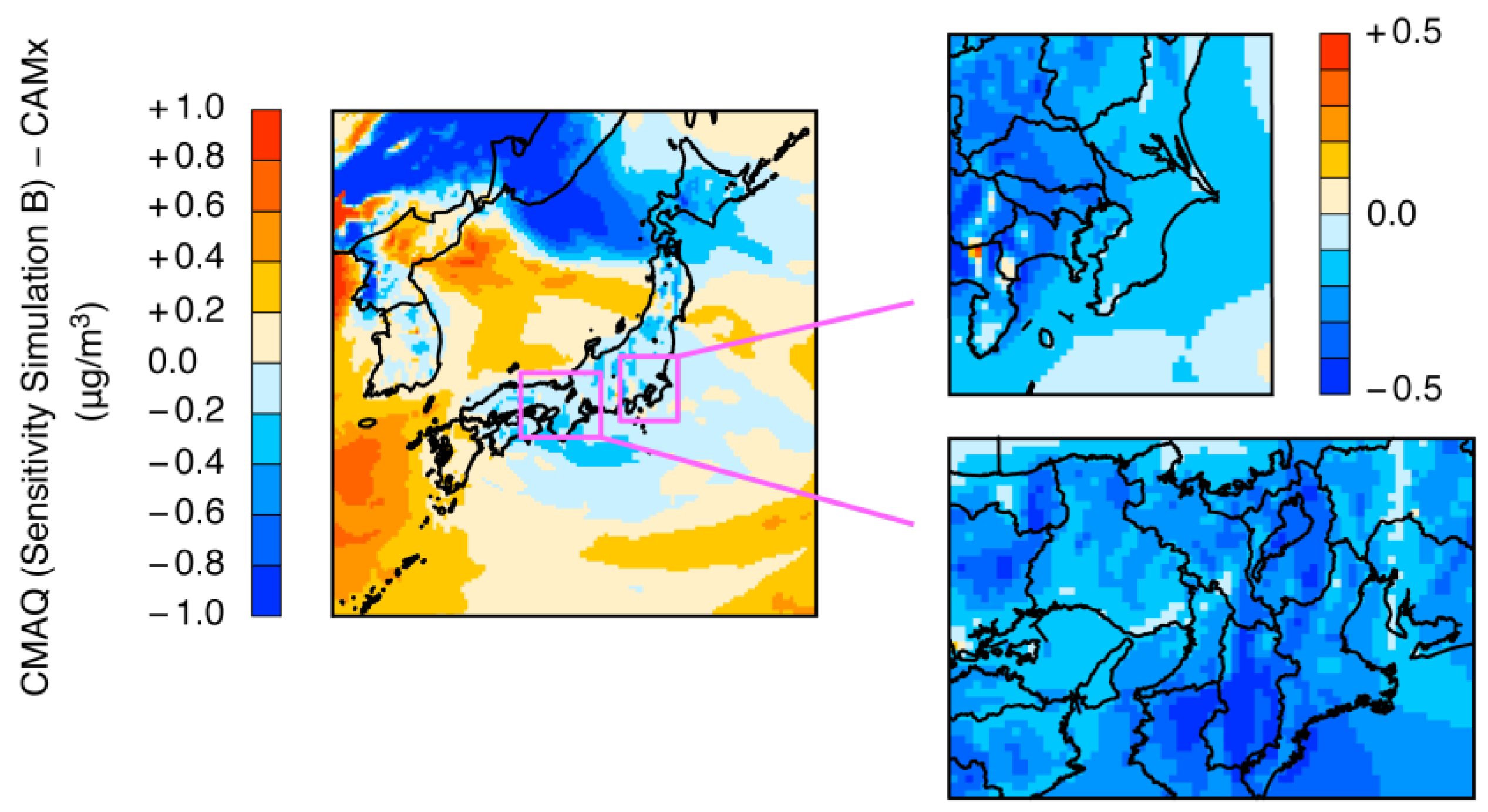
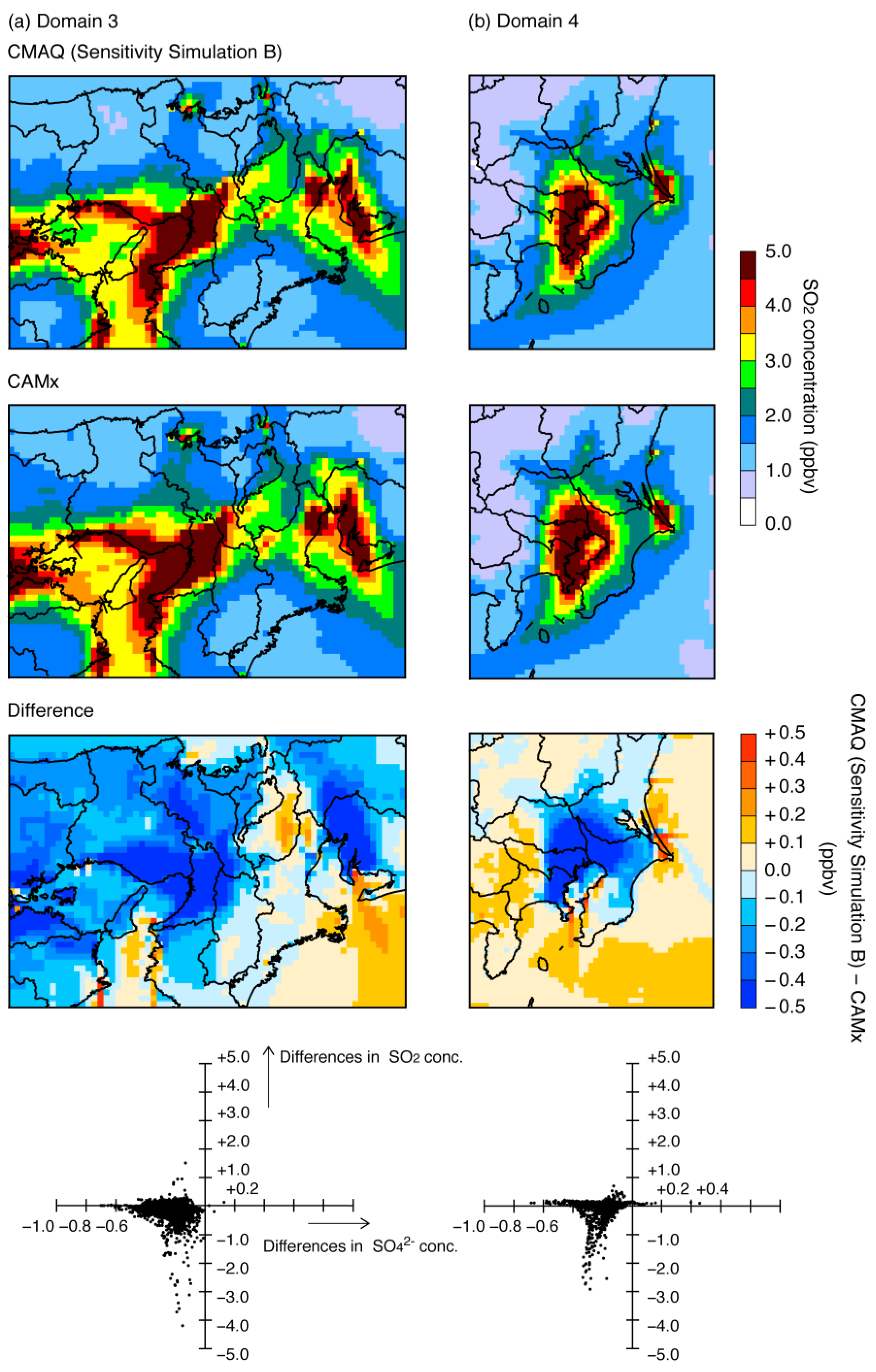
3.2. Differences between CMAQ and CAMx
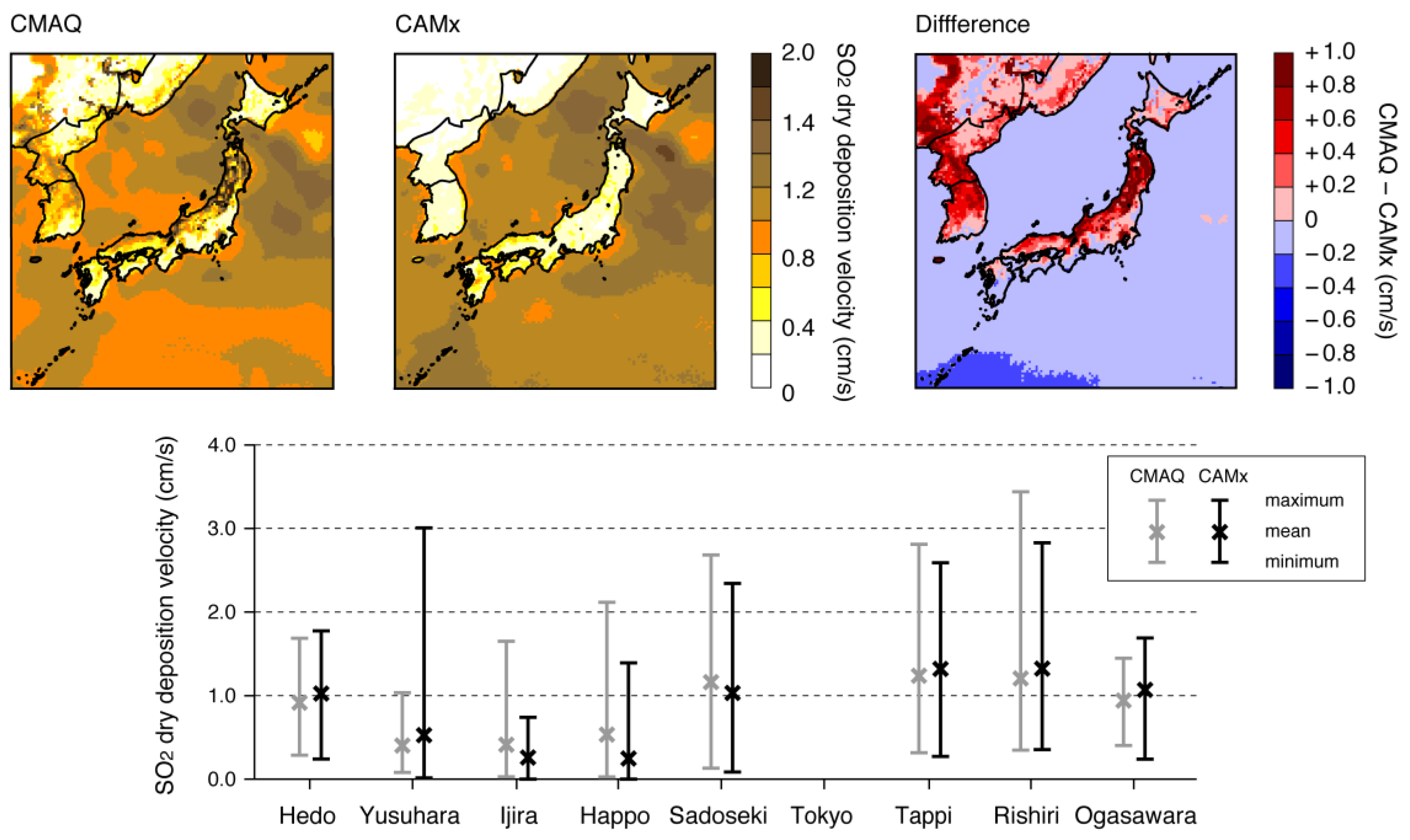
3.2.1. Dry Deposition of SO2
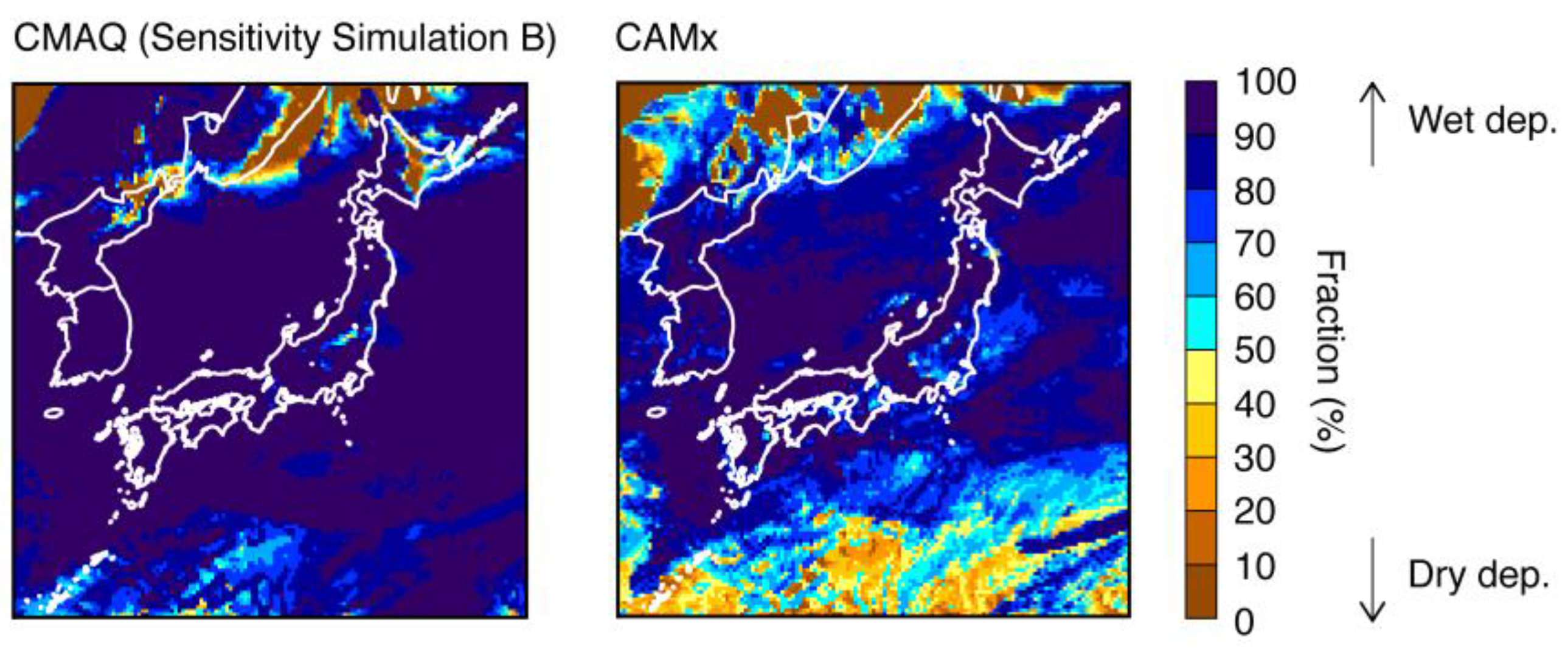
3.2.2. Wet Deposition of SO42−
4. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chatani, S.; Yamaji, K.; Sakurai, T.; Itahashi, S.; Shimadera, H.; Kitayama, K.; Hayami, H. Overview if model inter-comparison in Japan’s study for reference air quality modeling (J-STREAM). Atmosphere 2018, 9, 19. [Google Scholar] [CrossRef]
- Chatani, S.; Okumura, M.; Shimadera, H.; Yamaji, K.; Kitayama, K.; Matsunaga, S. Effects of a detailed vegetation database on simulated meteorological fields, biogenic VOC emissions, and ambient pollutant concentrations over Japan. Atmosphere 2018, 9, 179. [Google Scholar] [CrossRef]
- Itahashi, S.; Yamaji, K.; Chatani, S.; Hayami, H. Refinement of modeled aqueous-phase sulfate production via the Fe- and Mn-catalyzed oxidation pathway. Atmosphere 2018, 9, 132. [Google Scholar] [CrossRef]
- Fu, X.; Wang, S.; Zhao, B.; Xing, J.; Cheng, Z.; Liu, H.; Hao, J. Emission inventory of primary pollutants and chemical speciation in 2010 for the Yangtze River Delta region, China. Atmos. Environ. 2013, 70, 39–50. [Google Scholar] [CrossRef]
- US EPA Office of Research and Development. Community Multiscale Air Quality (CMAQ) Model Version 5.0.2; US EPA Office of Research and Development: Washington, DC, USA, 2014. [CrossRef]
- CMAQ v5.0 Sulfur Chemistry. Available online: Https://www.airqualitymodeling.org/index.php/CMAQv5.0_Sulfur_Chemistry (accessed on 1 August 2018).
- Cheng, Y.; Zheng, G.; Wei, C.; Mu, Q.; Zheng, B.; Wang, Z.; Gao, M.; Zhang, Q.; He, K.; Carmichael, G.; et al. Reactive nitrogen chemistry in aerosol water as a source of sulfate during haze events in China. Sci. Adv. 2016, 2, e1601530. [Google Scholar] [CrossRef]
- Song, S.; Gao, M.; Xu, W.; Shao, J.; Shi, G.; Wang, S.; Wang, Y.; Sun, Y.; McElroy, M.B. Fine-particle pH for Beijing winter haze as inferred from different thermodynamic equilibrium models. Atmos. Chem. Phys. 2018, 18, 7423–7438. [Google Scholar] [CrossRef]
- Lee, Y.N.; Schwartz, S.E. Precipitation Scavenging, Dry Deposition and Resuspension; Elsevier: New York, NY, USA, 1983; pp. 453–470. [Google Scholar]
- Clifton, C.L.; Altstein, N.; Huie, R.E. Rate constant for the reaction of nitrogen dioxide with sulfur(IV) over the pH range 5.3-13. Environ. Sci. Technol. 1998, 22, 586–589. [Google Scholar] [CrossRef]
- CAMx Overview. Available online: http://www.camx.com/about/default.aspx (accessed on 1 August 2018).
- Itahashi, S.; Uno, I.; Yumimoto, K.; Irie, H.; Osada, K.; Ogata, K.; Fukushima, H.; Wang, Z.; Ohara, T. Interannual variation in the fine-mode MODIS aerosol optical depth and its relationship to the changes in sulfur dioxide emissions in China between 2000 and 2010. Atmos. Chem. Phys. 2012, 12, 2631–2640. [Google Scholar] [CrossRef] [Green Version]
- Itahashi, S.; Uno, I.; Kim, S.-T. Source contributions of sulfate aerosol over East Asia estimated by CMAQ-DDM. Environ. Sci. Technol. 2012, 46, 6733–6741. [Google Scholar] [CrossRef]
- Itahashi, S.; Hayami, H.; Yumimoto, K.; Uno, I. Chinese province-scale source apportionments for sulfate aerosol in 2005 evaluated by the tagged tracer method. Environ. Pollut. 2017, 220, 1366–1375. [Google Scholar] [CrossRef]
- Itahashi, S.; Hatakeyama, S.; Shimada, K.; Tatsuta, S.; Taniguchi, Y.; Chan, C.K.; Kim, Y.-P.; Lin, N.-H.; Takami, A. Model estimation of sulfate aerosol source collected at Cape Hedo during an intensive campaign in October–November, 2015. Aerosol. Air Qual. Res. 2017, 17, 3079–3090. [Google Scholar] [CrossRef]
- Itahashi, S. Toward synchronous evaluation of source apportionments for atmospheric concentration and deposition of sulfate aerosol over East Asia. J. Geophys. Res. 2018, 123, 2927–2953. [Google Scholar] [CrossRef]
- Hong, Y.; Li, C.; Li, X.; Ma, Y.; Zhang, Y.; Zhou, D.; Wang, Y.; Liu, N.; Chang, X. Analysis of compositional variation and source characteristics of water-soluble ions in PM2.5 during several winter-haze pollution episodes in Shenyang, China. Atmosphere 2018, 9, 280. [Google Scholar] [CrossRef]
- Tesche, T.W.; Morris, R.; Tonnesen, G.; McNally, D.; Boylan, J.; Brewer, P. CMAQ/CAMx annual 2002 performance evaluation over the eastern US. Atmos. Environ. 2006, 40, 4906–4919. [Google Scholar] [CrossRef]
- Zhang, Y.; Olsen, K.M.; Wang, K. Fine-scale modeling of agricultural air quality over the Southeastern United States using two air quality models. Part I. Application and Evaluation. Aerosol. Air Qual. Res. 2013, 13, 1231–1252. [Google Scholar] [CrossRef]
- EANET. Technical Manual for Air Concentration Monitoring in East Asia. 2013. Available online: http://www.eanet.asia/product/manual/techacm.pdf (accessed on 3 September 2018).
- Boylan, J.W.; Russell, A.G. PM and light extinction model performance metrics, goals, and criteria for three-dimensional air quality models. Atmos. Environ. 2006, 40, 4946–4959. [Google Scholar] [CrossRef]
- Itahashi, S.; Uno, I.; Osada, K.; Kamiguchi, Y.; Yamamoto, S.; Tamura, K.; Wang, Z.; Kurosaki, Y.; Kanaya, Y. Nitrate transboundary heavy pollution over East Asia in winter. Atmos. Chem. Phys. 2017, 17, 3823–3843. [Google Scholar] [CrossRef] [Green Version]
- Yamaji, K.; (Kobe University, Kobe, Hyogo Prefecture, Japan). Personal communication, 2018.
- Pleim, J.E.; Xiu, A.; Finkelstein, P.L.; Otte, T.L. A coupled land-surface and dry deposition model and comparison to field measurements of surface heat, moisture, and ozone fluxes. Water Air Soil Pollut. 2001, 1, 243–252. [Google Scholar] [CrossRef]
- Zhang, L.; Brook, J.R.; Vet, R. A revised parameterization for gaseous dry deposition in air-quality models. Atmos. Chem. Phys. 2003, 3, 2067–2082. [Google Scholar] [CrossRef] [Green Version]
- Endo, T.; Yagoh, H.; Sato, K.; Matsuda, K.; Hayashi, K.; Noguchi, I.; Sawada, K. Regional characteristics of dry deposition of sulfur and nitrogen compounds at EANET sites in Japan from 2003 to 2008. Atmos. Environ. 2011, 45, 1259–1267. [Google Scholar] [CrossRef]
- EANET. Technical Manual for Wet Deposition Monitoring in East Asia. Available online: http://www.eanet.asia/product/manual/techwet.pdf (accessed on 3 September 2018).
- Appel, K.W.; Foley, K.M.; Bash, J.O.; Pinder, R.W.; Dennis, R.L.; Allen, D.J.; Pickering, K. A multi-resolution assessment of the community multiscale air quality (CMAQ) model v4.7 wet deposition estimates for 2002–2006. Geosci. Model Dev. 2011, 4, 357–371. [Google Scholar] [CrossRef]
- Ge, B.Z.; Wang, Z.F.; Xu, X.B.; Wu, J.B.; Yu, X.L.; Li, J. Wet deposition of acidifying substances in different regions of China and the rest of East Asia: Modeling with updated NAQPMS. Environ. Pollut. 2014, 187, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Vivanco, M.G.; Theobald, M.R.; Garcia-Gomez, H.; Garrido, J.L.; Prank, M.; Aas, W.; Adani, M.; Alyuz, U.; Andersson, C.; Bellasio, R.; et al. Model deposition of nitrogen and sulfur in Europe estimated by 14 air quality model systems: Evaluation, effect of changes in emissions and implications for habitat protection. Atmos. Chem. Phys. 2018, 18, 10199–10218. [Google Scholar] [CrossRef] [PubMed]
- Solazzo, E.; Hogrefe, C.; Colette, A.; Garcia-Vivanco, M.; Galmarini, S. Advanced error diagnostics of the CMAQ and Chimere modeling systems within the AQMEII3 model evaluation framework. Atmos. Chem. Phys. 2017, 17, 10435–10465. [Google Scholar] [CrossRef] [PubMed]
- Fahey, K.M.; Carlton, A.G.; Pye, H.O.T.; Baek, J.; Hutzell, W.T.; Stainier, C.O.; Baker, K.R.; Appel, K.W.; Jaoui, M.; Offenberg, J.H. A framework for expanding aqueous chemistry in the Community Multiscale Air Quality (CMAQ) model version 5.1. Geosci. Model Dev. 2017, 10, 1587–1605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Appel, K.W.; Napelenok, S.L.; Foley, K.M.; Pye, H.O.T.; Hogrefe, C.; Luecken, D.J.; Bash, J.O.; Roselle, S.J.; Pleim, J.E.; Foroutan, H.; et al. Description and evaluation of the Community Multiscale Air Quality (CMAQ) modeling version 5.1. Geosci. Model Dev. 2017, 10, 1073–1732. [Google Scholar] [CrossRef]
- Li, M.; Liu, H.; Geng, G.; Hong, C.; Liu, F.; Song, Y.; Tong, D.; Zheng, B.; Cui, H.; Man, H.; et al. Anthropogenic emission inventories in China: A review. Natl. Sci. Rev. 2017, 4, 834–866. [Google Scholar] [CrossRef]
- Krotkov, N.A.; McLinden, C.A.; Li, C.; Lamsal, L.N.; Celarier, E.A.; Marchenko, S.V.; Swartz, W.H.; Bucsela, E.J.; Joiner, J.; Duncan, B.N.; et al. Aura OMI observations of regional SO2 and NO2 pollution changes from 2005 to 2015. Atmos. Chem. Phys. 2016, 16, 4605–4629. [Google Scholar] [CrossRef]
- van der A, R.J.; Mijling, B.; Ding, J.; Koukouli, M.E.; Liu, F.; Li, Q.; Mao, H.; Theys, N. Cleaning up the air: Effectiveness of air quality policy for SO2 and NOx emissions in China. Atmos. Chem. Phys. 2017, 17, 1775–1789. [Google Scholar] [CrossRef]
- Itahashi, S.; Yumimoto, K.; Uno, I.; Hayami, H.; Fujita, S.; Pan, Y.; Wang, Y. A 15-year record (2001–2015) of the ratio of nitrate to non-sea-salt sulfate in precipitation over East Asia. Atmos. Chem. Phys. 2018, 18, 2835–2852. [Google Scholar] [CrossRef] [Green Version]
- Zheng, B.; Zhang, Q.; Zhang, Y.; He, K.B.; Wang, K.; Zheng, G.J.; Duan, F.K.; Ma, Y.L.; Kimoto, T. Heterogeneous chemistry: A mechanism missing in current models to explain secondary inorganic aerosol formation during January 2013 haze episode in North China. Atmos. Chem. Phys. 2015, 15, 2031–2049. [Google Scholar] [CrossRef]
- Li, G.; Bei, N.; Cao, J.; Huang, R.; Wu, J.; Feng, T.; Wang, Y.; Liu, S.; Quang, Z.; Tie, X.; et al. A possible pathway for rapid growth of sulfate during haze days in China. Atmos. Chem. Phys. 2017, 17, 3301–3316. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; Abbatt, J.P.D.; Chan, A.W.H. Novel pathway of SO2 oxidation in the atmosphere: Reactions with monoterpene ozonolysis intermediates and secondary organic aerosol. Atmos. Chem. Phys. 2018, 18, 5549–5565. [Google Scholar] [CrossRef]
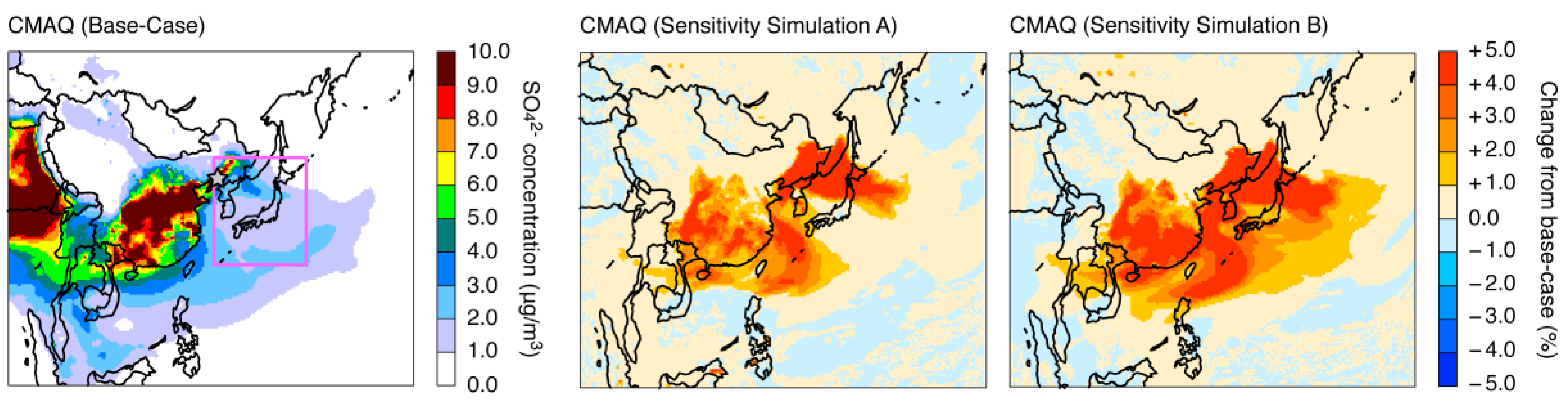
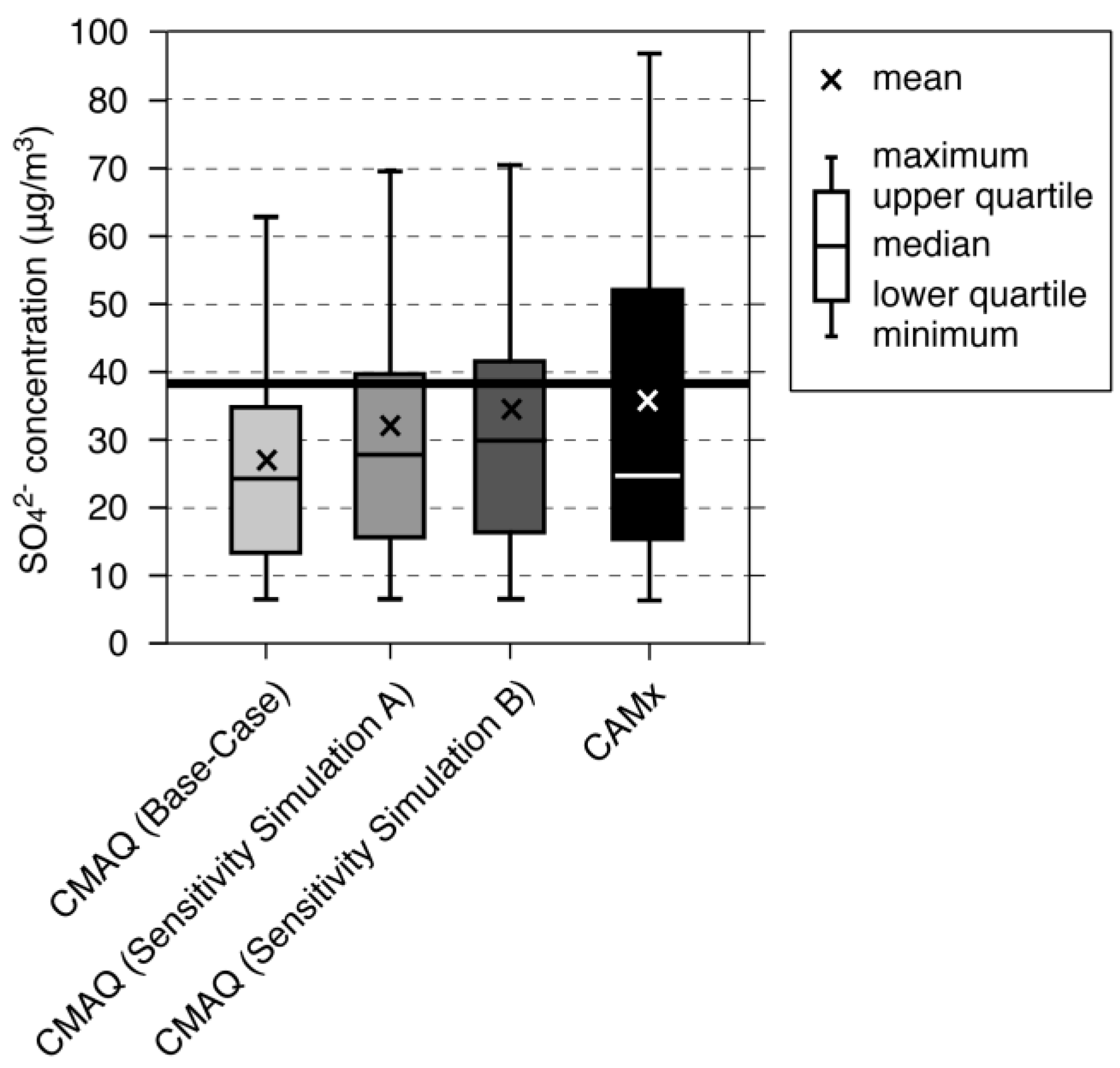
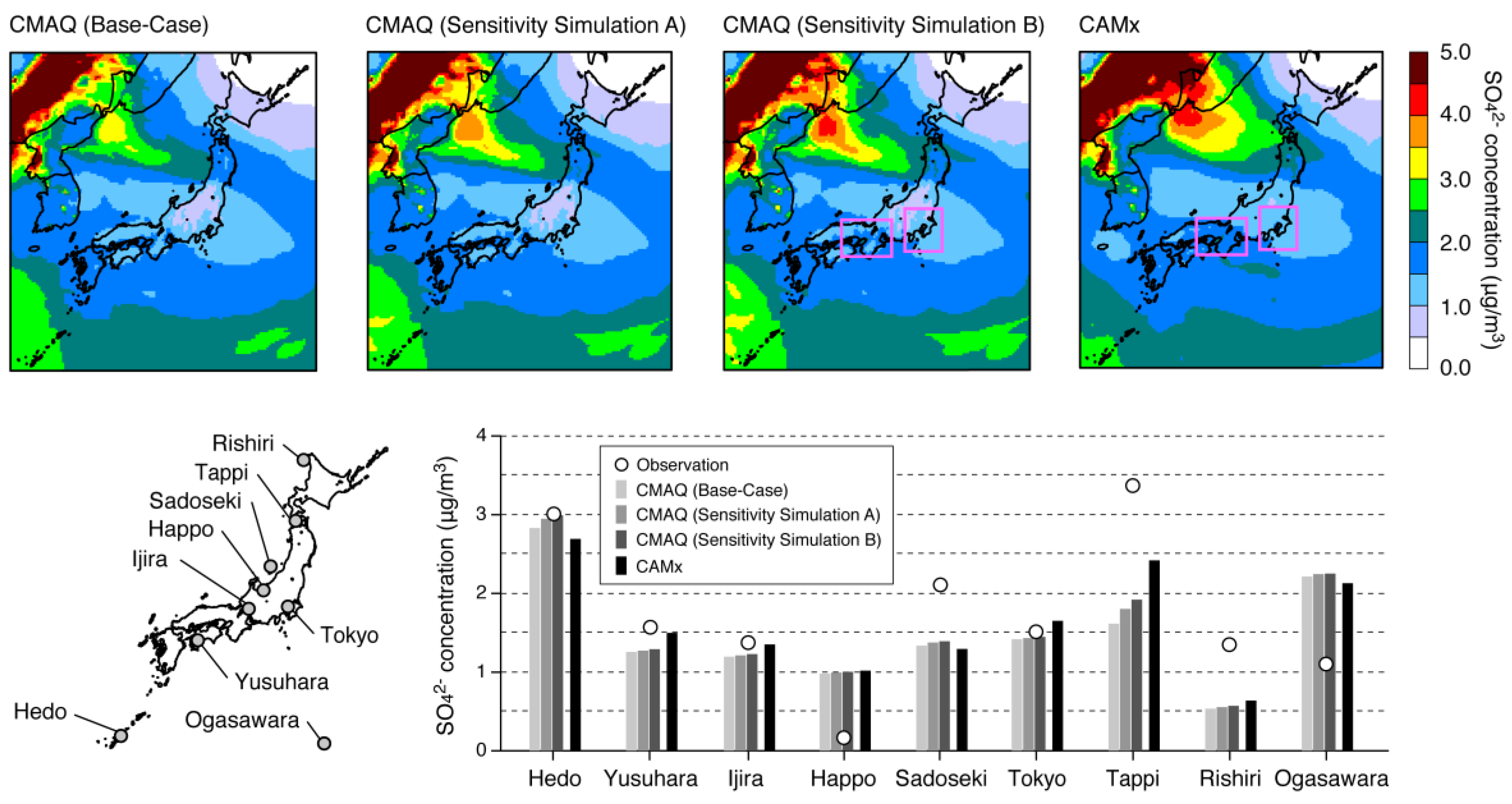
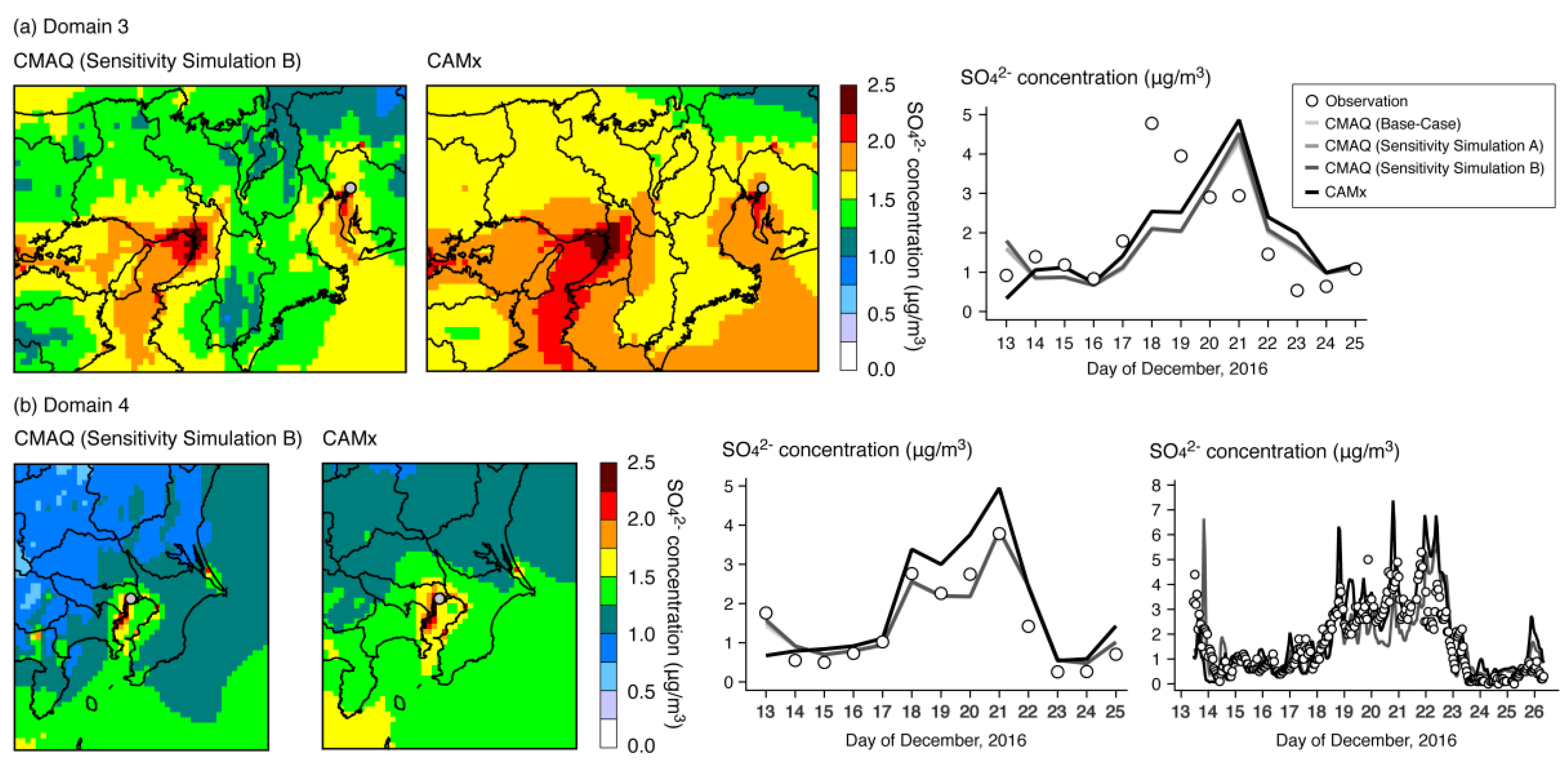

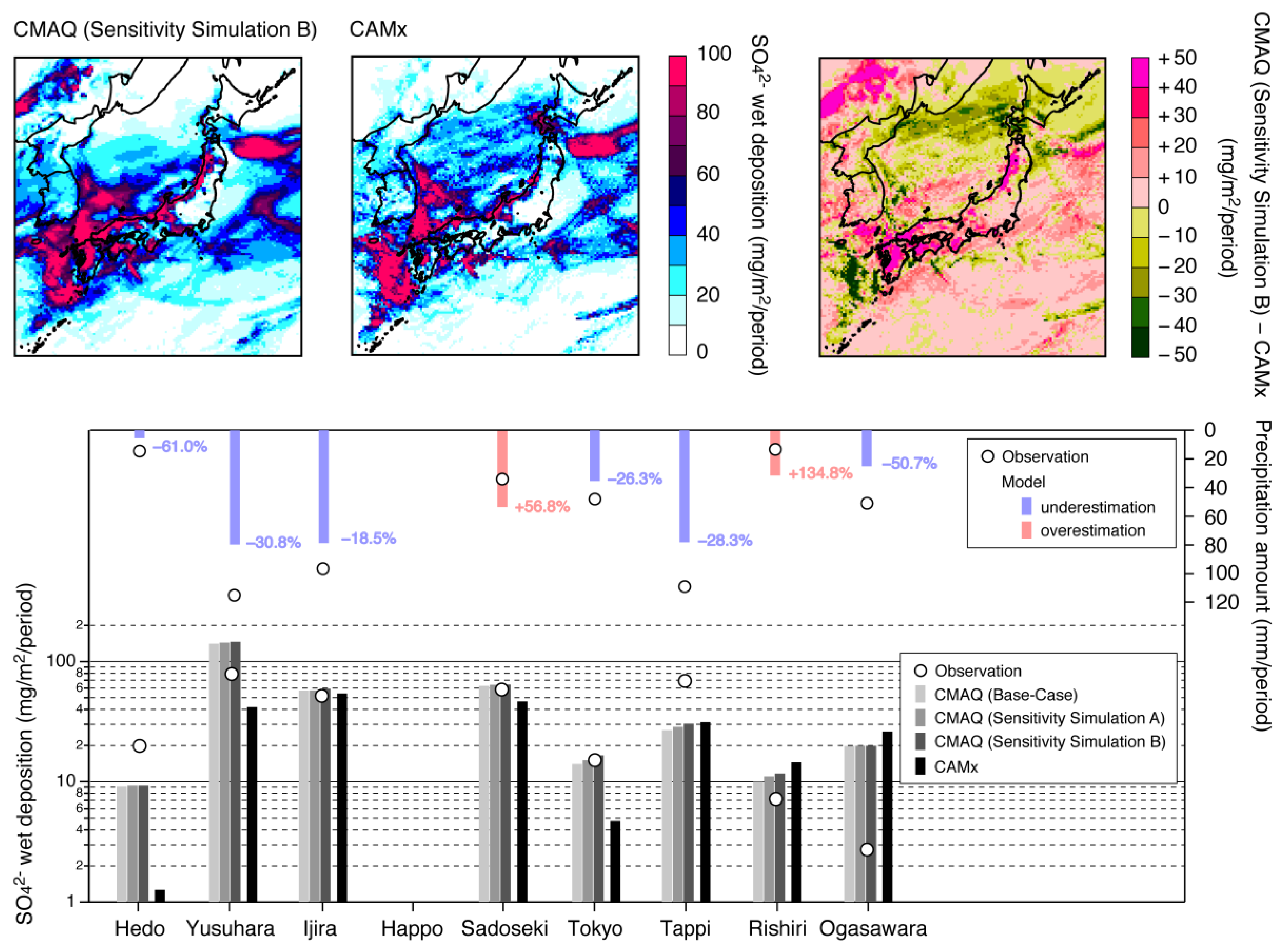
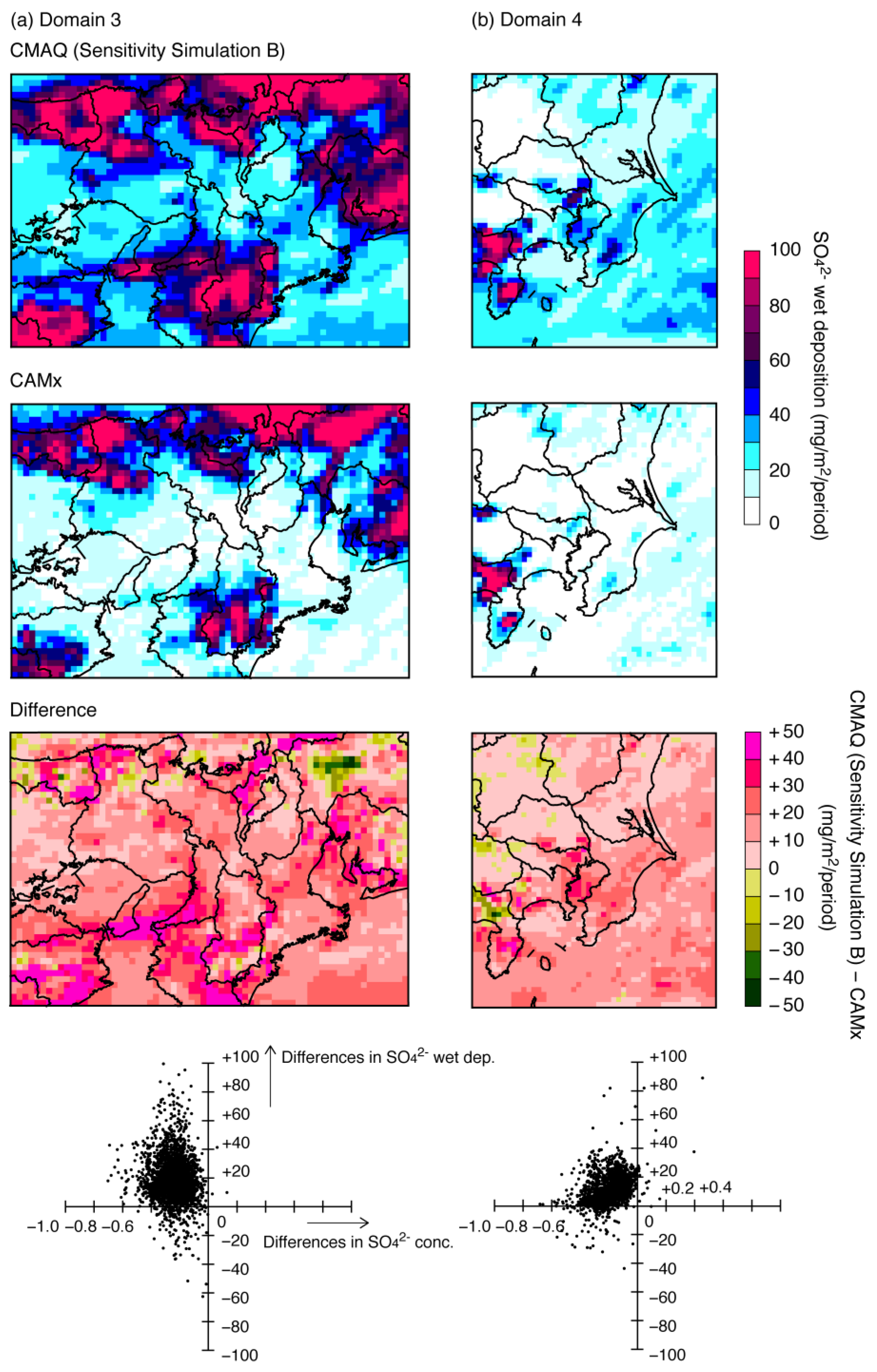
| Name | Description |
|---|---|
| Sensitivity simulation A | Fe and Mn solubilities are increased, and the rate constant expression of Fe- and Mn-catalyzed oxidation by O2 includes pH dependency. |
| Sensitivity simulation B | Same as sensitivity simulation A, but including the aqueous-phase reaction with NO2 (a total of six aqueous-phase reactions were treated). |
| CMAQ Base-Case Simulation | CMAQ Sensitivity Simulation A | CMAQ Sensitivity Simulation B | CAMx | |
|---|---|---|---|---|
| N | 9 | |||
| Mean (observations) [μg/m3] | 1.73 | |||
| Mean (model) [μg/m3] | 1.49 | 1.54 | 1.57 | 1.63 |
| R | 0.51 (p > 0.1) | 0.57 (p > 0.1) | 0.59 (p < 0.1) | 0.70 (p < 0.05) |
| MFB [%] | −4.6 | −1.8 | −0.1 | +5.1 |
| MFE [%] | 51.0 | 48.6 | 47.1 | 42.9 |
| CMAQ Base-Case Simulation | CMAQ Sensitivity Simulation A | CMAQ Sensitivity Simulation B | CAMx | ||
|---|---|---|---|---|---|
| Nagoya | N | 13 | |||
| Mean (observations) [μg/m3] | 1.88 | ||||
| Mean (model) [μg/m3] | 1.71 | 1.73 | 1.77 | 1.91 | |
| R | 0.58 (p < 0.05) | 0.57 (p < 0.05) | 0.55 (p < 0.05) | 0.64 (p < 0.05) | |
| MFB [%] | −2.1 | −0.9 | 0.9 | 1.5 | |
| MFE [%] | 43.7 | 44.0 | 45.1 | 42.9 | |
| Tokyo | N | 13 | |||
| Mean (observation) [ng/m3] | 1.44 | ||||
| Mean (model) [ng/m3] | 1.53 | 1.55 | 1.56 | 1.88 | |
| R | 0.94 (p < 0.001) | 0.94 (p < 0.001) | 0.94 (p < 0.001) | 0.93 (p < 0.001) | |
| MFB [%] | 18.5 | 19.4 | 20.3 | 30.9 | |
| MFE [%] | 28.8 | 28.3 | 28.3 | 44.6 | |
| Mukoujima | N | 292 | |||
| Mean (observation) [ng/m3] | 1.64 | ||||
| Mean (model) [ng/m3] | 1.54 | 1.55 | 1.56 | 1.88 | |
| R | 0.71 (p < 0.001) | 0.71 (p < 0.001) | 0.71 (p < 0.001) | 0.77 (p < 0.001) | |
| MFB [%] | 6.3 | 7.0 | 7.9 | 15.6 | |
| MFE [%] | 49.5 | 49.4 | 49.0 | 54.9 | |
| CMAQ Base-Case Simulation | CMAQ Sensitivity Simulation A | CMAQ Sensitivity Simulation B | CAMx | |
|---|---|---|---|---|
| N | 8 | |||
| Mean (observations) [mg/m2/period] | 37.83 | |||
| Mean (model) [mg/m2/period] | 42.59 | 43.64 | 44.70 | 27.55 |
| R | 0.77 (p < 0.05) | 0.77 (p < 0.05) | 0.77 (p < 0.05) | 0.71 (p < 0.05) |
| MFB [%] | 11.1 | 14.5 | 17.7 | −25.7 |
| MFE [%] | 53.4 | 53.4 | 55.3 | 84.2 |
| Mean (model) [mg/m2/period] | 54.76 | 56.04 | 57.37 | 33.63 |
| R | 0.67 (p > 0.05) | 0.68 (p > 0.05) | 0.68 (p > 0.05) | 0.57 (p > 0.05) |
| MFB [%] | 23.1 | 26.6 | 29.8 | −21.4 |
| MFE [%] | 60.1 | 59.3 | 59.5 | 72.6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Itahashi, S.; Yamaji, K.; Chatani, S.; Hisatsune, K.; Saito, S.; Hayami, H. Model Performance Differences in Sulfate Aerosol in Winter over Japan Based on Regional Chemical Transport Models of CMAQ and CAMx. Atmosphere 2018, 9, 488. https://doi.org/10.3390/atmos9120488
Itahashi S, Yamaji K, Chatani S, Hisatsune K, Saito S, Hayami H. Model Performance Differences in Sulfate Aerosol in Winter over Japan Based on Regional Chemical Transport Models of CMAQ and CAMx. Atmosphere. 2018; 9(12):488. https://doi.org/10.3390/atmos9120488
Chicago/Turabian StyleItahashi, Syuichi, Kazuyo Yamaji, Satoru Chatani, Kunihiro Hisatsune, Shinji Saito, and Hiroshi Hayami. 2018. "Model Performance Differences in Sulfate Aerosol in Winter over Japan Based on Regional Chemical Transport Models of CMAQ and CAMx" Atmosphere 9, no. 12: 488. https://doi.org/10.3390/atmos9120488





